Abstract
Two large studies compared posaconazole and fluconazole or itraconazole for prophylaxis in subjects undergoing allogeneic hematopoietic stem cell transplantation or subjects with acute myelogenous leukemia. To assess the impact of prophylaxis on colonization and the development of resistance in Saccharomyces yeasts, identification and susceptibility testing were performed with yeasts cultured at regular intervals from mouth, throat, and stool samples. Prior to therapy, 34 to 50% of the subjects were colonized with yeasts. For all three drugs, the number of positive Candida albicans cultures decreased during drug therapy. In contrast, the proportion of subjects with positive C. glabrata cultures increased by two- and fourfold in the posaconazole and itraconazole arms, respectively. Likewise, in the fluconazole arm the proportion of subjects with positive C. krusei cultures increased twofold. C. glabrata was the species that most frequently exhibited decreases in susceptibility, and this trend did not differ significantly between the prophylactic regimens. For the subset of subjects from whom colonizing C. glabrata isolates were recovered at the baseline and the end of treatment, approximately 40% of the isolates exhibited more than fourfold increases in MICs during therapy. Molecular typing of the C. albicans and C. glabrata isolates confirmed that the majority of the baseline and end-of-treatment isolates were closely related, suggesting that they were persistent colonizers and not newly acquired. Overall breakthrough infections by Candida species were very rare (∼1%), and C. glabrata was the colonizing species that was the most frequently associated with breakthrough infections.
Invasive fungal infections (IFIs) are a leading cause of morbidity and mortality in neutropenic patients with hematological malignancies and recipients of hematopoietic stem cell transplants (HSCT). Treatment of an established IFI is frequently very difficult, and the most effective agents have treatment-limiting toxicities (19). Consequently, antifungal prophylaxis is increasingly being used for patients at high risk of acquiring a fungal infection; the benefits of antifungal prophylaxis include reduced mortality as well as reduced health care costs (5, 6, 10, 12, 17, 18, 20). However, there are concerns that the prophylactic agent may select for less susceptible isolates. Changes in susceptibility can be caused by mutations in existing colonizing isolates, through overgrowth (by a previously minor species), or by de novo colonization by inherently less susceptible species. This concern is particularly relevant for Candida, since colonization of the oral mucosa, gastrointestinal tract, and skin are risk factors associated with the acquisition of invasive Candida infections (11, 13).
Two large randomized clinical trials compared orally administered posaconazole with either fluconazole or itraconazole for antifungal prophylaxis in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) or HSCT recipients receiving high-dose immunosuppressive therapy for graft-versus-host disease (GVHD) (5, 20). As part of these trials, sequential samples from nonsterile sites were collected and cultured to detect colonizing yeast isolates; this report details the analysis of those isolates. Specifically, the impact of azole prophylaxis on the prevalence, species transition, and development of resistance among colonizing yeast isolates and the association between colonization and infection were evaluated. The influence of the duration of prophylaxis and the differential impacts of azoles with markedly different antifungal spectra are also discussed.
(Part of this work was presented at the 46th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2006.)
MATERIALS AND METHODS
Subjects and study design.
Study 1 enrolled 600 allogeneic HSCT recipients (age 13 years or older) with acute or chronic GVHD (20). The subjects were randomly assigned to receive posaconazole (n = 301) at 200 mg three times daily or fluconazole (n = 299) at 400 mg once daily. Dosing was for up to 16 weeks; the median and the mean durations of dosing were 111 and 80 days, respectively. Study 2 enrolled 602 neutropenic subjects (age 13 years or older) with AML or MDS undergoing induction chemotherapy (5). The subjects were randomly assigned to receive posaconazole (n = 304) at 200 mg three times daily or a standard azole, fluconazole (n = 240) at 400 mg once daily or itraconazole (n = 58) at 200 mg twice daily. Prophylaxis was administered with each cycle of chemotherapy until complete remission or up to a maximum of 12 weeks; the median and the mean durations of dosing were 25 and 37 days, respectively.
Microbiology.
In study 1, clinical oral samples for surveillance cultures were obtained prior to the initiation of therapy (referred to as the baseline [BL]) and at weeks 2, 4, 8, 12, and 16 by use of an oral swish technique. In study 2, samples from the pharynx and stool were obtained for surveillance cultures at BL and weekly thereafter. Identification and susceptibility testing were performed at central reference laboratories. The isolates were identified by using CHROMagar Candida medium (Paris, France) and, when necessary, with the Vitek identification system with the yeast biochemical card (bioMérieux Vitek Inc, Hazelwood, MO). MIC testing was performed as described in Clinical and Laboratory Standards Institute (CLSI) document M27-A2 (4). The CLSI interpretive criteria for fluconazole and itraconazole were used: for susceptible, ≤8 and ≤0.125 μg/ml, respectively; for resistant, ≥64 and ≥1 μg/ml, respectively. For illustrative purposes, a susceptible breakpoint of ≤1 μg/ml was used for posaconazole. For the analysis of resistance development, the isolates in the BL cultures were defined as isolates cultured before or within 7 days prior to the start of therapy; the isolates in end-of-treatment (EOT) cultures were defined as isolates cultured less than 30 days prior to or within 7 days after the cessation of antifungal therapy.
Molecular analysis.
Molecular typing of Candida albicans and C. glabrata isolates was performed by multilocus sequence typing (MLST), as described previously (1). Dendrograms were generated by using the MLST website (www.mlst.net) at Imperial College London (developed by David Aanensen and funded by the Wellcome Trust). Control strains (C. glabrata C110, C454, and C624 and C. albicans C43 and C600) were from the Schering-Plough culture collection. DNA sequencing and measurement of gene expression levels in the C. glabrata isolates were performed by real-time PCR, as described previously. (2). Probes were designed to interrogate the CDR1, CDR2, and ERG11 genes and URA3 as the control gene. RNA was extracted from cells grown to mid-exponential phase in RPMI 1640 medium. Multiplex reactions were run with an 18S rRNA probe as an internal control, and the relative gene expression levels (the change in the threshold cycle [ΔCT] number) were calculated as ΔCT for test gene = CT for test gene − CT for 18S rRNA. For all probe sets there was a linear relationship between the signal and input cDNA over a 1,000-fold concentration range, and the CT values in these validation tests encompassed the range of values seen for the test samples. In each experiment, triplicate measurements were made, and each isolate was analyzed a minimum of three times.
RESULTS
Changes in colonization by yeasts in response to antifungal prophylaxis.
The proportions of subjects with positive Candida cultures at BL were similar for the two treatment groups: for posaconazole in studies 1 and 2, the numbers were 133 of 280 (51%) and 101 of 254 (40%), respectively, and for fluconazole in studies 1 and 2 (and also for itraconazole for study 2), the numbers were 129 of 266 (48%) and 88 of 258 (34%), respectively. C. albicans was by far the most prevalent colonizer at BL, followed by C. glabrata and C. krusei. The low numbers of other yeasts and Candida species precluded meaningful analysis, so data for those species are not included.
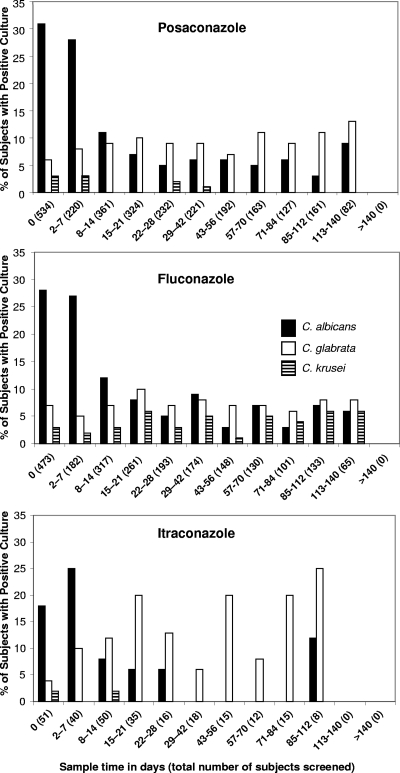
For all three prophylaxis regimens, the proportion of patients with positive C. albicans cultures decreased over the course of therapy (Fig. 1). In contrast, there was a slight increase in the proportion of positive C. glabrata cultures in the posaconazole-treated group (32 of 534 subjects at BL, 11 of 82 at EOT) but not in the fluconazole-treated group. There was a more pronounced increase in the proportion of positive C. glabrata cultures for subjects receiving itraconazole; however, the numbers of subjects were relatively small (2 of 51 subjects at BL, 2 of 8 at EOT). C. krusei, a species regarded as being inherently fluconazole resistant, was recovered twice as frequently at EOT (12 of 473 subjects at BL, 4 of 65 at EOT) from subjects receiving fluconazole but was not found in cultures at EOT from subjects receiving posaconazole or itraconazole.
FIG. 1.
Changes in the frequency of recovery of colonizing Candida species in subjects receiving antifungal prophylaxis. Shown are the numbers of positive cultures (data only for the three most prevalent Candida species are shown) identified in both studies while the subjects were receiving prophylaxis with posaconazole, fluconazole, or itraconazole.
Changes in susceptibility of colonizing isolates while receiving therapy.
Susceptibility testing of the surveillance isolates was performed at national reference laboratories. Since broth microdilution susceptibility testing is accurate to within ±1 doubling dilution, a more than fourfold difference in the MICs between isolates was considered significant.
For C. albicans isolates cultured from the posaconazole-treated subjects from either study, there was little to no change in the MIC90s between BL and EOT, and the highest MIC observed over the course of prophylaxis (maximum MIC) was lower at EOT than at BL (Table 1). For the same subjects, the MIC90s for C. glabrata also did not change; however, the MIC90s (but not the MIC50s) were two- to fourfold higher than those seen previously in a large survey of clinical isolates (14). For the fluconazole-treated subjects from study 2, the MIC90s for C. albicans also showed little change between BL and EOT, although for the same isolates the maximum MIC was much higher at EOT than at BL. In contrast, both the MIC90s and the maximum MIC for the EOT C. albicans isolates cultured from the fluconazole-treated subjects in study 1, who had a longer mean duration of dosing, exhibited significant increases, indicating that a small proportion of the isolates had developed resistance to all three azoles. The low numbers of subjects receiving itraconazole precluded meaningful analysis.
TABLE 1.
Changes in susceptibilities of the major colonizing Candida species in subjects receiving antifungal prophylaxis
| Study no., antifungal tested, and time of evaluation | Results for subjects receiving posaconazole |
Results for subjects receiving fluconazole |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
C. albicans |
C. glabrata |
C. krusei |
C. albicans |
C. glabrata |
C. krusei |
|||||||||||||||||||
| No. of subjects | MIC50 (μg/ml) | MIC90 (μg/ml) | Maximum MICb (μg/ml) | No. of subjects | MIC50 (μg/ml) | MIC90 (μg/ml) | Maximum MIC (μg/ml) | No. of subjects | MIC50 (μg/ml) | MIC90 (μg/ml) | Maximum MIC (μg/ml) | No. of subjects | MIC50 (μg/ml) | MIC90 (μg/ml) | Maximum MIC (μg/ml) | No. of subjects | MIC50 (μg/ml) | MIC90 (μg/ml) | Maximum MIC (μg/ml) | No. of subjects | MIC50 (μg/ml) | MIC90 (μg/ml) | Maximum MIC (μg/ml) | |
| Study 1, HSCT recipients | ||||||||||||||||||||||||
| Posaconazole | ||||||||||||||||||||||||
| BL | 60 | 0.016 | 0.06 | 16 | 15 | 0.5 | 4 | 8 | 9 | —a | — | 0.5 | 57 | 0.016 | 0.03 | 0.06 | 18 | 1 | 8 | 16 | 9 | 0.5 | ||
| EOT | 16 | 0.03 | 0.12 | 0.25 | 26 | 2 | 8 | 8 | 0 | — | — | 16 | 0.03 | 16 | 16 | 24 | 2 | 8 | 16 | 11 | 0.25 | 0.5 | 1 | |
| Fluconazole | ||||||||||||||||||||||||
| BL | 60 | 0.25 | 4 | 64 | 15 | 16 | 64 | 64 | 9 | — | — | 64 | 57 | 0.25 | 1 | 16 | 18 | 16 | 64 | 64 | 9 | 64 | ||
| EOT | 16 | 0.25 | 16 | 64 | 26 | 64 | 64 | 64 | 0 | — | — | 16 | 1 | 64 | 64 | 24 | 32 | 64 | 64 | 11 | 64 | 64 | 64 | |
| Itraconazole | ||||||||||||||||||||||||
| BL | 60 | 0.03 | 0.06 | 16 | 15 | 1 | 8 | 8 | 9 | — | — | 16 | 57 | 0.03 | 0.06 | 0.06 | 18 | 2 | 16 | 32 | 9 | 1 | ||
| EOT | 16 | 0.03 | 0.25 | 0.25 | 26 | 2 | 8 | 8 | 0 | — | — | 16 | 0.03 | 16 | 16 | 24 | 1 | 16 | 32 | 11 | 0.5 | 16 | 16 | |
| Study 2, subjects with AML or MDS | ||||||||||||||||||||||||
| Posaconazole | ||||||||||||||||||||||||
| BL | 103 | 0.015 | 0.015 | 0.06 | 16 | 0.25 | 2 | 4 | 6 | — | — | 0.25 | 74 | 0.015 | 0.015 | 0.06 | 14 | 0.5 | 2 | 8 | 3 | 0.25 | ||
| EOT | 19 | 0.015 | 0.015 | 0.03 | 36 | 0.5 | 4 | 8 | 0 | — | — | 14 | 0.015 | 0.015 | 8 | 18 | 0.5 | 1 | 2 | 7 | 0.25 | |||
| Fluconazole | ||||||||||||||||||||||||
| BL | 103 | 0.25 | 0.25 | 2 | 16 | 4 | 16 | 32 | 6 | — | — | 32 | 74 | 0.125 | 0.25 | 1 | 14 | 8 | 16 | 64 | 3 | 32 | ||
| EOT | 19 | 0.25 | 0.25 | 0.25 | 36 | 8 | 64 | 64 | 0 | — | — | 14 | 0.25 | 0.5 | 64 | 18 | 4 | 16 | 16 | 7 | 64 | |||
| Itraconazole | ||||||||||||||||||||||||
| BL | 103 | 0.015 | 0.03 | 0.03 | 16 | 0.25 | 8 | 8 | 6 | — | — | 0.5 | 74 | 0.015 | 0.015 | 0.125 | 14 | 0.5 | 2 | 8 | 3 | 0.5 | ||
| EOT | 19 | 0.015 | 0.03 | 0.03 | 36 | 0.5 | 8 | 8 | 0 | — | — | 14 | 0.015 | 0.015 | 8 | 18 | 0.5 | 2 | 8 | 7 | 1 | |||
When n is <10, no MIC50 or MIC90 is provided.
The highest MIC observed during the course of drug therapy.
Resistance development was also analyzed for the isolates from the subset of subjects who received at least 14 days of antifungal therapy and who had the same colonizing species at BL and EOT. When the data for both studies are combined, 47 subjects receiving fluconazole (26 colonized with C. albicans, 12 with C. glabrata, 6 with C. krusei, 3 with Saccharomyces cerevisiae, 1 with a Candida sp., and 1 with both C. albicans and S. cerevisiae) and 53 receiving posaconazole (25 colonized with C. albicans; 20 with C. glabrata; 5 with S. cerevisiae; and 1 each with C. dubliniensis, C. tropicalis, and C. inconspicua) met these criteria. Compared with the corresponding BL isolate, 9 (19%) and 12 (23%) of the EOT isolates from the fluconazole- and posaconazole-treated subjects, respectively, exhibited a more than fourfold increase in MIC for at least one of the azoles (Table 2). All but 1 of these 21 subjects were colonized with C. albicans or C. glabrata (the subject who was the exception was colonized with S. cerevisiae). As noted above, the majority of the isolates, particularly for those subjects receiving fluconazole, were recovered from subjects from study 1. For the posaconazole- and fluconazole-treated subjects, the proportions with EOT isolates exhibiting a more than fourfold increase in MICs were 45% and 42%, respectively, for C. glabrata and 8% and 15%, respectively, for C. albicans. The majority of the EOT C. glabrata isolates were resistant to all three azoles, while four of the six EOT C. albicans isolates remained susceptible to fluconazole and itraconazole (including the two isolates from subjects receiving posaconazole) and five were susceptible to posaconazole.
TABLE 2.
Isolates that exhibited more than fourfold increases in MICs cultured from subjects who received at least 14 days of antifungal prophylaxis and who had the same colonizing species at BL and EOT
| Prophylactic agent | No. of isolates with more than fourfold MIC increase/total no.a (species) | Subject no./sample sourceb/no. of days of prophylaxis | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|---|---|
| Posaconazole |
Fluconazole |
Itraconazole |
||||||
| BL | EOT | BL | EOT | BL | EOT | |||
| Posaconazole | 2/25 (C. albicans) | 262/mouth/115 | <0.015 | <0.015 | 0.25 | 2 | <0.015 | 0.06 |
| 301/mouth/114 | <0.015 | 0.03 | <0.125 | 0.25 | <0.015 | 0.25 | ||
| 9/20 (C. glabrata) | 1102/stool/26 | 0.25 | >8 | 4 | >64 | 1 | >8 | |
| 1318/stool/36 | 0.5 | 8 | 4 | >64 | 0.25 | >8 | ||
| 1187/stool/22 | 0.5 | 4 | 16 | 32 | 1 | 4 | ||
| 1497/pharynx/61 | 0.03 | 4 | 2 | >64 | 0.06 | 8 | ||
| 1491/pharynx/23 | 0.5 | 8 | 4 | >64 | 1 | 8 | ||
| 186/mouth/112 | 0.25 | 2 | 8 | 32 | 0.5 | 2 | ||
| 474/mouth/124 | 0.25 | 4 | 16 | 64 | 4 | 8 | ||
| 975/mouth/112 | 0.25 | 4 | 4 | 128 | 0.25 | 8 | ||
| 270/mouth/108 | 0.06 | 1 | 8 | 8 | 0.125 | 1 | ||
| 1/5 (S. cerevisiae) | 513/mouth/112 | 1 | 4 | 8 | 32 | 0.5 | 4 | |
| Fluconazole | 4/26 (C. albicans) | 031/mouth/109 | <0.015 | <0.015 | 16 | 128 | 0.03 | 0.06 |
| 462/mouth/112 | <0.015 | 0.06 | 8 | 8 | 0.06 | 0.03 | ||
| 042/mouth/112 | <0.015 | <0.015 | <0.125 | 1 | <0.015 | 0.06 | ||
| 928/mouth/112 | <0.015 | >8 | <0.125 | 128 | <0.015 | >8 | ||
| 5/12 (C. glabrata) | 1331/pharynx/21 | 2 | 8 | 8 | >64 | 1 | 8 | |
| 002/mouth/116 | 0.5 | 4 | 16 | 64 | 1 | >8 | ||
| 497/mouth/114 | 0.5 | 2 | 16 | 32 | 1 | 8 | ||
| 807/mouth/114 | 0.25 | >8 | 16 | 128 | 0.5 | >8 | ||
| 491/mouth/112 | 0.25 | 2 | 4 | 32 | 0.25 | 2 | ||
Only the subset of subjects with identical species at BL and EOT are included.
Mouth, study 1 (HSCT recipients); stool and pharynx, study 2 (subjects with AML or MDS).
Molecular characterization of isolates exhibiting a more than fourfold increase in MICs.
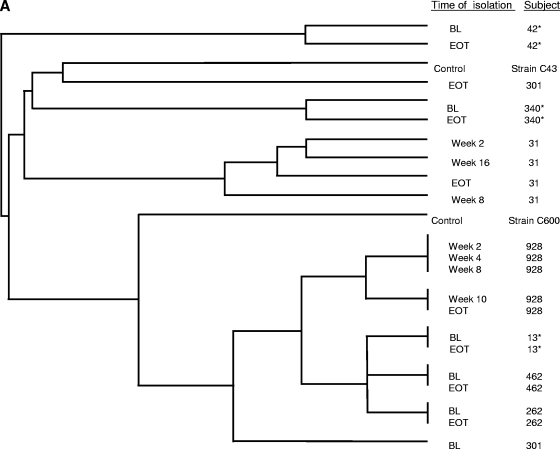
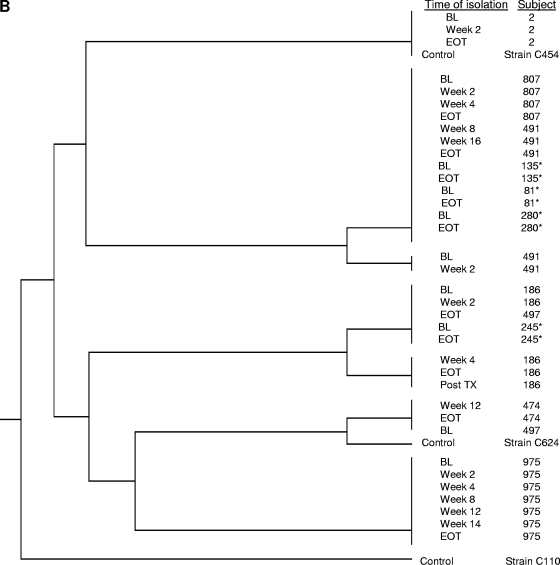
The relatedness between BL and EOT isolates, as well as (when available) isolates cultured at intervening time points, was assessed by MLST. The controls for the MLST analysis included five unrelated clinical isolates (C. glabrata strains C110, C454, and C624 and C. albicans strains C43 and C600), as well as matched BL and EOT isolates from seven subjects (three C. albicans isolates and four C. glabrata isolates) that did not show any MIC increases; the intrasubject isolates from the seven subjects clustered together (Fig. 2A and B).
FIG. 2.
MLST of C. albicans (A) and C. glabrata (B) isolates exhibiting decreases in susceptibility to azoles cultured from subjects receiving azole prophylaxis. Additional paired isolates that did not exhibit any changes in susceptibility (marked with asterisks) and clinical isolates unrelated to this study (marked “control”) were included as controls.
For the C. albicans isolates from subjects receiving posaconazole, the EOT isolates from subjects 262 and 301 were susceptible to posaconazole and fluconazole, while the EOT isolate from subject 301 was susceptible-dose dependent to itraconazole (Table 3). There were no Erg11p substitutions in the isolates from subject 301; both the BL and EOT isolates from subject 262 harbored substitutions (D116E and K128T) previously identified in azole-susceptible isolates (2). MLST analysis suggested that the two isolates from subject 262 were closely related, while those from subject 301 were unrelated (Fig. 2A).
TABLE 3.
Changes in levels of gene expression between BL and EOT isolates from subjects with a C. glabrata isolate that exhibited a more than fourfold MIC increase while receiving prophylaxis
| Prophylactic agent | Subject no. | No. of times tested | Fold increase/decrease in expression in EOT isolate vs that in BL isolate (SD) |
|||
|---|---|---|---|---|---|---|
| CDR1 | CDR2 | ERG11 | URA3 | |||
| Posaconazole | 186 | 3 | −1.5a (1.1) | −1.45 (1.1) | −1.4 (1.1) | −1.4 (1.1) |
| 975 | 4 | 2.25 (3.9) | 1.5 (3.0) | 1.6 (2.6) | 1.2 (3.2) | |
| Fluconazole | 002 | 5 | 1.5 (2.2) | −1.2 (2.0) | −1.2 (1.8) | −1.2 (2.6) |
| 497 | 5 | 1.3 (1.7) | −1.2 (1.6) | −1.2 (1.4) | −1.2 (1.6) | |
| 807 | 5 | 4.3 (2.1) | NDb | 1.1 (1.6) | 1.4 (2.5) | |
| 491 | 4 | 4.5 (2.5) | ND | 1.2 (1.4) | 1.0 (1.5) | |
A negative sign indicates that the expression level in the EOT isolate was lower than that measured in the BL isolate.
ND, not detected, as the signal was below the limit of detection.
For the C. albicans isolates from subjects receiving fluconazole, for subject 031 a K143R substitution in Erg11p, previously associated with reduced susceptibility to fluconazole, was identified in the EOT isolate but was absent from the azole-susceptible isolate recovered at week 2 (2). The BL and EOT isolates from subject 462 remained highly susceptible to posaconazole and itraconazole but had slightly elevated fluconazole MICs; both isolates harbored a substitution (Y132F) in Erg11p previously associated with reduced susceptibility to fluconazole (2). Despite the decrease in susceptibility to fluconazole over the course of therapy, the BL and EOT isolates from subject 042 remained highly susceptible to all azoles and were not further analyzed (other than by MLST). The EOT isolate from subject 928, which exhibited high-level resistance to all three azoles, had no substitutions in Erg11p but did have a homozygous nonsense mutation in codon 178 of ERG3; such mutations confer high-level azole resistance (3, 15). None of the azole-susceptible isolates cultured at weeks 2, 4, 8, and 10 harbored nonsense mutations in ERG3. MLST analysis indicated that for all four subjects detailed above, the intrasubject isolates were closely related.
For the C. glabrata isolates from subjects receiving posaconazole, isolates from subjects 270 and 474 were not available. No substitutions were identified in Erg11p from either BL or EOT isolates from subjects 186 and 975; and real-time PCR analysis did not reveal any significant differences in the levels of expression of CDR1, CDR2, or ERG11 (or URA3) between the BL and EOT isolates (Table 3). MLST analysis indicated that for both subjects, the intrasubject isolates (including isolates from intermediate time points) were closely related (Fig. 2B).
For the C. glabrata isolates from subjects receiving fluconazole, no substitutions in Erg11p were detected in any of the paired BL and EOT isolates from subjects 002, 497, 807, and 491. The level of expression of CDR1 was approximately fourfold higher in the EOT isolates than in the matched BL isolates from subjects 491 and 807 (Table 3). Interestingly, the level of CDR2 expression in these two isolates was below the limit of detection. No significant changes in the levels of expression were observed between the isolates from subjects 002 and 497. MLST analysis demonstrated that the intrasubject isolates from subjects 002, 807, and 491 were closely related, whereas the BL and EOT isolates from subject 497 clustered separately.
Breakthrough IFIs by colonizing species during and after prophylaxis.
In the two studies combined, there were eight proven Candida IFIs during treatment, four each in the posaconazole and fluconazole arms (5, 20). Two of the four IFIs from each treatment arm were attributed to the same species that was identified as a colonizer; for the remaining four subjects, there were no surveillance cultures available (Table 4). For one of the two posaconazole-treated subjects, both the colonizers and the breakthrough isolate were azole-resistant C. glabrata isolates; for the second subject, the breakthrough isolate was unavailable and the majority of the colonizing C. glabrata isolates appeared to be azole susceptible. For one of the two fluconazole-treated subjects, both the colonizers and the breakthrough isolate were identified to be fluconazole-resistant C. krusei isolates (both isolates were susceptible to posaconazole and itraconazole). The second subject was colonized by an azole-susceptible C. albicans isolate at BL, and the IFI occurred on day 2; the isolate was not available for MIC testing. None of the isolates causing IFIs detailed above (and in the section below) were available for MLST analysis, and therefore, it was not possible to assess the relationships between the surveillance isolates and those causing IFIs.
TABLE 4.
Subjects who experienced a breakthrough invasive fungal infection while receiving a study drug that was caused by the same Candida species identified as a colonizer
| Prophylactic agent | IFI and colonizing species | IFI onset (day)/duration of prophylaxis (days) | Results for isolates cultured from subjects with an invasive fungal infection |
|||
|---|---|---|---|---|---|---|
| Culture day/sourcea | MIC (μg/ml) |
|||||
| Posaconazole | Fluconazole | Itraconazole | ||||
| Posaconazole | C. glabrata | 44/48 | 29/stool | 1 | 8 | 1 |
| 29/pharynx | 8 | >64 | 8 | |||
| 36/pharynx | 1 | 8 | 1 | |||
| 42/stool | 1 | 8 | 1 | |||
| 44/blood | NAb | NA | NA | |||
| C. glabrata | 14/17 | 1/mouth | >8 | >64 | >8 | |
| 14/mouth | 8 | >64 | >8 | |||
| 14/blood | NA | NA | NA | |||
| 16/blood | >8 | NA | >8 | |||
| 19/mouth | 8 | >64 | >8 | |||
| Fluconazole | C. albicans | 2/2 | 1/mouth | <0.03 | <0.12 | <0.03 |
| C. krusei | 12/12 | 8/pharynx | 0.125 | >64 | 0.25 | |
| 8/stool | 0.125 | >64 | 0.25 | |||
| 12/blood | NA | NA | NA | |||
| 13/blood | 0.25 | >64 | 0.25 | |||
| 14/pharynx | 0.125 | 64 | 0.25 | |||
| 14/stool | 0.125 | 64 | 0.25 | |||
Cultures of samples from the mouth (study 1, HSCT recipients) and from the stool and pharynx (study 2, subjects with AML or MDS) were part of a surveillance program; blood cultures were performed in cases with an IFI.
NA, not available.
Colonization and breakthrough IFIs were also monitored after the discontinuation of prophylaxis in both studies. During this period, the majority of the subjects continued to receive antifungal therapy (but not posaconazole) at the discretion of their physicians. Nineteen Candida IFIs (9 and 10 from the posaconazole and the fluconazole arms, respectively) occurred during the follow-up period; colonization data were available for 13 subjects. For 2 of the 13 subjects, the colonizing Candida species was discordant with that causing the IFI, and for a third subject, no colonizer was observed. For the remaining 10 subjects, the colonizer and the isolate causing the IFI were the same species; there were four C. glabrata IFIs in each arm and one C. tropicalis and one C. krusei IFI in the posaconazole and fluconazole arms, respectively (Table 5). For 8 of the 10 subjects, the onset of the IFI occurred at least 3 weeks after EOT. The remaining two subjects received fluconazole; one had an IFI caused by a fluconazole-susceptible C. glabrata strain 3 days after the EOT (the subject did not receive additional antifungal therapy), and the second had an IFI caused by a fluconazole-resistant C. krusei 13 days after the EOT (the subject received voriconazole prophylaxis for 12 days prior to the IFI).
TABLE 5.
Subjects who experienced a breakthrough invasive fungal infection after removal from study drug that was caused by the same Candida species identified as a colonizer
| Prophylactic agent | IFI and colonizing species | Day of IFI onset/duration of prophylaxis (days)/time since last dose (days) | Results for isolates cultured from subjects with an invasive fungal infection |
|||
|---|---|---|---|---|---|---|
| Culture day/sourcea | MIC (μg/ml) |
|||||
| Posaconazole | Fluconazole | Itraconazole | ||||
| Posaconazole | C. glabrata | 75/33/42 | 59/mouth | 1 | 16 | 1 |
| 75/blood | NAb | NA | NA | |||
| C. glabrata | 165/138/27 | 35/mouth | 4 | 64 | 8 | |
| 60/mouth | 4 | >64 | 4 | |||
| 88/mouth | 2 | >64 | 2 | |||
| C. glabrata | 25/3/22 | 1/stool | 4 | 16 | 8 | |
| 3/stool | 0.5 | 8 | 1 | |||
| 23/stool | 0.125 | 4 | 0.125 | |||
| 25/blood | 0.5 | 8 | 2 | |||
| C. glabrata | 37/9/28 | 7/stool | 1 | 8 | 1 | |
| 9/urine | NA | NA | NA | |||
| 10/stool | 1 | 8 | 2 | |||
| 14/stool | NA | NA | NA | |||
| 37/blood | NA | NA | NA | |||
| C. tropicalis | 45/24/21 | −3/stool | <0.015 | 0.25 | 0.03 | |
| −3/pharynx | <0.015 | 0.5 | 0.03 | |||
| 2/stool | <0.015 | 0.25 | <0.015 | |||
| 2/pharynx | <0.015 | 0.25 | 0.03 | |||
| 9/stool | <0.015 | 0.5 | 0.03 | |||
| 45/blood | NA | NA | NA | |||
| Fluconazole | C. glabrata | 31/28/3 | 16/mouth | <0.5 | <2 | <1 |
| 31/mouth | <0.5 | <4 | <1 | |||
| C. glabrata | 143/116/27c | 1/mouth | 0.5 | 16 | 1 | |
| 12/mouth | 2 | 64 | 4 | |||
| 62/mouth | 4 | 64 | 8 | |||
| 85/mouth | 4 | 64 | 8 | |||
| 111/mouth | 4 | 64 | >8 | |||
| 143/blood | NA | NA | NA | |||
| C. glabrata | 168/113/55 | −2/mouth | 1 | 64 | 1 | |
| 15/mouth | 2 | 64 | 4 | |||
| 43/mouth | 2 | 64 | 2 | |||
| 176/mouth | 4 | >64 | 2 | |||
| C. glabrata | 172/114/58d | −2/mouth | 0.5 | 16 | 1 | |
| 56/mouth | 0.25 | 4 | 0.5 | |||
| 86/mouth | 1 | 16 | 2 | |||
| 114/mouth | 2 | 32 | 8 | |||
| 172/blood | NA | NA | NA | |||
| 177/mouth | 1 | 32 | 1 | |||
| C. krusei | 19/6/13 | 1/pharynx | 0.06 | 32 | 0.125 | |
| 6/stool | 0.125 | 64 | 0.5 | |||
| 6/pharynx | 0.06 | 32 | 0.5 | |||
| 16/stool | 0.25 | 64 | 0.25 | |||
| 16/pharynx | 0.25 | 64 | 0.5 | |||
| 19/blood | 0.06 | 32 | 0.25 | |||
Cultures of samples from the from mouth (study 1, HSCT recipients) and from the stool and pharynx (study 2, subjects with AML or MDS) were part of a surveillance program; blood and urine cultures were performed in cases with an IFI.
NA, not available.
Subject 002.
Subject 497.
Sequential surveillance isolates were available for many of the subjects described above, and in most cases there were no trends toward decreasing susceptibility. The two exceptions, subjects 002 and 497 (identified in Table 5), received fluconazole, and the IFI-causing isolates and the colonizers were C. glabrata. MLST analysis suggested that the BL and EOT isolates from subject 002 were closely related, whereas those from subject 497 appeared to be unrelated. The IFIs in those two subjects occurred 27 and 58 days after the last dose, even though both subjects had received caspofungin prophylaxis after the EOT and prior to the IFI.
DISCUSSION
A proportion of invasive Candida infections are believed to originate from colonizing isolates. The routes of infection include colonization of indwelling devices and transit of an intestinal mucosa damaged by GVHD and/or cytotoxic chemotherapy. Antifungal prophylaxis can reduce the incidence of candidemia through a reduction or the eradication of susceptible colonizing yeasts (7, 11, 13). However, even among high-risk subjects, IFIs are uncommon, and therefore, many subjects receive antifungal therapy without gaining an obvious benefit. A downside of this drug exposure is the possibility of selecting for less susceptible species/isolates. For example, fluconazole prophylaxis in neutropenic patients reduced the incidence of both colonization and IFIs caused by many Candida species but did not reduce the incidence of colonization or infections caused by inherently fluconazole-resistant species such as C. krusei or C. glabrata (8, 16, 21). Consistent with these prior data, in this study, antifungal prophylaxis with posaconazole, itraconazole, and fluconazole resulted in species-specific reductions in the numbers of colonizers, with the pattern of reductions mirroring the in vitro susceptibility profiles of the drugs. All three azoles decreased the number of positive C. albicans cultures, whereas only the broad-spectrum azoles, posaconazole and itraconazole, eradicated C. krusei. Although C. glabrata persisted as a colonizer irrespective of the prophylactic regimen, the proportion of subjects colonized by C. glabrata remained fairly constant, with only modest increases in the proportion of subjects receiving itraconazole and posaconazole colonized by C. glabrata and little to no change in the proportion of subjects in the fluconazole-treated group colonized by C. glabrata being observed.
The duration of prophylaxis differed markedly between the two studies: the median and the mean treatment durations were 111 and 80 days, respectively, for the HSCT and GVHD subjects and 25 and 27 days, respectively, for the neutropenic subjects. The treatment duration did not appear to affect the pattern of colonization, since the frequency of recovery of the three major species was similar in both studies (data not shown). However, the longer-duration therapy was associated with an increased frequency of recovery of C. albicans isolates with reduced susceptibilities to azoles from subjects receiving fluconazole (reflected in the MIC90s) but not those receiving posaconazole. These increases occurred in a minority of the C. albicans isolates, since the same trends were not seen when the MIC50s were examined. One explanation for the difference between these two azoles is that single substitutions in Erg11p have been documented to affect susceptibility to fluconazole, whereas multiple substitutions appear to be required to cause changes in susceptibility to posaconazole; thus, the genetic barrier to resistance development may be higher for posaconazole than for fluconazole (2, 9). Resistance development occurred far more frequently in C. glabrata than in any other species; the frequency did not differ between prophylactic agents or by study, suggesting that the duration of therapy was not a significant factor. Despite the elevated incidence of decreased susceptibility among the C. glabrata isolates, only two subjects colonized with C. glabrata isolates that became less susceptible during therapy experienced an IFI. In both instances, the IFI occurred at least 3 weeks after EOT, and during this period, the subjects were receiving caspofungin prophylaxis.
The ability to draw conclusions regarding the link between colonizers and breakthrough IFIs is limited by the low overall incidence of IFIs. Only four IFIs (of a total of eight) that occurred during treatment were caused by the same species identified as a prior colonizer. In two cases, both the colonizer and the cause of the IFI exhibited resistance to the prophylactic regimen. However, the isolates causing the IFIs were not available for additional analyses, and therefore, it was not possible to determine if the isolate causing the IFI and the colonizing isolate were related. The incidence of Candida IFIs increased after the subjects stopped receiving study drug. The frequency was the same for each prophylactic regimen, and most (16 of 19) IFIs occurred at least 3 weeks after the last drug dose. Surprisingly, at the time of the IFI, the majority of the subjects (at the discretion of their physicians) were receiving antifungal prophylaxis with other agents, including caspofungin, voriconazole, fluconazole, and amphotericin B (but not posaconazole).
In summary, breakthrough IFIs in subjects receiving antifungal prophylaxis with posaconazole, fluconazole, and itraconazole were rare. There was a concordance between the colonizing yeast species and the species causing the invasive infection; however, isolates that developed reduced susceptibility to the prophylactic regimen were no more likely to cause an IFI than susceptible isolates. The majority of breakthrough IFIs caused by yeasts were due to C. glabrata, suggesting that either the decreased susceptibility and/or the poor responsiveness of this species to treatment by azoles presents a greater risk factor for the progression to an invasive infection. Lastly, these data demonstrate that prophylaxis with a broad-spectrum antifungal agent like posaconazole, which, in comparison to fluconazole, provided an increased benefit in terms of reduced mold infections and survival (5, 20), did not exacerbate the development of resistance or cause a transition to a less susceptible species.
Acknowledgments
We thank David Aanensen (Imperial College London) for help with multilocus sequence typing.
Footnotes
Published ahead of print on 28 September 2009.
REFERENCES
- 1.Bougnoux, M. E., D. M. Aanensen, S. Morand, M. Théraud, B. G. Spratt, and C. d'Enfert. 2004. Multilocus sequence typing of Candida albicans: strategies, data exchange and applications. Infect. Genet. Evol. 4:243-252. [DOI] [PubMed] [Google Scholar]
- 2.Chau, A. S., C. A. Mendrick, F. J. Sabatelli, D. Loebenberg, and P. M. McNicholas. 2004. Application of real-time quantitative PCR to the molecular analysis of Candida albicans strains exhibiting reduced susceptibility to azoles. Antimicrob. Agents Chemother. 48:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chau, A. S., M. Gurnani, R. Hawkinson, M. Laverdière, A. Cacciapuoti, and P. M. McNicholas. 2005. Inactivation of the Δ56-desaturase attenuates virulence in Candida albicans. Antimicrob. Agents Chemother. 49:3646-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 2nd ed., M27-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Cornely, O. A., J. Maertens, D. J. Winston, J. Perfect, A. J. Ullmann, T. J. Walsh, D. Helfgott, J. Holowiecki, D. Stockelberg, Y.-T. Goh, M. Petrini, C. Hardalo, R. Suresh, and D. Angulo-Gonzalez. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348-359. [DOI] [PubMed] [Google Scholar]
- 6.Cornely, O. A., A Böhme, D. Buchheidt, H. Einsele, W. J. Heinz, M. Karthaus, S. W. Krause, W. Krüger, G. Maschmeyer, O. Penack, J. Ritter, M. Ruhnke, M. Sandherr, M. Sieniawski, J. J. Vehreschild, H. H. Wolf, and A. J. Ullmann. 2009. Primary prophylaxis of invasive fungal infections in patients with hematologic malignancies. Recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Haematologica 94:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh, A. Y., J. R. Köhler, K. T. Coggshall, N. Van Rooijen, and G. B. Pier. 2008. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 4:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laverdière, M., C. Rotstein, E. J. Bow, R. S. Roberts, S. Ioannou, D. Carr, and N. Moghaddam. 2000. Impact of fluconazole prophylaxis on fungal colonization and infection rates in neutropenic patients. J. Antimicrob. Chemother. 46:1001-1008. [DOI] [PubMed] [Google Scholar]
- 9.Li, X., N. Brown, A. S. Chau, J.-L. Lopez-Ribot, M. T. Ruesga, G. Quindos, C. A. Mendrick, R. S. Hare, D. Loebenberg, B. DiDomenico, and P. M. McNicholas. 2004. Changes in susceptibility to posaconazole in clinical isolates of Candida albicans. J. Antimicrob. Chemother. 53:74-80. [DOI] [PubMed] [Google Scholar]
- 10.Marr, K. A. 2008. Primary antifungal prophylaxis in hematopoietic stem cell transplant recipients: clinical implications of recent studies. Curr. Opin. Infect. Dis. 21:409-414. [DOI] [PubMed] [Google Scholar]
- 11.Nucci, M., and E. Anaissie. 2001. Revisiting the source of candidemia: skin or gut? Clin. Infect. Dis. 33:1959-1967. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan, A. K., A. Pandya, G. Papadopoulos, D. Thompson, A. Langston, J. Perfect, and M. C. Weinstein. 2009. Cost-effectiveness of posaconazole versus fluconazole or itraconazole in the prevention of invasive fungal infections among neutropenic patients in the United States. Value Health 12:666-673. [DOI] [PubMed] [Google Scholar]
- 13.Redding, S. W., K. A. Marr, W. R. Kirkpatrick, B. J. Coco, and T. F. Patterson. 2004. Candida glabrata sepsis secondary to oral colonization in bone marrow transplantation. Med. Mycol. 42:479-481. [DOI] [PubMed] [Google Scholar]
- 14.Sabatelli, F., R. Patel, P. A. Mann, C. A. Mendrick, C. C. Norris, R. Hare, D. Loebenberg, T. A. Black, and P. M. McNicholas. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanglard, D., F. Ischer, T. Parkinson, D. Falconer, and J. Bille. 2003. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob. Agents Chemother. 47:2404-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snydman, D. R. 2003. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest 123:500-503. [DOI] [PubMed] [Google Scholar]
- 17.Strasfeld, L., and D. M. Weinstock. 2006. Antifungal prophylaxis among allogeneic hematopoietic stem cell transplant recipients: current issues and new agents. Expert Rev. Anti-Infect. Ther. 4:457-468. [DOI] [PubMed] [Google Scholar]
- 18.Tamura, K., and R. Drew. 2008. Antifungal prophylaxis in adult hematopoietic stem cell transplant recipients. Drugs Today 44:515-530. [DOI] [PubMed] [Google Scholar]
- 19.Thompson, G. R., III, J. Cadena, and T. F. Patterson. 2009. Overview of antifungal agents. Clin. Chest Med. 30:203-215. [DOI] [PubMed] [Google Scholar]
- 20.Ullmann, A. J., J. H. Lipton, D. H. Vesole, P. Chandrasekar, A. Langston, S. R. Tarantolo, H. Greinix, W. Morais de Azevedo, V. Reddy, N. Boparai, L. Pedicone, H. Patino, and S. Durrant. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335-347. [DOI] [PubMed] [Google Scholar]
- 21.Wingard, J. R., W. G. Merz, M. G. Rinaldi, T. R. Johnson, J. E. Karp, and R. Saral. 1991. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 325:1274-1277. [DOI] [PubMed] [Google Scholar]