Abstract
Increasing evidence suggests that the growth/differentiation factors, GDFs 5, 6, and 7 in particular, may play a role in tendon and ligament biology. Mice with genetic mutations in Gdf5 have altered tendon composition and mechanical behavior, while animals with functional null mutations in Gdf7 have a more subtle tendon phenotype. The present study demonstrates for the first time that a null mutation in Gdf6 is associated with substantially lower levels of tail tendon collagen content (−33%) in four-week-old male mice, which has direct functional consequences for the mechanical integrity of the tissue (45-50% reduction in material properties). These data support a role for GDF6 in tendon matrix modeling.
INTRODUCTION
Over the last decade, increasing evidence has suggested that the growth/differentiation factors (GDFs), a subfamily of the bone morphogenetic proteins (BMPs), may play a role in tendon and ligament biology (Wolfman et al., 1997; Clark et al., 2001; Mikic et al., 2001; Helm et al., 2001; Chhabra et al., 2003; Fu et al., 2003; Chuen et al., 2004; Mikic, 2004; Mikic et al., 2006; Mikic et al., 2008). In particular, the discovery by Wolfman and colleagues that ectopically implanted GDF 5, 6, and 7 are capable of inducing tendon and ligament-like tissue in a rodent model opened the door to considering the use of these compounds to enhance tendon and ligament repair in a clinical setting (Wolfman et al., 1997). While it is clear that GDFs 5, 6, and 7 are capable of augmenting tendon/ligament repair in a variety of different animal and injury models using a range of different delivery mechanisms (Aspenberg and Forslund, 1999; Forslund and Aspenberg, 2001; Lou et al., 2001; Forslund and Aspenberg, 2002; Forslund and Aspenberg, 2003; Forslund et al., 2003; Rickert et al., 2005; Virchenko et al., 2005; Tashiro et al., 2006; Eliasson et al., 2008), surprisingly little is known about how these molecules function in vivo. To this end, three naturally occurring and/or genetically engineered mouse models have provided a unique opportunity to examine tendon phenotype and repair in the absence of GDF5, 6, and 7.
The best-characterized of these models to date is the GDF-5 deficient brachypodism mouse (see Mikic, 2004 for review). Eight week old male mice deficient in GDF5 have Achilles tendons with significantly less collagen (per DNA), are weaker and more compliant than control tendons, and have a propensity for more frequent mid-substance (rather than bony avulsion) failures of the Achilles tendon (Mikic et al., 2001). While tail tendons from animals of the same age exhibit no differences in material properties, glycosaminoglycan (GAG/DNA) or collagen content, the collagen fibrils themselves are somewhat irregularly shaped, and time-dependent stress-relaxation tests demonstrate that GDF5 deficient tail tendon fascicles relax more slowly and to a lesser extent than control fascicles (Clark et al., 2001). Additionally, after being subjected to full trans-section of the Achilles tendon, GDF5 −/− mice exhibit a one to two week delay in healing (Chhabra et al., 2003).
Young adult (16 week old) GDF-7 deficient male and female Achilles tendons exhibit a much more subtle phenotype, with a small, but statistically significant reduction in GAG/DNA, and a shift towards slightly smaller collagen fibrils, although these compositional and ultrastructural differences are not large enough to affect the material behavior of the tendons (Mikic et al., 2006). Tail tendons from animals of the same age showed no significant effect of GDF7 deficiency on any compositional parameter nor on any material property examined (Mikic et al., 2008). There is some evidence to suggest that the mild tendon phenotype in GDF7 −/− animals may be associated with overcompensation by the related family member, GDF5 (Mikic et al., 2006, 2008).
While a GDF6 deficient mouse line does exist (Settle et al., 2003), no characterization of its tendon phenotype has yet been published, in part because of the challenging nature of this animal model. Homozygous mutants are present in Mendelian ratios prenatally, but there is a dramatic increase in mortality postnatally, as the number of GDF6 −/− mice at the time of weaning is significantly less than 25% (Settle et al., 2003). In our own colony of mice from this same line, 4% of 4-week-old males are homozygous mutants, while less than 2% of females are GDF6 −/− animals (data from over 400 animals). Clearly, the mortality issues associated with this strain make it a challenge to produce adequate numbers for in-depth analysis, particularly in adult animals. Thus, the goal of the current study is to characterize tendon phenotype to the greatest extent possible in this, the only available GDF6 mouse line to date, before attempting to outcross onto a more robust background strain that might enable more comprehensive characterization of both intact and healing tendon in the absence of GDF6.
Although tissues and whole organs are not universally regarded as mechanical structures, the load bearing function of the tissues of the musculoskeletal system qualify them as such. In particular, the structural role of tendons is to transmit muscle forces to bone, thereby enabling movement of the skeletal elements of the vertebrate body plan. The structural strength (load to failure) and structural stiffness of a tendon are a function of its size (i.e. its cross-sectional area), as well as the intrinsic material properties of the tissue. These material properties are, in turn, a function of the composition of the tissue and the ultrastructural organization of its constituents: importantly, material properties are independent of tissue size. In the present study, we seek to establish whether GDF6 deficiency in mice produces a tendon phenotype by quantifying tail tendon composition and material properties, obtained via functional mechanical testing.
The experimental animals used for this study consisted of 4-week-old male mice deficient in GDF6 (−/−) and their wild type (+/+) littermates. Breeder pairs were obtained from a line of Gdf6 mutant mice generated in the Kingsley lab by intercrossing heterozygous carriers of the targeted Gdf6 mutation on a 129SV/J × C57BL/6J mixed background (Settle et al., 2003). Mutants had been generated by replacing a 1.74-kb fragment containing the entire mature signaling region of the Gdf6 gene with a positive selectable neomycin resistance cassette. A sample size of eight mice per group was used to assess tendon phenotype. Animals were sacrificed via CO2 inhalation in accordance with Institutional Animal Care and Use Guidelines, and animal body mass was immediately recorded.
The tail of a mouse contains four bundles of tendon fascicles that run along its length from base to tip. Immediately after sacrifice, each tail was cut at the base. One randomly chosen fascicle bundle was removed from each tail under sterile conditions, digested in 500μl of sterile papain solution for 18 hours at 60°C, and analyzed for DNA content, glycosaminoglycan (GAG) content, and hydroxyproline (OHP) content, indicative of total collagen, as described elsewhere in detail (Mikic et al., 2008). GAG and OHP values were normalized to DNA content to account for any differences in tissue volume. Both Achilles tendons from each animal were also processed for compositional analysis. Each tail, with the remaining three fascicle bundles intact, was immediately wrapped in saline-soaked gauze and frozen in a sealed bag at −20°C until the time of mechanical testing.
Detailed mechanical testing protocols are described elsewhere (Mikic et al., 2008). In brief, the three remaining fascicle bundles were removed from each tail and ten individual fascicles were then randomly selected, alternating between the three bundles, and diameters measured in triplicate while maintaining fascicle hydration with PBS throughout. Each fascicle was taped to a card frame to ensure a 20 mm gage length and placed between grips in a saline filled testing chamber attached to an Instron 5542 axial load frame (Instron Corp., Canton, MA). The card frame was cut immediately before loading began at one of two strain rates: 0.5%/sec (slow) or 50%/sec (fast). Five of the randomly chosen fascicles per tail were tested at each strain rate, and data from each of the five tests were averaged together to provide representative material properties at each strain rate for each mouse. Load and displacement data were converted to stress and strain by normalizing to fascicle cross sectional area (assuming a circular cross section) and initial gage length (20 mm), respectively. The following dependent parameters were calculated: (1) Ultimate Strength; (2) Yield Strength; (3) Yield Strain; (4) Ultimate Strain; (5) Post-yield Strain; (6) Modulus of Elasticity; and (7) Strain Energy Density. All dependent parameters were analyzed statistically with Statview software (SAS Institute Inc., Cary, NJ) using a one-factor ANOVA, with genotype as the independent variable and a cutoff value of p < 0.05 used for statistical significance. The tail from one additional mouse per group was processed for routine histology on 15μm frozen sections stained with Hematoxylin & Eosin.
RESULTS
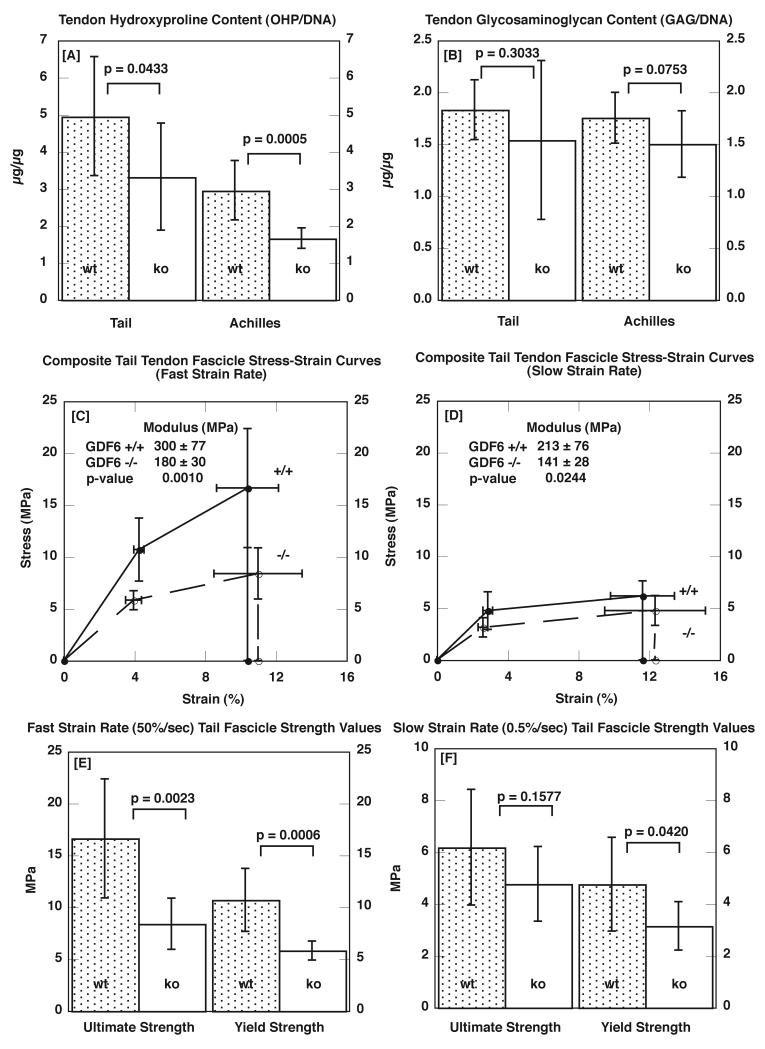
GDF6 deficient mice were 38% smaller than wild type animals (9.3 ± 1.9 grams Body Mass vs. 15.1 ± 1.3 grams, respectively; p < 0.0001). Although −/− tail tendon fascicle cross-sectional area was slightly smaller than that of wild types (−14%), this difference was not statistically significant. Tail tendons from GDF6 −/− animals had approximately 33% less total collagen, as indicated by hydroxyproline-per-DNA (Figure 1 A). While GAG/DNA content was 16% lower in GDF6 −/− tail tendons, this difference was not statistically significant (Figure 1 B). GDF6 deficiency had a similar effect on Achilles tendon composition, with −/− tendons exhibiting 44% less total collagen (Figure 1 A) and 15% less GAG/DNA (Figure 1 B).
Figure 1.
[A] Tendon hydroxyproline content (OHP/DNA), indicative of total collagen content, and [B] glycosaminoglycan content (GAG/DNA) for tail and Achilles tendons from four-week-old GDF 6 +/+ (wt) and −/− (ko) mice (n = 8 per group). Composite tail tendon fascicle stress-strain curves from [C] fast (50%/sec) and [D] slow (0.5%/sec) strain rate tensile tests to failure. GDF6 −/− data are shown with dashed lines, and +/+ data are shown with solid lines. Note that these figures are constructed using mean values of the yield stress and strain and ultimate stress and strain and are not meant to depict the exact shape of actual stress-strain failure curves for any single animal. Ultimate and yield strength values of GDF6 +/+ (wt) and −/− (ko) tail tendon fascicles tested at [E] fast and [F] slow strain rates. All data are shown as mean ± SD.
The significant reduction in collagen content observed with GDF6 deficiency was also associated with a significant reduction in tail tendon material properties at both fast and slow strain rates (Figure 1 C-F). At the fast strain rate, both the yield and ultimate tensile strengths of tail tendon fascicles were 45-50% lower in GDF6 −/− animals (Figure 1 E). In addition, the modulus of elasticity (a measure of material stiffness) was 40% lower than that of +/+ tail tendon fascicles, and the energy absorbed per unit volume (that is, Strain Energy Density), was 40% lower (p = 0.05). At the slower strain rate, material property values were 23-34% lower in −/− tendons, although the difference in ultimate strength was not statistically significant (Figure 1 F). At neither strain rate was a significant difference detected between genotypes in Yield Strain, Failure Strain, or Post-Yield Strain (data not shown). While insufficient tissue was available for mechanical testing of Achilles tendons, the comparable effect of GDF6 deficiency on tendon composition in tail and Achilles tendons suggests that the material properties of GDF6 −/− Achilles tendons would most likely be similarly compromised.
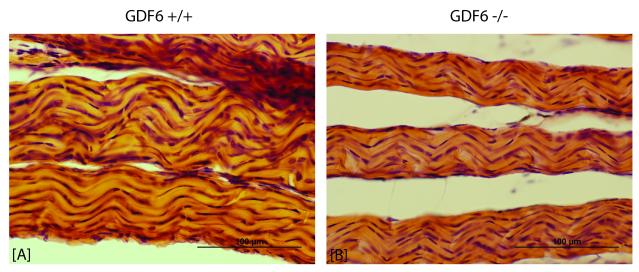
Histological comparison of tail tendon fascicles for one representative −/− and +/+ mouse per group confirmed a qualitatively smaller fascicle size in GDF6 deficient tail tendons (Figure 2). At the sub-fascicle level, −/− fibers appeared qualitatively smaller as well, with a more angular crimp compared to that of the +/+ fibers.
Figure 2.
Tendon fascicles from [A] GDF6 +/+ and [B] −/− tails (Hematoxylin & Eosin staining of 15μm frozen sections). Each image depicts three fascicles (the structural unit that was tested mechanically). At the sub-fascicle level, fibers can be seen with fibroblasts aligned along the fiber length. GDF6 deficient fascicles and fibers appear qualitatively smaller, with a more angular crimp compared to wild type.
DISCUSSION
This study demonstrates for the first time that GDF6 deficiency in four-week-old male mice results in a significant tendon phenotype, with −/− tendons containing substantially less collagen (per DNA) than wild type tendons. This reduction in collagen is associated with significantly compromised tendon material properties, as would be expected given that type I collagen is the primary tensile load bearing protein in tendon. Histologically, a qualitative difference in fiber size was noted between genotypes, which could potentially contribute to the observed differences in material properties (note that fascicle size differences were already accounted for in deriving material properties from the structural data obtained via fascicle tensile tests). Further, while a statistical analysis of fascicle cross-sectional area showed smaller fascicle size in −/− tail tendons (obtained under a dissecting microscope with an eye-piece micrometer prior to mechanical testing), these differences were not statistically significant. In sum, the data presented here support the hypothesis that the growth/differentiation factors play an import role in establishing normal tendon structure-function relationships, although the precise nature of this role remains to be determined.
GDF6 and its human homologue, BMP13, have been shown to be capable of inducing neotendon/ligament in rodent models when delivered as purified protein (Wolfman et al., 1997) or in adenoviral form (Helm et al., 2001). BMP13 has been immunohistochemically localized in adult human patellar tendon cells, specifically to ovoid tenoblasts rather than to the less metabolically active elongated interstitial tenocytes, and it is posited to play a role in matrix remodeling (Chuen et al., 2004). Further, recombinant human BMP13 has been shown to increase human tendon fibroblast proliferation in vitro, along with increasing the expression of procollagen types I and III (Fu et al., 2003). The reduced level of total collagen in tail and Achilles tendons seen in the present study in mice deficient in GDF6 is thus consistent with prior studies, and these data support a role for GDF6/BMP13 in the modeling of tendon extracellular matrix.
The present study has several limitations. First, the scope of this analysis is quite limited because of the challenges presented regarding postnatal mortality rates in this line of GDF6 −/− mice. Consequently, a limited number of (male only) animals were available for analysis, and no mechanical testing of the Achilles tendons was performed, nor was any tissue available for ultrastructural analysis of the collagen fibrils themselves. If the Gdf6 mutation can be successfully outcrossed onto a more robust background strain, a more complete analysis of intact tendon (tail and Achilles) at multiple ages (young and adult) in both males and females could be performed. Further, a more robust line would enable the examination of tendon healing processes in the absence of GDF6. Despite these limitations, however, the present study provides significant and conclusive evidence that GDF6 deficiency in young male mice is associated with a substantial reduction in tendon total collagen, which has direct functional consequences for the mechanical integrity of the tissue.
ACKNOWLEDGEMENTS
This publication was made possible by Grant Number AR049745 from the National Institutes of Health (NIH-NIAMS). We are greatly indebted to Dr. Douglas P. Mortlock of Vanderbilt University for generously providing the GDF6 breeder pairs and genotyping protocols.
LITERATURE CITED
- Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51–4. doi: 10.3109/17453679909000958. [DOI] [PubMed] [Google Scholar]
- Chhabra A, Tsou D, Clark RT, Gaschen V, Hunziker EB, Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826–35. doi: 10.1016/S0736-0266(03)00049-4. [DOI] [PubMed] [Google Scholar]
- Chuen FS, Chuk CY, Ping WY, Nar WW, Kim HL, Ming CK. Immunohistochemical characterization of cells in adult human patellar tendons. J Histochem Cytochem. 2004;52:1151–7. doi: 10.1369/jhc.3A6232.2004. [DOI] [PubMed] [Google Scholar]
- Clark RT, Johnson TL, Schalet BJ, Davis L, Gaschen V, Hunziker EB, Oldberg A, Mikic B. GDF-5 deficiency in mice leads to disruption of tail tendon form and function. Connect Tissue Res. 2001;42:175–86. doi: 10.3109/03008200109005648. [DOI] [PubMed] [Google Scholar]
- Eliasson P, Fahlgren A, Aspenberg P. Mechanical load and BMP signaling during tendon repair: a role for follistatin? Clin Orthop Relat Res. 2008;466:1592–7. doi: 10.1007/s11999-008-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund C, Aspenberg P. Tendon healing stimulated by injected CDMP-2. Med Sci Sports Exerc. 2001;33:685–7. doi: 10.1097/00005768-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Forslund C, Aspenberg P. CDMP-2 induces bone or tendon-like tissue depending on mechanical stimulation. J Orthop Res. 2002;20:1170–4. doi: 10.1016/S0736-0266(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Forslund C, Aspenberg P. Improved healing of transected rabbit Achilles tendon after a single injection of cartilage-derived morphogenetic protein-2. Am J Sports Med. 2003;31:555–9. doi: 10.1177/03635465030310041301. [DOI] [PubMed] [Google Scholar]
- Forslund C, Rueger D, Aspenberg P. A comparative dose-response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J Orthop Res. 2003;21:617–21. doi: 10.1016/S0736-0266(03)00010-X. [DOI] [PubMed] [Google Scholar]
- Fu SC, Wong YP, Chan BP, Pau HM, Cheuk YC, Lee KM, Chan KM. The roles of bone morphogenetic protein (BMP) 12 in stimulating the proliferation and matrix production of human patellar tendon fibroblasts. Life Sci. 2003;72:2965–74. doi: 10.1016/s0024-3205(03)00169-3. [DOI] [PubMed] [Google Scholar]
- Helm GA, Li JZ, Alden TD, Hudson SB, Beres EJ, Cunningham M, Mikkelsen MM, Pittman DD, Kerns KM, Kallmes DF. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J Neurosurg. 2001;95:298–307. doi: 10.3171/jns.2001.95.2.0298. [DOI] [PubMed] [Google Scholar]
- Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- Mikic B, Schalet BJ, Clark RT, Gaschen V, Hunziker EB. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. J Orthop Res. 2001;19:365–71. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
- Mikic B. Multiple effects of GDF-5 deficiency on skeletal tissues: implications for therapeutic bioengineering. Ann Biomed Eng. 2004;32:466–76. doi: 10.1023/b:abme.0000017549.57126.51. [DOI] [PubMed] [Google Scholar]
- Mikic B, Bierwert L, Tsou D. Achilles tendon characterization in GDF-7 deficient mice. J Orthop Res. 2006;24:831–41. doi: 10.1002/jor.20092. [DOI] [PubMed] [Google Scholar]
- Mikic B, Entwistle R, Rossmeier K, Bierwert L. Effect of GDF-7 deficiency on tail tendon phenotype in mice. J Orthop Res. 2008;26:834–9. doi: 10.1002/jor.20581. [DOI] [PubMed] [Google Scholar]
- Rickert M, Wang H, Wieloch P, Lorenz H, Steck E, Sabo D, Richter W. Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat Achilles tendon. Connect Tissue Res. 2005;46:175–83. doi: 10.1080/03008200500237120. [DOI] [PubMed] [Google Scholar]
- Settle SH, Jr, Rountree RB, Sinha A, Thacker A, Higgins K, Kingsley DM. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol. 2003;254:116–30. doi: 10.1016/s0012-1606(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Tashiro T, Hiraoka H, Ikeda Y, Ohnuki T, Suzuki R, Ochi T, Nakamura K, Fukui N. Effect of GDF-5 on ligament healing. J Orthop Res. 2006;24:71–9. doi: 10.1002/jor.20002. [DOI] [PubMed] [Google Scholar]
- Virchenko O, Fahlgren A, Skoglund B, Aspenberg P. CDMP-2 injection improves early tendon healing in a rabbit model for surgical repair. Scand J Med Sci Sports. 2005;15:260–4. doi: 10.1111/j.1600-0838.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–30. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YP, Fu SC, Cheuk YC, Lee KM, Wong MW, Chan KM. Bone morphogenetic protein 13 stimulates cell proliferation and production of collagen in human patellar tendon fibroblasts. Acta Orthop. 2005;76:421–7. [PubMed] [Google Scholar]