Abstract
Voriconazole and anidulafungin in combination are being investigated for use for the treatment of pulmonary aspergillosis. We determined the pulmonary disposition of these agents. Twenty healthy participants received intravenous voriconazole (at 6 mg/kg of body weight every 12 h [q12h] on day 1 and then at 4 mg/kg q12h) and anidulafungin (200 mg on day 1 and then 100 mg every 24 h) for 3 days. Five participants each were randomized for collection of bronchoalveolar lavage samples at times of 4, 8, 12, and 24 h. Drug penetration was determined by the ratio of the total drug area under the concentration-time curve during the dosing interval (AUC0-τ) for epithelial lining fluid (ELF) and alveolar macrophages (AM) to the total drug AUC0-τ in plasma. The mean (standard deviation) half-life and AUC0-τ were 6.9 (2.1) h and 39.5 (19.8) μg·h/ml, respectively, for voriconazole and 20.8 (3.1) h and 101 (21.8) μg·h/ml, respectively, for anidulafungin. The AUC0-τ values for ELF and AM were 282 and 178 μg·h/ml, respectively, for voriconazole, and 21.9 and 1,430 μg·h/ml, respectively, for anidulafungin. This resulted in penetration ratios into ELF and AM of 7.1 and 4.5, respectively, for voriconazole and 0.22 and 14.2, respectively, for anidulafungin. The mean total concentrations of both drugs in ELF and AM at 4, 8, 12, and 24 h remained above the MIC90/90% minimum effective concentration for most Aspergillus species. In healthy adult volunteers, voriconazole achieved high levels of exposure in both ELF and AM, while anidulafungin predominantly concentrated in AM.
Over the last few decades, the incidence of infections caused by Aspergillus spp. has steadily increased (17, 27). This increase parallels the rise in the numbers of immunocompromised patients who have been seen. Given the vulnerability of this patient population, Aspergillus infections cause a considerable number of deaths. The reported mortality rate for pulmonary aspergillosis, the most common presentation, ranges from 60 to 86% (9, 17).
Recent clinical practice guidelines recommend the use of voriconazole as the first-line therapy for patients with pulmonary aspergillosis (29). This recommendation stems from the predictable in vitro activity of voriconazole, coupled with its proven clinical efficacy in a randomized control trial (12, 18). The same guidelines note the potential utility of monotherapy or combination therapy with antifungal agents of different classes in patients whose infections are refractory to primary treatment. Secondary to their potent in vitro activity, unique mechanism of action, and favorable side effect profile, the echinocandin class of antifungals has become a popular option for combination therapy with voriconazole or polyenes. While at present caspofungin is the only echinocandin approved for use for the treatment of invasive aspergillosis (29), clinical trials with anidulafungin plus voriconazole are currently under way (see http://clinicaltrials.gov/).
Given that pulmonary aspergillosis is the most common presentation of invasive Aspergillus infections, it is important to understand the bronchopulmonary disposition of these agents. While a previously conducted pilot study shed at least some light on the penetration of voriconazole (5), no data exist for anidulafungin or a combination of anidulafungin and voriconazole. The purpose of this study was to characterize the pulmonary disposition of intravenous (i.v.) voriconazole and anidulafungin when they were given in combination to healthy adult volunteers.
MATERIALS AND METHODS
Study design.
This study was a prospective, open-label analysis of the plasma and intrapulmonary concentrations of voriconazole and anidulafungin at steady state. The study took place at the Clinical Research Center and Same Day Surgicenter at Hartford Hospital (Hartford, CT). The protocol was approved by the Hartford Hospital Institutional Review Board, and all participants provided written informed consent prior to screening in the study.
Participants.
A total of 20 healthy volunteer participants were included in the final analysis. The inclusion criteria required that each participant be greater than 18 years of age and free of any medical conditions, as determined in a prestudy evaluation performed within 28 days prior to study drug administration. This evaluation consisted of a detailed medical history, physical examination, and diagnostic testing (including determination of vital signs, electrocardiography, and blood/urine laboratory testing). The laboratory testing was repeated on the day prior to medication administration and again at the end of the study period. Participants were excluded for any of the following reasons: evidence of any clinically significant disease or illness; history of febrile illness within 5 days prior to the first study dose; positive urine drug screen; history of drug/alcohol abuse; habitual use of tobacco- or nicotine-containing products in excess of five cigarettes daily; participation in another investigation within 30 days; sitting blood pressure in excess of 140 mm Hg systolic or 90 mm Hg diastolic; pregnancy or nursing; use of prescription or nonprescription drugs, vitamins, and dietary supplements within 7 days of the first dose; use of hormonal contraception or hormone replacement therapy within 28 days of the first dose; blood donation within 56 days of dosing; known allergy or intolerance to the study medications, any other member of the echinocandin or triazole classes, lidocaine, or midazolam; a known hypersensitive pharyngeal reflex; and an inability to refrain from the consumption of alcohol, nicotine, tobacco, or caffeinated products during the study period.
Study medication.
Commercially available preparations of voriconazole and anidulafungin were supplied by Pfizer Inc. (New York, NY). Both medications were reconstituted, diluted, and stored according to the manufacturer's recommendations. The loading doses had a final volume of 560 ml, while the maintenance doses were 280 ml. All dosages were prepared by the Department of Pharmacy at Hartford Hospital and were administered within 12 h after preparation.
Dosing and sampling.
The participants received i.v. voriconazole loading doses of 6 mg/kg of body weight every 12 h on day 1 as 120-min infusions through a peripheral i.v. catheter, followed by maintenance doses of 4 mg/kg every 12 h on day 2 and a single 4-mg/kg dose on day 3 as 100-min infusions. Simultaneously, through a second peripheral i.v. site, the participants received an anidulafungin loading dose of 200 mg on day 1 as a 200-min infusion, followed by maintenance doses of 100 mg every 24 h on days 2 and 3 as 100-min infusions. Weight-based voriconazole doses were calculated from the actual body weight of each participant and were rounded to the nearest milligram. Duplicate blood samples were taken from a third peripheral i.v. catheter at 0 h (the start of the infusion); 100 min (the end of the infusion); and 2, 4, 8, 12, and 24 h after the start of drug administration for plasma drug concentration determination. Serum for urea analysis was collected at the start of the bronchoscopy and bronchoalveolar lavage (BAL) procedure.
Bronchoscopy and BAL.
The participants were randomized to undergo a single bronchoscopy with BAL at 4, 8, 12, or 24 h after the third dose. This provided data for five participants per BAL time point. After the participants fasted for 6 h, they were administered 4% aerosolized lidocaine in the nares and oropharynx and 2% lidocaine jelly in the nasal passageway within 30 min of bronchoscopy. Conscious sedation consisting of i.v. midazolam at 2 mg was reserved for participants undergoing a difficult bronchoscopy, as determined by the pulmonologist. A fiber optic bronchoscope (Olympus America Inc., Center Valley, PA) was inserted into the medial segment of the right middle lobe of the lung. Four 50-ml aliquots of 0.9% sodium chloride were separately instilled and immediately aspirated via the bronchoscope. After the first sample was discarded, the remaining aspirates were pooled and the volume was recorded. Duplicate 4-ml samples were taken from the pooled BAL fluid and sent to the clinical laboratory for a complete cell count, while the remaining fluid was placed on ice and transferred for processing.
Specimen processing.
The blood samples were centrifuged (1,000 × g at 4°C for 10 min) immediately after collection. The separated plasma was stored at −80°C until further analysis. The BAL fluid was centrifuged at 400 × g at 4°C for 10 min to separate the supernatant and the cell pellet. A sample of the supernatant was collected for urea concentration determination. To prevent the loss of drug due to the nonspecific binding of anidulafungin (observed in previous experiments), all containers were rinsed with methanol and pooled to create a 1:1 ratio of supernatant and methanol in the final samples. These samples were stored at −80°C until analysis. To lyse the cells, the cell pellet was resuspended in equal parts of 0.9% sodium chloride and methanol at a volume equal to 10% of the total pooled BAL fluid volume, and the mixture was stored at −80°C.
Antifungal concentration determination.
Plasma, BAL fluid supernatant, and BAL fluid cell pellet samples were assayed at PPD, Inc. (Richmond, VA), for voriconazole and anidulafungin by a validated high-performance liquid chromatography/tandem mass spectrometry method.
Plasma.
The voriconazole and anidulafungin concentrations in individual samples collected during the study period were determined separately. The methodology for plasma voriconazole concentration determination has recently been published (1). The dynamic range for the voriconazole assay was 10.0 to 5,000 ng/ml; the accuracy, expressed as the percent difference from the theoretical concentration in the quality control (QC) sample prepared with concentrations at 30.0, 250, 2,000, and 4,000 ng/ml, ranged from −2.89 to 5.37%; and the precision was ≤4.30%. The method used to measure the anidulafungin concentration in plasma is similar to the method described below for analysis of the BAL fluid supernatant and BAL fluid cell pellet samples. The plasma anidulafungin assay had a dynamic range of 50.0 to 20,000 ng/ml. The accuracy for the QC samples prepared with concentrations of 150, 750, 2,000, 6,000, and 15,000 ng/ml used during sample analysis ranged from 1.17 to 4.67%; and the precision, expressed as the coefficient of variation, was ≤4.80% for anidulafungin.
BAL fluid supernatant and alveolar cells.
A single method that measured the concentrations of both voriconazole and anidulafungin simultaneously in the BAL fluid supernatant and the BAL fluid cell pellet samples was validated. Due to a limited supply of BAL fluid aspirate, a surrogate matrix (0.9% sodium chloride solution) was used for all calibration standards, QC validations, and analyses of BAL fluid supernatant and cell pellet samples. The blank matrix was spiked with the appropriate concentration of each analyte for preparation of the calibration standards and QC samples, and then methanol in a 1:1 (vol/vol) ratio was added to each standard and QC sample. A 200-μl sample aliquot was fortified with 25 μl of 150 ng/ml of the internal standard (voriconazole-d3 for voriconazole and PF-04571511 for anidulafungin) working solution in a 96-well polypropylene plate. The plate was vortexed for 5 min, followed by centrifugation at 1,000 rpm for 5 min. The extract was chromatographed on an Xbridge C18 column (2.1 mm by 20 mm; particle diameter, 5 μm; Waters) with an injection volume of 35 μl. The mobile phases were 1:90:10:0.6 1.0 M ammonium bicarbonate-water-methanol-ammonium hydroxide (mobile phase A) and 1:5:95 1.0 M ammonium bicarbonate-water-acetonitrile (mobile phase B), where were run as a gradient. The high-performance liquid chromatography system consisted of an HP 1100 series pump and a CTC Analytics LCPAL autosampler interfaced to a Sciex API 4000 tandem mass spectrometer. For voriconazole and voriconazole-d3, the mass spectrometer operated in the multiple-reaction-monitoring, positive-ion mode and monitored the transition ions m/z 350.1 → 281.2 and 353.1 → 353.1, respectively. For anidulafungin and the internal standard PF-04571511, the mass spectrometer operated in the multiple-reaction-monitoring, negative-ion mode and monitored the transition ions m/z 1138.6 → 898.8 and 1149.7 → 909.6, respectively. The dynamic range of the curve was 1.00 to 1,000 ng/ml; and the concentrations of the QC samples were 3.00, 8.00, 30.0, 125, and 750 ng/ml. For voriconazole, the accuracy of the QC samples during sample analysis ranged from −1.78 to 5.43% and the precision was ≤12.1%. For anidulafungin, the accuracy of the QC samples ranged from −2.85 to 1.41% and the precision was ≤5.19%.
Urea concentration determination.
The urea concentrations in BAL fluid and serum collected simultaneously at the time of bronchoscopy were analyzed by a colorimetric enzymatic assay (Teco Diagnostics, Anaheim, CA) by a spectrophotometer detection method (Cary 50 series; Varian, Walnut Creek, CA). The assay was linear (R2 = 1.0) for the urea concentrations in both BAL fluid and serum over the range of 0.1 to 2.0 mg/dl. The intraday and interday variabilities of the QC samples (0.15 and 1.5 mg/dl in serum and the supernatant, respectively) were <5%.
Calculation of drug concentrations in ELF and AMs.
The volume of epithelial lining fluid (ELF) within the BAL fluid was calculated by the urea dilution method (26). The voriconazole and anidulafungin concentrations in ELF and alveolar macrophages (AMs) were calculated by methods previously described by our group (4, 20). The number of AMs within the BAL fluid was determined from the mean proportion present in the two manual cell counts, and the total volume of these cells in the cell pellet was calculated by using a mean AM volume of 2.42 μl/106 cells (14).
Pharmacokinetic analyses.
The pharmacokinetics of voriconazole and anidulafungin in plasma were estimated for each participant by noncompartmental methods. The area under the curve (AUC) from time zero to the end of the dosing interval (AUC0-τ) was calculated by use of the linear-log trapezoidal rule. The ends of the dosing intervals were 12 and 24 h for voriconazole and anidulafungin, respectively. The elimination rate constant (λz) was estimated as the slope of the best-fit linear regression line for the concentrations collected after the end of the infusion. The half-life for each participant was calculated as 0.693/λz. During these analyses, if the correlation coefficient of the linear regression line was less than 0.9, the participant-specific estimates for the half-life and the volume of distribution were not included in the summary statistics. The mean concentrations of voriconazole and anidulafungin in ELF and AMs for the five participants at each time point were used to calculate the total drug AUC0-τ in ELF and AMs. The penetration into ELF and AMs was calculated as the ratio of AUC0-τ in each compartment to the AUC0-τ in plasma. Total drug exposures were utilized for this calculation because the percentages of protein-bound drug within the individual compartments likely differ and are not currently known for ELF and AMs.
Safety assessment.
The safety and tolerability of voriconazole and anidulafungin were monitored by recording the adverse events that occurred throughout the duration of the study, coupled with a physical examination and diagnostic testing upon exit from the study.
RESULTS
Participants.
Twenty-one participants received at least one dose of the study medications, and 20 participants completed the analysis. Of these, 17 were male; 15 were Caucasian, 4 were Hispanic, and 1 was African American. Their mean (standard deviation [SD]) age and weight were 26.3 (6.7) years and 81.1 (12.6) kg, respectively.
Plasma pharmacokinetics.
The plasma pharmacokinetics of voriconazole and anidulafungin are listed in Table 1. The total drug mean concentration-time profiles for both agents are depicted in Fig. 1.
TABLE 1.
Steady-state plasma pharmacokinetics of i.v. voriconazole and anidulafungin in healthy volunteersa
| Drug (dose) | Cmax (μg/ml) | Tmax (h) | AUC0-τ (μg·h/ml) | Vss (liters) | CL (liters/h) | t1/2 (h) |
|---|---|---|---|---|---|---|
| Voriconazole (4 mg/kg) | 5.3 (1.8) | 1.8 (0.2) | 39.5 (19.8) | 109.7 (33.9) | 171.6 (86.2) | 6.9 (2.1) |
| Anidulafungin (100 mg) | 6.6 (1.6) | 1.9 (0.2) | 101.0 (21.8) | 30.8 (6.8) | 17.1 (3.1) | 20.8 (3.1) |
Data are presented as means (SDs). Cmax, maximum drug concentration; Tmax, time to maximum drug concentration; AUC0-τ, AUC for the dosing interval; Vss, volume of distribution at steady state; CL, total body clearance; t1/2, elimination half-life.
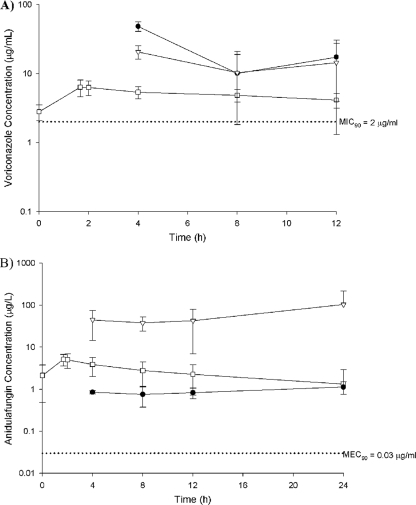
FIG. 1.
Mean ± SD steady-state total drug concentration-time profiles of voriconazole (A) and anidulafungin (B) in plasma (open squares), ELF (closed circles), and AMs (open triangles) in comparison with the MIC90 or the MEC90 (dotted line) for Aspergillus spp.
Pulmonary pharmacokinetics.
During bronchoscopy, the mean (SD) time required for the collection of all three BAL fluid aspirates was 2.9 (0.4) min. The mean cell counts, percentage of AMs, volume of ELF, and volume of lavage fluid did not differ statistically by bronchoscopy time point (Table 2).
TABLE 2.
BAL fluid, ELF, and alveolar cell recovery characteristicsa
| Time of bronchoscopy (h) | Cell count (no. of cells/ml) | % AMs | ELF vol (ml) | BAL fluid vol (ml) |
|---|---|---|---|---|
| 4 | 1.9 × 105 (6.1 × 104) | 83.5 (4.1) | 1.7 (0.4) | 80.2 (12.3) |
| 8 | 2.2 × 105 (1.3 × 105) | 74.9 (11.1) | 2.2 (1.2) | 64.8 (19.7) |
| 12 | 2.8 × 105 (1.8 × 105) | 73.7 (11.3) | 2.3 (1.4) | 92.6 (29.3) |
| 24 | 1.6 × 105 (9.7 × 104) | 68.9 (11.8) | 1.7 (0.4) | 86.6 (23.2) |
Data are presented as means (SDs).
The mean ELF and AM drug concentrations at each BAL collection fluid time point are shown in Table 3. For voriconazole, the concentrations in ELF and AMs were greater than those in plasma at 4, 8, and 12 h. For anidulafungin, the concentrations in AMs were greater than those in plasma and ELF at all time points at which they were measured. Regardless, the mean ELF, AM, and plasma total drug concentrations were above the MIC90 for voriconazole (2 μg/ml) and the 90% minimum effective concentration (MEC90) for anidulafungin (0.03 μg/ml) against Aspergillus spp. for the entire dosing interval (Fig. 1) (2, 15, 16, 18). Additionally, no individual patient had a voriconazole or anidulafungin concentration below the MIC90 or the MEC90 in any pulmonary compartment. The mean ± SD levels of penetration into ELF for voriconazole at 4, 8, and 12 h were 9.5 ± 2.3, 4.9 ± 2.8, and 7.7 ± 3.4, respectively; the mean ± SD levels of penetration into AMs at the same time points were 3.9 ± 0.6, 5.6 ± 1.9, and 5.9 ± 4.5, respectively. The mean ± SD levels of penetration into ELF for anidulafungin at 4, 8, 12, and 24 h were 0.15 ± 0.02, 0.15 ± 0.07, 0.20 ± 0.09, and 0.38 ± 0.14, respectively; the mean ± SD levels of penetration into AMs at the same time points were 7.0 ± 2.9, 7.8 ± 3.4, 9.2 ± 6.3, and 33.1 ± 34.6, respectively. The calculated AUC0-τ values in ELF and AMs were 282 and 178 μg·h/ml, respectively, for voriconazole and 21.9 and 1,430 μg·h/ml, respectively, for anidulafungin. On the basis of the total drug AUC0-τ, the overall levels of penetration into ELF and AMs were 7.1 and 4.5, respectively, for voriconazole and 0.22 and 14.2, respectively, for anidulafungin.
TABLE 3.
Steady-state concentrations of voriconazole and anidulafungin in plasma, ELF, and AMs
| Time of BAL (h) | Concn (μg/ml)a |
|||||
|---|---|---|---|---|---|---|
| Voriconazole |
Anidulafungin |
|||||
| Plasma | ELF | AMs | Plasma | ELF | AMs | |
| 4 | 5.3 (1.4) | 48.3 (7.6) | 20.6 (4.5) | 6.0 (1.5) | 0.9 (0.1) | 44.6 (30.1) |
| 8 | 1.7 (0.9) | 10.1 (10.8) | 10.3 (8.5) | 5.1 (0.8) | 0.8 (0.4) | 37.9 (13.8) |
| 12 | 2.2 (1.1) | 17.2 (13.3) | 14.4 (13.1) | 4.4 (1.0) | 0.8 (0.2) | 42.7 (35.8) |
| 24 | NR | NR | NR | 3.0 (0.5) | 1.1 (0.4) | 103.1 (110.7) |
Data are presented as means (SDs); values are estimated on the basis of the results for five participants per time point. NR, not reported.
Safety and tolerability.
In general, the combination of i.v. voriconazole and anidulafungin was well tolerated, and no serious adverse events were reported. The most commonly reported adverse events were mild and included headache (n = 6), visual disturbances (n = 6), and flu-like symptoms (n = 4). On laboratory testing at discharge, one participant experienced mild increases in liver function test results that returned to the baseline upon repeat testing. The bronchoscopy and BAL procedures were well tolerated by all participants, with one participant requiring conscious sedation.
DISCUSSION
Because of the high mortality rate and the relative increase in the incidence of invasive pulmonary fungal infections, the optimization of treatment modalities becomes imperative. An important consideration for each option is how well the antifungal agent(s) penetrates the target infection site and its disposition once it is there. In this report, we describe the first assessment of the bronchopulmonary penetration of i.v. voriconazole and anidulafungin given in combination. We found that voriconazole penetrated both ELF and AMs well, while anidulafungin predominately concentrated in AMs.
The steady-state plasma pharmacokinetics for both voriconazole and anidulafungin in this study were similar to those in previously published analyses of studies with healthy volunteers. We described a voriconazole half-life, AUC, and maximum drug concentration of 6.9 h, 39.5 μg·h/ml, and 5.3 μg/ml, respectively, which correlated well with values of 5 to 8 h, 29.5 μg·h/ml, and 5.4 μg/ml, respectively, presented previously (24, 25). For anidulafungin, while our AUC and maximum drug concentration of 101 μg·h/ml and 6.6 μg/ml, respectively, were similar to those reported previously (120 μg·h/ml and 7.9 μg/ml, respectively), our half-life of 20 h was shorter than the 40 h previously reported from a single study with healthy volunteers (11). Instead, the half-life observed in our healthy volunteers was very similar to the 25.9 h reported in a population pharmacokinetic analysis of patients (10). While it was not the purpose of this study, nor should it have any bearing on the observations made relative to pulmonary penetration, it should be noted that the use of a 24-h sampling schedule is not ideal for the full characterization of the plasma pharmacokinetics of any drug with a half-life near or above 24 h, which may explain the differences observed between the studies with healthy volunteers.
With respect to the pulmonary disposition, voriconazole penetrated well into both extracellular (i.e., ELF) and intracellular (i.e., AM) compartments, with the concentrations in each of those compartments exceeding those in plasma for most of the time points (Table 3). A previous study conducted with patients receiving oral voriconazole after lung transplantation found similar results for ELF (5). In that population, the point penetration ratio into ELF ranged from 2 to 28, and the mean was 11. Despite obvious differences in study design and study participants, this correlated well with our observed range of 2.5 to 12.4 throughout the dosing interval. That study did not assess the AM voriconazole concentrations. In contrast, when assessing the pulmonary pharmacokinetics of two other triazoles, itraconazole and posaconazole, Conte and colleagues found the ELF concentrations to be less than the plasma concentrations, while the AM concentrations were greater (6, 7).
The pulmonary disposition of anidulafungin was quite different between the intra- and extracellular compartments, as the level of penetration into AMs was much greater than that into ELF. A similar study conducted with micafungin given to healthy volunteers at 150 mg daily found comparable results (19). On the basis of the AUC, the penetration ratio of micafungin was found to be 0.05 for ELF and 1.1 for AMs. Moreover, a case report evaluating the intrapulmonary penetration of caspofungin in a lone lung transplant patient also found the penetration into ELF to be very low (i.e., ELF concentrations were below the lower limit of quantification at all time points), while the concentrations in AMs were many times those in plasma and the mean penetration ratio was 10.9 (3). Although it is unknown how exactly these echinocandins enter the alveolar cells, it is clear that preferential penetration into AMs is a class effect. Relative to other echinocandins, such a high level of penetration into AMs with anidulafungin was not unexpected. A recent tissue distribution study with rats noted anidulafungin to have the highest level of penetration into lung tissue (although the study did not assess whether the drug was predominantly intra- or extracellular) compared with similar rat studies with micafungin and caspofungin (8). These observations are consistent with the fact that among the echinocandins anidulafungin has the largest volume of distribution. As such, the absolute AM concentrations for the doses studied were approximately threefold greater for anidulafungin over the dosing interval than for micafungin in healthy volunteers (19). The clinical significance of these observations is unknown.
When the mean concentrations of anidulafungin in AMs are assessed (Fig. 1B), it is important to note that the mean increase seen at the 24-h time point is attributable to the BAL fluid data for a single participant, in whom the concentration in that participant was at least three times the concentrations in the remaining four participants. Likewise, the calculated ratio of penetration into AMs for this single participant was three times that into the AMs for the other participants at 24 h. While a certain degree of variability is expected when pharmacokinetic studies are conducted with humans, it is unclear why the level of penetration in this participant varied so greatly from that in the others. Reanalysis of the results after exclusion of the data for this participant did not alter the conclusions, although the degree of penetration into AMs did decrease slightly from 14.2 to 11.0.
While we have described the significant penetration of both drugs into AMs and it is relatively well documented that AMs are the first line of defense against inhaled conidia in both human and murine ex vivo studies (13, 31), it is unclear if this trait translates to clinical efficacy in the treatment of pulmonary aspergillosis. It should also be noted that although the level of anidulafungin penetration into ELF was rather low in comparison to that into AMs, the total drug concentrations remained above the MEC90 for Aspergillus spp. (0.03 μg/ml) throughout the entire dosing interval (2, 15, 18). Unfortunately, relevant pharmacodynamic relationships between anidulafungin exposure at the infection site and efficacy for determination of whether the magnitude of these concentrations would be adequate are not available.
As mentioned above, a randomized, multicenter, clinical trial evaluating the efficacy of the combination of anidulafungin and voriconazole compared with that of voriconazole alone for the treatment of pulmonary aspergillosis is currently under way. While the results of that trial should provide a more definitive answer as to the benefit of combination therapy, previous in vitro and in vivo models have shown mixed results. Using MIC as an endpoint for determination, one study found combinations of voriconazole and anidulafungin to be synergistic against 18/26 Aspergillus sp. isolates (23), while another found the combination to be mostly indifferent (21). Yet another study that applied Bliss independence drug interaction analysis found the combination to be synergistic in vitro against one Aspergillus fumigatus isolate that was then tested in vivo. Using a rabbit model of pulmonary aspergillosis, the authors found voriconazole and anidulafungin at 5 mg/kg in combination to be synergistic, while they found combinations with 10 mg/kg of anidulafungin to be antagonistic (22). Lastly, a study evaluating a simulated human AUC in rats with pulmonary aspergillosis found a nonsignificant increase in the rate of survival in those treated with anidulafungin and voriconazole in combination compared with that in those treated with voriconazole alone (28). When different models of infection are compared, it is important to consider that the penetration into different pulmonary compartments may vary substantially among species.
We found i.v. therapy with the combination of voriconazole and anidulafungin to be well tolerated, with only mild adverse events being reported by the group of healthy volunteers studied. Much as was the case in a previous assessment of oral voriconazole and i.v. anidulafungin administered in combination, we found the reported adverse events to be similar to those reported for each medication in independent analyses (11, 12, 24, 25, 30).
In conducting the analyses for this study, a few assumptions that could have affected the reported results were made. The first assumption was that when the ratios of the penetration of both drugs into ELF and AMs were determined, we compared the total drug exposure in each compartment to the total drug exposure in plasma. We made this assumption for a few reasons. First, the types of proteins and the amount of each available in the various matrices (i.e., ELF and AM) are unknown and may differ from those in plasma. Second, the affinities of the drugs to the available proteins could also be different, all of which would potentially result in various degrees of protein-bound drug within each compartment. Moreover, the clinical significance of protein binding within each compartment is also unknown. Lastly, this approach represents the most conservative estimate of penetration. In blood, voriconazole is 58% protein bound and anidulafungin is approximately 99% protein bound; therefore, use of the free fraction would increase the penetration ratios by 2- and 100-fold, respectively.
The second assumption was made when the concentrations of both antifungal agents within AMs were determined. We assumed that the recovered drug was found only from within the hystiocytes and monocytes. While these cells made up the majority of the cells present within the BAL fluid, it is possible that drug within the other immune cell lines was expelled during the lysis procedure. Finally, this analysis was conducted with healthy volunteers, and further studies with patients, particularly those with aspergillosis, are needed to evaluate if the pulmonary disposition of the drugs is altered in the presence of infection and comorbidities.
In conclusion, we determined the pulmonary penetration of i.v. voriconazole and anidulafungin in healthy volunteers. We found that voriconazole penetrates both ELF and AMs very well, while anidulafungin was predominately distributed intracellularly. The total drug concentrations of both voriconazole and anidulafungin in all three compartments were above the MIC90/MEC90 for Aspergillus spp. throughout the entire dosing interval. These data support ongoing clinical investigations of the use of voriconazole and anidulafungin in combination for the treatment of pulmonary aspergillosis.
Acknowledgments
We acknowledge the following individuals for their assistance with the conduct of this study (listed alphabetically): Susan Albino, David Marshall, Debra Sanchez, and Lee Steere. We also thank the entire staff of the Center for Anti-Infective Research and Development.
Funding for this study was supplied by Pfizer Inc. D.P.N. and J.L.K. have both received research grants from Pfizer Inc. A.F.F. and P.H.C. are employees of Pfizer Inc.
Footnotes
Published ahead of print on 21 September 2009.
REFERENCES
- 1.Andrews, E., B. D. Damle, A. Fang, G. Foster, P. Crownover, R. LaBadie, and P. Glue. 2008. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br. J. Clin. Pharmacol. 65:531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh. 2008. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob. Agents Chemother. 52:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhardt, O., S. Ellis, H. Burhenne, V. Kaever, J. Hadem, J. T. Kielstein, and T. Welte. 2009. High caspofungin levels in alveolar cells of a lung transplant patient with suspected pulmonary aspergillosis. Int. J. Antimicrob. Agents 34:491-492. [DOI] [PubMed] [Google Scholar]
- 4.Capitano, B., H. M. Mattoes, E. Shore, A. O'Brien, S. Braman, C. Sutherland, and D. P. Nicolau. 2004. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 125:965-973. [DOI] [PubMed] [Google Scholar]
- 5.Capitano, B., B. A. Potoski, S. Husain, S. Zhang, D. L. Paterson, S. M. Studer, K. R. McCurry, and R. Venkataramanan. 2006. Intrapulmonary penetration of voriconazole in patients receiving an oral prophylactic regimen. Antimicrob. Agents Chemother. 50:1878-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte, J. E., Jr., J. A. Golden, J. Kipps, M. McIver, and E. Zurlinden. 2004. Intrapulmonary pharmacokinetics and pharmacodynamics of itraconazole and 14-hydroxyitraconazole at steady state. Antimicrob. Agents Chemother. 48:3823-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte, J. E., Jr., J. A. Golden, G. Krishna, M. McIver, E. Little, and E. Zurlinden. 2009. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob. Agents Chemother. 53:703-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damle, B., M. Stogniew, and J. Dowell. 2008. Pharmacokinetics and tissue distribution of anidulafungin in rats. Antimicrob. Agents Chemother. 52:2673-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 10.Dowell, J. A., W. Knebel, T. Ludden, M. Stogniew, D. Krause, and T. Henkel. 2004. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J. Clin. Pharmacol. 44:590-598. [DOI] [PubMed] [Google Scholar]
- 11.Dowell, J. A., J. Schranz, A. Baruch, and G. Foster. 2005. Safety and pharmacokinetics of coadministered voriconazole and anidulafungin. J. Clin. Pharmacol. 45:1373-1382. [DOI] [PubMed] [Google Scholar]
- 12.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim-Granet, O., B. Philippe, H. Boleti, E. Boisvieux-Ulrich, D. Grenet, M. Stern, and J. P. Latge. 2003. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 71:891-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, J. D., W. L. Hand, J. B. Francis, N. King-Thompson, and R. W. Corwin. 1980. Antibiotic uptake by alveolar macrophages. J. Lab. Clin. Med. 95:429-439. [PubMed] [Google Scholar]
- 15.Lass-Florl, C., A. Alastruey-Izquierdo, M. Cuenca-Estrella, S. Perkhofer, and J. L. Rodriguez-Tudela. 2009. In vitro activities of various antifungal drugs against Aspergillus terreus: global assessment using the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 53:794-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lass-Florl, C., A. Mayr, S. Perkhofer, G. Hinterberger, J. Hausdorfer, C. Speth, and M. Fille. 2008. Activities of antifungal agents against yeasts and filamentous fungi: assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 52:3637-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 18.Messer, S. A., G. J. Moet, J. T. Kirby, and R. N. Jones. 2009. Activity of contemporary antifungal agents, including the novel echinocandin anidulafungin, tested against Candida spp., Cryptococcus spp., and Aspergillus spp.: report from the SENTRY Antimicrobial Surveillance Program (2006 to 2007). J. Clin. Microbiol. 47:1942-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicasio, A. M., P. R. Tessier, D. P. Nicolau, R. F. Knauft, J. Russomanno, E. Shore, and J. L. Kuti. 2009. Bronchopulmonary disposition of micafungin in healthy adult volunteers. Antimicrob. Agents Chemother. 53:1218-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong, C. T., P. K. Dandekar, C. Sutherland, C. H. Nightingale, and D. P. Nicolau. 2005. Intrapulmonary concentrations of telithromycin: clinical implications for respiratory tract infections due to Streptococcus pneumoniae. Chemotherapy 51:339-346. [DOI] [PubMed] [Google Scholar]
- 21.Perkhofer, S., D. Jost, M. P. Dierich, and C. Lass-Florl. 2008. Susceptibility testing of anidulafungin and voriconazole alone and in combination against conidia and hyphae of Aspergillus spp. under hypoxic conditions. Antimicrob. Agents Chemother. 52:1873-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petraitis, V., R. Petraitiene, W. W. Hope, J. Meletiadis, D. Mickiene, J. E. Hughes, M. P. Cotton, T. Stergiopoulou, M. Kasai, A. Francesconi, R. L. Schaufele, T. Sein, N. A. Avila, J. Bacher, and T. J. Walsh. 2009. Combination therapy in treatment of experimental pulmonary aspergillosis: in vitro and in vivo correlation of the concentration and dose dependent interaction between anidulafungin and voriconazole by Bliss independence drug interaction analysis. Antimicrob. Agents Chemother. 53:2382-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philip, A., Z. Odabasi, J. Rodriguez, V. L. Paetznick, E. Chen, J. H. Rex, and L. Ostrosky-Zeichner. 2005. In vitro synergy testing of anidulafungin with itraconazole, voriconazole, and amphotericin B against Aspergillus spp. and Fusarium spp. Antimicrob. Agents Chemother. 49:3572-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purkins, L., N. Wood, P. Ghahramani, K. Greenhalgh, M. J. Allen, and D. Kleinermans. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purkins, L., N. Wood, K. Greenhalgh, M. D. Eve, S. D. Oliver, and D. Nichols. 2003. The pharmacokinetics and safety of intravenous voriconazole-a novel wide-spectrum antifungal agent. Br J. Clin. Pharmacol. 56(Suppl. 1):2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnell, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 27.Richardson, M. D. 2005. Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 56(Suppl. 1):i5-i11. [DOI] [PubMed] [Google Scholar]
- 28.van de Sande, W. W., R. A. Mathot, M. T. ten Kate, W. van Vianen, M. Tavakol, B. J. Rijnders, and I. A. Bakker-Woudenberg. 2009. Combination therapy of advanced invasive pulmonary aspergillosis in transiently neutropenic rats using human pharmacokinetic equivalent doses of voriconazole and anidulafungin. Antimicrob. Agents Chemother. 53:2005-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard, and T. F. Patterson. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]
- 30.Walsh, T. J., P. Pappas, D. J. Winston, H. M. Lazarus, F. Petersen, J. Raffalli, S. Yanovich, P. Stiff, R. Greenberg, G. Donowitz, M. Schuster, A. Reboli, J. Wingard, C. Arndt, J. Reinhardt, S. Hadley, R. Finberg, M. Laverdiere, J. Perfect, G. Garber, G. Fioritoni, E. Anaissie, and J. Lee. 2002. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 346:225-234. [DOI] [PubMed] [Google Scholar]
- 31.Wasylnka, J. A., and M. M. Moore. 2002. Uptake of Aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infect. Immun. 70:3156-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]