Abstract
Surra is an animal pathogenic protozoan infection, caused by Trypanosoma evansi, that develops into a fatal wasting disease. Control measures rely on diagnosis and treatment. However, with the continuous emergence of drug resistance, this tactic is failing, and the pressing need for new chemotherapeutic agents is becoming critical. With the introduction of novel aromatic diamidines, a new category of antitrypanosomal drugs was discovered. Nevertheless, their efficacy within a T. evansi-infected mouse model was not known. In total, 30 compounds previously selected based on their in vitro activity were tested in a T. evansi mouse model of infection. Six of the compounds were capable of curing T. evansi-infected mice at drug doses as low as 0.5 and 0.25 mg/kg of body weight administered for 4 consecutive days, and they were more effective than the standard drugs suramin, diminazene, and quinapyramine. After all selection criteria were applied, three diamidine compounds (DB 75, DB 867, and DB 1192) qualified as lead compounds and were considered to have the potential to act as preclinical candidates against T. evansi infection.
Surra is an animal pathogenic protozoan infection, caused by Trypanosoma evansi, that develops into a fatal wasting disease. It is mechanically transmitted by biting flies of the genera Tabanus, Stomoxys, and Lyperosia. No intermediate hosts and no insect developmental cycles have been identified yet (8). Control measures for protozoan diseases such as Surra rely on treatment and vector control and, to a much lesser extent, on chemoprophylaxis. In the case of Surra, drugs containing antitrypanosomal properties usually are administered once an infection already has been established (7, 17). However, with the continuous emergence of drug resistance, this tactic is failing, and the pressing need for new, alternative chemotherapeutic agents is becoming critical (16, 20). Moreover, the prospect of a vaccination against T. evansi infection appears nonexistent, largely based upon the phenomenon of the antigenic variation of trypanosomes in general.
With the introduction of novel aromatic diamidines, a new category of antitrypanosomal drugs was discovered (9, 18, 21). The dicationic spacer between the active moieties remains the same, yet the active groups are altered to include structure-activity relationship information obtained from previous compounds. Several of these dicationic compounds have shown great efficacy in vitro against both human and animal pathogenic trypanosome species. A previous study of these diamidine compounds enabled the positive selection of diamidine compounds exhibiting high in vitro activity against both a Chinese T. evansi reference strain and a P2 transporter knockout strain, which have low cytotoxicity and low in vivo toxicity (15 and K. Gillingwater, A. Kumar, M. A. Ismail, R. K. Arafa, C. E. Stephens, D. W. Boykin, and R. Brun, submitted for publication). The 50% inhibitory concentrations (IC50s) of the lead compounds tested ranged from 10.5 to 1.7 ng/ml. The IC50s of the four existing agents are 87.6, 12.5, 1.1, and 0.1 ng/ml for suramin, diminazene, cymelarsan, and quinapyramine, respectively (Gillingwater et al., submitted). Since diamidines are actively taken up by transporters, any alterations to these transporters will affect the compounds' modes of action, selectivity, and development of drug resistance. By having tested the compounds against a P2 knockout strain, compounds reliant on P2 transportation alone can be identified.
The aim of this study was to investigate 30 previously selected diamidine compounds in vivo in a T. evansi mouse model of infection.
MATERIALS AND METHODS
Trypanosome strain.
The Trypanosoma evansi STIB 806K strain was used for all in vivo experiments performed in this study. It was isolated from a water buffalo in China in 1983 (15). This strain was cloned and adapted to axenic culture (2).
Mice.
Female NMRI mice, weighing 22 to 25 g, were used for the in vivo experiments. All mice were specific pathogen free (SPF) and were maintained in standard Macrolon type II cages at 22°C and with a relative humidity of 60 to 70%. Water and pelleted food was provided ad libitum. All in vivo experiments performed complied with the regulations set out by the Swiss Federal Veterinary Office.
Standard trypanocidal drugs.
Suramin (Germanin; Bayer, Leverkusen, Germany), diminazene aceturate (D-7770; Sigma, St. Louis, MO), cymelarsan (MelCy; Rhône Mérieux, Toulouse, France), and quinapyramine sulfate (Trypacide; May & Baker, Lagos, Nigeria) were used as the standard drugs in this study.
Diamidine compounds.
The diamidine compounds were synthesized previously in the laboratories of David Boykin and Richard Tidwell. For the in vivo experiments, the diamidine compounds were selected by means of their previously demonstrated in vitro activity against T. evansi strains (several reference strains and a P2 knockout strain) and the absence of toxicity shown in preliminary toxicity trials performed in vivo for doses of up to 100 mg/kg of body weight (Gillingwater et al., submitted). Of the 67 compounds tested, 69% showed no acute toxicity at doses of 100 mg/kg.
Culture medium.
Bloodstream-form trypanosomes were cultivated in minimum essential medium (MEM) (a powder; no. 11400-033; GIBCO-BRL) with Earle's salts supplemented with 25 mM HEPES, 1 g/liter additional glucose, 2.2 g/liter NaHCO3, and 10 ml/liter MEM nonessential amino acids (50× concentration). The medium then was further supplemented according to Baltz et al. (2) by adding 1% of a 1.2 mM stock of 2-mercaptoethanol, 1% of a stock consisting of 100 mM sodium pyruvate and 50 mM hypoxanthine, and 15% heat-inactivated horse serum. The complete medium is called Baltz MEM (BMEM).
Stock solutions and dilutions.
A 1.5-mg amount of each compound was weighed out in powder form and dissolved in 15 ml of sterile distilled water to provide a 0.1 mg/ml stock solution. From these stock solutions, further compound dilutions were made depending on the dose being tested. Stock solutions and compound dilutions were made fresh on the day of administration and for each experiment.
In vivo experiments.
Female NMRI mice were divided into groups of four mice each. Mice were infected with 104 parasites in 0.25 ml BMEM with in vitro cultures of the STIB 806K T. evansi reference strain using an intraperitoneal (i.p.) route of infection. A parasitemia of 106 parasites per ml blood was allowed to develop for 72 h before treatment. All treatment was given i.p. on days 3 to 6 postinfection. Thereafter, the parasitemia was checked twice a week for the first 2 weeks and once per week thereafter using a tail blood examination technique until day 60 postinfection. This 2-month experimental follow-up period after treatment was used to account for any possible relapses. Subsequently, any surviving and aparasitemic mice were considered cured. Untreated mice survive, on average, 7 days postinfection.
RESULTS
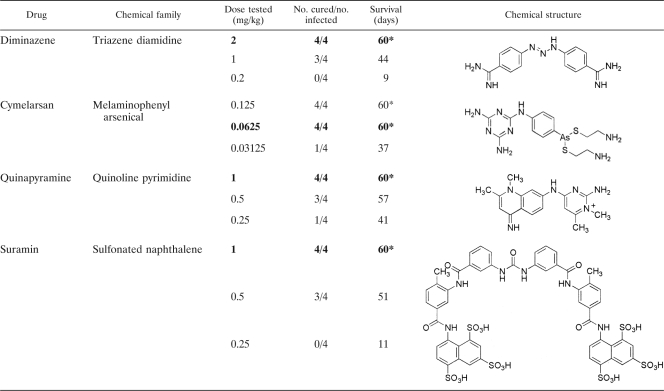
The four standard drugs currently in use against Trypanosoma evansi infection and 30 selected diamidine compounds were tested in vivo in this study. The results of the in vivo investigations for the four standard drugs can be seen in Table 1.
TABLE 1.
In vivo investigations of four standard drugs given on 4 consecutive days to NMRI mice infected with T. evansi (STIB 806K)a
The asterisk indicates that all mice were cured at this dose. Numbers in boldface indicate the lowest curative dose of that drug.
The standard diamidine drug, diminazene aceturate, was capable of curing only 3/4 mice at a 1 mg/kg dose administered for 4 consecutive days. Twice this amount (2 mg/kg) actually was required to achieve the lowest curative dose. Cymelarsan demonstrated the highest efficacy, with a lowest curative dose of 0.0625 mg/kg administered for 4 consecutive days. At a dose of 1 mg/kg administered for 4 consecutive days, both quinapyramine and suramin were able to cure 4/4 mice. However, as the dose was reduced to 0.5 and 0.25 mg/kg, only 3/4 and 1/4 mice were cured, respectively, for quinapyramine, and only 3/4 and 0/4 mice were cured, respectively, for suramin.
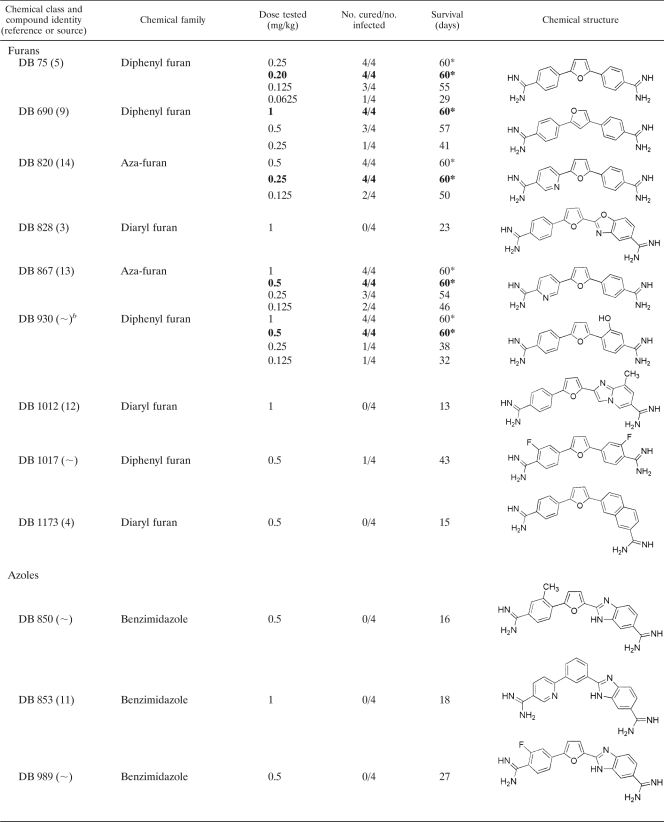
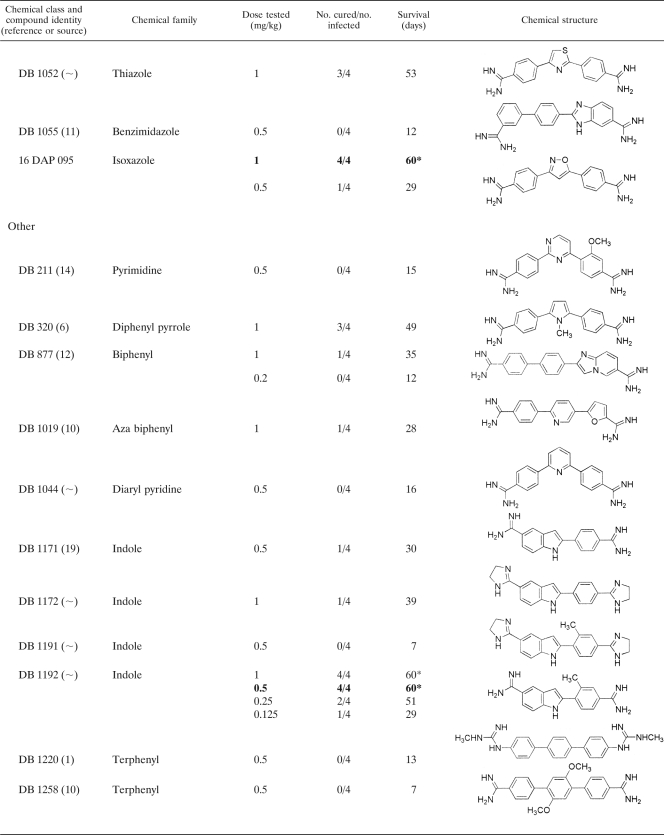
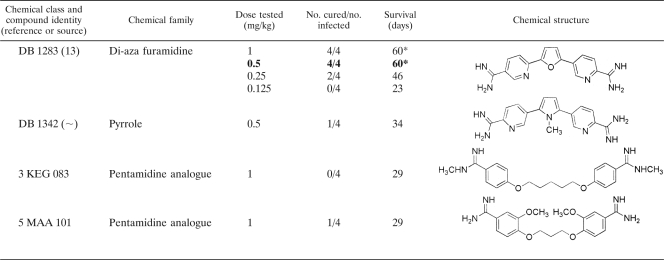
Thirty selected diamidine compounds were investigated for in vivo efficacy in a mouse model. The various doses tested, showing the number of mice cured above the number originally infected, with the average survival results (in days), can be seen in Table 2. Four compounds, DB 867, DB 930, DB 1192, and DB 1283, produced minimum curative doses of 0.5 mg/kg administered for 4 consecutive days. The compounds DB 820 and DB 75 produced the lowest curative doses of 0.25 and 0.20 mg/kg, respectively. At these relatively low doses, 4/4 mice were cured, and all six compounds were more effective at curing T. evansi-infected mice than the standard drugs suramin, diminazene, and quinapyramine. The lowest curative dose of cymelarsan (0.0625 mg/kg administered for 4 consecutive days) remains four to eight times lower than the lowest curative doses of these six diamidine compounds.
TABLE 2.
In vivo investigations of selected diamidine compounds given on 4 consecutive days to NMRI mice infected with T. evansi (STIB 806K)a
The asterisk indicates that all mice were cured at this dose. Numbers in boldface indicate the lowest curative dose of that drug.
The synthesis for this compound has yet to be published.
In summary, only these six diamidine compounds (DB 75, DB 820, DB 867, DB 930, DB 1192, and DB 1283) showed a better efficacy in the mouse model than three of the four standard drugs, and these qualify for further investigation in a larger animal model.
DISCUSSION
The aim of this study was to investigate 30 selected diamidine compounds that have been found to be highly active in vitro against the reference STIB 806K Trypanosoma evansi strain. These diamidine compounds were chosen not only for their antitrypanosomal activity against T. evansi strains but also for their reduced potential to develop drug resistance, as determined by previous activity against a genetic P2 transporter knockout strain (TbAT1 K.O.) (Gillingwater et al., submitted). Many diamidines are known to be taken up into the parasite via the P2 transporter, so testing against a strain known not to possess a P2 transporter enables the elimination of such compounds. Compounds that still are actively taken up efficiently by the P2 knockout strain demonstrate alternative uptake pathways and hence may still be effective against drug-resistant populations. The low cytotoxicity against mammalian cells and the absence of toxicity within a mouse model also are important criteria for the specific selection of these diamidine compounds (Gillingwater et al., submitted). By determining the in vivo chemotherapeutic efficacy of these 30 compounds, it was possible to further select the most efficient, nontoxic compounds, which eventually could be developed into alternatives to the four standard drugs currently in use.
The standard drugs were able to cure 4/4 mice at drug doses of 1 mg/kg (for suramin and quinapyramine), 2 mg/kg (for diminazene), and 0.0625 mg/kg (for cymelarsan) administered for 4 consecutive days. In this mouse model, the diamidine diminazene appears to have the least efficacy among all of the standard drugs currently on the market. In contrast, cymelarsan demonstrates the best efficacy, yet the costs of this drug, which are higher than those of other trypanocidal drugs (approximately $10 for a camel), coupled with its limited availability throughout the world (it is available only in the Middle East and Africa), overshadow its beneficial properties.
From the 30 compounds tested, 8 (27%) cured at 1 mg/kg administered for 4 consecutive days and 6 (20%) cured at 0.5 mg/kg administered for 4 consecutive days. Since the general aim of this study was to discover potential lead compounds possessing greater activity than the standard drugs, only the best six compounds (providing cure at 0.5 mg/kg) were selected.
Another selection criterion for clinical candidate compounds capable of entering into clinical trials included the cost assessment and large-scale production of a compound. Given that an alternative compound against T. evansi infection would significantly benefit many communities within developing countries, an ideal candidate should be cheap. Therefore, an element of cost-effectiveness was applied to establish which compounds contained the most advantageous properties for large-scale production. Compounds requiring expensive starting materials, as well as compounds involving complex and numerous synthetic steps, would increase the cost of goods. It was recognized that some of the compounds included a lengthy and complicated synthesis, which would pose problems of practicality and cost. DB 930 and DB 1283 have a complicated synthesis, which would increase the overall costs of these compounds, and as a result, they were not selected for continued investigation.
The aza-furan compound DB 820 currently is under investigation as a potential clinical candidate for human African trypanosomiasis. The selection and safety criteria for drugs intended for human use differ from those for drugs intended for veterinary use. Even though a common drug for both human and veterinary use would appear economically beneficial, it would be rendered useless in both cases should the parasites involved become resistant to it. Hence, compounds already under investigation for human use were avoided in this study. Consequently, DB 820 was removed from the list of candidates for further in vivo studies. DB 75 was once in a situation similar to that of DB 820, but the further development of the prodrug DB 289 was stopped in the meantime.
The remaining three compounds (DB 75, DB 867, and DB 1192) were capable of curing T. evansi-infected mice, at doses of 0.5 mg/kg or lower, more effectively than the standard drugs suramin, diminazene, and quinapyramine. Additionally, these selected compounds are cost-efficient in terms of synthesis and currently are not undergoing examination as lead compounds against human trypanosomiasis. Although DB 75 did not qualify for all of the specified criteria by the time these experiments were carried out, this diamidine compound was included for further investigations, since its prodrug, DB 289, no longer is being developed as a human drug. In summary, three selected diamidine compounds remain (DB 75, DB 867, and DB 1192) that warrant continued investigation as potential clinical candidates against T. evansi infection in a larger animal model.
Acknowledgments
We thank James E. Hall and Susan Kilgore Jones for providing detailed information on the compound structures and properties and Pascale Steiger and Karin Gysin for their expert help in animal maintenance.
Footnotes
Published ahead of print on 28 September 2009.
REFERENCES
- 1.Arafa, R. K., M. A. Ismail, M. Munde, W. D. Wilson, T. Wenzler, R. Brun, and D. W. Boykin. 2008. Novel linear triaryl guanidines, N-substituted guanidines and potential prodrugs as antiprotozoal agents. Eur. J. Med. Chem. 43:2901-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltz, T., D. Baltz, C. Giroud, and J. Crockett. 1985. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 4:1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batista-Parra, A., S. Venkitachalam, W. D. Wilson, and D. W. Boykin. 2003. Synthesis of 2-(5-amidinobenzoxazol-2-yl)-5-(4-amidinophenyl)-furan and -thiophene. Heterocycles 60:1367-1376. [Google Scholar]
- 4.Chackal-Catoen, S., Y. Miao, W. D. Wilson, T. Wenzler, R. Brun, and D. W. Boykin. 2006. Dicationic DNA-targeted antiprotozoal agents: naphthalene replacement of benzimidazole. Bioorg. Med. Chem. 14:7434-7445. [DOI] [PubMed] [Google Scholar]
- 5.Das, B. P., and D. W. Boykin. 1977. Synthesis and antiprotozoal activity of 2,5-bis-(4-guanylphenyl)furans. J. Med. Chem. 20:531-536. [DOI] [PubMed] [Google Scholar]
- 6.Das, B. P., and D. W. Boykin. 1977. Synthesis and antiprotozoal activity of 2,5-bis-(4-guanylphenyl)thiophenes and pyrroles. J. Med. Chem. 20:1219-1221. [DOI] [PubMed] [Google Scholar]
- 7.Dávila, A. M., and R. A. Silva. 2000. Animal trypanosomiasis in South America; current status, partnership and information technology. Ann. N. Y. Acad. Sci. 916:199-212. [DOI] [PubMed] [Google Scholar]
- 8.Foil, L. D. 1989. Tabanids as vectors of disease agents. Parasitol. Today 5:88-96. [DOI] [PubMed] [Google Scholar]
- 9.Francesconi, I., W. D. Wilson, F. A. Tanious, J. E. Hall, B. C. Bender, R. R. Tidwell, D. McCurdy, and D. W. Boykin. 1999. 2,4-Diphenyl furan diamidines as novel anti-Pneumocystis carinii pneumonia agents. J. Med. Chem. 42:2260-2265. [DOI] [PubMed] [Google Scholar]
- 10.Ismail, M. A., R. K. Arafa, R. Brun, T. Wenzler, Y. Miao, W. D. Wilson, C. Generaux, A. Bridges, J. E. Hall, and D. W. Boykin. 2006. Synthesis, DNA affinity and antiprotozoal activity of linear dications: terphenyl diamidines and analogues. J. Med. Chem. 49:5324-5332. [DOI] [PubMed] [Google Scholar]
- 11.Ismail, M. A., R. Brun, T. Wenzler, Y. Miao, W. D. Wilson, and D. W. Boykin. 2004. Dicationic biphenyl benzimidazole derivatives as antiprotozoal agents. Bioorg. Med. Chem. 12:5405-5413. [DOI] [PubMed] [Google Scholar]
- 12.Ismail, M. A., R. Brun, T. Wenzler, F. A. Tanious, W. D. Wilson, and D. W. Boykin. 2004. Novel dicationic imidazo(1,2-a)pyridines and 5,6,7,8 tetrahydro-imidazo(1,2-a)pyridines as antiprotozoal agents. J. Med. Chem. 47:3658-3664. [DOI] [PubMed] [Google Scholar]
- 13.Ismail, M., R. Brun, F. Tanious, W. D. Wilson, and D. W. Boykin. 2003. Synthesis and anti-protozoal activity of aza analogs of furamidine. J. Med. Chem. 46:4761-4769. [DOI] [PubMed] [Google Scholar]
- 14.Kumar, A., D. W. Boykin, W. D. Wilson, S. K. Jones, B. K. Bender, C. C. Dykstra, J. E. Hall, and R. R. Tidwell. 1996. Anti-Pneumocystis carinii pneumonia activity of dicationic 2,4-diarylpyrimidines. Eur. J. Med. Chem. 31:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lun, Z. R., R. Allingham, R. Brun, and S. M. Lanham. 1992. The isoenzyme characteristics of Trypanosoma evansi and Trypanosoma equiperdum isolated from domestic stocks in China. Ann. Trop. Med. Parasitol. 86:333-340. [DOI] [PubMed] [Google Scholar]
- 16.Reid, S. A. 2002. Trypanosoma evansi control and containment in Australasia. Trends Parasitol. 18:219-224. [DOI] [PubMed] [Google Scholar]
- 17.Seidl, A. F., A. S. Moraes, and R. A. Silva. 2001. Trypanosoma evansi control and horse mortality in the Brazilian Pantanal. Mem. Inst. Oswaldo Cruz. 96:599-602. [DOI] [PubMed] [Google Scholar]
- 18.Soeiro, M. N., E. M. De Souza, C. E. Stephens, and D. W. Boykin. 2005. Aromatic diamidines as antiparasitic agents. Expert Opin. Investig. Drugs 14:957-972. [DOI] [PubMed] [Google Scholar]
- 19.Tidwell, R. R., J. D. Geratz, O. Dann, G. Volz, D. Zeh, and H. Loewe. 1978. Diarylamidine derivatives with one or both of the aryl moieties consisting of an indole or indole like ring. J. Med. Chem. 21:613-623. [DOI] [PubMed] [Google Scholar]
- 20.Touratier, L. 2000. Challenges of non-tsetse transmitted animal trypanosomoses (NTTAT). An outline and some perspectives. Ann. N. Y. Acad. Sci. 916:237-239. [DOI] [PubMed] [Google Scholar]
- 21.Wilson, W. D., B. Nguyen, F. A. Tanious, A. Mathis, J. E. Hall, C. E. Stephens, and D. W. Boykin. 2005. Dications that target the DNA minor groove: compound design and preparation, DNA interactions, cellular distribution and biological activity. Curr. Med. Chem. Anticancer Agents 5:389-408. [DOI] [PubMed] [Google Scholar]