Abstract
The effect of hepatic impairment on fosamprenavir/ritonavir pharmacokinetics was investigated. Sixty human immunodeficiency virus type 1-infected subjects, including 13, 20, and 10 subjects with mild, moderate, and severe hepatic impairment, respectively, and a comparator group of 17 subjects with normal hepatic function, were enrolled. Subjects with normal hepatic function received fosamprenavir at 700 mg plus ritonavir at 100 mg twice daily, whereas subjects with hepatic impairment received adjusted doses in anticipation of increased exposures. For subjects with mild hepatic impairment, the studied regimen of fosamprenavir 700 mg twice daily plus ritonavir 100 mg once daily delivered 17% higher values for the maximum plasma amprenavir concentration at the steady state (Cmax), 22% higher values for the area under the plasma concentration versus time curve over the dosing interval at the steady state [AUC(0-τ)], similar values for the concentration at the end of the dosing interval (Cτ), and 114% higher unbound Cτ values. For subjects with moderate hepatic impairment, the studied dosage regimen of fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily delivered 27% lower plasma amprenavir Cmax values, 27% lower AUC(0-24) values, 57% lower Cτ values, and 21% higher unbound amprenavir Cτ values. For subjects with severe hepatic impairment, the studied dosage regimen of fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily delivered 19% lower plasma amprenavir Cmax values, 23% lower AUC(0-24) values, 38% lower Cτ values, and similar unbound amprenavir Cτ values. With a reduced ritonavir dosing frequency of 100 mg once daily, the plasma ritonavir AUC(0-24) values were 39% lower, similar, and 40% higher for subjects with mild, moderate, and severe hepatic impairment, respectively. The results of the study support the use of reduced fosamprenavir/ritonavir doses or dosing frequencies in the treatment of patients with hepatic impairment. No significant safety issues were identified; however, plasma amprenavir and ritonavir exposures were more variable in subjects with hepatic impairment, and those patients should be closely monitored for safety and virologic response.
Among the estimated 40 million persons infected with human immunodeficiency virus (HIV) worldwide, an estimated 2 to 4 million are chronically infected with hepatitis B virus (HBV) and an estimated 4 to 5 million are chronically infected with hepatitis C virus (HCV) (1). The prevalence of HBV and HCV coinfection in HIV-infected subjects is correlated with intravenous drug use as an HIV risk factor; the prevalence is above 40% in some southern European countries (2, 5, 7). Those with hepatitis infection often have some degree of liver impairment. For those with chronic HCV infection alone, the estimated rate of progression to cirrhosis is 2 to 20% over 20 years (1).
Dosing recommendations for the hepatically impaired are available for several protease inhibitors, although most exclude those with severe impairment and/or include safety precautions. Until recently, unboosted amprenavir was the only protease inhibitor indicated for use in the treatment of HIV-infected patients with severe hepatic impairment; indeed, all antiretroviral agents other than selected nucleosides are contraindicated for this difficult-to-treat population. Thus, more options are clearly needed.
Fosamprenavir is the prodrug of the HIV type 1 (HIV-1) protease inhibitor amprenavir and is often used in combination with low-dosage ritonavir to increase plasma amprenavir concentrations by inhibiting amprenavir CYP3A4-mediated metabolism. We studied fosamprenavir/ritonavir combinations administered to HIV-infected subjects with mild, moderate, and severe hepatic impairment as well as to control subjects with normal hepatic function for 2 weeks. Because amprenavir is highly bound to plasma proteins (including albumin and α1-acid glycoprotein [AAG]) that are synthesized in the liver, plasma unbound amprenavir concentrations and percent unbound were evaluated in the present study. The primary goals of this study were to evaluate the impact of hepatic impairment on amprenavir and ritonavir pharmacokinetics (PK) and to determine dosing recommendations for this patient population.
MATERIALS AND METHODS
Study design and dosing.
This study was conducted at 5 study centers in the United States and Puerto Rico and 14 study centers in Spain during the period from November 2004 through November 2007. These study centers received Institutional Review Board or International Ethics Committee approval to enroll subjects into the study prior to initiation, and subjects provided written informed consent prior to enrollment in the study.
This phase I, open-label, parallel-group, multicenter study was originally designed to enroll HIV-infected, treatment-naïve or -experienced subjects with mild (number of subjects, ∼10) and moderate (∼10) hepatic impairment as defined by Child-Pugh classification (4) as well as a control group with normal hepatic function. The protocol was later modified to enroll subjects with severe (number of subjects, ∼10) hepatic impairment and another control group with normal hepatic function. Subjects with moderate or severe hepatic impairment were matched with HIV-infected subjects with normal hepatic function on the basis of gender, age (±5 years), and weight (±5 kg). Eligible subjects were required to have exhibited indications of stable HIV disease for at least 3 months and to be receiving either no current therapy or antiretroviral therapy consisting of nucleoside or nucleotide reverse transcriptase inhibitors with or without a protease inhibitor(s) other than atazanavir or tipranavir. The hepatically impaired subjects and their matched controls were started on or switched their protease inhibitor to fosamprenavir and ritonavir for 14 days of treatment, either alone or along with background nucleoside or nucleotide reverse transcriptase inhibitors; treatment with concomitant protease inhibitors or nonnucleoside inhibitors was prohibited. Given the a priori knowledge of the impact of hepatic impairment on plasma amprenavir exposure (8) and ritonavir exposure (3), doses were adjusted for each group in accordance with the level of hepatic impairment. Subjects with normal hepatic function received the standard regimen of fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily. Subjects with mild hepatic impairment received fosamprenavir at 700 mg twice daily, those with moderate hepatic impairment were randomized to receive either fosamprenavir at 300 mg twice daily or fosamprenavir at 700 mg once daily, and subjects with severe hepatic impairment received fosamprenavir at 300 mg twice daily; for all subjects with hepatic impairment, the frequency of ritonavir coadministration was reduced to 100 mg once daily. Fosamprenavir 700-mg doses were administered as tablets, whereas doses of less than 700 mg were administered as an oral suspension (50 mg/ml). A prior relative bioavailability study had demonstrated bioequivalence between the fosamprenavir oral suspension and tablet formulations after single-dose administration, whereas, at the steady state, the fosamprenavir oral suspension delivered equivalent plasma amprenavir values for the area under the plasma concentration versus time curve over the dosing interval at steady state [AUC(0-τ)] and for the concentration at the end of the dosing interval (Cτ) and a 23% higher maximum concentration at the steady state (Cmax) than the tablet formulation (unpublished data). All ritonavir doses were administered as capsules. When ritonavir was administered once daily, it was coadministered with the morning dose of fosamprenavir. The study drugs were administered at least 2 h before or after a meal, but grape or apple juice was allowed to improve the palatability of the fosamprenavir oral suspension formulation.
Subjects.
Male and nonpregnant, nonlactating female subjects 18 to 65 years of age, with body mass indices of 19 to 35 kg/m2 and clinically stable HIV disease for at least 3 months prior to enrollment, were eligible for the study. To be classified as hepatically impaired, subjects were required to have had a known medical history of liver disease and previous confirmation of liver fibrosis by liver biopsy or a macroscopic evaluation result by laparoscopy, computerized tomography scan, magnetic resonance imaging, or ultrasonography consistent with chronic liver disease associated with an unambiguous medical history. Hepatically impaired subjects with a Child-Pugh score of 5 to 6 were classified as exhibiting mild impairment, those with a score of 7 to 9 as exhibiting moderate impairment, and those with a score of 10 to 15 as exhibiting severe impairment (4).
Potential subjects whose results showed the presence of grade 3 alanine aminotransferase (ALT) or aspartate aminotransferase (AST) or any grade 4 laboratory abnormality in a screening assessment were excluded from the study. In addition, potential subjects with hepatic impairment who had received atazanavir treatment within the preceding 2 weeks, had evidence of alcohol abuse within the preceding 2 months, or had acute or exacerbating hepatitis were excluded. Control subjects with normal hepatic function who were HBV or HCV positive, had a history of alcohol abuse or illicit drug use within the preceding 6 months, or had a positive alcohol test result at screening were excluded. Investigational products were not allowed; in addition, concurrent medications were not allowed in cases in which a significant interaction was expected to result in a potential safety issue, alter immune system function, or impact the PK results of the study.
PK and safety assessments.
Subjects underwent predose (trough) PK sampling on the mornings of days 3, 7, 10, and 13; morning doses of the study drugs were administered at the study center (observed dosing) on those days. On the evening of day 13, study site staff reminded subjects (e.g., by telephone) to take their dose of the study drugs that evening. On day 14, serial PK samples were collected predose (within 15 min prior to administration of the next dose) and at 0.25, 0.50. 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, and 12 h after the administration of fosamprenavir and ritonavir. Subjects with hepatic impairment returned to the study center on the morning of day 15 for collection of a 24-h PK sample. Additional PK samples were collected on day 14 for measurement of unbound amprenavir concentrations at 2 and 12 h after dosing for the regimens of fosamprenavir administered twice daily and at 2 and 24 h after dosing for the regimen of fosamprenavir administered once daily. Subject safety was assessed by ascertainment of adverse events (graded using the Division of AIDS Table for Grading Severity of Adult Adverse Experiences, August 1992 [http://rcc.tech-res.com/safetyandpharmacovigilance/]), clinical laboratory tests, and plasma HIV-1 RNA and CD4 lymphocyte count.
PK sampling procedures and analytical methods.
PK samples were obtained from a forearm vein and collected into potassium EDTA anticoagulation tubes for measurement of total plasma fosamprenavir, amprenavir, and ritonavir concentrations or sodium citrate anticoagulation tubes for measurement of unbound amprenavir concentrations. Samples were immediately inverted 8 to 10 times to mix the anticoagulant with the whole blood and then placed on ice or in a refrigerator. Plasma was separated by refrigerated (4°C) centrifugation at 2,000 × g for 10 min within 1 h of sample collection. Supernatant plasma was transferred to polypropylene tubes and stored at −20°C or at a lower temperature until analysis.
The bioanalytical method used to measure fosamprenavir and amprenavir concentrations and the methods used for measurement of unbound amprenavir concentrations were each validated using quality control (QC) samples at five concentration levels, in replicates of six measurements on each occasion, on three separate occasions. The QC sample acceptance criteria for each analytical run were that no more than one-third of the QC samples should be beyond ±15% of the actual concentration and at least 50% of the QC samples at each concentration must be within ±15% of the actual concentration.
Fosamprenavir and amprenavir were extracted from 50 μl of human plasma by protein precipitation using acetonitrile containing [13C6]fosamprenavir and [13C6]amprenavir as internal standards. Extracts were then analyzed using high-performance liquid chromatography coupled to tandem mass spectrometry with a TurboIonSpray interface and multiple-reaction monitoring. Ritonavir was extracted from 100 μl of human plasma by protein precipitation using acetonitrile containing [2H215N13C6]ritonavir as an internal standard. Extracts were then analyzed using high-performance liquid chromatography coupled to tandem mass spectrometry with a TurboIonSpray interface and multiple-reaction monitoring. Plasma concentrations of study drugs were determined using a standard calibration curve constructed with standard solutions prepared with human plasma. The lower limit of quantification (LLQ) for fosamprenavir was 0.005 μg/ml, and the higher limit of quantification (HLQ) was 1 μg/ml; the LLQ was 0.010 μg/ml and the HLQ was 5 μg/ml for both amprenavir and ritonavir. The calibration curves were linear over these concentration ranges. For the study, the values for within- and between-run precision (percent coefficient of variation) for fosamprenavir were ≤8.2% and ≤6.1%, respectively, for amprenavir were ≤6.2% and 6.5%, respectively, and for ritonavir were ≤3.9% and 5.5%, respectively. The accuracy (percent bias) for fosamprenavir was between −1.4% and −0.6%, for amprenavir was between −5.3% and 3.3%, and for ritonavir was between −5.5% and −1.4%.
Unbound amprenavir from human plasma samples was isolated using centrifugal filtration. Subsequently, amprenavir and the corresponding internal standard, [13C6]amprenavir, were extracted from 100 μl of plasma ultra (protein-free)-filtrate by solid-phase extraction. Extracts were then analyzed using high-performance liquid chromatography coupled to tandem mass spectrometry with a TurboIonSpray interface and multiple-reaction monitoring. The LLQ for amprenavir was 0.0005 μg/ml, and the HLQ was 1 μg/ml. The calibration curves were linear over this concentration range. For the study, the between-run precision (percent coefficient of variation) for amprenavir was ≤6.6%. The accuracy (percent bias) for amprenavir was between −9.0% and −7.7%.
PK and statistical analyses.
Plasma amprenavir and ritonavir PK parameters were calculated based on actual sample collection times recorded during the study using the noncompartmental 200 model of Winnonlin Professional software, version 4.1 (Pharsight Corporation, Mountain View, CA). The values recorded for the Cmax were the actual observed values. The Cτ was calculated as the average of predose concentrations collected at the steady state. The AUC(0-τ) was calculated by a combination of linear (for ascending concentrations) and logarithmic (for descending concentrations) trapezoidal methods. To facilitate the comparison of regimens administered over different dosing intervals, the AUC over 24 h [AUC(0-24)] was calculated by multiplying the AUC(0-τ) for the twice-daily regimen (i.e., the 12-h dosing interval) by 2 and leaving the AUC(0-τ) for the once-daily regimen (i.e., the 24-h dosing interval) unchanged. To facilitate the comparison of the groups to which different fosamprenavir doses were administered, plasma amprenavir Cmax, AUC(0-τ), and Cτ values were normalized to a 700-mg dose of fosamprenavir by multiplying the individual PK parameter value by 700 and dividing it by the size of the dose administered in milligrams. Apparent clearance following oral dosing (CL/F) was calculated as the administered dose/AUC(0-τ). The values recorded for the unbound amprenavir concentration in plasma at 2 h after dosing (unbound C2 h) and at the end of the dosing interval at the steady state (unbound Cτ) were the actual observed values, and the percent unbound amprenavir at those time points was calculated as the unbound concentration divided by the corresponding total concentration and multiplied by 100.
Plasma amprenavir and ritonavir PK parameters were compared between hepatic impairment groups and subjects with normal hepatic function by analysis of variance, considering the group as a fixed effect. The ratio of geometric least-square means and associated 90% confidence intervals was estimated for each comparison. Achievement of steady-state plasma amprenavir and ritonavir concentrations was assessed by calculating the 90% confidence interval of the slope of the linear regression of predose concentrations versus the day for each group.
The relationship between the amprenavir dose-normalized AUC(0-τ) [DN-AUC(0-τ)] and the Child-Pugh score and clinical laboratory assessments of levels of albumin, bilirubin, prothrombin time, cholinesterase, ammonia, ALT, and AST was assessed by Pearson correlation and multivariate stepwise regression analysis, adjusting for baseline measurements. The Child-Pugh score was treated as a continuous variable, and subjects with normal hepatic function were assigned a score of 0. The ritonavir AUC(0-τ) was assessed for the same relationships.
RESULTS
Subject demographics.
Sixty subjects received study drugs, and 55 completed the study. The majority of subjects were white (97%) and male (85%); mean age ranged between 42 and 44 years and mean body mass indices ranged between 23.6 and 26.6 kg/m2 across the groups (Table 1). Most subjects (40/43 [91%]) with hepatic impairment showed evidence of coinfection with HCV; 3 subjects with hepatic impairment were coinfected with HBV. One subject with normal hepatic function was coinfected with HCV. At the prebaseline time point (day −4), no subjects with mild or moderate hepatic impairment had encephalopathy; by contrast, two of those with severe hepatic impairment had grade 2 encephalopathy at the baseline. Ascites was graded at 2 or higher for 90% (9/10) of subjects with severe hepatic impairment at the baseline compared with only 35% (7/20) of subjects with moderate hepatic impairment and no subjects with mild hepatic impairment. Among all subjects, 48 (80%) reported taking nucleoside/nucleotide reverse transcriptase inhibitors and 57 (95%) reported taking other concurrent medications during the study.
TABLE 1.
Demographic and baseline characteristics
| Characteristic | Treatment group value(s)a |
|||||
|---|---|---|---|---|---|---|
| Mild HI (FPV 700 mg BID + RTV 100 mg QD) (n = 13) | Moderate HI (FPV 300 mg BID + RTV 100 mg QD) (n = 10) | Moderate HI (FPV 700 mg QD + RTV 100 mg QD) (n = 10) | Normal hepatic function (FPV/RTV 700/100 mg BID) (n = 10)b | Severe HI (FPV 300 mg BID + RTV 100 mg QD) (n = 10) | Normal hepatic function (FPV/RTV 700/100 mg BID) (n = 7)c | |
| Mean (range) age (yr) | 42.2 (34-50) | 44.0 (39-49) | 43.7 (38-54) | 43.4 (34-49) | 42.8 (38-53) | 43.9 (33-48) |
| No. (%) male | 12 (92) | 8 (80) | 8 (80) | 8 (80) | 9 (90) | 6 (86) |
| Mean (range) BMI (kg/m2) | 23.62 (19.7-32.4) | 24.25 (22.1-33.7) | 24.81 (19.7-34.3) | 25.89 (19.7-34.9) | 24.95 (20.4-33.0) | 26.59 (20.7-31.6) |
| No. (%) white | 13 (100) | 10 (100) | 9 (90) | 9 (90) | 10 (100) | 7 (100) |
| No. (%) hepatitis B surface antigen positive | 0 | 2 (20) | 1 (10) | 0 | 1 (10) | 0 |
| No. (%) hepatitis C antibody positive | 13 (100) | 10 (100) | 9 (90) | 1 (10) | 8 (80) | 0 |
| No. (%) in CDC C classificationd | 5 (38) | 5 (50) | 4 (40) | 4 (40) | 3 (30) | 2 (29) |
| Median (range) baseline CD4+ cell count (cells/mm3) | 404 (79-1,281) | 226 (36-638) | 202 (111-525) | 392 (78-877) | 260 (90-420) | 470 (90-1,280) |
| Mean (range) albumin (g/liter)e | 35.0 (29.0-42.0) | 33.3 (30.0-44.0) | 34.0 (26.0-43.0) | 44.1 (40.0-47.0) | 26.1 (21-30) | 44.7 (43.0-47.0) |
| Mean (range) α1-acid glycoprotein (g/liter)f | 0.53 (0.27-0.86) | 0.45 (0.30-0.62) | 0.44 (0.26-0.077) | 1.02 (0.71-2.04) | 0.66 (0.22-2.36) | 0.90 (0.55-1.27) |
FPV, fosamprenavir; RTV, ritonavir; QD, once daily; BID, twice daily; HI, hepatic impairment; BMI, body mass index.
Normal hepatic function group matched to subjects with moderate hepatic impairment receiving fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily for sex, age (±5 years), and body mass index (±5 kg) values.
Normal hepatic function group matched to subjects with severe hepatic impairment for sex, age (±5 years), and body mass index (±5 kg) values.
CDC C classification as per http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm.
Day −4.
Day 1.
A total of 53 subjects were included in the statistical analysis of the PK data, including 10 subjects with mild hepatic impairment, 18 subjects with moderate hepatic impairment, 8 subjects with severe hepatic impairment, and 17 subjects with normal hepatic function; 3 subjects were excluded from the statistical analysis of the PK data because of dosing error, incorrect cohort assignment, or protocol violation (administration of nevirapine).
PK.
Plasma fosamprenavir concentrations were generally quantifiable between 0.25 and 1 h after dosing for more than 50% of subjects with hepatic impairment. In contrast, plasma fosamprenavir concentrations were below the limit of quantification at all time points for the majority of subjects with normal hepatic function. Fosamprenavir concentrations were low in all groups (the highest fosamprenavir concentration was 0.033 μg/ml), and fosamprenavir concentrations were below the LLQ (<0.005 μg/ml) in all samples after the 4-h postdose sampling time.
Mild hepatic impairment.
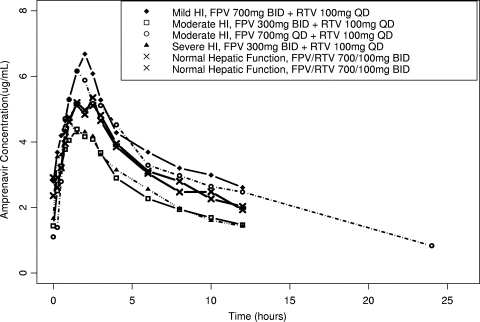
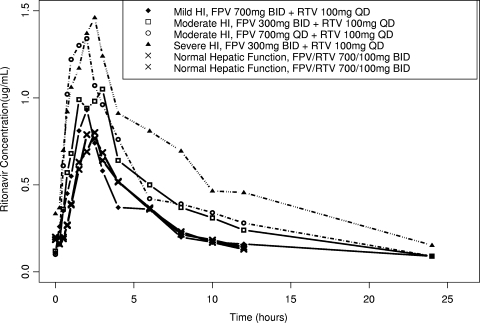
Mild hepatic impairment had a minor impact on total plasma amprenavir and ritonavir exposure, as demonstrated by an 18% average reduction in plasma amprenavir and ritonavir CL/F values, equating to 22 to 23% higher plasma amprenavir and ritonavir AUC(0-τ) values compared to subjects with normal hepatic function. An average of a 17% higher plasma amprenavir Cmax value, similar amprenavir Cτ values, and 114% higher plasma unbound amprenavir Cτ values were observed for subjects with mild hepatic impairment compared to subjects with normal hepatic function (Fig. 1; Tables 2 and 3). With a reduced ritonavir dosing frequency of 100 mg administered once daily for subjects with mild hepatic impairment, the observed ritonavir AUC(0-24) was an average of 39% lower and ritonavir Cτ was 60% lower compared to the values observed for subjects with normal hepatic function receiving fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily (Fig. 2; Tables 4 and 5).
FIG. 1.
Mean amprenavir concentration-time profiles. HI, hepatic impairment; FPV, fosamprenavir; RTV, ritonavir; BID, twice daily; QD, once daily.
TABLE 2.
Summary of amprenavir PK parameter estimates, unbound amprenavir concentrations, and unbound percentagesa
| Treatment group | Geometric mean [95% confidence interval] (% coefficient of variation of the geometric mean) |
Geometric mean [95% confidence interval] (% coefficient of variation) for amprenavir |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose normalized (DN) |
Observed |
Observed |
Observed unbound amprenavir concn and % unbound |
|||||||||
| DN-AUC(0-τ) (μg·h/ml) | DN-Cmax (μg/ml) | DN-Cτ (μg/ml) | AUC(0-τ) (μg·h/ml) | AUC(0-24) (μg·h/ml) | Cmax (μg/ml) | Cτ (μg/ml) | CL/F (ml/min) | C2h (μg/ml) | Cτ (μg/ml) | 2-h % unbound | Cτ % unbound | |
| Mild HI (FPV 700 mg BID + RTV 100 mg QD) (n = 10) | 46.6 [39.0, 55.5] (25) | 7.04 [5.72, 8.66] (30) | 2.38 [1.80, 3.15] (40) | 46.6 [39.0, 55.5] (25) | 93.1 [78.1, 111.0] (25) | 7.04 [5.72, 8.66] (30) | 2.38 [1.80, 3.15] (40) | 215 [180, 256] (25) | 0.56 [0.42, 0.75] (41) | 0.26e [0.19, 0.36] (42) | 8.92 [7.68, 10.4] (21) | 10.9e [8.88, 13.3] (25) |
| Moderate HI (FPV 300 mg BID + RTV 100 mg QD) (n = 10) | 64.8 [46.5, 90.4] (49) | 10.2 [7.18, 14.5] (52) | 2.63 [1.73, 3.99] (64) | 27.8 [19.9, 38.7] (49) | 55.6 [39.9, 77.5] (49) | 4.38 [3.08, 6.22] (52) | 1.13 [0.74, 1.71] (64) | 154 [111, 215] (49) | 0.35d [0.20, 0.61] (82) | 0.15d [0.08, 0.26] (88) | 10.1d [7.49, 13.5] (40) | 12.5d [9.98, 15.5] (29) |
| Moderate HI (FPV 700 mg QD + RTV 100 mg QD) (n = 8) | 57.8 [42.1, 79.3] (39) | 6.68 [5.14, 8.70] (32) | 0.93 [0.53, 1.62] (75) | 57.8 [42.1, 79.3] (39) | 57.8 [42.1, 79.3] (39) | 6.68 [5.14, 8.70] (32) | 0.93 [0.53, 1.62] (75) | 173 [126, 238] (39) | 0.67g [0.56, 0.80] (20) | 0.07f [0.04, 0.14] (69) | 11.8g [8.81, 15.8] (32) | 9.26f [7.37, 11.6] (22) |
| Normal hepatic function (FPV/RTV 700/100 mg BID) (n = 10)b | 38.1 [31.6, 46.0] (27) | 6.00 [4.97, 7.25] (27) | 2.62 [2.14, 3.21] (29) | 38.1 [31.6, 46.0] (27) | 76.3 [63.3, 92.0] (27) | 6.00 [4.97, 7.25] (27) | 2.62 [2.14, 3.21] (29) | 262 [217, 316] (27) | 0.35d [0.30, 0.41] (21) | 0.12d [0.10, 0.15] (24) | 7.53d [6.10, 9.29] (28) | 6.16d [4.52, 8.38] (42) |
| Severe HI (FPV 300 mg BID + RTV 100 mg QD) (n = 8) | 70.8 [52.8, 94.9] (36) | 11.2 [8.22, 15.3] (38) | 3.17 [1.99, 5.05] (60) | 30.3 [22.6, 40.7] (36) | 60.7 [45.3, 81.4] (36) | 4.80 [3.52, 6.54] (38) | 1.36 [0.85, 2.16] (60) | 141 (105, 189) [36] | 0.34g [0.22, 0.53] (51) | 0.12 [0.05, 0.26] (122) | 8.37g [6.57, 10.7] (27) | 9.37 [6.24, 14.1] (52) |
| Normal hepatic function (FPV/RTV 700/100 mg BID) (n = 7)c | 39.4 [34.5, 45.0] (14) | 5.94 [5.05, 6.99] (18) | 2.19 [1.82, 2.64] (20) | 39.4 [34.5, 45.0] (14) | 78.8 [69.1, 89.9] (14) | 5.94 [5.05, 6.99] (18) | 2.19 [1.82, 2.64] (20) | 254 (222, 290) [14] | 0.44f [0.30, 0.65] (38) | 0.12h [0.07, 0.20] (43) | 8.97f [5.14, 15.7] (57) | 5.69h [3.72, 8.69] (35) |
FPV, fosamprenavir; RTV, ritonavir; QD, once daily; BID, twice daily; HI, hepatic impairment; unbound C2h, unbound amprenavir concentration at 2 h after dosing; unbound Cτ, unbound amprenavir concentration at the end of the dosing interval at the steady state.
Normal hepatic function group matched to subjects with moderate hepatic impairment receiving fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily for sex, age (± 5 years), and body mass index (± 5 kg).
Normal hepatic function group matched to subjects with severe hepatic impairment for sex, age (± 5 years), and body mass index (± 5 kg).
n = 9.
n = 8.
n = 6.
n = 7.
n = 5.
TABLE 3.
Comparison of amprenavir PK parameters, unbound amprenavir concentrations, and unbound percentages between groupsa
| Treatment group | Geometric least-squares mean ratio (90% confidence interval) vs normal hepatic functionb |
Geometric least-squares mean ratio (90% confidence interval) vs normal hepatic functionb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DN PK parameter |
Observed PK parameter |
Observed PK parameter |
Unbound amprenavir concn and % unbound amprenavir between groups |
|||||||||
| DN-AUC(0-τ)e | DN-Cmax | DN-Cτ | AUC(0-τ) | AUC(0-24)f | Cmax | Cτ | CL/F | Unbound C2h | Unbound Cτ | 2-h % unbound | Cτ % unbound | |
| Mild HI (FPV 700 mg BID + RTV 100 mg QD)c | 1.22 (0.94, 1.59) | 1.17 (0.90, 1.53) | 0.91 (0.63, 1.32) | 1.22 (0.94, 1.59) | 1.22 (0.94, 1.59) | 1.17 (0.90, 1.53) | 0.91 (0.63, 1.32) | 0.82 (0.63, 1.07) | 1.62 (1.16, 2.28) | 2.14 (1.29, 3.56) | 1.18 (0.94, 1.50) | 1.77 (1.37, 2.27) |
| Moderate HI (FPV 300 mg BID + RTV 100 mg QD)c | 1.70 (1.31, 2.21) | 1.70 (1.30, 2.22) | 1.00 (0.69, 1.45) | 0.73 (0.56, 0.95) | 0.73 (0.56, 0.95) | 0.73 (0.56, 0.95) | 0.43 (0.30, 0.62) | 0.59 (0.45, 0.76) | 1.02 (0.72, 1.44) | 1.21 (0.74, 1.98) | 1.33 (1.05, 1.69) | 2.02 (1.58, 2.58) |
| Moderate HI (FPV 700 mg QD + RTV 100 mg QD)c | 1.51 (1.15, 2.00) | 1.11 (0.84, 1.48) | 0.35 (0.24, 0.53) | 1.51 (1.15, 2.00) | 0.76 (0.57, 1.00) | 1.11 (0.84, 1.48) | 0.35 (0.24, 0.53) | 0.66 (0.50, 0.87) | 1.93 (1.33, 2.80) | 0.60 (0.34, 1.03) | 1.57 (1.21, 2.02) | 1.50 (1.15, 1.98) |
| Severe HI (FPV 300 mg BID + RTV 100 mg QD)d | 1.80 (1.40, 2.31) | 1.88 (1.44, 2.47) | 1.45 (0.97, 2.15) | 0.77 (0.60, 0.99) | 0.77 (0.60, 0.99) | 0.81 (0.62, 1.06) | 0.62 (0.42, 0.92) | 0.56 (0.43, 0.72) | 0.77 (0.50, 1.18) | 0.96 (0.42, 2.17) | 0.93 (0.62, 1.40) | 1.65 (1.05, 2.58) |
FPV, fosamprenavir; RTV, ritonavir; QD, once daily; BID, twice daily; HI, hepatic impairment; unbound C2h, unbound amprenavir concentration at 2 h after dosing; unbound Cτ, unbound amprenavir concentration at the end of the dosing interval at the steady state.
Subjects with normal hepatic function received fosamprenavir/ritonavir at 700/100 mg twice daily.
Comparison made to normal hepatic function group matched to subjects with moderate hepatic impairment receiving fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily for sex, age (± 5 years), and body mass index (± 5 kg).
Comparison made to normal hepatic function group matched to subjects with severe hepatic impairment for sex, age (± 5 years), and body mass index (± 5 kg).
Comparison of DN-AUC(0-τ) values shows the impact of each level of hepatic impairment on plasma amprenavir exposure (i.e., assuming subjects received the same dose and dosing interval).
Comparison of AUC(0-24) values shows differences in observed amprenavir exposure over the same interval of time.
FIG. 2.
Mean ritonavir concentration-time profiles. HI, hepatic impairment; FPV, fosamprenavir; RTV, ritonavir; BID, twice daily; QD, once daily.
TABLE 4.
Summary of ritonavir PK parameter estimates
| Treatment groupa | Geometric mean [95% confidence interval] (% coefficient of variation of the geometric mean) |
||||
|---|---|---|---|---|---|
| AUC(0-τ) (μg·h/ml) | AUC(0-24) (μg·h/ml) | Cmax (μg/ml) | Cτ (μg/ml) | CL/F (ml/min) | |
| Mild HI (FPV 700 mg BID + RTV 100 mg QD) (n = 10) | 4.59 [2.75, 7.67] (75) | 4.59 [2.75, 7.67] (75) | 0.80 [0.44, 1.45] (92) | 0.07 [0.03, 0.14] (121) | 363 [217, 605] (75) |
| Moderate HI (FPV 300 mg BID + RTV 100 mg QD) (n = 10) | 7.21 [4.92, 10.6] (57) | 7.21 [4.92, 10.6] (57) | 1.10 [0.68, 1.77] (75) | 0.09 [0.05, 0.14] (76) | 231 [158, 338] (57) |
| Moderate HI (FPV 700 mg QD + RTV 100 mg QD) (n = 8) | 8.26 [5.28, 12.9] (58) | 8.26 [5.28, 12.9] (58) | 1.26 [0.61, 2.60] (107) | 0.08 [0.04, 0.16] (95) | 202 [129, 316] (58) |
| Normal hepatic function (FPV/RTV 700/100 mg BID) (n = 10)b | 3.75 [2.52, 5.57] (60) | 7.49 [5.04, 11.1] (60) | 0.77 [0.48, 1.26] (77) | 0.17 [0.12, 0.23] (50) | 445 [299, 662] (60) |
| Severe HI (FPV 300 mg BID + RTV 100 mg QD) (n = 8) | 10.8 [5.72 20.5] (89) | 10.8 [5.72 20.5] (89) | 1.26 [0.57, 2.78] (121) | 0.23 [0.15, 0.35] (53) | 154 (81.1, 291) [89] |
| Normal hepatic function (FPV/RTV 700/100 mg BID) (n = 7)c | 3.88 [2.37 6.34] (57) | 7.75 [4.74, 12.7] (57) | 0.77 [0.38, 1.56] (90) | 0.17 [0.12, 0.24] (41) | 430 (263, 703) [57] |
FPV, fosamprenavir; RTV, ritonavir; QD, once daily; BID, twice daily; HI, hepatic impairment.
Normal hepatic function group matched to subjects with moderate hepatic impairment receiving fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily for sex, age (± 5 years), and body mass index (± 5 kg).
Normal hepatic function group matched to subjects with severe hepatic impairment for sex, age (± 5 years), and body mass index (± 5 kg).
TABLE 5.
Comparison of ritonavir PK parameters between groups
| Treatment groupa | Geometric least-squares mean ratio (90% confidence interval) vs normal hepatic functionb |
||||
|---|---|---|---|---|---|
| AUC(0-τ)e | AUC(0-24)f | Cmax | Cτ | CL/F | |
| Mild HI (FPV 700 mg BID + RTV 100 mg QD)c | 1.23 (0.78, 1.92) | 0.61 (0.39, 0.96) | 1.03 (0.58, 1.84) | 0.40 (0.22, 0.70) | 0.82 (0.52, 1.27) |
| Moderate HI (FPV 300 mg BID + RTV 100 mg QD)c | 1.93 (1.25, 2.98) | 0.96 (0.62, 1.49) | 1.42 (0.81, 2.50) | 0.53 (0.31, 0.93) | 0.52 (0.34, 0.80) |
| Moderate HI (FPV 700 mg QD + RTV 100 mg QD)c | 2.20 (1.39, 3.50) | 1.10 (0.69, 1.75) | 1.62 (0.89, 2.95) | 0.49 (0.27, 0.89) | 0.45 (0.29, 0.72) |
| Severe HI (FPV 300 mg BID + RTV 100 mg QD)d | 2.80 (1.52, 5.16) | 1.40 (0.76, 2.58) | 1.64 (0.74, 3.64) | 1.38 (0.91, 2.09) | 0.36 (0.19, 0.66) |
FPV, fosamprenavir; RTV, ritonavir; QD, once daily; BID, twice daily; HI, hepatic impairment.
Subjects with normal hepatic function received fosamprenavir/ritonavir at 700/100 mg twice daily.
Comparison made to normal hepatic function group matched to subjects with moderate hepatic impairment receiving fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily for sex, age (± 5 years), and body mass index (± 5 kg).
Comparison made to normal hepatic function group matched to subjects with severe hepatic impairment for sex, age (± 5 years), and body mass index (± 5 kg).
Comparison of AUC(0-τ) values shows the impact of each level of hepatic impairment on plasma ritonavir exposure (i.e., assuming subjects received the same dosing interval).
Comparison of AUC(0-24) values shows difference in observed ritonavir exposure over the same interval of time.
Moderate hepatic impairment.
Moderate hepatic impairment had a significant impact on total plasma amprenavir and ritonavir exposure, as demonstrated by an average of a 34 to 41% reduction in plasma amprenavir CL/F values and a 48 to 55% reduction in plasma ritonavir CL/F values, equating to 51 to 70% higher plasma amprenavir DN-AUC(0-τ) and 93 to 120% higher plasma ritonavir AUC(0-τ) values compared to the values observed for subjects with normal hepatic function (Fig. 1, Table 2, Table 3, Fig. 2, Table 4, and Table 5). Because a significant increase in plasma amprenavir and ritonavir exposure for subjects with moderate hepatic impairment was expected, those subjects received reduced doses or reduced dosing frequencies of both drugs. With the use of a reduced ritonavir dosing frequency of 100 mg administered once daily for subjects with moderate hepatic impairment, the observed ritonavir AUC(0-24) values were similar and the ritonavir Cτ value was approximately 50% lower compared to the values observed for subjects with normal hepatic function receiving fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily (Table 4 and Table 5). For subjects with moderate hepatic impairment, the regimen of fosamprenavir at 700 mg once daily plus ritonavir at 100 mg once daily (i.e., a reduced dosing frequency of both drugs) delivered averages of 11% higher plasma amprenavir Cmax values, 24% lower amprenavir AUC(0-24) values, 65% lower amprenavir Cτ values, and 40% lower unbound amprenavir Cτ values compared to subjects with normal hepatic function receiving fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily (Table 2 and Table 3). For subjects with moderate hepatic impairment, the studied regimen of fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily (i.e., reduced dose of fosamprenavir and reduced dosing frequency of ritonavir) delivered averages of 27% lower plasma amprenavir Cmax values, 27% lower amprenavir AUC(0-24) values, 57% lower amprenavir Cτ values, and 21% higher unbound amprenavir Cτ values compared to subjects with normal hepatic function receiving fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily (Table 2 and Table 3).
Severe hepatic impairment.
Severe hepatic impairment had a significant impact on total plasma amprenavir and ritonavir exposure, as demonstrated by an average of a 44% reduction in plasma amprenavir CL/F and 64% reduction in plasma ritonavir CL/F values, equating to 80% higher in plasma amprenavir DN-AUC(0-τ) and 180% higher in plasma ritonavir AUC(0-τ) values (Fig. 1, Table 2, Table 3, Fig. 2, Table 4, and Table 5). Because a significant increase in plasma amprenavir and ritonavir exposure was expected for subjects with severe hepatic impairment, those subjects received reduced doses or reduced dosing frequencies of both drugs. With the use of a reduced ritonavir dosing frequency of 100 mg once daily in subjects with severe hepatic impairment, the observed ritonavir AUC(0-24) value was on average 40% higher and the ritonavir Cτ value was 38% higher compared to subjects with normal hepatic function receiving fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily (Table 4 and Table 5). For subjects with severe hepatic impairment, the studied dosage regimen of fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily (i.e., reduced dose of fosamprenavir and reduced dosing frequency of ritonavir) delivered an average 19% lower plasma amprenavir Cmax value, 23% lower amprenavir AUC(0-24) value, 38% lower amprenavir Cτ value, and similar unbound amprenavir Cτ value compared to subjects with normal hepatic function receiving fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily (Table 2 and Table 3).
Relationships between unbound and total amprenavir Cτ values and between drug exposure and markers of hepatic impairment.
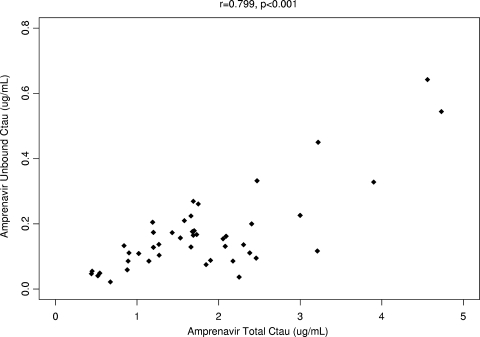
Levels of unbound amprenavir in plasma and total Cτ values were highly correlated (r = 0.799 [P < 0.001]), as shown in Fig. 3; a similar correlation was observed between unbound and total amprenavir concentrations at 2 h after dosing (r = 0.748 [P < 0.001]).
FIG. 3.
Unbound amprenavir Cτ versus total Cτ.
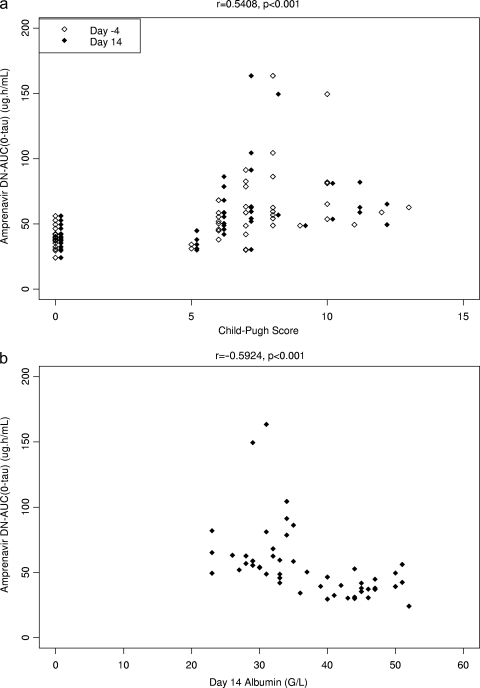
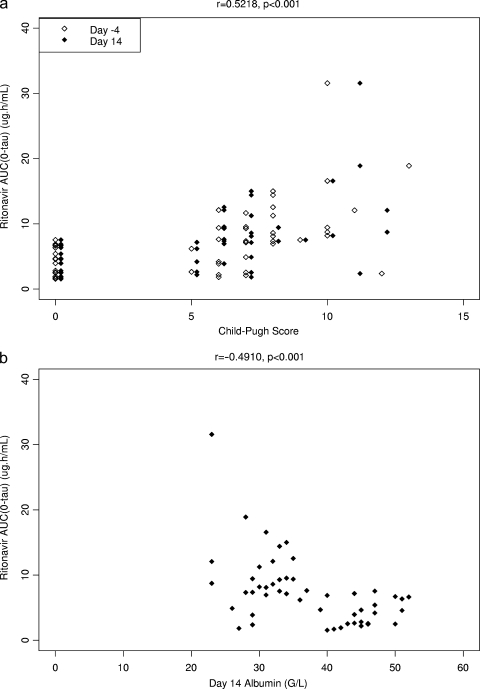
Concerning the relationship between drug exposure and markers of hepatic function, the strongest correlations for the amprenavir DN-AUC(0-τ) values were with the Child-Pugh score (r = 0.5408 [P < 0.001]) and levels of cholinesterase (r = −0.5935 [P < 0.001]) and albumin (r = −0.5924 [P < 0.001]) (Fig. 4a and b). The strongest correlations for the ritonavir AUC(0-τ) values were with the Child-Pugh score (r = 0.5218 [P < 0.001]) and levels of cholinesterase (r = −0.4941 [P < 0.001]), albumin (r = −0.4910 [P < 0.001]) (Fig. 5a and b), and bilirubin (r = 0.4989 [P < 0.001]). Markers of hepatic function such as the Child-Pugh score and levels of albumin, bilirubin, and cholinesterase were highly correlated with each other. In stepwise regression analysis, only the baseline albumin level was significantly associated with amprenavir DN-AUC(0-τ) values, and the relationship was inverse (Fig. 4b).
FIG. 4.
(a) Amprenavir (DN to fosamprenavir at 700 mg) AUC(0-τ) versus Child-Pugh score. Subjects with normal hepatic function were assigned a Child-Pugh score of 0. (b) Amprenavir (DN to fosamprenavir at 700 mg) AUC(0-τ) versus albumin concentration.
FIG. 5.
(a) Ritonavir AUC(0-τ) versus Child-Pugh score. Subjects with normal hepatic function were assigned a Child-Pugh score of 0. (b) Ritonavir AUC(0-τ) versus albumin concentration.
Safety. (i) Clinical adverse events.
During this 14-day study, the antiretroviral regimen that included fosamprenavir/ritonavir was generally well tolerated by subjects with hepatic impairment and by those with normal hepatic function.
Eleven of the 13 subjects with mild hepatic impairment completed the study; 1 subject withdrew due to an adverse event (gastritis) after 1 day of dosing with fosamprenavir/ritonavir and another decided to withdraw from the study for personal reasons after 8 days of dosing. Four subjects within this cohort experienced a total of four grade 3 to 4 or serious adverse events, one of which was considered by the investigator to have been study drug related (grade 3 gastritis leading to withdrawal). Adverse events not considered to be related to the study drugs comprised increased creatine phosphokinase levels, hepatic encephalopathy, and suicidal ideation (the latter two conditions were considered serious adverse events).
Of the 20 subjects with moderate hepatic impairment, all completed the study. Three subjects within the two cohorts experienced grade 3 to 4 or serious adverse events, of which one was considered by the investigator to have been at least possibly related to the study drugs (grade 3 elevation of AST). Adverse events not considered to be related to study medications included respiratory infection and ankle edema. One subject with normal hepatic function experienced pneumonia, which was not considered to have been drug related.
Of the 10 subjects enrolled with severe hepatic impairment, 7 completed the study. Two subjects withdrew due to adverse events (one with grade 1 vomiting deemed related to the study drugs and one due to worsening hepatic encephalopathy attributed by the investigator to surreptitious ingestion of the benzodiazepine chloracepate). The third of the subjects who did not complete the study was withdrawn due to a protocol violation. Three subjects experienced grade 3 to 4 or serious adverse events, none of which was attributed to study medications. These included the case of encephalopathy described above, a case of grade 4 exacerbation of chronic liver failure (comprising worsening of baseline hepatic encephalopathy and an increase in prothrombin time), and cholecystitis that occurred after the study drugs had been discontinued.
(ii) Liver chemistry changes.
Treatment-emergent grade 3 or higher toxicities in liver chemistries were uncommon, with none recorded for ALT among subjects with mild, moderate, or severe hepatic impairment. Mean bilirubin concentrations decreased from day 1 to day 14 of the study in all groups (−11.3 U/liter in the cohort with mild hepatic impairment, −15.7 and −13.9 U/liter for members of the cohort with moderate hepatic impairment who were administered the study drugs twice daily and once daily, respectively, and −13.4 U/liter in the cohort with severe hepatic impairment). No significant changes were noted in AAG and albumin concentrations from day 1 to day 14.
DISCUSSION
Hepatic impairment can alter drug disposition and exposure through various mechanisms such as the shunting of blood past the liver, impaired metabolizing activity of the hepatocytes, impaired biliary excretion, and reduced protein binding. Given that amprenavir is metabolized by CYP3A4 and is ≥90% bound to plasma proteins, multiple mechanisms could be involved in the altered amprenavir disposition. Both total and unbound plasma concentrations were increased in subjects with hepatic impairment. For example, comparisons between the severe hepatic impairment and normal hepatic function groups yielded geometric least squares mean ratios of 0.62 for total plasma Cτ (1.42 for DN Cτ) and 0.96 for unbound plasma Cτ (approximately 2.2 for DN unbound Cτ). Because both total and unbound plasma amprenavir concentrations were increased in subjects with hepatic impairment, the metabolism of amprenavir was reduced in this population, which could have been due to impaired hepatocyte metabolic activity and the shunting of blood past the liver (i.e., bypassing the site of metabolism).
In a previous study of unboosted amprenavir, subjects with moderate hepatic impairment had 28% higher plasma amprenavir Cmax and 2.5-fold-higher AUC to infinity [AUC(0-∞)] values and subjects with severe hepatic impairment had 96% higher plasma amprenavir Cmax and 4.5-fold-higher AUC(0-∞) values compared to subjects with normal hepatic function (8). Since the study involved only single-dose administration, Cτ values were not collected; however, mean plasma amprenavir concentrations at 12 h after administration of a single dose of amprenavir were increased approximately 2.5-fold for subjects with moderate hepatic impairment and 3.5-fold for subjects with severe hepatic impairment (unpublished data). Compared to the impact of ritonavir coadministration on amprenavir PK, where the amprenavir Cmax value increased 51% and the AUC(0-τ) and Cτ values increased 3.4-fold and 12.7-fold, respectively (9), moderate hepatic impairment had a lesser impact and severe hepatic impairment had a greater impact on amprenavir Cmax and AUC values, whereas both moderate and severe hepatic impairment had a lesser impact on amprenavir Cτ values than ritonavir coadministration.
Even though ritonavir coadministration results in large increases in plasma amprenavir exposure, the present study demonstrated increased amprenavir exposure in subjects with hepatic impairment receiving ritonavir-boosted fosamprenavir. The increase in amprenavir exposure observed in the boosted setting was less than that observed for unboosted amprenavir. For example, for subjects with moderate hepatic impairment, amprenavir AUC(0-τ) values increased 51 to 70% in the ritonavir-boosted setting versus 2.5-fold in the unboosted amprenavir study; similarly, for subjects with severe hepatic impairment, amprenavir AUC(0-τ) values increased 80% in the ritonavir-boosted setting versus 4.5-fold in the unboosted amprenavir study. Ritonavir exposure [AUC(0-τ)] also increased 93 to 120% for subjects with moderate hepatic impairment and 180% for subjects with severe hepatic impairment, and the increases were of a magnitude similar to those reported for lopinavir/ritionavir (3).
A previously reported study also demonstrated increased amprenavir and ritonavir exposures in eight HIV-infected subjects with cirrhosis who were receiving fosamprenavir/ritonavir (6), but specific recommendations could not be made for each level of hepatic impairment. The study presented herein prospectively enrolled subjects with mild, moderate, and severe hepatic impairment to allow dosing recommendations for each level of hepatic impairment. In addition, unbound amprenavir concentrations were measured in order to better guide dosing recommendations.
For subjects with mild hepatic impairment, the studied regimen of fosamprenavir administered at 700 mg twice daily plus ritonavir administered at a reduced frequency of 100 mg once daily delivered acceptable amprenavir and ritonavir exposures and did not lead to any increased safety concerns. Therefore, fosamprenavir administered at 700 mg twice daily plus ritonavir administered at 100 mg once daily is an acceptable regimen for this population.
The lower amprenavir AUC(0-24), amprenavir Cτ, and unbound Cτ values observed for subjects with moderate hepatic impairment receiving fosamprenavir at 700 mg once daily plus ritonavir at 100 mg once daily suggest that the regimen consisting solely of once-daily drug administration is not a good option for this population. For subjects with moderate hepatic impairment, the studied regimen of a reduced dose of fosamprenavir administered at 300 mg twice daily plus ritonavir administered at a reduced frequency of 100 mg once daily delivered average values of 27% lower plasma amprenavir Cmax, 27% lower amprenavir AUC(0-τ), 57% lower amprenavir Cτ, and 21% higher unbound amprenavir Cτ, while the results of daily exposure to ritonavir were similar to those seen with subjects with normal hepatic function receiving fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily. A regimen of fosamprenavir at 450 mg twice daily plus ritonavir at 100 mg once daily is predicted to deliver plasma amprenavir AUC(0-τ) values similar to those observed for subjects with normal hepatic function receiving fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily. Although total amprenavir Cτ values are predicted to be 35% lower, unbound amprenavir Cτ values are predicted to be 87% higher. Considering the higher PK variability observed for subjects with hepatic impairment, the regimen consisting of administration of fosamprenavir at 450 mg twice daily plus ritonavir at 100 mg once daily would allow a higher proportion of subjects with moderate hepatic impairment to achieve unbound amprenavir Cτ values in the range of those observed for subjects with normal hepatic function receiving the standard dosage regimen of fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily.
For subjects with severe hepatic impairment, the studied regimen of reduced doses of fosamprenavir administered at 300 mg twice daily plus ritonavir administered at a reduced frequency of 100 mg once daily delivered on average 19% lower plasma amprenavir Cmax, 23% lower amprenavir AUC(0-τ), 38% lower amprenavir Cτ, and similar plasma unbound amprenavir Cτ values. These amprenavir results were consistent with those seen with the moderate hepatic impairment group receiving the same fosamprenavir-plus-ritonavir regimen. However, subjects with severe hepatic impairment had an average 40% higher daily exposure to ritonavir compared to subjects with normal hepatic function receiving fosamprenavir at 700 mg twice daily plus ritonavir at 100 mg twice daily, whereas ritonavir AUC(0-24) values for the moderate hepatic impairment group and for subjects with normal hepatic function were similar. The studied dosage regimen of fosamprenavir at 300 mg twice daily plus ritonavir at 100 mg once daily is acceptable for the patient population with severe hepatic impairment, although close monitoring of clinical status is warranted for this difficult-to-treat population.
The correlation between total and unbound plasma amprenavir Cτ values was expected and supports the use of total plasma amprenavir concentrations for therapeutic drug monitoring in patients with hepatic impairment. The relationship between plasma amprenavir DN-AUC(0-τ) values and Child-Pugh scores is consistent with findings from previous studies of amprenavir (8) and fosamprenavir/ritonavir (6). We also found correlations between amprenavir DN-AUC(0-τ) values and other markers of hepatic function such as cholinesterase and albumin concentrations. Plasma ritonavir AUC(0-τ) values also correlated with Child-Pugh scores and other markers of hepatic function. The present study did not evaluate the impact of hepatitis coinfection in the absence of cirrhosis, though others have shown a minimal impact of chronic hepatitis in the absence of cirrhosis (6).
This study had several limitations. Most importantly, the duration of therapy in this short-term PK study precludes definitive conclusions regarding the long-term safety of the recommended fosamprenavir/ritonavir regimen. In addition, most of the subjects were white men; whether these findings may be extrapolated to women and other races is unknown.
In conclusion, this study forms the basis of recommendations for the use of reduced fosamprenavir/ritonavir doses or dosing frequencies for patients with mild, moderate, or severe hepatic impairment and thus provides a new option for the treatment of this important patient group. The modified fosamprenavir/ritonavir dosage regimens were generally well tolerated in this short-term study; observed adverse events were generally attributable to the underlying hepatic impairment of the subject. Plasma amprenavir and ritonavir exposures were more variable in subjects with hepatic impairment, however; these patients should thus be closely monitored for safety and virologic responses.
Acknowledgments
We gratefully acknowledge Jose-Ramón Arribas, Rafael Rubio, Fedrico Pulido, Vicente Estrada, Juan Berenguer, Miguel Muniain, Arturo Prieto, Edwin DeJesus, Anthony Mills, Anthony La Marca, and Miguel Pascual-Bernaldez for assistance in the conduct of the study. We gratefully acknowledge Rashida Rana for manuscript preparation and all subjects who participated in the study.
The study was supported by GlaxoSmithKline.
Footnotes
Published ahead of print on 10 August 2009.
REFERENCES
- 1.Alter, M. 2006. Epidemiology of viral hepatitis and HIV co-infection. J. Hepatol. 44(Suppl. 1):S6-S9. [DOI] [PubMed] [Google Scholar]
- 2.Crum, N. F., R. Riffenburgh, S. Wegner, B. K. Agan, S. Tasker, K. M. Spooner, A. W. Armstrong, S. Fraser, and M. Wallace on behalf of the Triservice AIDS Clinical Consortium. 2006. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J. Acquir. Immune Defic. Syndr. 41:194-200. [DOI] [PubMed] [Google Scholar]
- 3.Peng, J. Z., F. Pulido, S. J. Kemmis Causemaker, J. Li, A. Lorenzo, C. Cepeda, J. A. Garcia Cabanillas, B. DaSilva, S. C. Brun, and J. Arribas. 2006. Pharmacokinetics of lopinavir/ritonavir in HIV/hepatitis C virus coinfected subjects with hepatic impairment. J. Clin. Pharmacol. 46:265-274. [DOI] [PubMed] [Google Scholar]
- 4.Pugh, R. N. H., I. M. Murray-Lyon, J. L. Dawson, M. C. Pietroni, and R. Williams. 1973. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 60:646-649. [DOI] [PubMed] [Google Scholar]
- 5.Rockstroh, J., D. Konopnicki, V. Soriano, O. Kirk, F. Antunes, B. Knysz, C. Tural, S. D. Wit, A. Mocroft, J. Lundgren, and the EuroSIDA study group. 2004. Hepatitis B hepatitis C in the EuroSIDA cohort: prevalence and effect on mortality, AIDS progression and response to HAART, abstr. 799. Abstr. 11th Conf. Retrovir. Opportunistic Infect., San Francisco, CA.
- 6.Seminari, E., A. De Bona, G. Gentilini, L. Galli, G. Schira, N. Gianotti, C. Eberti-Foppa, A. Soldarini, F. Dorigatti, A. Lazzarin, and A. Castagna. 2007. Amprenavir and ritonavir plasma concentrations in HIV-infected patients treated with fosamprenavir/ritonavir with various degrees of liver impairment. J. Antimicrob. Chemother. 60:831-836. [DOI] [PubMed] [Google Scholar]
- 7.Soriano, V., M. Puoti, M. Sulkowski, S. Mauss, P. Cacoub, A. Cargnel, D. Dieterich, A. Hatzakis, and J. Rockstroh. 2004. Care of patients with hepatitis C and HIV co-infection. AIDS 18:1-12. [DOI] [PubMed] [Google Scholar]
- 8.Veronese, L., J. Rautaureau, B. M. Sadler, C. Gillotin, J. Petite, B. Pillegand, M. Delvaux, C. Masliah, S. Fosse, Y. Lou, and D. Stein. 2000. Single-dose pharmacokinetics of amprenavir, a human immunodeficiency virus type 1 protease inhibitor, in subjects with normal or impaired hepatic function. Antimicrob. Agents Chemother. 44:821-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wire, M. B., K. L. Baker, L. S. Jones, M. J. Shelton, Y. Lou, G. Thomas, and M. Berrey. 2006. Ritonavir increases plasma amprenavir (APV) exposure to a similar extent when coadministered with either fosamprenavir or APV. Antimicrob. Agents Chemother. 50:1578-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]