Abstract
Tigecycline (TGC) is an extended-spectrum antibiotic with activity against Staphylococcus aureus, including methicillin (meticillin)-resistant S. aureus strains, which are well-recognized pathogens in nosocomial pneumonia. The objective of this study was to characterize the exposure-response relationship for TGC against S. aureus in an immunocompromised BALB/c murine pneumonia model. Six S. aureus isolates were studied, and the TGC MICs for those isolates ranged from 0.125 to 0.5 mg/liter. The pharmacokinetics (PK) of TGC in serum and bronchoalveolar lavage (BAL) fluid were evaluated, as was the level of protein binding of the compound in this murine species. Administration of TGC at 1.56 to 150 mg/kg of body weight/day in single or two to three divided doses was used in the efficacy studies. TGC displayed linear PK and had a mean half-life of 10.9 ± 2.5 h. Efficacy was highly correlated with the area under the free concentration-time curve (fAUC)/MIC (r2 = 0.93). The 80% and 50% effective exposure indexes and the stasis exposure index were similar between the isolates (means ± standard deviations, 3.04 ± 1.12, 1.84 ± 1.3, and 1.9 ± 1.5, respectively). Maximal efficacy was predicted at a 2.85-log10-CFU reduction. TGC appeared to accumulate in the interstitial space, as the ratios of the fAUC from 0 to 8 h of epithelial lining fluid to plasma were 7.02, 15.11, and 23.95 for doses of 12.5, 25, and 50 mg/kg, respectively. TGC was highly effective in this murine pneumonia model. In light of current MIC distributions, the fAUC/MIC targets that we defined against S. aureus are readily achievable in humans given conventional doses of TGC.
Staphylococcus aureus has long been recognized as an important cause of infection, and the emergence of S. aureus strains with the methicillin (meticillin)-resistant phenotype (methicillin-resistant S. aureus [MRSA]) has further complicated management. Both community-acquired MRSA (CA-MRSA) and hospital-acquired MRSA (HA-MRSA) strains have been associated with severe and difficult-to-treat infections. While the most common site of staphylococcal infection is the skin and skin structures, the surveillance of 8,792 invasive MRSA cases in the United States showed that pneumonia is the second most common clinical manifestation of MRSA infection (13.3% overall; 14% of the strains were CA-MRSA and 28% were HA-MRSA) (9).
Tigecycline (TGC) is a broad-spectrum glycylcycline with efficacy against gram-positive and gram-negative bacteria, including drug-resistant bacteria such as MRSA. The MIC90 of TGC against methicillin-susceptible S. aureus (MSSA) and MRSA strains is reported to be ≤0.25 mg/liter (6). TGC is approved by the FDA for use for the treatment of complicated skin and skin structure infections and complicated intra-abdominal infections. Pneumonia is an important clinical manifestation of infection with drug-resistant bacteria; therefore, many in vivo and in vitro studies of TGC for the treatment of lower respiratory infection are ongoing (data available at http://www.clinicaltrialssearch.org/tigecycline_versus_imipenemcilastatin_for_the_treatment_of_subjects_with_nosocomial_pneumonia.html and http://www.medicalnewstoday.com/articles/53035.php).
Previously, we demonstrated the efficacy of TGC against Acinetobacter spp. in a murine pneumonia model (10) and against S. aureus in a murine thigh infection model (3). We also found that TGC penetrated well into lung tissue, as displayed by high concentrations in bronchoalveolar (BAL) fluid (4). In the present study, we aimed to explore the exposure-response relationship for TGC against S. aureus in an immunocompromised BALB/c murine pneumonia model.
MATERIALS AND METHODS
Test antimicrobial agents.
Standard analytical-grade TGC (lot RB5603; expiration date, September 2009; Wyeth, Madison, NJ) was used for all in vitro and in vivo experiments. For all animal studies, the TGC powder was weighed and reconstituted with normal saline to achieve the desired concentrations immediately prior to each experiment. The solution was used within 30 min of reconstitution.
Microorganisms.
Six isolates of S. aureus (ATCC 29213, two HA-MRSA isolates, and three CA-MRSA isolates) were utilized in the study. The MIC of TGC for all organisms was determined in triplicate by the microdilution method, according to the guidelines of the CLSI (1). The modal MIC was utilized in all pharmacodynamic assessments.
Lung infection (pneumonia) model.
Specific-pathogen-free, female BALB/c mice were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, IN), and were utilized throughout these experiments. This study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee. The animals were maintained and used in accordance with the recommendations of the National Research Council and were provided food and water ad libitum. The mice were rendered transiently neutropenic by intraperitoneal injections of cyclophosphamide at 250 and 100 mg/kg of body weight at 4 days and 1 day prior to inoculation, respectively.
The S. aureus isolates were frozen at −80°C in skim milk and were subcultured twice onto blood agar medium. For inoculation, a suspension of the test organism was prepared from the second subculture, which had been incubated at 37°C for 20 to 24 h, and was adjusted to a 0.5 to 1 McFarland turbidity standard in a 3% mucin solution (3.0 × 108 CFU/ml). The bacterial density of the final inoculum was confirmed by serial dilution and culture of an aliquot from each inoculum. The animals were lightly anesthetized with isoflurane. Pneumonia was induced by instilling 0.05 ml of the bacterial suspension into the mouth of the mice and completely blocking the nasal cavity of the animal, thus resulting in bacterial inhalation through the mouth to the lungs.
Pharmacokinetic studies.
The animals were prepared as described in the section describing the pneumonia model. Four infected groups of BALB/c mice (six mice per time point, eight sampling times) were dosed with a single 0.2-ml subcutaneous dose of 6.25, 12.5, 25, or 50 mg/kg of TGC. The animals were euthanized by CO2 exposure, followed by cervical dislocation prior to sample collection. Blood was obtained from each group of six mice at 0.25, 0.5, 1, 1.5, 2, 4, 6, and 8 h after drug administration and then centrifuged to acquire serum. Following blood collection, BAL fluid was obtained from the same mice by use of a normal saline lavage at times of 0.25, 1, 4, and 8 h after drug administration. The BAL fluid was used to determine the epithelial lining fluid (ELF) concentrations by a previously described method (7). All serum and ELF samples were stored in polypropylene tubes at −80°C until analysis. The TGC concentrations in murine serum and ELF were determined by a validated high-performance liquid chromatography assay (11) at the Center for Anti-Infective Research and Development, Hartford Hospital. The interday and intraday coefficients of variation of the assay were <5%. Portions of the serum and ELF samples were retained and utilized for urea determinations. The interday and intraday coefficients of variation of this assay were also <5%. The TGC concentrations in ELF (TGCELF) were calculated by using the following formula: TGCBAL × (ureaserum/ureaBAL), where ureaserum and ureaBAL are the concentrations of urea in the serum and BAL fluid, respectively, and TGCBAL is the concentration of TGC in BAL fluid. The areas under the concentration-time curves (AUCs) from 0 to 8 h (AUC0-8s) were calculated by using the trapezoidal rule. The TGC detected in BAL fluid was assumed to be free drug, whereas all serum exposures were adjusted to the free-drug equivalents on the basis of the findings of the protein binding experiments that were undertaken.
Protein binding studies.
Protein binding studies were conducted in triplicate by the ultrafiltration method with Amicon Centrifree micropartition devices with a 30,000-molecular-weight cutoff (Millipore, Bedford, MA). An aqueous stock solution of the compound containing 1 mg/ml of TGC was prepared in normal saline. Dilutions were made in freshly collected mouse serum to yield final concentrations of 1.5, 3, 6, and 15 μg/ml. These concentrations were selected such that the range incorporated the peak serum concentration profile of the doses to be utilized in the pharmacodynamic studies. Each of the serum solutions was heated at 37°C in a shaking water bath for 10 min. Exactly 0.9 ml of each serum solution was transferred into three ultrafiltration devices, and the devices were centrifuged at 1,000 × g for 45 min at 10°C to generate an ultrafiltrate volume of approximately 250 μl. The nonspecific binding of TGC to the filter device was previously assessed and was found to be negligible (3).
The percent protein binding with each concentration prepared was calculated by using the following equation: [(S − SUF)/S] × 100, where S is the TGC concentration in the initial serum solutions and SUF is the concentration in the ultrafiltrate.
Given the concentration-dependent protein binding noted previously (4) and herein, a sigmoidal maximum-effect (Emax) model explaining the relationship between the level of protein binding and the TGC concentration was constructed. Using this model, we calculated the free-drug exposures for each of the doses used in the bacterial density studies by uniformly correcting the concentration-time profile with the percentage of free drug noted at the peak serum drug concentration for each given dose (3).
Therapeutic efficacy of TGC.
To assess the in vivo bactericidal activity of TGC against the S. aureus isolates, treatment was initiated 6 h after inoculation. Mice were given TGC at a dose of 1.56, 3.13, 6.25, 12.5, 25, 50, 100, or 150 mg/kg/day in single or two to three divided doses. All doses were given subcutaneously. Control animals received sterile normal saline in the same volume (0.2 ml) and on the same schedule as the most frequently administered active-drug regimen. Untreated control animals (six per group) were killed just prior to the initiation of antibiotic treatment (0 h) and after 24 h, along with all drug-treated animal groups. After the animals were killed (euthanasia by CO2 exposure followed by cervical dislocation), all lobes of the lung were removed and homogenized in normal saline. Serial dilutions of the homogenate were plated onto blood agar for determination of the numbers of CFU. For the purposes of these studies, efficacy (the change in bacterial density) was calculated as the change in the numbers of bacterial CFU obtained for treated mice after 24 h compared with the numbers of CFU in the control animals at 0 h.
Pharmacodynamic analysis.
For each S. aureus isolate, a dose-response curve was constructed by plotting the change in the log10 CFU versus the ratio of the area under the free AUC to the MIC (fAUC/MIC) by using a sigmoid Emax model (WinNonlin, version 5.0.1; Pharsight, Mountain View, CA). This allowed determination of the effective exposure indexes (EIs; i.e., the exposure value required to produce 80% of the maximal effect [EI80], the exposure value required to produce 50% of the maximal effect [EI50], and the exposure value required to produce stasis]. Only ƒAUC/MIC was assessed in this study, as this pharmacodynamic parameter has previously been determined to be the most closely correlated to efficacy in other in vivo studies with this compound (3, 14).
RESULTS
The in vitro potencies of TGC and the other compounds tested against the S. aureus isolates used in the pharmacodynamic studies are displayed in Table 1. The TGC MICs for the S. aureus isolates ranged from 0.125 to 0.5 mg/liter.
TABLE 1.
MICs of TGC and other clinically utilized compounds for the Staphylococcus aureus isolates studied
| Compounda | MIC (mg/liter) |
|||||
|---|---|---|---|---|---|---|
| MSSA ATCC 29213 | CA-MRSA 144 | CA-MRSA 156 | CA-MRSA 147 | HA-MRSA 56 | HA-MRSA 152 | |
| LZD | 2 | 2 | 2 | 2 | 8 | 2 |
| VAN | 1 | 1 | 1 | 1 | 1 | 1 |
| ERY | 1 | >32 | >32 | >32 | 32 | 1 |
| CLI | 0.125 | >16 | ≤0.5 | 0.125 | 0.25 | 0.125 |
| LVX | 0.25 | 0.5 | 8 | 0.25 | 0.25 | |
| TMP-SXT | 0.125 | 0.25 | ≤0.5 | 0.125 | 0.06 | 0.06 |
| DOX | 0.5 | 0.5 | 2 | 8 | 0.5 | |
| TGCb | 0.25 | 0.25 | 0.125 | 0.25 | 0.5 | 0.25 |
LZD, linezolid; VAN, vancomycin; ERY, erythromycin; CLI, clindamycin; LVX, levofloxacin; TMP-SXT, trimethoprim-sulfamethoxazole; DOX, doxycycline.
The FDA-approved breakpoint of TGC for susceptibility is an MIC of ≤0.5 mg/liter.
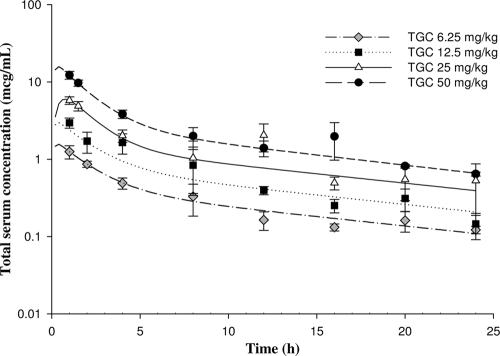
Figure 1 displays the total serum drug concentration-time profiles of TGC after various single subcutaneous doses; the values of the pharmacokinetic parameters are summarized in Table 2. The range of the total AUC from 0 to 24 h (AUC0-24) was 10.4 to 103.5 mg·h/liter for the dosing regimens used during the pharmacodynamic analyses. The level of TGC protein binding was found to be concentration dependent, with 85.0%, 87.2%, 93.7%, and 95.9% of the TGC administered at concentrations of 1.5, 3, 6, and 15 mg/liter, respectively, being protein bound. Correction for the level of protein binding resulted in fAUC0-24s of 0.3 to 6.48 mg·h/liter for the dosing regimens used.
FIG. 1.
Total concentrations of TGC after various single subcutaneous doses in S. aureus-infected mice.
TABLE 2.
Pharmacokinetic parameter values for TGC after a single subcutaneous dose in S. aureus-infected micea
| Dosing regimen (mg/kg) | Cmax (μg/ml) | Tmax (h) | AUC0-24 (mg·h/liter) | Half-life (h) | V (liters/kg) | Protein binding (%) |
|---|---|---|---|---|---|---|
| 6.25 | 1.57 | 0.34 | 9.98 | 12.40 | 3.44 | 85.0 |
| 12.5 | 3.09 | 0.27 | 16.52 | 7.13 | 3.62 | 87.2 |
| 25 | 5.99 | 0.74 | 37.49 | 12.43 | 2.77 | 93.7 |
| 50 | 15.8 | 0.35 | 72.52 | 11.52 | 2.62 | 95.9 |
Cmax, maximum concentration of drug in serum; Tmax, time to achieve the maximum concentration of drug in serum; V, volume of distribution.
The TGC concentrations in the ELF samples were greater than their corresponding concentrations in the serum samples for each dose and at all time points. The AUC0-8s in ELF after doses of 12.5, 25, and 50 mg/kg were 11.0, 20.4, and 41.9 mg·h/liter, respectively; and the penetration ratios, which consisted of the fAUC0-8 for ELF to the fAUC0-8 for plasma, were 7.02, 15.11, and 23.95, respectively.
The mean starting (0-h) bacterial density in the lungs of the control mice was 7.69 × 105 CFU. The bacterial density increased 1.76 log10 CFU, on average (range, 1.03 to 2.3 log10 CFU), in the untreated control group at 24 h. The observed mean maximal reduction in the numbers of CFU in the TGC-treated animals after 24 h of exposure was 2.11 log10 CFU (range, 1.78 to 2.39 log10 CFU). This value was very similar to the mean maximal reductions defined by the Emax model (i.e., 2.85 log killing) in the six S. aureus isolates tested (Table 3).
TABLE 3.
fAUC/MICs for corresponding effective EIs of TGC against S. aureus isolates in an immunocompromised murine pneumonia model
| S. aureus strain | r2 |
fAUC/MIC |
Predicted maximum log10 CFU reduction | ||
|---|---|---|---|---|---|
| EI80 | EI50 | Stasis | |||
| MSSA ATCC 29213 | 0.97 | 2.68 | 2.33 | 2.38 | 3.36 |
| CA-MRSA 144 | 0.99 | 2.64 | 2.29 | 2.17 | 2.26 |
| CA-MRSA 156 | 0.92 | 5.22 | 3.91 | 4.18 | 2.90 |
| CA-MRSA 147 | 0.80 | 3.12 | 0.69 | 0.41 | 2.57 |
| HA-MRSA 56 | 0.99 | 2.11 | 0.38 | 0.43 | 3.26 |
| HA-MRSA 152 | 0.96 | 2.48 | 1.43 | 1.51 | 3.45 |
| Mean | 0.93 | 3.04 | 1.84 | 1.88 | 2.85 |
| SD | 0.08 | 1.12 | 1.29 | 1.49 | 0.41 |
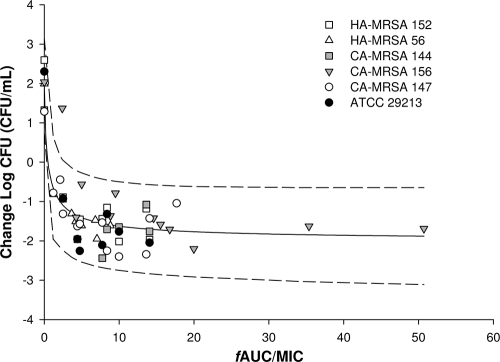
The relationship between the antimicrobial activities of TGC and the fAUC/MIC was assessed for each individual S. aureus isolate. The mean correlation coefficient (r2) of the fitted curves was 0.93 (range, 0.802 to 0.991) (Table 3).
Table 3 also displays the individually generated EI80, EI50, and stasis exposure values for the six S. aureus isolates studied. The mean values from the individual isolates were very similar to mean value defined in the composite curve (Fig. 2), with the EI80, EI50, and stasis values being 3.04, 1.84, and 1.88, respectively.
FIG. 2.
Composite assessment of TGC's antibacterial effect versus fAUC/MIC (mean ± 95% confidence interval) for six S. aureus isolates.
DISCUSSION
Pneumonia is the second most common clinical manifestation of S. aureus and invasive MRSA infections (9). The appropriate antimicrobial agent for the treatment of such infections should possess not only activity against this organism but also the ability to penetrate into the site of infection. In vivo data describing the efficacy of TGC for the treatment of various staphylococcal infections exist (3, 8, 12, 18). In addition, data describing the efficacy of TGC against nonstaphylococcal pneumonia are available (10, 17). Taken collectively, it seems reasonable to suggest that that TGC could be an option for the treatment of staphylococcal pneumonia. We explored the pharmacodynamic profile of TGC against S. aureus in a murine model of pneumonia as a bridge to the treatment of pneumonia in humans.
We found TGC to be highly active against the six S. aureus isolates tested in this pneumonia model. This appears to be consistent with the findings presented in a lone case report of the clinical efficacy of TGC against MRSA pneumonia complicated by sepsis in a 57-year-old man who had recently undergone liver transplantation and who was receiving immunosuppressive agents. The patient was successfully treated with TGC after he failed both vancomycin and linezolid treatments (15).
On the basis of our Emax model, we described an average maximal reduction in the numbers of CFU of 2.85 log units at 24 h after inoculation in TGC-treated mice. When these results are compared with those of our previous study (3), in which we used the same staphylococcal isolates in a murine thigh infection model, the maximal reductions noted in the pneumonia model were greater than those noted in the thigh infection model (1.9 log CFU), while the starting inocula and the growth after 24 h in the untreated mice were very similar. Moreover, the effective EIs required in the pneumonia model (mean EI80, EI50, and stasis values, 3.0, 1.8, and 1.9, respectively) were lower than those required in the thigh infected mice (5.1, 2.2, and 2.4, respectively) and likely reflect enhanced penetration into the lung.
We demonstrated TGC concentrations in ELF to be greater than those in serum at each sampling time point. Moreover, we noted that the AUC for ELF ranged from 7 to 24 times the fAUC for serum, depending on the dose. This observation was also made in a previously conducted bronchopulmonary pharmacokinetic analysis by our group, in which AUC-based TGC penetration ratios in mice with lung infections ranged from 12.9 to 23.3 (5). While the previously noted thigh infection study did not describe the level of TGC penetration into thigh tissue, a blister fluid penetration study with healthy volunteers found decreased levels of exposure for tissue relative to those for blood (16). This comparison seems to be reasonable, given the similarities in ELF penetration noted between our murine data with the lowest dose (12.5 mg/kg) and the ELF penetration reported by Conte et al. in the study with human volunteers (2). In that study, volunteers given standard doses of TGC were found to have an ELF penetration ratio of 1.31 when the total drug AUC in serum was used. After correction for 79% protein binding (13), the ratio of the AUC for ELF to the fAUC for serum was 6.28. While the correlation between ELF TGC concentrations and clinical efficacy is unclear, in our murine model we demonstrated a high degree of ELF penetration to be consistent with enhanced bacterial killing.
In light of the current distributions of TGC MICs against S. aureus (MIC90, 0.12 mg/ml for MSSA strains and 0.12 to 0.25 mg/ml for MRSA strains) (6), the fAUC/MIC targets that we defined are readily achievable in humans given conventional doses of TGC (19).
In summary, we demonstrated that the TGC exposures in ELF are many times those in serum. This penetration correlated with an increased maximal reduction in the numbers of CFU in murine lungs by the use of exposures lower than those required in a thigh infection model. While further clinical studies of TGC against staphylococcal pneumonia are needed to fully assess its clinical utility, our data support the potential role of the compound in the setting of pneumonia.
Acknowledgments
We thank Pamela Tessier, Debora Santini, Lindsay Tuttle, Jennifer Hull, Henry Christensen, and Christina Sutherland for their assistance with the conduct of the animal experimentation and the analytical determinations of TGC.
This study was supported by a grant from Wyeth Research.
D.P.N. is on the Wyeth speaking bureau and has received research support for projects other than this study. None of the other authors has anything to declare.
Footnotes
Published ahead of print on 8 September 2009.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. CLSI document M7-A7 (26)2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 2.Conte, J. E. Jr., J. A. Golden, M. G. Kelly, et al. 2005. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int. J. Antimicrob. Agents 25:523-529. [DOI] [PubMed] [Google Scholar]
- 3.Crandon, J. L., M. A. Banevicius, and D. P. Nicolau. 2009. Pharmacodynamics of tigecycline against phenotypically diverse Staphylococcus aureus isolates in a murine thigh model. Antimicrob. Agents Chemother. 53:1165-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crandon, J. L., A. Kim, and D. P. Nicolau. 2009. Tigecycline penetration into the epithelial lining fluid of infected and uninfected murine lungs, abstr. P-228. Abstr. Am. College Clin. Pharm./Eur. Soc. Clin. Pharm. Int. Congr. Clin. Pharm. [DOI] [PubMed]
- 5.Crandon, J. L., A. Kim, and D. P. Nicolau. 2009. Comparison of tigecycline penetration into the epithelial lining fluid of infected and uninfected murine lungs. J. Antimicrob. Chemother. 64:837-839. [DOI] [PubMed] [Google Scholar]
- 6.Dowzicky, M. J., and C. H. Park. 2008. Update on antimicrobial susceptibility rates among gram-negative and gram-positive organisms in the United States: results from the Tigecycline Evaluation and Surveillance Trial (TEST) 2005 to 2007. Clin. Ther. 30:2040-2050. [DOI] [PubMed] [Google Scholar]
- 7.Du, X., C. Li, H. K. Sun, C. H. Nightingale, and D. P. Nicolau. 2005. A sensitive assay of amoxicillin in mouse serum and broncho-alveolar lavage fluid by liquid-liquid extraction and reversed-phase HPLC. J. Pharm. Biomed. Anal. 39:648-652. [DOI] [PubMed] [Google Scholar]
- 8.Ellis-Grosse, E. J., T. Babinchak, N. Dartois, et al. 2005. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin. Infect. Dis. 41(Suppl. 5):S341-S353. [DOI] [PubMed] [Google Scholar]
- 9.Klevens, R. M., M. A. Morrison, J. Nadle, et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763. [DOI] [PubMed] [Google Scholar]
- 10.Koomanachai, P., A. Kim, and D. P. Nicolau. 2009. Pharmacodynamic evaluation of tigecycline against Acinetobacter baumannii in a murine pneumonia model. J. Antimicrob. Chemother. 63:982-987. [DOI] [PubMed] [Google Scholar]
- 11.Li, C., C. A. Sutherland, C. H. Nightingale, and D. P. Nicolau. 2005. Quantitation of tigecycline, a novel glycylcycline [corrected] by liquid chromatography. J. Chromatogr. B 819:201. [DOI] [PubMed] [Google Scholar]
- 12.Meagher, A. K., J. A. Passarell, K. Liolios, et al. 2007. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 51:1939-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muralidharan, G., M. Micalizzi, J. Speth, et al. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicasio, A. M., J. L. Crandon, and D. P. Nicolau. 2009. In vivo pharmacodynamic profile of tigecycline against phenotypically diverse Escherichia coli and Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 53:2756-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saner, F. H., M. Heuer, P. M. Rath, et al. 2006. Successful salvage therapy with tigecycline after linezolid failure in a liver transplant recipient with MRSA pneumonia. Liver Transpl. 12:1689-1692. [DOI] [PubMed] [Google Scholar]
- 16.Sun, H. K., C. T. Ong, A. Umer, et al. 2005. Pharmacokinetic profile of tigecycline in serum and skin blister fluid of healthy subjects after multiple intravenous administrations. Antimicrob. Agents Chemother. 49:1629-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaseanu, C., C. Bergallo, O. Teglia, et al. 2008. Integrated results of 2 phase 3 studies comparing tigecycline and levofloxacin in community-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 61:329-338. [DOI] [PubMed] [Google Scholar]
- 18.van Ogtrop, M. L., D. Andes, T. J. Stamstad, et al. 2000. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 44:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wart, S. A., J. S. Owen, E. A. Ludwig, et al. 2006. Population pharmacokinetics of tigecycline in patients with complicated intra-abdominal or skin and skin structure infections. Antimicrob. Agents Chemother. 50:3701-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]