Abstract
Posaconazole is a potent broad-spectrum triazole antifungal. Little is known about the prevalence and risk factors for low plasma posaconazole concentrations (PPCs). We retrospectively reviewed all adult patients whose PPCs were measured after at least 5 days of treatment between April 2006 and July 2008 at the Hôpital Necker Enfants Malades. A low PPC was defined as a concentration lower than 500 ng/ml. Fifty-four patients were included: 36 receiving prophylactic (200 mg three times a day) and 18 receiving curative (400 mg twice a day) posaconazole therapy. The prevalence of low PPCs was 44% (16/36) in the prophylaxis group and 22% (4/18) in the curative-treatment group. In the prophylaxis group, low PPCs tended to be more frequent in cases of digestive disease (62.5% versus 30%; P = 0.051) and were significantly more frequent among patients with diarrhea (71.4% versus 27%; P = 0.009) or mucositis (100% versus 33%; P = 0.004). In the curative-treatment group, low PPCs were significantly more frequent in cases of diarrhea (75% versus 7%; P = 0.018). In the prophylaxis group, the only two patients who subsequently developed invasive fungal infections exhibited low PPCs. The only adverse event was hepatotoxicity for 2/54 patients (3.7%), which was not related to high plasma drug concentrations. In conclusion, low PPC is common, significantly more frequent in cases of diarrhea or mucositis, and potentially associated with subsequent invasive fungal infection. Therapeutic drug monitoring of posaconazole is therefore mandatory for immunosuppressed adults, at least for those with gastrointestinal disorders.
Posaconazole (PSZ) is a potent broad-spectrum triazole antifungal agent that has in vitro and clinical activity against a wide variety of fungal pathogens, including pathogenic yeasts (15) and molds (7). It is currently used for curative treatment of invasive fungal infection (IFI), mostly invasive aspergillosis and refractory mucosal candidiasis (22, 23), and for antifungal prophylaxis for patients with graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) (21) or with neutropenia after induction chemotherapy for acute myeloid leukemia or myelodysplastic syndrome (4). PSZ is available only as an oral formulation and has a long elimination half-life (over 24 h) (1). A number of factors have been demonstrated to impact PSZ absorption, including food (and fat specifically), gastric pH (and the use of proton pump inhibitors), the integrity of the mucosa, and the frequency of administration (due to a saturable absorption) (14).
Recent data have suggested that the efficacy and tolerance of voriconazole (another broad-spectrum oral azole drug) were increased with therapeutic drug monitoring (TDM) for patients with invasive mycoses (17, 23). In contrast, little is known about the potential benefit of TDM of PSZ. We therefore conducted a monocentric retrospective study involving a total of 54 adults to assess the prevalence of low plasma PSZ concentrations (PPC) in cases of prophylaxis or curative treatment, and we analyzed host characteristics associated with low PPC.
(This work was presented in part at the 19th European Congress of Clinical Microbiology and Infectious Diseases, Helsinki, Finland, 16 to 19 May 2009, and at the 17th Congress of The International Society for Human and Animal Mycology, Tokyo, Japan, 25 to 29 May 2009.)
MATERIALS AND METHODS
Study setting and patient enrollment.
We retrospectively reviewed all adult patients whose PPC were measured after at least 5 days of PSZ therapy between April 2006 and July 2008 at the Hôpital Necker Enfants Malades. The daily dosage of PSZ for prophylaxis or curative treatment was 200 mg three times a day (t.i.d.) or 400 mg twice a day (b.i.d.), respectively. A drug assay was performed using a previously published high-performance chromatography-UV detection method (2). Blood samples were performed at steady state. We retrospectively reviewed the medical records of these patients; clinical and biological data were obtained at the initiation of PSZ treatment. If the PPC was measured later than a month after PSZ initiation, the most recent clinical and biological data before PPC measurement were collected.
Data collection and variables of interest.
Low PPC were defined as concentrations below 500 ng/ml. This cutoff has been chosen according to a recent review by Andes et al. (1) and because of the results of a study evaluating prophylaxis with PSZ in cases of severe GVHD. In the latter study, median PPC were lower for the five patients with IFI than for the 241 patients without IFI (611 versus 922 ng/ml), suggesting a clinical benefit of targeting a PPC above 500 ng/ml (13). Nevertheless, the optimal PPC target in cases of prophylaxis or curative treatment has not been fully defined yet.
In cases of repeated measurements of PPC, the first value was used for statistical analysis. Evidence of one of the following was defined as digestive disease: digestive GVHD attested to by pathological examination, granulomatous colitis (in cases of chronic granulomatous disease [CGD]), viral colitis, bacterial colitis, or vincristine toxicity (paralytic ileus). Mucositis was clinically defined, and these data were not included in the list of digestive diseases. Diarrhea was defined as ≥3 stools/day that were abundant enough to be reported in medical and nurse files.
Hepatic cholestasis was defined by an alkaline phosphatase level above 390 IU/liter. Hepatic cytolysis was defined by an alanine aminotransferase (ALT) level above 120 IU/liter. Neutropenia was defined by a concentration of polymorphonuclear cells below 500/mm3. IFI was defined as proven, probable, or possible, according to the 2008 European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) (8). Clinical outcome in cases of curative treatment was defined as a complete response, a partial response, stable, or treatment failure.
Statistical analysis.
Continuous data are presented as means (± standard deviations [SD]) or medians (ranges), and categorical data are presented as numbers (proportions). Bivariable analyses were performed using Fisher's exact test and the chi-square test to compare categorical variables, and Student t tests were performed to compare continuous variables. PPC were nonnormally distributed and then compared between groups using the Wilcoxon rank sum test. Spearman's rank correlations were used to test the association between PPC measurements and continuous variables. A P value of <0.05 was considered statistically significant. All statistical analyses were performed by using the R software package (http://www.R-project.org).
RESULTS
Characteristics of patients.
A total of 54 patients (38 men and 16 women) were included, with a mean age of 48.7 (±15) years, a mean body mass index (BMI) of 23.2 (±4.0) kg/m2, a mean creatinine clearance of 89 (±34) ml/min, a median ALT level of 35 (range, 5 to 473) IU/liter, and a median alkaline phosphatase level of 97 (range, 21 to 1,097) IU/liter. Underlying diseases are described in Table 1.
TABLE 1.
Underlying diseases of the 54 adult patients with TDM of PSZ
| Underlying condition | No. (%) of patients |
|---|---|
| Hematological malignancya | 37 (69) |
| Acquired immunodeficiency | |
| AIDS | 2 (3.7) |
| Kidney transplantation | 1 (1.8) |
| Primary immunodeficiency | |
| CGD | 3 (5.5) |
| Common variable immunodeficiency | 3 (5.5) |
| Other | |
| Chronic bronchopulmonary disease | 1 (1.8) |
| Diabetes | 1 (1.8) |
| None | 6 (11) |
Hematological malignancies (with the numbers of patients in parentheses) comprised acute myeloid leukemia (9/37), acute lymphoid leukemia (8/37), lymphoma (3/37), chronic lymphocytic leukemia (1/37), idiopathic aplasia (1/37), myeloma (6/37), myeloproliferative syndrome (4/37), and myelodysplastic syndrome (5/37). Of the 37 patients with hematological malignancies, 28 (52%) had allogeneic HSCT with GVHD, 2 (4%) had allogeneic HSCT without GVHD, and 7 (13%) did not have allogeneic HSCT.
Thirty-six patients received antifungal prophylaxis (200 mg t.i.d.) for allogeneic HSCT (75%), mostly complicated by GVHD (n = 28), hematological malignancy with prolonged neutropenia (19%), or constitutive immunodeficiency (6%).
Eighteen patients received curative PSZ therapy (400 mg b.i.d.) for IFI (14/18 [78%]), chronic necrotizing pulmonary aspergillosis (1/18 [5.5%]), refractory mycetoma (1/18 [5.5%]), refractory mucosal candidiasis due to Candida albicans (1/18 [5.5%]), or refractory subcutaneous dermatophytic disease (1/18 [5.5%]). In cases of IFI, fungal pathogens were Aspergillus fumigatus (6/14 [42.8%]), Aspergillus flavus (1/14 [7.1%]), zygomycetes (3/14 [21.4%]), Fusarium spp. (2/14 [14.3%]), Trichophyton rubrum (1/14 [7.1%]), and C. albicans (1/14 [7.1%]). Madurella mycetomatis was responsible for the single case of refractory mycetoma. Twenty-two patients exhibited digestive diseases (16 and 6 in the prophylaxis and curative-treatment groups, respectively), including digestive GVHD (14/22 [63.5%]), granulomatous colitis (3/22 [14%]), viral colitis (2/22 [9%]), bacterial colitis (2/22 [9%]), and vincristine toxicity (paralytic ileus) (1/22 [4.5%]).
A total of 159 PPC measurements were performed (98 in cases of prophylaxis and 61 in cases of curative treatment), with a median of 2.9 (range, 1 to 11) per patient. Three values were beyond the lower limit of quantification of the analytical method (i.e., 50 ng/ml), so median values of the measured concentrations were determined based on the 156 quantifiable results. The first PPC measurement was obtained after a median of 21.5 days (range, 5 to 849 days) after the start of therapy. The median PPC was 875 ng/ml (range, 30 to 4,290 ng/ml). For 23 (42%) patients, PPC was measured only once.
Low PPC in cases of prophylaxis.
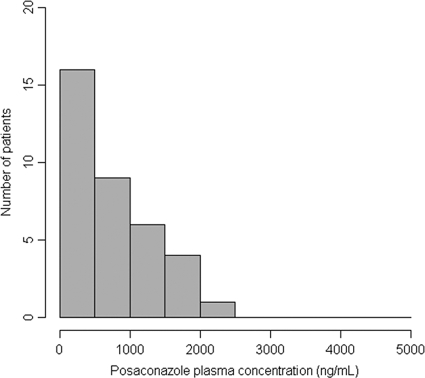
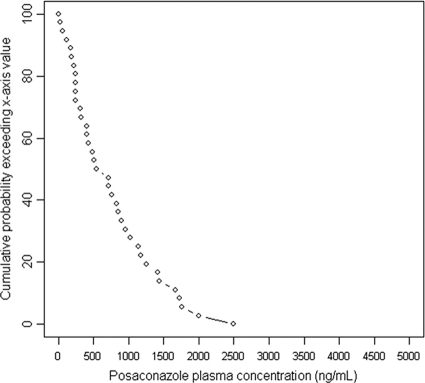
The prevalence of low PPC was 44% (16/36) in the prophylaxis group (Table 2). The distributions of the first PPC measurements for patients receiving prophylaxis are shown in Fig. 1 and 2. Low PPC tended to be more frequent among patients with digestive disease (62.5% versus 30%; P = 0.051). Low PPC were significantly more frequent among patients with diarrhea (71% versus 27%; P = 0.009) or mucositis (100% versus 33%; P = 0.004). Low PPC tended to be more prevalent among women than among men (64% versus 36%; P = 0.16). The mean BMI tended to be lower for patients with low PPC (21.6 ± 3.0 versus 24.3 ± 4.2 kg/m2; P = 0.055). Patients with low PPC tended to be younger (44.1 ± 17.3 years old versus 52.5 ± 11.6 years old; P = 0.095). Other parameters, such as allogeneic HSCT, use of proton pump inhibitors, GVHD, and renal and hepatic functions, were not associated with low PPC (data not shown).
TABLE 2.
Characteristics of the 54 adult patients with TDM of PSZ
| Characteristic | Value for patients receiving: |
Value for all patients (n = 54) | |||||
|---|---|---|---|---|---|---|---|
| Prophylaxis (n = 36) |
Curative treatment (n = 18) |
||||||
| PPC, <500 ng/ml | PPC, ≥500 ng/ml | P | PPC, <500 ng/ml | PPC, ≥500 ng/ml | P | ||
| No. (%) | 16 (44) | 20 (56) | 4 (22) | 14 (78) | |||
| Mean (SD) age (yr) | 44.1 (17.6) | 52.5 (11.6) | 0.095 | 31 (7.3) | 44 (16.3) | 0.15 | 48.7 (15.0) |
| Mean (SD) BMI (kg/m2) | 21.6 (3.0) | 24.3 (4.2) | 0.055 | 15.8 (5.5) | 22.6 (4.0) | 0.07 | 23.2 (4.0) |
| No. (%) female | 7 (44) | 4 (20) | 0.16 | 1 (25) | 4 (28) | 1 | 16 (30) |
| Mean (SD) creatinine clearance (ml/min) | 89 (30) | 91 (25) | 0.92 | 116 (47.6) | 78 (45.0) | 0.16 | 89 (34) |
| Median (range) ALT level (IU/liter) before treatment | 29.5 (5-473) | 42.5 (6-151) | 0.35 | 38 (6-56) | 27 (13-59) | 1 | 35 (5-473) |
| No. (%) with: | |||||||
| Digestive disease | 10 (63) | 6 (30) | 0.051 | 3 (75) | 3 (21) | 0.083 | 22 (41) |
| Diarrhea | 10 (63) | 4 (20) | 0.0093 | 3 (75) | 1 (7) | 0.018 | 18 (33) |
| Mucositis | 6 (37.5) | 0 | 0.0041 | 0 | 0 | 6 (11) | |
| Allogeneic HSCT | 13 (81) | 14 (70) | 0.7 | 1 (25) | 4 (28) | 1 | 32 (59) |
| GVHD | 12 (75) | 13 (65) | 0.7 | 1 (25) | 3 (21) | 1 | 29 (54) |
FIG. 1.
Numbers of patients receiving prophylaxis with PPC in each range at the first measurement.
FIG. 2.
Curve showing the probability (vertical axis) for a patient receiving prophylaxis to have a first PPC above the value on the x axis.
When PPC was considered as a continuous variable, it tended to be associated with mucositis (355 ng/ml [range, 60 to 480 ng/ml] for patients with mucositis versus 795 ng/ml [range, 30 to 2,500 ng/ml] for those without mucositis; P = 0.056), diarrhea (360 ng/ml [range, 60 to 1,140 ng/ml] for patients with diarrhea versus 840 ng/ml [range, 30 to 2,500 ng/ml] for those without diarrhea; P = 0.1), and albumin level (rho = 0.30; P = 0.077).
In the prophylaxis group, only two patients experienced possible IFI (according to the EORTC/MSG criteria) with pulmonary involvement, and both exhibited low PPC (310 and 190 ng/ml). They experienced IFI 8 and 11 months, respectively, after the introduction of PSZ. Of the 16 patients with low PPC, only 5 had a second PPC measurement. The first PPC were 190, 120, 400, 250, and 480 ng/ml, and the second PPC were 280, 430, 550, 1,500, and 590 ng/ml, respectively. The second PPC measurements were performed 124, 55, 21, 31, and 39 days after the first measurements, respectively. None of these patients had therapeutic optimization. In cases of PPC above 500 ng/ml (20 patients), only one patient had therapeutic optimization, and his initial PPC was 540 ng/ml. After the PSZ dosage was increased to 200 four times a day, his PPC was 670 ng/ml (18 days later), and the third PPC measured was 940 ng/ml after the amount of fat in his meal was increased.
The median PPC in cases of prophylaxis was 630 ng/ml (range, 30 to 2,500 ng/ml).
Low PPC in cases of curative treatment.
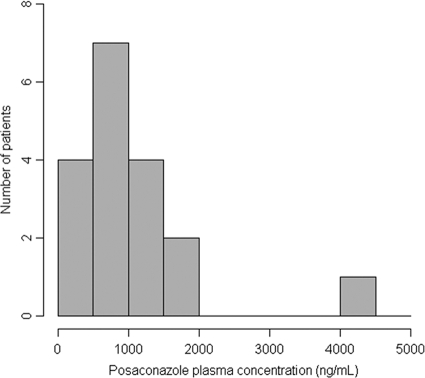
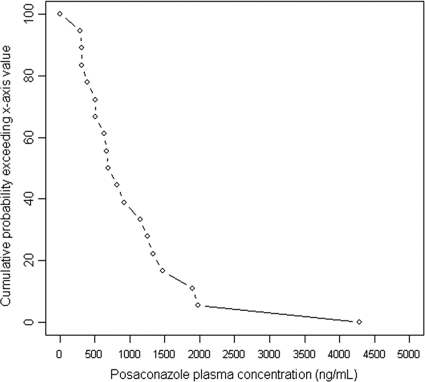
The prevalence of low PPC was 22% (4/18) in the curative-treatment group (Table 2). Sixty-one percent of PPC were below 1,000 ng/ml, and 66% were below 1,200 ng/ml. The distributions of the first PPC measurements for patients receiving curative treatment are shown in Fig. 3 and 4. Low PPC tended to be more frequent among patients with digestive disease (50% versus 8%; P = 0.08) and were more frequent among those with diarrhea (75% versus 7%; P = 0.018). The mean age of patients with low PPC tended to be lower (30.3 ± 7.1 versus 43.1 ± 16.4 years old; P = 0.15); the BMI tended to be lower for patients with low PPC (15.8 ± 5.5 versus 22.6 ± 4.1 kg/m2; P = 0.073); and creatinine clearance tended to be lower in the group without low PPC (78 ± 45 versus 116 ± 48 ml/min; P = 0.17).
FIG. 3.
Numbers of patients receiving curative treatment with PPC in each range at the first measurement.
FIG. 4.
Curve showing the probability (vertical axis) for a patient receiving curative treatment to have a first PPC above the value on the x axis.
Other parameters, such as gender, use of proton pump inhibitors, and renal and hepatic functions, were not associated with low PPC (data not shown).
When PPC was considered as a continuous variable, it was associated with diarrhea (350 ng/ml [range, 310 to 510 ng/ml] for patients with diarrhea versus 1,035 ng/ml [range, 290 to 4,290 ng/ml] for those without diarrhea; P = 0.014), age (rho = 0.57; P = 0.013), and albumin level (rho = 0.56; P = 0.024) and tended to be associated with the presence of a digestive disease (450 ng/ml [range, 310 to 1,470 ng/ml] for patients with digestive diseases versus 1,035 ng/ml [range, 290 to 4,290 ng/ml] for those without digestive disease; P = 0.075).
Two patients exhibited granulomatous colitis associated with CGD. Both had PPC below 1,200 ng/ml (510 and 670 ng/ml, respectively). After therapeutic optimization, including a posology increase (to 400 mg t.i.d.) for one patient and an increase in the fat content of the meal for the other, their PPC reached 1,100 and 1,250 ng/ml, respectively.
The median PPC in cases of curative treatment was 755 ng/ml (range, 290 to 4,290 ng/ml).
Efficacy of curative treatment.
In the context of curative treatment, there were 8 (44%) complete responses, 4 (22%) partial responses, 2 (11%) stable diseases, and 2 (11%) treatment failures, and 2 (11%) patients died from another cause before being evaluated. Two patients with low PPC had complete responses; one died from another cause before being evaluated; and one exhibited stable disease. For one of the patients who had a complete response but whose first PPC was low (310 ng/ml), a second measurement of PPC performed after therapeutic optimization (administration of PSZ during a meal containing fat) showed 1,910 ng/ml. The second patient did not have another PPC measurement.
Four patients experienced treatment failure or stability of their IFI. Their PPC were 290, 670, 1,150, and 1,330 ng/ml. The PSZ dosage was increased for the patient whose PPC was 290 ng/ml, but his PPC was not measured again. The patient whose first PPC was 670 ng/ml had another PPC measurement, showing 1,250 ng/ml, after therapeutic optimization (administration of PSZ during a meal containing fat). The other two patients did not have any therapeutic modifications. The two patients who experienced treatment failure received PSZ for IFI caused by A. fumigatus and T. rubrum, respectively.
Toxicity.
The only adverse event recorded was grade 4 hepatotoxicity (>10 times the normal ALT value) for 2/54 patients (3.7%). Those patients exhibited PPC measurements of 1,410 ng/ml and 30 ng/ml.
DISCUSSION
PSZ is the most recently approved oral triazole (16) for which a saturable absorption has been reported. Of note, the bioavailability of PSZ can be increased by splitting its administration during the day (5, 9). However, many factors, such as food, gastric pH, and mucosal disease, affect the absorption of PSZ, which may explain the important interindividual pharmacokinetic variability observed in patients (11, 20, 21).
In a large study examining the prophylactic use of PSZ for 600 HSCT recipients with GVHD (21), PSZ concentrations were lower for 18 patients without IFI who experienced diarrhea on the day of sampling than for 223 patients without diarrhea. In this study, lower PPC were also associated with acute GVHD. The influence of diarrhea on the PSZ concentration was confirmed by the pharmacokinetic subanalysis of another prospective, randomized trial comparing PSZ to standard azoles (fluconazole and itraconazole) in cases of prolonged neutropenia. This study found that the mean PSZ concentration at steady state was 37% lower for patients with diarrhea (12). The effect of diarrhea on the plasma drug concentration appeared to increase with severity. In our study, we found no relationship between low PPC and GVHD per se but a significant association with diarrhea.
We also found that mucositis was strongly associated with low PPC. Clinical mucositis thus clearly seems to reflect larger damage involving the digestive tract. Overall, our TDM, combined with data from recent clinical trials, strongly supports the recommendation that patients exhibiting diarrhea and/or mucositis should be monitored for PPC during the entire period of digestive disturbances.
Based on the major prophylactic effect of PSZ (reduction of the occurrence of IFI), it is difficult to validate a clear correlation between low PPC and less-effective primary prophylaxis. However, in the study evaluating prophylaxis with PSZ in cases of severe GVHD, median PPC were lower for the five patients with IFI than for the 241 patients without IFI (611 versus 922 ng/ml) (13). This difference was not statistically significant, possibly due to the small number of patients who developed infections. Nevertheless, such a difference had not been found in the study of prolonged neutropenia, since the PPC of the six patients with IFI were not different from the PPC of those who did not develop IFI. In this study, a survival benefit of PSZ prophylaxis was observed, although 75% of the patients had average PSZ levels of 319 ng/ml or above (4, 12).
This finding is surprising considering the in vitro activity of PSZ against Aspergillus spp. Indeed, using the methods of the Clinical and Laboratory Standards Institute (CLSI), Sabatelli et al. assessed the in vitro activity of PSZ against approximately 19,000 clinical strains of yeasts and molds (18a). The MIC at which 90% of isolates were inhibited was 500 ng/ml for all Aspergillus spp. (16). Pfaller et al. confirmed this finding except for Aspergillus niger and Aspergillus versicolor (the MIC at which 90% of isolates were inhibited was 1,000 ng/ml) (18).
The efficacy of prophylactic PSZ even in cases of low PPC could be explained by the relatively high pulmonary PSZ concentrations. Indeed, in a randomized study performed with healthy adults (3), PSZ concentrations in plasma and pulmonary epithelial lining fluid were similar, but higher PSZ concentrations were found in alveolar cells (AC), with an AC/plasma ratio ranging from 27.3 to 44.3. Therefore, although it remains difficult to establish an optimal threshold for PPC adequate for effective prophylaxis, the available published data and our present results suggest that a minimal PPC above 500 ng/ml might be satisfactory. The target PPC for prophylaxis could, furthermore, depend on the clinical context, which could not be evaluated in the present work. Recently, an FDA document recommended achieving a PPC above 700 ng/ml (10). Thompson et al. retrospectively reviewed all PPC measurements performed in their reference laboratory and found that 66.8% were <611 ng/ml (19). Further studies to evaluate the relationship between PPC and the prophylactic efficacy of PSZ are warranted.
For patients receiving curative treatment, Andes et al. (1) suggested a PPC target between 500 and 1,500 ng/ml. A relationship between PSZ concentration and clinical outcome was suggested in a study investigating the efficacy of PSZ for refractory invasive aspergillosis. The response rate increased with the average PSZ concentration at steady state; the higher success rate of 70% corresponded to PPC above 1,250 ng/ml (23). Such a precise relationship could not be investigated in our study due to the low number of patients under curative treatment. Twenty-two percent of PPC were below 500 ng/ml; 61% were below 1,000 ng/ml; and 66% were below 1,200 ng/ml. Two of the four patients who experienced treatment failure or stability of the disease had high PPC, clearly emphasizing that factors other than PPC impact the success of treatment for IFI.
This study has a number of limitations, including its retrospective design and the low number of patients included in each group. Furthermore, 23 (42%) patients had a single PPC measurement, and therapeutic optimization was not standardized. Nevertheless, these results suggest that TDM is mandatory for patients with a high risk of low PPC, such as those presenting with diarrhea and/or mucositis. PPC can be increased by taking the drug concomitantly with food, and an increase in exposure by a factor of 4 is also possible with a high-fat meal (6). Establishing a precise correlation between low PPC and prophylaxis or curative treatment failure will require additional clinical data.
Acknowledgments
O. Lortholary is a member of the speaker's bureaus of Pfizer, Schering, Merck Sharp & Dohme, Astellas, and Gilead Sciences. F. Lanternier is a member of the speaker's bureaus of Schering and Gilead Sciences. V. Jullien is a member of the speaker's bureau of Schering. The other authors declare no conflict of interest.
Footnotes
Published ahead of print on 14 September 2009.
REFERENCES
- 1.Andes, D., A. Pascual, and O. Marchetti. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chhun, S., E. Rey, A. Tran, O. Lortholary, G. Pons, and V. Jullien. 2007. Simultaneous quantification of voriconazole and posaconazole in human plasma by high-performance liquid chromatography with ultra-violet detection. J. Chromatogr. B 852:223-228. [DOI] [PubMed] [Google Scholar]
- 3.Conte, J. E., Jr., J. A. Golden, G. Krishna, M. McIver, E. Little, and E. Zurlinden. 2009. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob. Agents Chemother. 53:703-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornely, O. A., J. Maertens, D. J. Winston, J. Perfect, A. J. Ullmann, T. J. Walsh, D. Helfgott, J. Holowiecki, D. Stockelberg, Y. T. Goh, M. Petrini, C. Hardalo, R. Suresh, and D. Angulo-Gonzalez. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348-359. [DOI] [PubMed] [Google Scholar]
- 5.Courtney, R., S. Pai, M. Laughlin, J. Lim, and V. Batra. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtney, R., D. Wexler, E. Radwanski, J. Lim, and M. Laughlin. 2004. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenca-Estrella, M., M. C. Arendrup, E. Chryssanthou, E. Dannaoui, C. Lass-Florl, P. Sandven, A. Velegraki, and J. L. Rodriguez-Tudela. 2007. Multicentre determination of quality control strains and quality control ranges for antifungal susceptibility testing of yeasts and filamentous fungi using the methods of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin. Microbiol. Infect. 13:1018-1022. [DOI] [PubMed] [Google Scholar]
- 8.De Pauw, B., T. J. Walsh, J. P. Donnelly, D. A. Stevens, J. E. Edwards, T. Calandra, P. G. Pappas, J. Maertens, O. Lortholary, C. A. Kauffman, D. W. Denning, T. F. Patterson, G. Maschmeyer, J. Bille, W. E. Dismukes, R. Herbrecht, W. W. Hope, C. C. Kibbler, B. J. Kullberg, K. A. Marr, P. Munoz, F. C. Odds, J. R. Perfect, A. Restrepo, M. Ruhnke, B. H. Segal, J. D. Sobel, T. C. Sorrell, C. Viscoli, J. R. Wingard, T. Zaoutis, and J. E. Bennett. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezzet, F., D. Wexler, R. Courtney, G. Krishna, J. Lim, and M. Laughlin. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211-220. [DOI] [PubMed] [Google Scholar]
- 10.FDA. 17 February 2009, accession date. Posaconazole. FDA briefing document. http://www.aapsj.org/abstracts/AM_2007/AAPS2007-000993.PDF.
- 11.Gubbins, P. O., G. Krishna, A. Sansone-Parsons, S. R. Penzak, L. Dong, M. Martinho, and E. J. Anaissie. 2006. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob. Agents Chemother. 50:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishna, G., M. AbuTarif, F. Xuan, M. Martinho, D. Angulo, and O. A. Cornely. 2008. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28:1223-1232. [DOI] [PubMed] [Google Scholar]
- 13.Krishna, G., M. Martinho, P. Chandrasekar, A. J. Ullmann, and H. Patino. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627-1636. [DOI] [PubMed] [Google Scholar]
- 14.Krishna, G., A. Moton, L. Ma, M. M. Medlock, and J. McLeod. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53:958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lortholary, O., E. Dannaoui, D. Raoux, D. Hoinard, A. Datry, A. Paugam, J. L. Poirot, C. Lacroix, and F. Dromer. 2007. In vitro susceptibility to posaconazole of 1,903 yeast isolates recovered in France from 2003 to 2006 and tested by the method of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 51:3378-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagappan, V., and S. Deresinski. 2007. Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent. Clin. Infect. Dis. 45:1610-1617. [DOI] [PubMed] [Google Scholar]
- 17.Pascual, A., T. Calandra, S. Bolay, T. Buclin, J. Bille, and O. Marchetti. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201-211. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2008. In vitro survey of triazole cross-resistance among more than 700 clinical isolates of Aspergillus species. J. Clin. Microbiol. 46:2568-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Sabatelli, F., R. Patel, P. A. Mann, C. A. Mendrick, C. C. Norris, R. Hare, D. Loebenberg, T. A. Black, and P. M. Nicholas.. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson, G. R., III, M. G. Rinaldi, G. Pennick, S. A. Dorsey, T. F. Patterson, and J. S. Lewis II. 2009. Posaconazole therapeutic drug monitoring: a reference laboratory experience. Antimicrob. Agents Chemother. 53:2223-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullmann, A. J., O. A. Cornely, A. Burchardt, R. Hachem, D. P. Kontoyiannis, K. Topelt, R. Courtney, D. Wexler, G. Krishna, M. Martinho, G. Corcoran, and I. Raad. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullmann, A. J., J. H. Lipton, D. H. Vesole, P. Chandrasekar, A. Langston, S. R. Tarantolo, H. Greinix, W. Morais de Azevedo, V. Reddy, N. Boparai, L. Pedicone, H. Patino, and S. Durrant. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335-347. [DOI] [PubMed] [Google Scholar]
- 22.Vazquez, J. A., D. J. Skiest, H. Tissot-Dupont, J. L. Lennox, N. Boparai, and R. Isaacs. 2007. Safety and efficacy of posaconazole in the long-term treatment of azole-refractory oropharyngeal and esophageal candidiasis in patients with HIV infection. HIV Clin. Trials 8:86-97. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, T. J., I. Raad, T. F. Patterson, P. Chandrasekar, G. R. Donowitz, R. Graybill, R. E. Greene, R. Hachem, S. Hadley, R. Herbrecht, A. Langston, A. Louie, P. Ribaud, B. H. Segal, D. A. Stevens, J. A. van Burik, C. S. White, G. Corcoran, J. Gogate, G. Krishna, L. Pedicone, C. Hardalo, and J. R. Perfect. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2-12. [DOI] [PubMed] [Google Scholar]