Abstract
Candida dubliniensis commonly shows paradoxical or trailing growth effects in vitro in the presence of echinocandins. We tested the in vitro activities of anidulafungin, caspofungin, and micafungin against clinical isolates of C. dubliniensis and evaluated the efficacy of these drugs in two murine models of systemic infection. The three echinocandins were similarly effective in the treatment of experimental disseminated infections with C. dubliniensis strains showing or not showing abnormal growth in vitro.
Candida dubliniensis is a species closely related to C. albicans. It can cause invasive infections in immunocompromised patients (5, 11, 12). Data on the antifungal management of C. dubliniensis infections are scarce; however, in vitro fluconazole resistance and one possible case of relapse after caspofungin (CAS) treatment have been reported (14, 15). Several recent studies have reported a high prevalence among the strains of C. dubliniensis of abnormal growth in vitro characterized by paradoxical or trailing effects observed in the presence of echinocandins (6, 9). It is unknown if these effects are related to a low efficacy of these drugs in vivo. Here we have tested the activities of anidulafungin (AFG), CAS, and micafungin (MFG) against seven strains of C. dubliniensis (Table 1). We have tested these drugs and compared their efficacies between strains that showed or did not show paradoxical growth in vitro in a murine model of infection.
TABLE 1.
In vitro antifungal activities of AFG, CAS, and MFG against seven strains of C. dubliniensis
| Strain | Body site (origin) | AFG |
CAS |
MFG |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50a (μg/ml) |
P rangeb (μg/ml) |
MFCc | MIC50 (μg/ml) |
P range (μg/ml) |
MFC | MIC50 (μg/ml) |
P range (μg/ml) |
MFC | ||||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |||||
| 9453 | Blood (Ireland) | <0.03 | <0.03 | 4-16 | >16 | 0.25 | 0.25 | 8-16 | Trailingd | >16 | 0.125 | 0.125 | >16 | |||
| 9456 | Oral (Spain) | <0.03 | <0.03 | 8-16 | >16 | 0.25 | 0.25 | 8-16 | 2-16 | >16 | 0.06 | 0.125 | >16 | |||
| 10031 | Oral (Spain) | <0.03 | <0.03 | >16 | 0.125 | 0.25 | 8-16 | Trailingd | >16 | 0.125 | 0.125 | >16 | ||||
| 10032 | Oral (Ireland) | <0.03 | <0.03 | 2 | 0.06 | 0.06 | 4 | <0.03 | 0.125 | 4 | ||||||
| 10033 | Oral (Spain) | <0.03 | 0.06 | 4-16 | 2 | 0.25 | 0.25 | 8-16 | 2-16 | >16 | 0.125 | 0.25 | 2 | |||
| 10034 | Oral (Spain) | <0.03 | <0.03 | 4-16 | >16 | 0.125 | 0.25 | 16 | 1-16 | >16 | 0.06 | 0.125c | 2-16 | >16 | ||
| 10035 | Oral (Israel) | <0.03 | <0.03 | 0.125 | 0.06 | 0.06 | 8-16 | 0.5 | 0.03 | 0.03 | 0.25 | |||||
MIC50 corresponds to 50% inhibition of growth.
P range, range of antifungal concentrations where a paradoxical growth effect was observed.
MFC corresponds to ≥99.9% reduction in the CFU/ml count.
Fungal growth was observed over the entire range of drug concentrations, and the P range was not determined.
Susceptibility in vitro was determined by using a reference method (4). The minimal fungicidal concentration (MFC) was defined as a 99.9% or greater reduction in the number of CFU/ml (8). A hemocytometer was used to adjust the desired fungal inocula for both in vitro and in vivo studies.
Male OF1 mice were used, and the procedure standards approved by the Animal Welfare Committee of the Universitat Rovira i Virgili were followed. Immunocompetent mice were challenged intravenously (i.v.) with 1.5 × 107 CFU of strain FMR 10032 or FMR 10034, and immunosuppressed mice were challenged with 1.5 × 105 CFU. Immunosuppressed mice received 200 mg/kg of cyclophosphamide intraperitoneally plus 150 mg/kg of 5-fluorouracil i.v. on the day of infection (10). Groups of 10 mice were randomly established for survival and tissue burden studies with both strains. The different groups of immunocompetent mice were treated with AFG, CAS, or MFG at 1 or 10 mg/kg of body weight/dose i.v. once daily. Immunosuppressed mice received AFG, CAS, or MFG at 1 mg/kg of body/weight/dose also i.v. once daily. Control animals received no treatment. All treatments began 24 h after challenge. Due to the early deaths occurring in the immunocompetent mouse model of infection, therapies lasted 3 days. The later occurrence of deaths in the immunosuppressed mouse model of infection allowed the administration of therapies for 5 days. For survival studies, mice were checked daily for 15 days. For tissue burden studies, mice were killed 1 day after the completion of treatment. Spleens and kidneys were aseptically removed, and the entire organs were homogenized in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated on Sabouraud dextrose agar, incubated at 35°C, and examined daily for 3 days.
Mean survival times were estimated by the Kaplan-Meier method and compared among groups by using the log rank test. Colony counts in tissue burden studies were analyzed by using the Mann-Whitney U test, and a P value of ≤0.05 was considered statistically significant.
A paradoxical growth effect was observed in four (57%) C. dubliniensis isolates for AFG, six (85%) for CAS, and one for MFG (14%) (Table 1). Based on these results, two strains were selected for in vivo studies: FMR 10032, which showed low MFCs and no abnormal growth with any of the drugs tested, and FMR 10034, which showed high MFCs and paradoxical growth with all of the drugs tested.
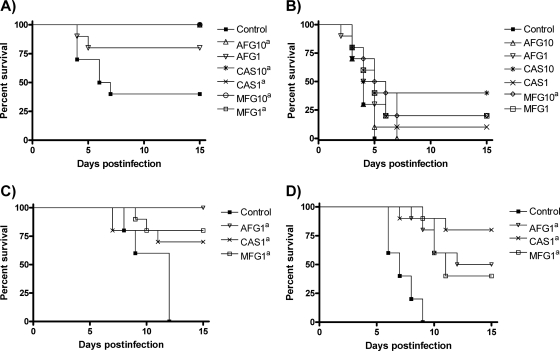
Figure 1 and Table 2 show the results of in vivo studies with immunocompetent mice. For strain FMR 10032, all of the treatments significantly prolonged survival, except AFG at 1 mg/kg. For strain FMR 10034, only MFG at 10 mg/kg significantly prolonged survival but no statistically significant differences were observed among the treatments. For both strains, all of the drugs and doses significantly reduced the fungal burdens in both organs, except for MFG and AFG at 1 mg/kg in the kidneys for strain FMR 10034, both also being less effective than the other treatments in these organs. AFG at 10 mg/kg was more effective than the other treatments in reducing the fungal load of strain FMR 10032 in the spleen.
FIG. 1.
Cumulative mortality of immunocompetent mice infected with C. dubliniensis FMR 10032 (A) and FMR 10034 (B) and immunosuppressed mice infected with C. dubliniensis FMR 10032 (C) and FMR 10034 (D). AFG1 and AFG10, AFG at 1 and 10 mg/kg/day. CAS1 and CAS10, CAS at 1 and 10 mg/kg/day. MFG1 and MFG10, MFG at 1 and 10 mg/kg/day. A superscript lowercase letter a indicates a P value of <0.05 versus the control.
TABLE 2.
Effects of antifungal treatments on the tissue fungal burdens of mice infected with C. dubliniensis FMR 10032 and FMR 10034
| Isolate (immunosuppression, inoculum [CFU/mouse]) and drug (dose [mg/kg/day]) | Log10 mean CFU count (SEM) |
|
|---|---|---|
| Kidney | Spleen | |
| FMR 10032 (none, 1.5 × 107) | ||
| None | 8.12 (0.10) | 6.18 (0.12) |
| AFG (10) | 2.95 (0.14)a,b | 2.64 (0.16)a,c |
| AFG (1) | 5.65 (0.17)a | 4.21 (0.22)a |
| CAS (10) | 3.08 (0.10)a,b | 3.38 (0.14)a |
| CAS (1) | 3.41 (0.15)a,b | 3.56 (0.10)a |
| MFG (10) | 3.23 (0.16)a,b | 3.58 (0.14)a |
| MFG (1) | 4.25 (0.18)a | 3.97 (0.19)a |
| FMR 10034 (none, 1.5 × 107) | ||
| None | 6.60 (0.18) | 5.60 (0.07) |
| AFG (10) | 4.63 (0.12)a,b | 4.25 (0.09)a |
| AFG (1) | 6.33 (0.14) | 4.54 (0.14)a |
| CAS (10) | 4.09 (0.18)a,b | 3.89 (0.22)a |
| CAS (1) | 4.50 (0.15)a,b | 4.21 (0.14)a |
| MFG (10) | 4.69 (0.13)a,b | 4.18 (0.17)a |
| MFG (1) | 6.33 (0.14) | 4.79 (0.15)a |
| FMR 10032 (CPO + 5FU,d 1.5 × 105) | ||
| None | 8.03 (0.08) | 6.58 (0.12) |
| AFG (1) | 5.75 (0.28)a | 2.62 (0.25)a |
| CAS (1) | 1.77 (0.15)a,b | 1.26 (0.31)a,b |
| MFG (1) | 5.47 (0.12)a | 2.62 (0.21)a |
| FMR 10034 (CPO + 5FU, 1.5 × 105) | ||
| None | 7.86 (0.08) | 6.31 (0.14) |
| AFG (1) | 5.94 (0.25)a | 2.96 (0.20)a |
| CAS (1) | 3.39 (0.14)a,b | 2.08 (0.12)a,b |
| MFG (1) | 6.47 (0.08)a | 3.26 (0.08)a |
P < 0.05 versus the control.
P < 0.05 versus AFG at 1 mg/kg and MFG at 1 mg/kg.
P < 0.05 versus the other treatments.
CPO + 5FU, cyclophosphamide (200 mg/kg) and 5-fluorouracil (150 mg/kg) administered on the day of infection.
Figure 1 and Table 2 also show the in vivo results obtained with immunosuppressed mice. For both strains, all of the drugs significantly prolonged survival and were effective in reducing the fungal burdens in the spleen and kidneys. CAS was significantly better than the other treatments at reducing the fungal burdens of both strains in both organs.
The low echinocandin MICs observed for all of the strains tested agree with previous in vitro data on activity against C. dubliniensis (9, 13). Paradoxical growth has been observed previously, mainly in the presence of CAS and MFG but rarely with AFG (6, 9). By contrast, we observed here a paradoxical growth effect in the presence of AFG and CAS but in only one strain for MFG.
In immunocompetent mice, all of the echinocandins were effective in reducing the tissue burdens of both strains and in improving the survival of animals challenged with strain FMR 10032. Only MFG at 10 mg/kg was able to significantly improve the survival of the animals challenged with strain FMR 10034, although this might be because of the higher virulence of this strain and the short-term treatments used in this model rather than a lower efficacy of the drugs against this strain.
The smaller inoculum and the longer treatment period used in the immunosuppressed mouse model allowed the observation of good responses to all of the drugs tested against both strains.
The low dose of CAS reduced the fungal tissue burden in the kidneys of immunocompetent mice in the same way as the three echinocandins did at doses 10 times higher. This agrees with the previously described longer persistence of CAS in the kidneys relative to the other two echinocandins (1, 2, 7).
Clemons et al. (3) reported no correlation between abnormal growth in vitro and reduced efficacy in vivo of the echinocandins against C. albicans. Although the data presented here represent only a few strains of C. dubliniensis, our data for this species are similar to those of Clemons et al. (3) for C. albicans. Overall, echinocandins were effective in the treatment of invasive murine candidiasis caused by C. dubliniensis, but further investigation is needed to improve therapies for this kind of infection.
Acknowledgments
We thank Núria Pilas, Catalina Nuñez, and Pilar Hernández for their technical assistance.
This work was partially supported by a grant from the Fondo de Investigaciones sanitarias from the Ministerio de Sanidad y Consumo of Spain (PI 050031) and by a grant from the Departamento de Educación, Universidades e Investigación, from the Gobierno Vasco (project GIC07 123-IT-222-07).
Footnotes
Published ahead of print on 28 September 2009.
REFERENCES
- 1.Andes, D. R., D. J. Diekema, M. A. Pfaller, K. Marchillo, and J. Bohrmueller. 2008. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:3497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., D. J. Diekema, M. A. Pfaller, R. A. Prince, K. Marchillo, J. Ashbeck, and J. Hou. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemons, K. V., M. Espiritu, R. Parmar, and D. A. Stevens. 2006. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob. Agents Chemother. 50:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard—third edition. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Fanci, R. 2009. Breakthrough Candida dubliniensis fungemia in an acute myeloid leukemia patient during voriconazole therapy successfully treated with caspofungin. J. Chemother. 21:105-107. [DOI] [PubMed] [Google Scholar]
- 6.Fleischhacker, M., C. Radecke, B. Schulz, and M. Ruhnke. 2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur. J. Clin. Microbiol. Infect. Dis. 27:127-131. [DOI] [PubMed] [Google Scholar]
- 7.Hajdu, R., R. Thompson, J. G. Sundelof, B. A. Pelak, F. A. Bouffard, J. F. Dropinski, and H. Kropp. 1997. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isham, N. C., and M. A. Ghannoum. 2007. Voriconazole and caspofungin cidality against non-albicans Candida spp. Infect. Dis. Clin. Pract. 15:250-253. [Google Scholar]
- 9.Jacobsen, M. D., J. A. Whyte, and F. C. Odds. 2007. Candida albicans and Candida dubliniensis respond differently to echinocandin antifungal agents in vitro. Antimicrob. Agents Chemother. 51:1882-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariné, M., C. Serena, B. Fernández-Torres, F. J. Pastor, and J. Guarro. 2005. Activities of flucytosine, fluconazole, amphotericin B, and micafungin in a murine model of disseminated infection by Candida glabrata. Antimicrob. Agents Chemother. 49:4757-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marriott, D., M. Laxton, and J. Harkness. 2001. Candida dubliniensis candidemia in Australia. Emerg. Infect. Dis. 7:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mubareka, S., D. C. Vinh, and S. E. Sanche. 2005. Candida dubliniensis bloodstream infection: a fatal case in a lung transplant recipient. Transplant. Infect. Dis. 7:146-149. [DOI] [PubMed] [Google Scholar]
- 13.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinjon, E., G. P. Moran, D. C. Coleman, and D. J. Sullivan. 2005. Azole susceptibility and resistance in Candida dubliniensis. Biochem. Soc. Trans. 33:1210-1214. [DOI] [PubMed] [Google Scholar]
- 15.van Hal, S. J., D. Stark, J. Harkness, and D. Marriott. 2008. Candida dubliniensis meningitis as delayed sequela of treated C. dubliniensis fungemia. Emerg. Infect. Dis. 14:327-329. [DOI] [PMC free article] [PubMed] [Google Scholar]