Abstract
Leishmaniasis is parasitic disease that is an important problem of public health worldwide. Intramuscularly administered glucantime and pentostam are the most common drugs used for treatment of this disease, but they have significant limitations due to toxicity and increasing resistance. A recent breakthrough has been the introduction of orally administered miltefosine for the treatment of visceral, cutaneous, and mucocutaneous leishmaniasis, but the relative high cost and concerns about teratogenicity have limited the use of this drug. Searching for alternative drugs, we previously demonstrated that the antiarrhythmic drug amiodarone is active against Leishmania mexicana promastigotes and intracellular amastigotes, acting via disruption of intracellular Ca2+ homeostasis (specifically at the mitochondrion and the acidocalcisomes of these parasites) and through inhibition of the parasite's de novo sterol biosynthesis (X. Serrano-Martín, Y. García-Marchan, A. Fernandez, N. Rodriguez, H. Rojas, G. Visbal, and G. Benaim, Antimicrob. Agents Chemother. 53:1403-1410, 2009). In the present work, we found that miltefosine also disrupts the parasite's intracellular Ca2+ homeostasis, in this case by inducing a large increase in intracellular Ca2+ levels, probably through the activation of a plasma membrane Ca2+ channel. We also investigated the in vitro and in vivo activities of amiodarone and miltefosine, used alone or in combination, on L. mexicana. It was found that the drug combination had synergistic effects on the proliferation of intracellular amastigotes growing inside macrophages and led 90% of parasitological cures in a murine model of leishmaniasis, as revealed by a PCR assay using a novel DNA sequence specific for L. mexicana.
Leishmaniasis is a parasitic disease present in tropical and subtropical areas that generates high mortality and morbidity (15). The first line of treatment, based on pentavalent antimonials, is unsatisfactory due to high toxicity and the requirement of long periods of parenteral treatment. On the other hand, miltefosine, an orally active alkyl-lysophospholipid with potent anti-Leishmania activity, represents a major advance in the treatment of leishmaniasis (4). Nevertheless, miltefosine presents a series of adverse effects such as teratogenicity and potential development of resistance (4).
It has been suggested that miltefosine acts in Leishmania mexicana by inhibiting the alkyl-specific acyl coenzyme A-acyltransferase involved in the synthesis de novo of phosphatidylcholine (9), whereas in Trypanosoma cruzi and Leishmania donovani, miltefosine acts by inhibiting the Bremer-Greenberg pathway, specifically at level of phosphatidylethanolamine N-methyl-transferase (8, 17). In contrast, this drug inhibits phosphatidylcholine biosynthesis in humans by blocking phosphocholine citidyltransferase (24).
In a search for new alternative therapies, we previously demonstrated that amiodarone, a commonly used antiarrhythmic drug, affects the viability of T. cruzi epimastigotes and amastigotes (2), as well as L. mexicana promastigotes and amastigotes (20). In both cases, amiodarone induces an increase in the intracellular Ca2+ concentration ([Ca2+]i) by disrupting the Ca2+ regulation in the mitochondrion and the acidocalcisomes and also by blocking the ergosterol biosynthesis of these parasites (2, 20).
It has also been shown that miltefosine is able to affect intracellular Ca2+ homeostasis in human epithelial KB cells (7) and human myeloid leukemia cell lines (23). In this work, we sought to investigate the effects of miltefosine on the [Ca2+]i in L. mexicana and its potential synergistic effects with amiodarone, using in in vitro and in vivo assays.
MATERIALS AND METHODS
Drugs.
Amiodarone {(2-butyl-3-benzofuranyl)-[4-[2-(diethylamino)ethoxi]-3,5-diiodophenyl]methanone hydrochloride} and miltefosine (hexadecylphosphocholine) were obtained from Sigma Aldrich (St. Louis, MO). All other reagents were of the highest purity available.
Determination of in vitro antiproliferative activity.
L. mexicana promastigotes were cultured in RPMI 1640 medium (Gibco) supplemented with 10% inactivated fetal bovine serum (FBS) in constant agitation at 29°C as described previously (20). L. mexicana susceptibility to amiodarone and miltefosine was evaluated by following up the proliferation of parasites in the absence or presence of these drugs, counting living parasites every day in a Neubauer chamber in triplicate cultures. Drugs were added to the growth medium 24 h after starting the cultures with 106 parasites/ml. At least three independent experiments were performed for each drug or drug combination.
Determination of FIC index and isobologram construction.
For the fractional inhibitory concentration (FIC) and isobologram experiments, we used promastigotes and amastigote-infected macrophages, as previously reported (20). Briefly, L. mexicana promastigotes were maintained at 29°C in RPMI 1640 (Gibco) medium supplemented with 10% FBS, and the J774G8 mouse macrophages were maintained at 37°C and 5% CO2 in RPMI 1640 (Gibco) medium supplemented with 10% FBS. For parasite infection, a proportion of 10 promastigotes to 1 macrophage was employed and the infected cells were incubated for 24 h at 37°C and 5% CO2. To determine the effects of amiodarone and miltefosine on intracellular amastigotes, the percentage of infection was determined at 72 h posttreatment, under a light microscope, using Giemsa stain. At least three independent experiments were performed for each condition. Isobologram construction and calculation of the FIC were carried out as described by Hallender et al. (6). Briefly, FIC was determined as the combined concentration divided by the single concentration. The interaction index was calculated as ∑FIC = (MIC combination A/MIC alone A) + (MIC combination B/MIC alone B), where MIC is the MIC that produces total death of parasite population in the culture, with A representing the amiodarone concentration and B the miltefosine concentration.
[Ca2+]i determinations.
For the [Ca2+]i measurements, the fluorescent ratiometric Ca2+ indicator Fura 2 was used, because its excitation spectrum depends on the concentration of the cation, while its emission peak remains invariable. Parasites were loaded with Fura 2 as previously described (20). Briefly, parasites (4 × 106 promastigotes/1.5 ml of culture medium) were centrifuged at 600 × g for 2.5 min and then washed twice with the following loading buffer: 137 mM NaCl, 4 mM KCl, 1.5 mM KH2PO4, 8.5 mM Na2HPO4, 11 mM glucose, 1 mM CaCl2, 0.8 mM MgSO4, and 20 mM HEPES-NaOH, pH 7.4. The parasite pellet was resuspended in the same buffer, and a mixture of 6 μM Fura 2-AM and 2.4 mM probenecid was added. Parasites were then incubated at 29°C for 45 min under continuous agitation. Following two washes with the loading buffer without Fura 2-AM, fluorescence measurements were performed in a Perkin-Elmer LS-55 spectrofluorimeter provided with an acquisition system for excitation ratio measurements at 29°C with continuous agitation in a stirred cuvette. [Ca2+]is were evaluated by applying the equation [Ca2+]i = Kd × (R − Rmin/Rmax − R) × Fmin (380)/Fmax (380), where Kd is the dissociation constant of Fura 2 (244 nM) as reported by Grynkiewicz et al. (5); R is the fluorescent emission ratio obtained after excitation at 340 nm/380 nm; Rmax and Fmax are the ratio of excitation fluorescence at 340 nm/380 nm and the fluorescence of Fura 2 at 380 nm, respectively, under saturated Ca2+ concentrations; and Rmin and Fmin are the ratio of excitation fluorescence at 340 nm/380 nm and the fluorescence of Fura 2 at 380 nm, respectively, in the absence of Ca2+. Maximum and minimum values were obtained after the addition of 30 μM digitonin, which allows the flow of Ca2+ to the interior of the cell. Then, 8 mM EGTA was added to chelate all remaining Ca2+ outside the cells (5).
In vivo studies.
Drugs were tested in vivo using the murine model of leishmaniasis described previously (19) with minor modifications. Briefly, parasites were used and maintained by passage in hamsters every 8 weeks. Groups of 10 female BALB/c mice (6 weeks old, 25 g of weight) were inoculated (day 0) in the left hind footpad with 100 μl of phosphate-buffered saline (PBS) containing 1 × 106 amastigotes obtained from donor hamsters. One day after the inoculation, mice were randomly divided into 8 groups of 10 mice each and treatment was initiated 7 days after inoculation, when infection was well established and lesions were present. Each experimental group was treated daily with the drugs for 21 consecutive days, as follows: group 1 was treated with methylcellulose plus Tween 80 (0.2% [vol/vol]) used as vehicle, group 2 with glucantime at 100 mg/kg/day, group 3 with amiodarone at 25 mg/kg/day, group 4 with amiodarone at 50 mg/kg/day, group 5 with miltefosine at 10 mg/kg/day, group 6 with miltefosine at 20 mg/kg/day, group 7 with amiodarone at 25 mg/kg/day plus miltefosine at 10 mg/kg/day, and group 8 with amiodarone at 50 mg/kg/day plus miltefosine at 20 mg/kg/day. Glucantime was administered by the intraperitoneal route; all other drugs and the vehicle were administered orally through a stomach tube. Animals were observed 56 days after the end of treatment and then sacrificed to assess the parasite load in the infected footpad. Lesion growth was determined by measuring the diameter of both rear feet by direct reading with an electronic vernier scale. The size of the lesion in millimeters was calculated by subtracting the measurement of the uninfected foot from that of the infected foot. Measurements were made weekly and were continued for 84 days. For each experimental condition, the mean and standard error were calculated.
Parasite load determination. (i) Inoculation in cultures.
Biopsy sections (of ∼0.5 cm of diameter) from control and treated mice were placed in PBS, and after homogenization, 200 μl of the homogenate was added to 10 ml of culture medium supplemented with 10% FBS. Parasite growth was followed by microscopy for 3 weeks. Also, a smear impression from tissue sections was Giemsa stained and fixed with methanol to detect amastigotes or infected macrophages under light microscope.
(ii) PCR assay.
DNA samples were prepared as previously described (16) and used in a novel Leishmania multiplex PCR assay based on a methodology previously described for these parasites (11). The assay combines the amplification of a 260-bp fragment from a DNA sequence specific for a Leishmania subgenus and a 900-bp fragment from the coding region of the β-tubulin gene. The PCR was conducted in a final volume of 50 μl and with the reaction mixture containing PCR buffer (200 mM Tris-HCl, pH 8.4, and 500 mM KCl), 80 ng DNA, 1.25 U Platinum Taq DNA, 2 mM MgCl2, 2 mM each deoxynucleoside triphosphate (dNTP), and 50 pmol of each primer. The sequences of the Leishmania subgenus-specific oligonucleotide primers are as follows: F1, 5′-TGTGGGTGTGGGTGTGTGTGTATG-3′ (melting temperature [Tm],74°C); and B2, 5′-CGTGAAGAACATACAAAGCCTCCC-3′ (Tm, 72°C). The sequences of the β-tubulin primer set are as follows: Tub 1, 5′-ATGCGTGAGATCGTTTCC-3′ (Tm, 60°C); and Tub 2, 5′-GGCGGCCTGCATCAT-3′ (Tm, 62°C). The PCR was performed in an MJ Research PTC100 thermocycler, comprising 5 min of preincubation at 95°C followed by 40 cycles of 1 min at 95°C, 1 min at 60°C, and 2 min at 72°C, with a final extension for 5 to 10 min at 72°C. Each assay contained a positive control (0.10 μg parasite DNA) and a negative control (reaction mixture without DNA). PCR products were analyzed by electrophoresis on a 1% agarose gel with 1× TAE (4 mM Tris-acetate, 1 mM EDTA, pH 8).
RESULTS
Susceptibility of Leishmania to amiodarone and miltefosine.
In this work, we studied the effect of amiodarone on promastigotes alone and in combination with miltefosine. As found previously (20), amiodarone produced a marked reduction in the viability of L. mexicana promastigotes, with a 50% inhibitory concentration (IC50) of 900 nM and a MIC of 5 μM. On the other hand miltefosine induced a dose-dependent effect on the parasites' proliferation, with an IC50 of 8 μM, and a MIC of 15 μM, both results being similar to that reported by Soto and Berman (21). In order to test whether the efficacy of the drugs was enhanced when used in combination, we constructed an isobologram from the MICs obtained for the drugs used alone or in combination: the results are shown in Fig. 1, where a weak synergism can be observed (FIC, 0.74).
FIG. 1.
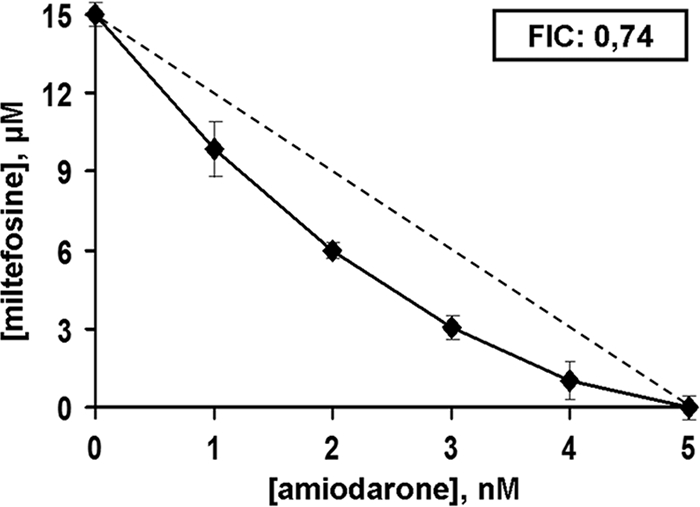
Representative isobologram of in vitro interaction between amiodarone and miltefosine against L. mexicana promastigotes. The combined effect of amiodarone plus miltefosine upon L. mexicana promastigote viability is shown. FIC value was calculated as described in Materials and Methods. The dotted line corresponds to the predicted positions of the experimental points for a simple additive effect. This experiment was performed at least three times for each condition, and results are expressed as means ± standard errors.
We also studied the effects of both drugs on the clinically relevant form of the parasite, using amastigote-infected macrophages. We obtained an IC50 of 8 nM and a MIC of 20 nM for amiodarone and an IC50 of 1 μM and a MIC of 6 μM for miltefosine. When the drugs were used in combination, the results indicated clear synergism, as shown by a concave isobologram and an FIC value of 0.49 (Fig. 2).
FIG. 2.
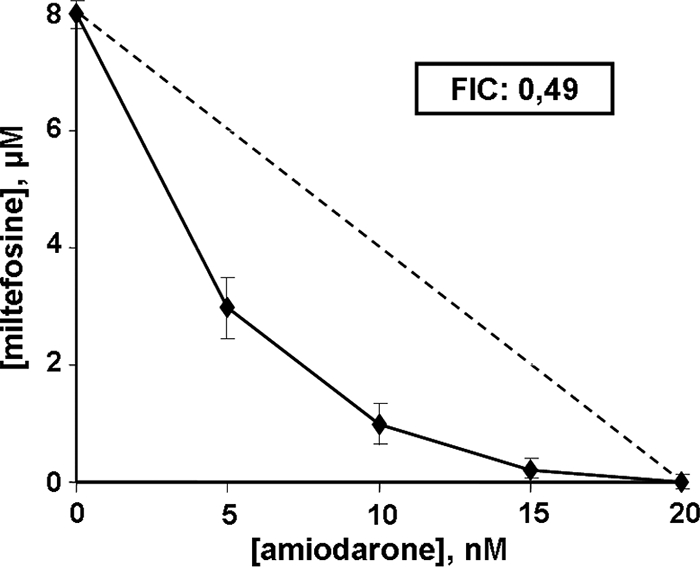
Representative isobologram of in vitro interaction between amiodarone and miltefosine against intracellular L. mexicana amastigotes. The combined effect of amiodarone plus miltefosine upon L. mexicana amastigote viability is shown. The FIC value was calculated as described in Materials and Methods. The dotted line corresponds to the predicted positions of the experimental points for a simple additive effect. This experiment was performed at least three times for each condition, and results are expressed as means ± standard errors.
Effect of miltefosine on the [Ca2+]i of L. mexicana promastigotes.
L. mexicana promastigotes were initially loaded with the Ca2+ indicator Fura 2. Figure 3A shows that the addition of 2.5 μM (a sublethal concentration) miltefosine induced a marked increase in [Ca2+]i in promastigotes, when the assay was performed in the presence of extracellular Ca2. Under this condition, digitonin induced a further large increase in fluorescence (not shown), which is due to the permeabilization of the plasma membrane to Ca2+ and Fura 2. This result rules out the possibility that miltefosine induces an increase in Fura 2 permeability of the plasma membrane.
FIG. 3.
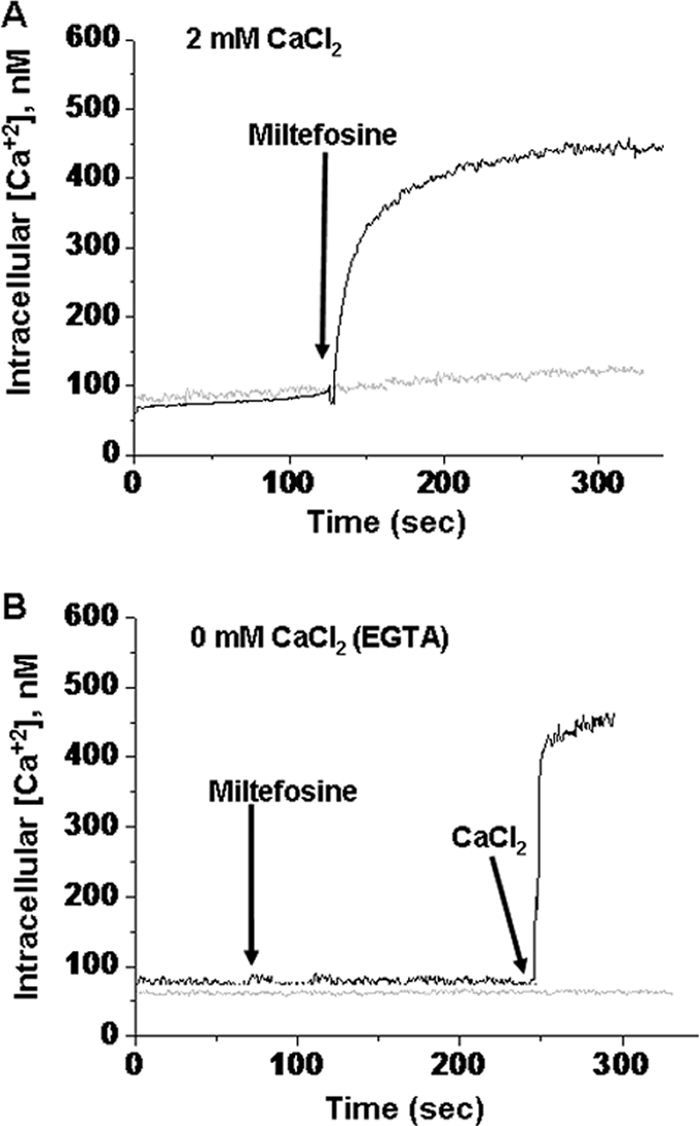
Effect of miltefosine on the [Ca2+]i of L. mexicana promastigotes. Populations of L. mexicana promastigotes (4 × 106 cells in 1.5 ml) were loaded with Fura 2 (using 6 μM Fura 2-AM) in a loading buffer, and [Ca2+]i was calculated as described in Materials and Methods. (A) Effect of 2.5 μM miltefosine on the parasite cytoplasmic Ca2+ concentration in the presence of 2 mM Ca2+. The gray line represents the same experiment without miltefosine. (B) Effect of 2.5 μM miltefosine on promastigotes loaded with Fura 2 in the absence of external Ca2+ (1 mM EGTA). The gray line represents the same experiment without any addition.
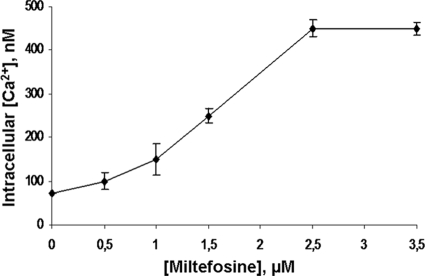
In order to study if the observed increase in the [Ca2+]i was due to an influx of this cation from the extracellular milieu or if, instead, the cytoplasmic Ca2+ was elevated due to a release of the ion from intracellular compartments, we performed the same experiment, but in the absence of extracellular Ca2+ (in the presence of EGTA). Under this condition, miltefosine was unable to induce an increase in the [Ca2+]i (Fig. 3B). This result demonstrated that the rise in the [Ca2+]i level induced by miltefosine resulted from the entry of extracellular Ca2+, probably through a Ca2+ channel at the plasma membrane of these parasites activated by the drug. Figure 4 shows a dose-response curve related to the effect of miltefosine on the [Ca2+]i. It can be seen that a plateau was reached with 2.5 μM of the drug. Concerning this effect of miltefosine on the [Ca2+]i, it has been demonstrated that, at least in one cancer cell line, namely, epidermoid carcinoma KB, miltefosine is also able to increase the [Ca2+]i (7). Remarkably, the effect of miltefosine on the increase in the [Ca2+]i observed in the present work for L. mexicana was at least 10 times more potent than in epidermoid carcinoma KB cells (25 μM). We have also tested the effect of miltefosine on other human cancer cell lines such as LoVo (colon cancer) and CarC (skin cancer), showing that miltefosine is also able to increase the [Ca2+]i in these cells, but only using 50 μM of the drug, while 2.5 μM had no effects (data not shown).
FIG. 4.
Dose-response curve of the effect of miltefosine on the [Ca2+]i of L. mexicana promastigotes. Populations of L. mexicana promastigotes (4 × 106 cells in 1.5 ml) were loaded with Fura 2 (using 6 μM Fura 2-AM) in a loading buffer, and [Ca2+]i was calculated as described in Materials and Methods. Each point represent the mean ± standard error of at least three independent experiments.
Effect of amiodarone and miltefosine on the course of infection in BALB/c mice.
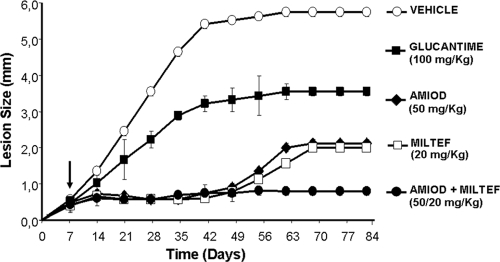
We analyzed the anti-Leishmania efficacy of amiodarone and miltefosine, used alone or in combination, in a murine model of cutaneous leishmaniasis. BALB/c mice were infected with L. mexicana amastigotes on the footpad and then treated with amiodarone or miltefosine alone or in combination, according to the protocol described in Materials and Methods. Treatment evaluation showed that amiodarone at 50 mg/kg/day or miltefosine at 20 mg/kg/day given orally was more effective than glucantime (100 mg/kg/day intraperitoneal), fully preventing the development of lesions during the course of the treatment (Fig. 5). However, when the pressure of any of the drugs disappeared, a relapse of the infection was observed, as suggested by a sustained increase of the lesion size, indicating therapeutic failure (Fig. 5). In contrast, mice treated with the drug combination (amiodarone plus miltefosine at 50 and 20 mg/kg/day, respectively) showed a permanent control of the lesion size. The presence of parasites on infected mice after treatment was evaluated at day 84, using biopsy samples from the lesions and a novel multiplex PCR assay developed during this work (Table 1). All control (untreated) infected mice showed positive results by this method. Miltefosine was the most effective drug when given alone, with 50 to 60% of parasitological cures, depending on the drug concentration. Interestingly, the combination treatment at low doses of the individual drugs (amiodarone at 25 mg/kg/day plus miltefosine at 10 mg/kg/day) led to high (70%) values of apparent parasite elimination in comparison with the effects of the drugs given alone (Table 1), while the drug combination at higher concentrations (50 mg/kg/day of amiodarone plus 20 mg/kg/day of miltefosine) led to parasitological cure in 90% of the infected animals (Table 1 and Fig. 6).
FIG. 5.
Lesion evolution of mice infected with L. mexicana amastigotes, treated with amiodarone and miltefosine (alone or in combination). Shown are weekly measurements of lesion size (mm) versus time in days of mice infected with L. mexicana amastigotes and treated with amiodarone, miltefosine, glucantime, or different combinations of amiodarone plus miltefosine. Notice that the combination of amiodarone at 50 mg/kg/day plus 20 mg/kg/day miltefosine avoids the evolution of lesions. The arrow indicates the beginning of treatment.
TABLE 1.
Conditions and healing tests applied to L. mexicana-infected mice treated with amiodarone and miltefosinea
| Group (n = 10) | Treatment group | Dose of amiodarone or miltefosine (mg/kg body wt) | No. of mice negative/total by: |
||
|---|---|---|---|---|---|
| Culture | Giemsa staining | PCR | |||
| 1 | Drug vehicle | None | 0/10 | 0/10 | 0/10 |
| 2 | Glucantime | 100 | 6/10 | 3/10 | 2/10 |
| 3 | Amiodarone | 25 | 2/10 | 0/10 | 0/10 |
| 4 | Amiodarone | 50 | 5/10 | 2/10 | 2/10 |
| 5 | Miltefosine | 10 | 8/10 | 7/10 | 5/10 |
| 6 | Miltefosine | 20 | 8/10 | 7/10 | 6/10 |
| 7 | Amiodarone + miltefosine | 25/10 (amiodarone/miltefosine) | 10/10 | 10/10 | 7/10 |
| 8 | Amiodarone + miltefosine | 50/20 (amiodarone/miltefosine) | 10/10 | 10/10 | 9/10 |
Female BALB/c mice (body weight, 25 g) were infected with L. mexicana amastigotes (1 × 106). Drug treatment was started 7 days later, at the doses and frequencies indicated, followed by culture of lesion homogenates as described in Materials and Methods.
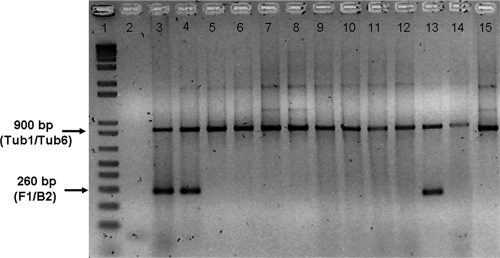
FIG. 6.
Parasitological evaluation of infected BALB/c mice treated with amiodarone plus miltefosine by a Leishmania multiplex PCR assay. PCRs were performed as described in Materials and Methods. Lane 1, molecular weight marker (1 Kb Plus; Invitrogen); lane 2, PCR mixture without DNA (negative control); lane 3, L. mexicana genomic DNA (positive control); lane 4, untreated lesion DNA from infected mouse footpad; lanes 5 to 14, DNA from lesions of infected mouse footpads treated with 50 mg/kg/day amiodarone plus 20 mg/kg/day miltefosine for 49 days; lane 15, genomic DNA from a healthy footpad. Arrows indicate the β-tubulin amplification products (900 bp) and the specific fragment from L. mexicana amplified with the F1 and B2 primers (260 bp). The absence of a 260-bp band indicates the absence of Leishmania in the lesion.
DISCUSSION
Despite its registration as an anti-Leishmania drug in several countries, the mechanism of action of miltefosine is not yet fully understood. Taking into account that this drug is able to induce an increase in the [Ca2+]i in some cancer cell lines and considering the well-known relevance of calcium homeostasis in Leishmania spp. (1), in this work we investigated the possible effect of miltefosine on Ca2+ regulation in these parasites and found that the drug generates a large and rapid increase in [Ca2+]i in L. mexicana promastigotes, a previously unreported finding that could explain the synergistic antiparasitic effects of the drug when used in combination with amiodarone. The specific increment in the [Ca2+]i induced by miltefosine is probably due to the activation of a not-yet-characterized plasma membrane Ca2+ channel. Concerning this point, there are some reports related to the existence of Ca2+ channels in Leishmania spp. Thus, an increase in the cytosolic Ca2+ levels through the activation of nonselective cation channel induced by oxidative stress in L. donovani has been demonstrated (12), while Mehta and Shaha (10) demonstrated that some metalloids induced death in L. donovani promastigotes through activation of a T-type Ca2+ channel. Interestingly, Verma and Dey (22) concluded that miltefosine induces apoptosis-like death in L. donovani, a process that is associated with altered Ca2+ homeostasis (3). Thus, it is conceivable that the disruption of Ca2+ homeostasis generated by miltefosine in L. mexicana could induce apoptotic-like phenomena seriously affecting the parasite's viability.
Based on these and previous findings (20), we evaluated the effect of the combination of miltefosine and amiodarone on the proliferation of L. mexicana in vitro and in vivo. Drug interactions were assessed through the construction of isobolograms for the in vitro effects on both promastigote and amastigote forms of the parasite. It was interesting to note that while the FIC value obtained for the promastigotes showed only a weak synergism, the FIC value of 0.49 obtained for intracellular amastigotes, the clinically relevant forms, indicated clear synergism. It has been previously demonstrated that miltefosine acts synergistically with other drugs used in combination on Leishmania, such as sodium stibogluconate (18), but the synergy observed with this combination in intracellular amastigotes was relatively low (FIC, 0.75). The studies on infected mice obtained in the present work showed a marked control of the lesion size during treatment with any of the drugs given alone (at 50 mg/kg/day/day for amiodarone or 20 mg/kg/day/day for miltefosine, given for 21 days), but the effects were only suppressive (leishmanistatic), as a clear relapse of the infections was observed when the drug pressure stopped. In contrast, the combined administration of the drug combination at the high dose levels led to curative (leishmanicidal) action, since no relapse of the lesion after drug withdrawal was observed and a high percentage of parasitological cures (90%) were verified by PCR. The level of cures obtained in this study with the combination of amiodarone plus miltefosine was higher than that (80%) reported for the treatment with amiodarone (50 mg/kg/day) plus posaconazole (20 mg/kg/day) in NMRI mice infected with T. cruzi (2).
In this work, we also developed a novel multiplex PCR assay using as targets the coding region of the β-tubulin gene, which is a highly conserved DNA sequence, and a novel Leishmania DNA sequence, L280, specific for Leishmania species, such as L. mexicana. The assay is straightforward and the sensitivity is, on average, 100 pg DNA, as determined by serial dilution experiments. The method proved to be very useful for tracking the cure of infected animals upon treatment with the different drugs used in this study and should be easily applicable in future investigations of experimental chemotherapy of leishmaniasis.
In closing, it is worthwhile to mention the results of a recent study on alternative therapies for human leishmaniasis treatment in which a patient with L. mexicana and T. cruzi mixed infection was treated with amiodarone (14). The amiodarone treatment (1,600 mg/day for the first 4 days and 800 mg/day for the next 21 days) led to the clinical cure of a borderline diffuse leishmaniasis in this patient (14). Based on the demonstration of the intrinsic activity of amiodarone against trypanosomatid parasites in experimental animals and human patients, it is possible that this drug could be used in combination with bonafide antiparasitic drugs to combat these infections at low doses, thus reducing its known adverse side effects such as cardiotoxicity, thyroid dysfunction, and pulmonary fibrosis (13). Accordingly, the present study represents the first evidence that the combination amiodarone plus miltefosine acts synergistically in controlling L. mexicana parasitic infections in vivo.
Acknowledgments
This work was supported by grants from Fondo Nacional de Ciencia, Tecnología e Investigación (FONACIT), Venezuela G-2001000637, and Consejo de Desarrollo Científico y Humanístico de la Universidad Central de Venezuela (C.D.C.H.-U.C.V.) (PI 03-00-7380-2008) to G.B. X.S.-M. is a recipient of a fellowship from the Academia de Ciencias, Fisicas, Matematicas y Naturales, de Venezuela.
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Benaim, G. 1996. Intracellular calcium signaling and regulation in Leishmania, p. 89-106. In F. Tapia, G. Caceres-Dittmar, and M. A. Sanchez (ed.), Molecular and immune mechanism in the pathogenesis of cutaneous leishmania. R. G. Landes, Co., Medical Intelligence Unit, Austin, TX.
- 2.Benaim, G., J. Sanders, Y. Garcia-Marchan, C. Colina, R. Lira, A. Caldera, G. Payares, C. Sanoja, J. Burgos, A. Leon-Rossell, J. Concepcion, A. Schijman, M. Levin, E. Oldfield, and J. Urbina. 2006. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J. Med. Chem. 49:892-899. [DOI] [PubMed] [Google Scholar]
- 3.Colina, C., A. Flores, H. Rojas, A. Acosta, M. R. Garrido, A. Israel, C. Castillo, R. DiPolo, and G. Benaim. 2005. Ceramide increases cytoplasmic Ca2+ concentration in Jurkat T cells by liberation of calcium from intracellular stores and activation of a store-operated calcium channel. Arch. Biochem. Biophys. 436:333-345. [DOI] [PubMed] [Google Scholar]
- 4.Croft, S., and J. Engel. 2006. Miltefosine—discovery of the antileishmanial activity of phospholipid derivatives. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl. 1):S4-S8. [DOI] [PubMed] [Google Scholar]
- 5.Grynkiewicz, G., M. Poenie, and R. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450. [PubMed] [Google Scholar]
- 6.Hallander, H. O., K. Dornbusch, L. Gezelius, K. Jacobson, and I. Karlsson. 1982. Synergism between aminoglycosides and cephalosporins with antipseudomonal activity: interaction index and killing curve method. Antimicrob. Agents Chemother. 22:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henke, J., J. Engelmann, B. Kutscher, G. Nssner, J. Engel, R. Voegeli, and D. Leibfritz. 1999. Changes of intracellular calcium, fatty acids and phospholipids during miltefosine-induced apoptosis monitored by fluorescence- and 13C NMR-spectroscopy. Anticancer Res. 19:4027-4032. [PubMed] [Google Scholar]
- 8.Lira, R., L. M. Contreras, R. Santa-Rita, and J. A. Urbina. 2001. Mechanism of action of antiproliferative alkyl-lysophospholipids against the protozoan parasite Trypanosoma cruzi. Potentiation of in vitro activity by the sterol biosynthesis inhibitor ketoconazole. J. Antimicrob. Chemother. 47:537-546. [DOI] [PubMed] [Google Scholar]
- 9.Lux, H., N. Heise, T. Klenner, D. T. Hart, and F. R. Opperdoes. 2000. Ether-lipid (alkyl-phospholipids analog) metabolism and the mechanism of action of ether-lipid analogs in Leishmania. Mol. Biochem. Parasitol. 111:1-14. [DOI] [PubMed] [Google Scholar]
- 10.Mehta, A., and C. Shaha. 2006. Mechanism of metalloid-induced death in Leishmania spp.: role of iron, reactive oxygen species, Ca2+, and glutathione. Free Radic. Biol. Med. 15:1857-1868. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza-León, A., L. Luis, O. Fernandes, E. Cupolillo, and L. Garcia. 2002. Molecular markers for species identification in the Leishmania subgenus Viannia. Trans. R. Soc. Trop. Med. Hyg. 96:65-70. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee, S., M. Das, G. Sudhandiran, and C. Shaha. 2002. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J. Biol. Chem. 227:24717-24727. [DOI] [PubMed] [Google Scholar]
- 13.Opie, L. H., and B. J. Gersh. 2005. Antiarrhythmic drugs and strategies, p. 218-274. In L. H. Opie and B. J. Gersh (ed.), Drugs for the heart, 6th ed. Elsevier, Inc., Philadelphia, PA.
- 14.Paniz-Mondolfi, A., A. Perez-Alvarez, O. Reyes-Jimenez, G. Socorro, O. Zerpa, D. Slova, and J. L. Concepción. 2008. Concurrent Chagas disease and borderline disseminated cutaneous leishmaniasis: the role of amiodarone as an antitrypanosomatidae drug. Ther. Clin. Risk Manag. 4:659-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reithinger, R., J.-C. Dujardin, C. Pirmez, B. Alexander, and S. Brooker. 2007. Cutaneous leishmaniasis. Lancet Infect. Dis. 7:581-596. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez, N., H. De Lima, C. M. Aguilar, A. Rodriguez, D. Barker, and J. Convit. 2002. Molecular epidemiology of cutaneous leishmaniasis in Venezuela. Trans. R. Soc. Trop. Med. Hyg. 96:101-109. [DOI] [PubMed] [Google Scholar]
- 17.Rokotomanga, M., S. Blanc, K. Gaudin, P. Chaminade, and P. M. Loiseau. 2007. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 51:1425-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifert, K., and S. L. Croft. 2006. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob. Agents Chemother. 50:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serrano-Martín, X., G. Payares, and A. Mendoza-León. 2006. Glibenclamide, a blocker of K+ATP channels, shows antileishmanial activity in experimental cutaneous leishmaniasis. Antimicrob. Agents Chemother. 50:4214-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serrano-Martín, X., Y. García-Marchan, A. Fernandez, N. Rodriguez, H. Rojas, G. Visbal, and G. Benaim. 2009. Amiodarone destabilizes intracellular Ca2+ homeostasis and the biosynthesis of sterols in Leishmania mexicana. Antimicrob. Agents Chemother. 53:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soto, J., and J. Berman. 2006. Treatment of New World cutaneous leishmaniasis with miltefosine. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl. 1):S34-S40. [DOI] [PubMed] [Google Scholar]
- 22.Verma, N. K., and C. S. Dey. 2004. Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob. Agents Chemother. 48:3010-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, Y. Z., Y. B. Chang, C. Xing, and D. Fu. 1998. The interference effects of hexadecylphosphocholine on proliferation and membrane phospholipid metabolism in human myeloid leukemia cell lines. Int. J. Tissue React. 20:101-107. [PubMed] [Google Scholar]
- 24.Wright, M. M., A. G. Howe, and V. Zeremberg. 2004. Cell membranes and apoptosis: role of cardiolipin, phosphatidylcholine and anticancer lipid analogues. Biochem. Cell Biol. 82:18-26. [DOI] [PubMed] [Google Scholar]