Abstract
Polyamine levels are greatly increased in alveolar macrophages (AMs) during Pneumocystis pneumonia (PCP), leading to increased production of H2O2, which causes AMs to undergo apoptosis. One of the mechanisms by which polyamine levels in AMs are elevated is enhanced uptake of exogenous polyamines. In this study, the possibility of targeting polyamine uptake as a treatment for PCP was examined. Four anthracene- and one benzene-polyamine conjugates that are potential polyamine transport inhibitors, including N1-anthracen-9-ylmethyl-butane-1,4-diamine; N-(4-aminobutyl)-N-anthracen-9-ylmethylbutane-1,4-diamine; N-[4-(4-aminobutylamino)butyl]-N-anthracen-9-ylmethylbutane-1,4-diamine; N-(4-amino-butyl)-N′-(10-{[4-(4-amino-butylamino)butylamino]-methyl}anthracen-9-ylmethyl)butane-1,4-diamine (44-Ant-44); and benzene-polyamine conjugate N-(4-amino-butyl)-N′-(4-{[4-(4-amino-butylamino)butylamino]-methyl}benzyl)butane-1,4-diamine (44-Bn-44), were tested. Compounds 44-Ant-44 and 44-Bn-44 were found to have a very low toxicity to AMs in vitro and were evaluated for their therapeutic effect on PCP in vivo. Sprague-Dawley rats infected with P. carinii for 28 days were intranasally instilled with 50 μl of a 1 mM solution of 44-Bn-44 or 44-Ant-44 every 2 days. Twenty-one days after initiation of the treatment, three to five rats from each group were sacrificed and examined for lung pathology, organism burden, and apoptosis of AMs. Both 44-Bn-44 and 44-Ant-44 reduced organism burdens; however, only 44-Ant-44 decreased the severity of the infection with reduced lung inflammation, increased clearance of exudates, increased air space, and decreased apoptosis of AMs. 44-Ant-44 also significantly prolonged the survival of treated animals. These results suggest that polyamine uptake is a potential target for treatment of PCP.
Pneumocystis pneumonia (PCP) is the leading opportunistic disease in AIDS patients. PCP can be life threatening, as it may cause extensive pulmonary injury, impaired gas exchange, and respiratory failure. The first-line drug for both prophylaxis and treatment of PCP is a combination of trimethoprim and sulfamethoxazole (TMP-SMZ) (40). TMP and SMZ inhibit dihydrofolate reductase and dihydropteroate synthase (DHPS), respectively (18). However, TMP-SMZ may cause adverse effects, such as fever and rash (37), and TMP-SMZ-resistant Pneumocystis strains have emerged (28), probably due to point mutations at codon 55 or 57 of the DHPS gene of Pneumocystis jirovecii (18, 41). Clindamycin-primaquine, atovaquone, and pentamidine are alternative therapies for PCP; however, they are also associated with the issues of side effects.
Another potential target for treatment of PCP is polyamine synthesis (8, 9, 14, 32, 36, 39). Polyamines are essential organic compounds for cell growth (20, 26). The rate-limiting enzyme of polyamine synthesis is ornithine decarboxylase (ODC), which is usually increased in proliferating cells. α-Difluoromethylornithine (DFMO, also known as eflornithine) is a potent ODC inhibitor with low cytotoxicity. DFMO was first developed as an anticancer drug and was shown to be effective for treatment of trypanosomiasis (2, 29). It also has activity against Plasmodium falciparum (30), Giardia lamblia (13), Eimeria tenella (16), and Trichomonas vaginalis (47). DFMO has also been used to treat PCP in humans, with success rates ranging from 33 to 57% (14, 32, 36, 38, 39). This partial success suggests that targeting polyamine synthesis alone is not sufficient for treatment of PCP.
Our recent studies revealed that polyamine levels in alveolar macrophages (AMs) are greatly increased during PCP, resulting in an elevated apoptosis rate and impaired Pneumocystis clearance activity by AMs (23). We also found that the increased intracellular polyamine levels were at least partially due to increased uptake of exogenous polyamines (25). Based on these findings, we hypothesized that polyamine uptake can be a target for treatment of PCP and tested five potential polyamine transport (PAT) inhibitors. Results showed that compound 44-Ant-44 was effective against PCP in a rat model.
MATERIALS AND METHODS
PAT inhibitors.
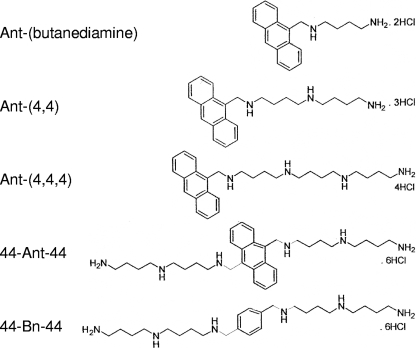
The anthracene-polyamine conjugates N1-anthracen-9-ylmethyl-butane-1,4-diamine [Ant-(butanediamine)]; N-(4-aminobutyl)-N-anthracen-9-ylmethylbutane-1,4-diamine [Ant-(4,4)]; N-[4-(4-aminobutylamino)butyl]-N-anthracen-9-ylmethylbutane-1,4-diamine [Ant-(4,4,4)]; N-(4-amino-butyl)-N′-(10-{[4-(4-amino-butylamino)butylamino]-methyl}anthracen-9-ylmethyl)butane-1,4-diamine (44-Ant-44); and benzene-polyamine conjugate N-(4-amino-butyl)-N′-(4-{[4-(4-amino-butylamino)butylamino]-methyl}benzyl)butane-1,4- diamine (44-Bn-44) were synthesized and characterized as previously described (21, 43-45). Their structures are shown in Fig. 1, and 50% inhibitory concentrations (IC50s) for CHO and CHO-MG cells are listed in Table 1.
FIG. 1.
Structures of PAT ligands.
TABLE 1.
IC50 values of polyamine derivatives in CHO and CHO-MG cells
| Compound | IC50 (μM) |
IC50 ratioa | |
|---|---|---|---|
| CHO-MG | CHO | ||
| Ant-(butanediamine) | 7.6 ± 0.4 | 7.7 ± 0.5 | 1 |
| Ant-(4,4) | 66.7 ± 4.1 | 0.45 ± 0.1 | 148 |
| Ant-(4,4,4) | 33.2 ± 1.7 | 10.6 ± 0 | 3.1 |
| 44-Ant-44 | >100 | 0.045 ± 0.003 | >2,222 |
| 44-Bn-44 | 50.2 ± 3.8 | 0.074 ± 0.01 | 677 |
CHO-MG/CHO IC50 ratio is a measure of polyamine transporter selectivity. Compounds with high IC50 ratios are highly selective ligands of polyamine transporters and effective inhibitors of PAT.
Rat PCP model.
P. carinii infection in rats was established as described previously (4). Briefly, female Sprague-Dawley rats (Harlan, Indianapolis, IN) approximately 120 g in weight were immunosuppressed by giving them 1.8 mg/liter dexamethasone continuously in drinking water to reduce the number of CD4+ T lymphocytes. One week after initiation of dexamethasone treatment, rats were anesthetized by intramuscular injection of 100 μl ketamine mixture (80 mg/ml ketamine hydrochloride, 0.38 mg/ml atropine, and 1.76 mg/ml acepromazine) and then transtracheally inoculated with 7.5 × 106 trophic-form P. carinii organisms. Each rat was given 10,000 units of penicillin (Butler, Dublin, OH) intramuscularly once a week to prevent bacterial infections. Rats in this study were divided into four groups: (i) Dex, which includes immunosuppressed, uninfected control rats; (ii) Dex-Pc, which includes immunosuppressed rats infected with P. carinii organisms; (iii) 44-Ant-44, which includes Dex-Pc rats treated with the compound 44-Ant-44; (iv) 44-Bn-44, which includes Dex-Pc rats treated with 44-Bn-44. Each rat in the PAT inhibitor treatment groups was given 50 μl of a 1 mM solution (approximately 0.5 mg/kg of body weight) of the compound intranasally every 2 days starting at the 28th day of infection. All animal studies were approved and supervised by the Indiana University Animal Care and Use Committee.
Cytotoxicity assay.
Toxicity of PAT inhibitors to AMs was determined by the trypan blue exclusion assay. AMs were isolated as described previously (25). One million AMs from normal rats were placed in 2 ml of serum-free DMEM medium in one well of a 12-well plate and incubated with a test compound at various concentrations. After 3 h of incubation, cells were stained with 0.4% trypan blue solution (Invitrogen, Carlsbad, CA), and the numbers of viable cells (unstained cells) were determined. Since AMs may lose their viability quickly in serum-free media, a later time point was not used.
Pulmonary pathology.
Rats were anesthetized by intramuscular injection of 100 μl of the ketamine mixture described above and then sacrificed. The thoracic cavity was exposed by dissection. The whole lung was excised and then fixed overnight with 4% paraformaldehyde in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4 [pH 7.4]). Sections of paraffin-embedded lung were stained with hematoxylin and eosin (H&E) and then examined by light microscopy. The degree of pulmonary inflammation was graded on a scale of 0 to 5 as previously described (35): grade 0, no lesions; grade 1, minimal lymphocytic inflammation in perivascular and peribronchiolar regions; grade 2, moderate perivascular and peribronchiolar inflammation with a slight increase in the number of inflammatory cells, including AMs, lymphocytes, eosinophils, and multinucleated giant cells; grade 3, profound perivascular and peribronchiolar inflammation with moderately increased numbers of inflammatory cells; grade 4, severe perivascular and peribronchiolar inflammation with markedly increased numbers of inflammatory cells; grade 5, severe perivascular and peribronchiolar inflammation with effacement of alveolar parenchyma and small airways by sheets of inflammatory cells.
P. carinii burden.
The numbers of P. carinii cysts in the lung sections were enumerated after staining with the modified Grocott's methenamine silver stain (catalog number HT100A; Sigma, St. Louis, MO). Organism burden was graded on a scale of 0 to 4 as previously described (35). Briefly, grade 0 indicated no cysts; grade 1 indicated scattered cysts; grade 2 indicated cysts in most alveoli surrounding tertiary bronchioles; grade 3 indicated cysts throughout alveoli in many regions; grade 4 indicated cysts and foamy exudates throughout alveoli in most regions.
Apoptosis assay.
Macrophage apoptosis was determined using the DeadEnd fluorometric terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) system (Promega, Madison, WI). Briefly, deparaffinized and rehydrated rat lung sections on slides were digested with 20 μg/ml of proteinase K at room temperature for 10 min. After washing with phosphate-buffered saline, the sections were incubated with terminal deoxynucleotidyl transferase and fluorescein-12-dUTP at 37°C for 1 h to label DNA fragments at their 3′ ends. Unincorporated fluorescein-12-dUTPs were removed by washing the slides two times with 2× saline sodium citrate buffer. Slides were mounted with ProLong Gold antifade reagent containing 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen), which stains DNA. Our previous results showed that AM was the major cell type undergoing apoptosis in the lungs of animals with PCP; therefore, TUNEL-positive cells were considered macrophages. The percentage of macrophages undergoing apoptosis was determined by the ratio of TUNEL-positive cells to DAPI-positive cells in a total of 10 randomly selected 100× fields on each slide.
Statistical analysis.
One-way analysis of variance with Bonferroni's test was used for statistical analysis of results from multiple groups of experiments. Survival curve was analyzed by the Kaplan-Meier method with the log rank test. The difference was considered significant if the P value was <0.05. All statistical analyses were performed with the PASW Statistics 17 software (formerly SPSS).
RESULTS
Cytotoxicity of PAT inhibitors.
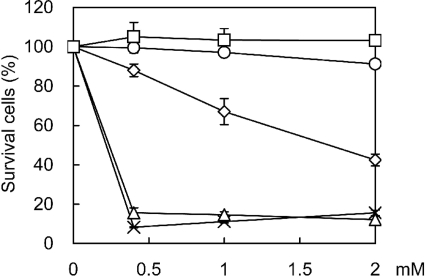
Since compounds Ant-(butanediamine), Ant-(4,4), Ant-(4,4,4), 44-Ant-44, and 44-Bn-44 had not been used in vivo, their toxicity to AMs from normal rats was first determined. Trypan blue exclusion assays showed that less than 16% of the cells were viable after 3 h of incubation with 0.4 mM of Ant-(butanediamine) or Ant-(4,4), indicating that these two compounds were toxic to AMs. Compound Ant-(4,4,4) was less toxic, as 88% of the cells remained viable after 3 h of treatment with the lowest concentration (0.4 mM) and 42% with the highest concentration (2 mM). There was no significant change in viability after the cells were treated for 3 h with compound 44-Ant-44 or 44-Bn-44 at a concentration of up to 2 mM, indicating that these two compounds had little toxicity to AMs (Fig. 2).
FIG. 2.
Cytotoxicity of PAT inhibitors. AMs from normal rats were incubated with PAT inhibitors 44-Bn-44 (□), 44-Ant-44 (○), Ant-(4,4,4) (⋄), Ant-(4,4) (×), and Ant-(butanediamine) (▵) at the indicated concentrations for 3 h and then examined for viability by the trypan blue exclusion method.
Effects of compounds 44-Ant-44 and 44-Bn-44 on survival of rats with PCP.
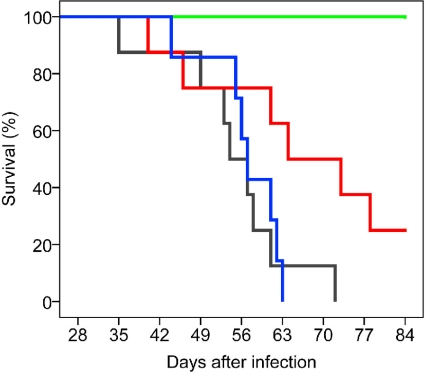
Since compounds 44-Ant-44 and 44-Bn-44 showed no significant cytotoxicity in vitro, their therapeutic effects on PCP were examined. Rats that had been infected with P. carinii (Dex-Pc rats) for 28 days were treated intranasally with 50 μl of a 1 mM solution of 44-Ant-44 (n = 8) or 44-Bn-44 (n = 7) every 2 days. Immunosuppressed and uninfected Dex rats (n = 4) and untreated Dex-Pc rats (n = 8) were included in this study as controls. All Dex rats survived the entire 84 days of the study, while untreated Dex-Pc rats died at different times (Fig. 3). Survival results analyzed by the Kaplan-Meier statistical method revealed that 44-Ant-44 significantly prolonged the survival of rats with PCP (P = 0.032), whereas 44-Bn-44 had minimal effects (P = 0.783).
FIG. 3.
Effects of PAT inhibitors in vivo. Rats with PCP were treated with 44-Ant-44 (n = 8) (red) or 44-Bn-44 (n = 7) (blue) by intranasal instillation of 50 μl of a 1 mM solution of either compound every 2 days starting at the 28th day after infection. Untreated Dex-Pc (n = 8) (gray) and uninfected Dex (n = 4) (green) rats were used as controls. Kaplan-Meier analysis showed that 44-Ant-44 significantly prolonged the survival of PCP rats (P = 0.032).
Effects of compounds 44-Ant-44 and 44-Bn-44 on lung pathology and organism burden.
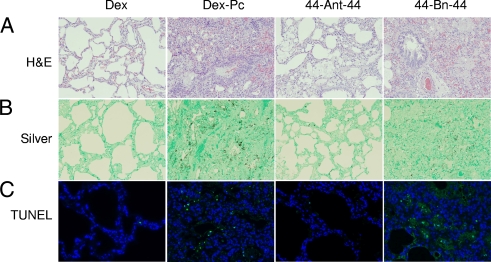
Light microscopy of H&E-stained lung sections from Dex-Pc rats treated for 21 days with the compounds revealed that 44-Ant-44 treatment increased open alveolar space, reduced alveolar septum thickness, reduced inflammation, and decreased foamy exudates in alveoli compared to those from untreated Dex-Pc rats. Lung sections from 44-Bn-44-treated Dex-Pc rats showed a slight decrease in inflammatory cell infiltration but no apparent changes in alveolar structure and foamy exudates. The overall inflammatory scores for untreated, 44-Ant-44-treated, and 44-Bn-44-treated Dex-Pc rats were determined to be 3.63 ± 0.48, 2.3 ± 0.27, and 3.17 ± 0.29, respectively, on a scale of 0 to 5 (Fig. 4A). These results indicated that 44-Ant-44 substantially reduced the pulmonary damage caused by P. carinii infection (P < 0.001) but that 44-Bn-44 had little effect (P = 0.587). The overall organism burden on a scale of 0 to 4 of 44-Ant-44-treated Dex-Pc rats was determined to be 0.8 ± 0.27, whereas those of untreated and 44-Bn-44-treated Dex-Pc rats were 3 ± 0 and 2.17 ± 0.29, respectively (Fig. 4B). These results indicated that both 44-Ant-44 (P < 0.001) and 44-Bn-44 (P = 0.001) decreased the organism burden in P. carinii-infected lungs, but 44-Ant-44 worked much better than 44-Bn-44.
FIG. 4.
Therapeutic activity of PAT inhibitors. The effects of 44-Ant-44 and 44-Bn-44 on PCP-mediated pulmonary pathology, P. carinii burden, and macrophage apoptosis were examined after 3 weeks of treatment. (A) Pulmonary pathology of H&E-stained lung sections. (B) P. carinii cyst burden analyzed by Grocott's methenamine silver staining. (C) TUNEL assay for apoptotic macrophages. Results shown are representative images of three 44-Bn-44-treated, five 44-Ant-44-treated, and four untreated Dex and Dex-Pc rats. Magnification, ×100.
Effects of compounds 44-Ant-44 and 44-Bn-44 on apoptosis of AMs.
Results of TUNEL assays on lung sections from untreated, 44-Ant-44-treated, and 44-Bn-44-treated Dex-Pc rats revealed that 21 days of treatment with 44-Ant-44 reduced the number of apoptotic AMs by approximately 80% in the lungs (P < 0.001) (Fig. 4C). In contrast, 44-Bn-44 did not significantly reduce the apoptosis rates of AMs.
DISCUSSION
Polyamines are essential for cell growth. Increased growth rates of cells are often accompanied by increased intracellular polyamine levels, as seen in many cancers (12). The major cause of elevated polyamine levels is increased synthesis due to increased levels of ODC. Therefore, inhibition of ODC activity with DFMO is a potential therapy for cancer. Unfortunately, clinical trials showed that DFMO has little or no effect against cancers (24, 31, 42). Possible causes for this treatment failure may include decreased polyamine catabolism (27) and increased polyamine uptake (17) that compensated for the decreased polyamine synthesis during DFMO therapy.
Although DFMO therapy has not been successful against cancer, it was found to be very effective against African trypanosomiasis caused by Trypanosoma brucei subspecies (2). This could be due to the huge difference between the half-life of T. brucei subspecies (more than 10 h) and that of mammalian ODC (10 to 20 min). The slower turnover rate of T. brucei subspecies ODC may render it more sensitive to DFMO (6). Another possibility is the lack of a system in T. brucei subspecies to acquire polyamines from exogenous sources (3). The accumulation of S-adenosylmethionine during DFMO treatment is also a possibility, as this may cause abnormal methylation of intracellular components (3), making T. brucei subspecies more susceptible to DFMO.
DFMO has also been shown to inhibit the growth of P. carinii in short-term cultures at a concentration of 1 mM (11) and used to treat PCP in humans with various success rates (33 to 57%) (14, 32, 36, 38, 39). This partial success suggests that antipolyamine therapy can be effective against PCP, but inhibiting polyamine synthesis alone may not be sufficient. Since polyamines are present in both Pneumocystis and host cells, the involvement of host polyamine synthesis and metabolism systems should also be considered in the antipolyamine therapy against PCP. Our previous studies have found that AM apoptosis is a cause of disease progression during PCP (22) and that this apoptosis is triggered by increased polyamine levels due to both increased synthesis and uptake of polyamines (25). These findings may explain the limited success of DFMO therapy against PCP, because DFMO inhibits only polyamine synthesis, not uptake.
In this study, we used anthracene- and benzene-polyamine conjugates, which target the polyamine transporter with high affinity and inhibit uptake of native polyamines. Two compounds, 44-Ant-44 and 44-Bn-44, were tested in vivo, and 44-Ant-44 was found to significantly reduce organism burden (P < 0.001) (Fig. 4B), decrease lung inflammation (P < 0.001) (Fig. 4A), and prolong the survival of treated PCP animals (P = 0.032) (Fig. 3). We believe that the effectiveness of this treatment is due to the decreased rate of apoptosis of AMs (P < 0.001) (Fig. 4C), as PAT inhibitors would block polyamine uptake and thus decrease the levels of intracellular polyamines and H2O2. As a result, AMs survive to clear the organisms, thus reducing organism burden and lung inflammation, leading to longer survival of animals as shown previously (22). Although AMs were the intended target in this 44-Ant-44 therapy, it is possible that 44-Ant-44 also affected PAT in P. carinii, which actively excretes acetylated polyamines (27).
PAT inhibitors can be structurally classified into two major types: polyamine polymer and polyamine-lysine conjugate. The homodimers of putrescine (10) and spermine (15, 19) and polymer of spermine (1) have been shown to effectively inhibit PAT. Simple lysine-spermine conjugate (7, 46) and lipophilic lysine-spermine (5) also exhibit PAT inhibition activity. 44-Ant-44, the compound shown to have anti-PCP activity in this study, is structurally similar to a spermidine dimer cross-linked by a 9,10-bis-substituted anthraldehyde.
In a recent report, 44-Ant-44 was shown to have the highest PAT inhibition activity in a screen of CHO cells (21, 33). This screen utilized two CHO cell lines: the PAT-active wild-type CHO K-1 line and the PAT-defective CHO-MG line. Comparison of conjugate cytotoxicity in these two CHO lines provided an important tool to detect selective conjugate delivery via the polyamine transporter. A conjugate with high utilization of the polyamine transporter would be very toxic to CHO cells but less so to CHO-MG cells. Highly selective polyamine transporter ligands should give high (CHO-MG/CHO) IC50 ratios. As shown in Table 1, 44-Ant-44 and 44-Bn-44 were the most PAT selective of the series, with CHO-MG/CHO IC50 ratios of >600. One potential explanation for why 44-Ant-44 was superior to the benzyl derivative, 44-Bn-44, is cellular metabolism. For example, a benzyl-homospermidine compound was shown to be metabolized to homospermidine in cell culture, whereas the anthryl-homospermidine was not (45). This is rationalized due to significant steric constraints near the benzylic position of the anthryl platform, which may impede the action of amine oxidases (33).
Although inhibition of polyamine uptake with compound 44-Ant-44 showed impressive results, it did not cure the disease. This is not surprising nor unexpected, as the increase in both ODC levels and the uptake of exogenous polyamines accounts for the increased polyamine levels in AMs during PCP. Studies using both ODC (DFMO) and PAT (44-Ant-44) inhibitors to treat PCP are being conducted. This combination therapy is expected to be a more effective PCP treatment, as a synergistic effect has been shown in cancer therapy (5, 7). Because of a limited supply of the compound, only one dosage and one treatment condition were tested in the current study. Additional dosages remain to be tested to determine whether a lower dose also works or whether a higher dose of the compound would be more effective. Other methods of drug delivery, such as placing the drug in drinking water, also need to be explored. Successful development of this combination therapy will have a great impact on cancer therapy, as cancers also have increased polyamine synthesis and uptake (12, 34).
Acknowledgments
The study was supported by grant R01 AI062259 from the National Institutes of Health.
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Aziz, S. M., M. P. Gosland, P. A. Crooks, J. W. Olson, and M. N. Gillespie. 1995. A novel polymeric spermine conjugate inhibits polyamine transport in pulmonary artery smooth muscle cells. J. Pharmacol. Exp. Ther. 274:181-186. [PubMed] [Google Scholar]
- 2.Bacchi, C. J., H. C. Nathan, S. H. Hutner, P. P. McCann, and A. Sjoerdsma. 1980. Polyamine metabolism: a potential therapeutic target in trypanosomes. Science 210:332-334. [DOI] [PubMed] [Google Scholar]
- 3.Bacchi, C. J., and N. Yarlett. 2002. Polyamine metabolism as chemotherapeutic target in protozoan parasites. Mini Rev. Med. Chem. 2:553-563. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett, M. S., J. A. Fishman, S. F. Queener, M. M. Durkin, M. A. Jay, and J. W. Smith. 1988. New rat model of Pneumocystis carinii infection. J. Clin. Microbiol. 26:1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns, M. R., G. F. Graminski, R. S. Weeks, Y. Chen, and T. G. O'Brien. 2009. Lipophilic lysine-spermine conjugates are potent polyamine transport inhibitors for use in combination with a polyamine biosynthesis inhibitor. J. Med. Chem. 52:1983-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrillo, C., S. Cejas, M. Cortes, C. Ceriani, A. Huber, N. S. Gonzalez, and I. D. Algranati. 2000. Sensitivity of trypanosomatid protozoa to DFMO and metabolic turnover of ornithine decarboxylase. Biochem. Biophys. Res. Commun. 279:663-668. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y., R. S. Weeks, M. R. Burns, D. W. Boorman, A. Klein-Szanto, and T. G. O'Brien. 2006. Combination therapy with 2-difluoromethylornithine and a polyamine transport inhibitor against murine squamous cell carcinoma. Int. J. Cancer 118:2344-2349. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson, A. B., Jr., M. Saric, and R. W. Grady. 1990. Deferoxamine and eflornithine (dl-α-difluoromethylornithine) in a rat model of Pneumocystis carinii pneumonia. Antimicrob. Agents Chemother. 34:1833-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarkson, A. B., Jr., D. E. Williams, and C. Rosenberg. 1988. Efficacy of dl-α-difluoromethylornithine in a rat model of Pneumocystis carinii pneumonia. Antimicrob. Agents Chemother. 32:1158-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covassin, L., M. Desjardins, D. Soulet, R. Charest-Gaudreault, M. Audette, and R. Poulin. 2003. Xylylated dimers of putrescine and polyamines: influence of the polyamine backbone on spermidine transport inhibition. Bioorg. Med. Chem. Lett. 13:3267-3271. [DOI] [PubMed] [Google Scholar]
- 11.Cushion, M. T., D. Stanforth, M. J. Linke, and P. D. Walzer. 1985. Method of testing the susceptibility of Pneumocystis carinii to antimicrobial agents in vitro. Antimicrob. Agents Chemother. 28:796-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerner, E. W., and F. L. Meyskens, Jr. 2004. Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer 4:781-792. [DOI] [PubMed] [Google Scholar]
- 13.Gillin, F. D., D. S. Reiner, and P. P. McCann. 1984. Inhibition of growth of Giardia lamblia by difluoromethylornithine, a specific inhibitor of polyamine biosynthesis. J. Protozool. 31:161-163. [DOI] [PubMed] [Google Scholar]
- 14.Gilman, T. M., Y. J. Paulson, C. T. Boylen, P. N. Heseltine, and O. P. Sharma. 1986. Eflornithine treatment of Pneumocystis carinii pneumonia in AIDS. JAMA 256:2197-2198. [PubMed] [Google Scholar]
- 15.Graminski, G. F., C. L. Carlson, J. R. Ziemer, F. Cai, N. M. Vermeulen, S. M. Vanderwerf, and M. R. Burns. 2002. Synthesis of bis-spermine dimers that are potent polyamine transport inhibitors. Bioorg. Med. Chem. Lett. 12:35-40. [DOI] [PubMed] [Google Scholar]
- 16.Hanson, W. L., M. M. Bradford, W. L. Chapman, Jr., V. B. Waits, P. P. McCann, and A. Sjoerdsma. 1982. α-Difluoromethylornithine: a promising lead for preventive chemotherapy for coccidiosis. Am. J. Vet. Res. 43:1651-1653. [PubMed] [Google Scholar]
- 17.Heston, W. D., D. Kadmon, D. F. Covey, and W. R. Fair. 1984. Differential effect of α-difluoromethylornithine on the in vivo uptake of 14C-labeled polyamines and methylglyoxal bis(guanylhydrazone) by a rat prostate-derived tumor. Cancer Res. 44:1034-1040. [PubMed] [Google Scholar]
- 18.Huang, L., K. Crothers, C. Atzori, T. Benfield, R. Miller, M. Rabodonirina, and J. Helweg-Larsen. 2004. Dihydropteroate synthase gene mutations in Pneumocystis and sulfa resistance. Emerg. Infect. Dis. 10:1721-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber, M., J. G. Pelletier, K. Torossian, P. Dionne, I. Gamache, R. Charest-Gaudreault, M. Audette, and R. Poulin. 1996. 2,2′-Dithiobis(N-ethyl-spermine-5-carboxamide) is a high affinity, membrane-impermeant antagonist of the mammalian polyamine transport system. J. Biol. Chem. 271:27556-27563. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi, K., and K. Kashiwagi. 2000. Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 271:559-564. [DOI] [PubMed] [Google Scholar]
- 21.Kaur, N., J. G. Delcros, J. Imran, A. Khaled, M. Chehtane, N. Tschammer, B. Martin, and O. Phanstiel IV. 2008. A comparison of chloroambucil- and xylene-containing polyamines leads to improved ligands for accessing the polyamine transport system. J. Med. Chem. 51:1393-1401. [DOI] [PubMed] [Google Scholar]
- 22.Lasbury, M. E., P. J. Durant, C. A. Ray, D. Tschang, R. Schwendener, and C. H. Lee. 2006. Suppression of alveolar macrophage apoptosis prolongs survival of rats and mice with Pneumocystis pneumonia. J. Immunol. 176:6443-6453. [DOI] [PubMed] [Google Scholar]
- 23.Lasbury, M. E., S. Merali, P. J. Durant, D. Tschang, C. A. Ray, and C. H. Lee. 2007. Polyamine-mediated apoptosis of alveolar macrophages during Pneumocystis pneumonia. J. Biol. Chem. 282:11009-11020. [DOI] [PubMed] [Google Scholar]
- 24.Levin, V. A., K. R. Hess, A. Choucair, P. J. Flynn, K. A. Jaeckle, A. P. Kyritsis, W. K. Yung, M. D. Prados, J. M. Bruner, S. Ictech, M. J. Gleason, and H. W. Kim. 2003. Phase III randomized study of postradiotherapy chemotherapy with combination alpha-difluoromethylornithine-PCV versus PCV for anaplastic gliomas. Clin. Cancer Res. 9:981-990. [PubMed] [Google Scholar]
- 25.Liao, C. P., M. E. Lasbury, S. H. Wang, C. Zhang, P. J. Durant, Y. Murakami, S. Matsufuji, and C. H. Lee. 2009. Pneumocystis mediates overexpression of antizyme inhibitor resulting in increased polyamine levels and apoptosis in alveolar macrophages. J. Biol. Chem. 284:8174-8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luk, G. D., G. Goodwin, L. J. Marton, and S. B. Baylin. 1981. Polyamines are necessary for the survival of human small-cell lung carcinoma in culture. Proc. Natl. Acad. Sci. USA 78:2355-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merali, S. 1999. Pneumocystis carinii polyamine catabolism. J. Biol. Chem. 274:21017-21022. [DOI] [PubMed] [Google Scholar]
- 28.Meshnick, S. R. 1999. Drug-resistant Pneumocystis carinii. Lancet 354:1318-1319. [DOI] [PubMed] [Google Scholar]
- 29.Milord, F., J. Pepin, L. Loko, L. Ethier, and B. Mpia. 1992. Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping sickness. Lancet 340:652-655. [DOI] [PubMed] [Google Scholar]
- 30.Müller, I. B., R. Das Gupta, K. Luersen, C. Wrenger, and R. D. Walter. 2008. Assessing the polyamine metabolism of Plasmodium falciparum as chemotherapeutic target. Mol. Biochem. Parasitol. 160:1-7. [DOI] [PubMed] [Google Scholar]
- 31.O'Shaughnessy, J. A., L. M. Demers, S. E. Jones, J. Arseneau, P. Khandelwal, T. George, R. Gersh, D. Mauger, and A. Manni. 1999. Alpha-difluoromethylornithine as treatment for metastatic breast cancer patients. Clin. Cancer Res. 5:3438-3444. [PubMed] [Google Scholar]
- 32.Paulson, Y. J., T. M. Gilman, P. N. Heseltine, O. P. Sharma, and C. T. Boylen. 1992. Eflornithine treatment of refractory Pneumocystis carinii pneumonia in patients with acquired immunodeficiency syndrome. Chest 101:67-74. [DOI] [PubMed] [Google Scholar]
- 33.Phanstiel, O., IV, N. Kaur, and J. G. Delcros. 2007. Structure-activity investigations of polyamine-anthracene conjugates and their uptake via the polyamine transporter. Amino Acids 33:305-313. [DOI] [PubMed] [Google Scholar]
- 34.Roy, U. K., N. S. Rial, K. L. Kachel, and E. W. Gerner. 2008. Activated K-RAS increases polyamine uptake in human colon cancer cells through modulation of caveolar endocytosis. Mol. Carcinog. 47:538-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudmann, D. G., A. M. Preston, M. W. Moore, and J. M. Beck. 1998. Susceptibility to Pneumocystis carinii in mice is dependent on simultaneous deletion of IFN-gamma and type 1 and 2 TNF receptor genes. J. Immunol. 161:360-366. [PubMed] [Google Scholar]
- 36.Sahai, J., and A. J. Berry. 1989. Eflornithine for the treatment of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome: a preliminary review. Pharmacotherapy 9:29-33. [DOI] [PubMed] [Google Scholar]
- 37.Slatore, C. G., and S. A. Tilles. 2004. Sulfonamide hypersensitivity. Immunol. Allergy Clin. N. Am. 24:477-490, vii. [DOI] [PubMed] [Google Scholar]
- 38.Smego, R. A., Jr., S. Nagar, B. Maloba, and M. Popara. 2001. A meta-analysis of salvage therapy for Pneumocystis carinii pneumonia. Arch. Intern. Med. 161:1529-1533. [DOI] [PubMed] [Google Scholar]
- 39.Smith, D. E., S. Davies, J. Smithson, I. Harding, and B. G. Gazzard. 1992. Eflornithine versus cotrimoxazole in the treatment of Pneumocystis carinii pneumonia in AIDS patients. AIDS 6:1489-1493. [DOI] [PubMed] [Google Scholar]
- 40.Thomas, C. F., Jr., and A. H. Limper. 2007. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat. Rev. Microbiol. 5:298-308. [DOI] [PubMed] [Google Scholar]
- 41.Valerio, A., E. Tronconi, F. Mazza, A. Cargnel, G. Fantoni, and C. Atzori. 2006. DHPS-mutated isolates of Pneumocystis jirovecii from HIV-infected individuals: analysis of related ITS genotypes. J. Eukaryot. Microbiol. 53(Suppl. 1):S108-S109. [DOI] [PubMed] [Google Scholar]
- 42.Vlastos, A. T., L. A. West, E. N. Atkinson, I. Boiko, A. Malpica, W. K. Hong, and M. Follen. 2005. Results of a phase II double-blinded randomized clinical trial of difluoromethylornithine for cervical intraepithelial neoplasia grades 2 to 3. Clin. Cancer Res. 11:390-396. [PubMed] [Google Scholar]
- 43.Wang, C., J. G. Delcros, J. Biggerstaff, and O. Phanstiel, IV. 2003. Molecular requirements for targeting the polyamine transport system. Synthesis and biological evaluation of polyamine-anthracene conjugates. J. Med. Chem. 46:2672-2682. [DOI] [PubMed] [Google Scholar]
- 44.Wang, C., J. G. Delcros, J. Biggerstaff, and O. Phanstiel, IV. 2003. Synthesis and biological evaluation of N1-(anthracen-9-ylmethyl)triamines as molecular recognition elements for the polyamine transporter. J. Med. Chem. 46:2663-2671. [DOI] [PubMed] [Google Scholar]
- 45.Wang, C., J. G. Delcros, L. Cannon, F. Konate, H. Carias, J. Biggerstaff, R. A. Gardner, and O. Phanstiel, IV. 2003. Defining the molecular requirements for the selective delivery of polyamine conjugates into cells containing active polyamine transporters. J. Med. Chem. 46:5129-5138. [DOI] [PubMed] [Google Scholar]
- 46.Weeks, R. S., S. M. Vanderwerf, C. L. Carlson, M. R. Burns, C. L. O'Day, F. Cai, B. H. Devens, and H. K. Webb. 2000. Novel lysine-spermine conjugate inhibits polyamine transport and inhibits cell growth when given with DFMO. Exp. Cell Res. 261:293-302. [DOI] [PubMed] [Google Scholar]
- 47.Yarlett, N., and C. J. Bacchi. 1988. Effect of dl-alpha-difluoromethylornithine on polyamine synthesis and interconversion in Trichomonas vaginalis grown in a semi-defined medium. Mol. Biochem. Parasitol. 31:1-9. [DOI] [PubMed] [Google Scholar]