Abstract
9-(S)-[3-Hydroxy-2-(phosphonomethoxy)propyl]adenine [(S)-HPMPA] has been reported to have antischistosomal activity. Ether lipid esters of (S)-HPMPA and cidofovir (CDV) have greatly increased activities in antiviral assays and in lethal animal models of poxvirus diseases. To see if ether lipid esters of CDV and (S)-HPMPA enhance antischistosomal activity, we tested their alkoxyalkyl esters using Schistosoma mansoni worm killing in vitro. Hexadecyloxypropyl (HDP)-cyclic-(S)-HPMPA and HDP-cyclic-CDV exhibited significant in vitro antischistosomal activities and may offer promise alone or in combination with praziquantel.
Schistosomiasis is the second most prevalent parasitic disease worldwide after malaria, with about 200 million human beings infected in 74 countries. It is estimated that 779 million people are at risk of contracting schistosomiasis and more than 200 million individuals are infected, with more than half of them suffering from disease-associated symptoms (18, 29, 34). Severe disease manifestations are exhibited in about 20 million individuals (30). The annual mortality rate due to schistosomiasis in sub-Saharan Africa might be as high as 280,000 (31). Chemotherapeutic measures have been the mainstay for control of schistosomiasis (12), and since the 1970s, praziquantel (PZQ) has become the drug of choice against the three major human species of schistosomes, Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum (10, 13). PZQ is a relatively safe, orally administered drug that leads to reduction in the prevalence of schistosomiasis (3, 28). Mass drug administration programs currently rely heavily on PZQ for the control of schistosome-induced morbidity. However, with only one drug of choice for treatment and with the possibility of development of parasite resistance, the present situation is dangerous. There is a real and pressing need for discovering alternatives to the only available antischistosomal drug worldwide (5).
Acyclic nucleoside phosphonates are a group of biologically active compounds which have been developed primarily as antivirals (15). The S-enantiomer of 9-(3-hydroxy-2-phosphonyl-methoxypropyl)adenine [(S)-HPMPA] is of particular interest because it has a broad spectrum of antiviral activity (8) as well as in vivo activity against Plasmodium falciparum and Plasmodium berghei (murine models for human malaria) (27). The compound showed a trypanocidal activity against all extracellular trypanosomes and some of the intracellular hemoflagellates (9). We previously reported antischistosomal activity for (S)-HPMPA (4), as it caused significant reductions in vivo in worm loads, tissue egg loads, and the frequency of egg developmental stages. Most prominently, (S)-HPMPA treatment resulted in the nearly complete disappearance of mature and immature eggs (4). In vitro, (S)-HPMPA did not significantly affect the muscle tension of S. mansoni worms regardless of the concentration tested (4). In this report we have evaluated the antischistosomal activity of various alkoxyalkyl esters of (S)-HPMPA, CDV, and other acyclic nucleoside phosphonate compounds to assess their potential antischistosomal activities.
Chemistry.
Analogs of (S)-HPMPA, including the 3-(hexadecyloxy)propyl 9-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine (HDP) and 2-(octadecyloxy)ethyl 9-(S)-[3-hydroxy-2-(phosphono-methoxy)propyl]adenine (ODE) esters as well as the HDP ester of cyclic-(S)-HPMPA were tested in vitro for their potential antischistosomal activity based on adult Schistosoma mansoni worm killing. Also tested were HDP-cyclic CDV, ODE-HPMPG, ODE-HPMP-diaminopurine (ODE-HPMPDAP), praziquantel (PZQ), and dimethyl sulfoxide (DMSO) controls. HDP-(S)-HPMPA and ODE-(S)-HPMPA were synthesized as described previously (2). HDP-(S)-HPMPA was cyclized by reaction with N,N-dicyclohexylcarbodiimide and purified by silica gel column chromatography. HDP-cyclic-CDV was synthesized as described by Beadle et al. (1), and the ODE esters of diaminopurine and guanosine were synthesized and purified as described by Valiaeva et al. (30a). All compounds were greater than 98% pure as determined by 1H nuclear magnetic resonance, 31P nuclear magnetic resonance, elemental combustion analysis, and liquid chromatography/mass spectrometry.
Antischistosomal activities of test compounds based on in vitro schistosome worm killing.
Syrian golden hamsters (Mesocrietus auratus) weighing 100 to 120 g were obtained from the Schistosome Biological Supply Center, Theodor Bilharz Research Institute. Schistosoma mansoni cercariae (Egyptian strain CD) were used to infect the hamsters with 350 cercariae each by abdominal skin exposure (21). Praziquantel (Shin Poong Pharmaceutical Co., South Korea) and the respective acyclic nucleoside phosphonate analogs were prepared as 5 mM stock solutions in aqueous DMSO. Immediately before use, the stock solutions were diluted with complete medium to the concentrations indicated. S. mansoni-infected hamsters were sacrificed and worms harvested from the portomesenteric vessels (11). Twelve to 16 worms were placed in duplicate petri dishes, and fresh RPMI 1640 medium (glutamine, 20% fetal calf serum, and antibiotics [streptomycin, penicillin, and gentamicin]) containing the indicated concentration of test compounds was added. The worms were incubated overnight in a CO2 incubator, washed thrice with saline, and fresh medium without drug was added and the incubation was continued overnight in the CO2 incubator. On the second day, worm motility was observed and the medium was again changed and the incubation continued. On day 5 the numbers of living and dead worms were recorded. Negative controls using pure medium alone or medium with DMSO and positive control media containing various concentrations of praziquantel were similarly evaluated. At the end of the observation period worms were examined in a laminar flow hood for their motility and appearance by using a stereomicroscope, and the final recording of percent worm mortality was assessed (the number of dead worms [contracted and opaque] relative to the total number of worms).
S. mansoni killing results.
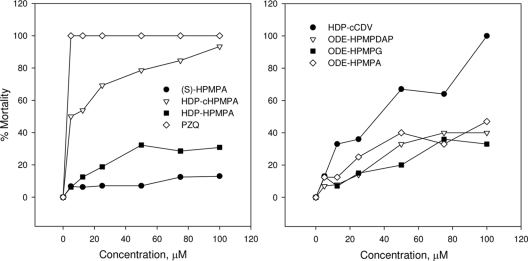
The percentages of Schistosoma mansoni worm killing in vitro under the influences of different test compounds at different concentrations versus untreated and DMSO negative controls and positive controls treated with PZQ were determined (Table 1 and Fig. 1). Controls and DMSO-treated controls had no observed mortality. PZQ was the most effective compound studied, with 100% worm mortality found between 5 and 100 μM drug. (S)-HPMPA was the least active compound, with a 50% effective concentration (EC50) of >100 μM. Covalent addition of an HDP ester group increased the antischistosomal activity somewhat to a mortality of 30.8% at 100 μM, the highest concentration tested. The most active compound was the HDP ester of cyclic-(S)-HPMPA, which had an EC50 of 5.0 μM and achieved 93.3% mortality at 100 μM. The progressive increases in antischistosomal activity caused by successive modifications of (S)-HPMPA by HDP esterification and cyclization can be appreciated best by examination of the left panel of Fig. 2 and Table 2.
TABLE 1.
Schistosoma mansoni worm mortality versus concentration of ether lipid esters of acyclic nucleoside phosphonates
| S. mansoni treatment group | % Worm mortality (no. dead/total exposed) at indicated phosphonate concn (μM) |
EC50 | |||||
|---|---|---|---|---|---|---|---|
| 100 | 75 | 50 | 25 | 12.5 | 5 | ||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | |
| Praziquantel | 100 | 100 | 100 | 100 | 100 | 100 | 0.22 |
| HDP-cyclic-(S)-HPMPA | 93.3 (14/15) | 84.6 (11/13) | 78.6 (11/14) | 69.2 (9/13) | 53.8 (7/13) | 50 (8/16) | 5.0 |
| HDP-(S)-HPMPA | 30.8 (4/13) | 28.6 (4/14) | 32.3 (10/31) | 18.8 (3/16) | 12.5 (2/16) | 6.25 (1/16) | >100 |
| ODE-(S)-HPMPA | 47 (7/15) | 33 (5/15) | 40 (6/15) | 25 (4/16) | 12.5 (2/16) | 12.5 (2/16) | >100 |
| ODE-(S)-HPMPG | 33 (5/15) | 36 (5/14) | 20 (3/15) | 15 (2/13) | 7 (1/14) | 13 (2/15) | >100 |
| ODE-HPMP-DAP | 40 (6/15) | 40 (6/15) | 33 (4/12) | 14 (2/14) | 8 (1/12) | 7 (1/14) | >100 |
| HDP-cyclic-CDV | 100 (15/15) | 64 (9/14) | 67 (8/12) | 36 (5/14) | 33 (5/15) | 13 (2/15) | 28.0 |
| (S)-HPMPA | 13 (2/15) | 12.5 (2/16) | 7 (1/14) | 7 (1/14) | 6.25 (1/16) | 6.7 (1/15) | >100 |
FIG. 1.
Structures of selected analogs of (S)-HPMPA and CDV.
FIG. 2.
Effects of (S)-HPMPA and HDP-(S)-HPMPA, HDP-(S)-cyclic-HPMPA, and PZQ (left) and HDP-cyclic-CDV, ODE-HPMPA, ODE-HPMPG, and ODE-HPMPDAP (right) on adult schistosome worm mortality following a 24-h exposure in vitro. Results are expressed as the percent mortality in groups of 13 to 16 worms exposed to drugs for 24 h as described in the text.
TABLE 2.
Effects of phosphonate negative charge on antischistosomal activities of (S)-HPMPA and two analogs
| Compound | Charge | EC50 (mM) | Max. % worm mortality |
|---|---|---|---|
| (S)-HPMPA | −2 | >100 | 13.3 |
| HDP-(S)-HPMPA | −1 | >100 | 30.8 |
| HDP-cyclic-(S)-HPMPA | 0 | 5.0 | 93.3 |
ODE esters of (S)-HPMPA, (S)-HPMPG, and (S)-HPMPDAP had moderate activities similar to that of HDP-(S)-HPMPA, with worm mortality of 33% to 47% at the highest concentration tested. HDP-cyclic-CDV showed substantial antischistosomal activity, with an EC50 of 28 μM and 100% mortality at 100 μM (Table 2 and Fig. 2, right panel). All acyclic nucleoside phosphonate analogs were less active than PZQ, which had an EC50 of 0.22 μM.
Discovery of new antischistosomal drugs depends on both in vitro whole parasite screens and S. mansoni-infected animal models of disease. The in vitro worm killing screen is advantageous because it allows rapid screening of many compounds at several drug concentrations. ODE-(S)-HPMPA and ODE-CDV were previously shown to have greatly increased antiviral activities versus unmodified (S)-HPMPA and CDV (2, 6, 14, 16, 17, 19, 20, 22-26, 32, 35) primarily due to greatly increased cell uptake and conversion to the active metabolite (7). In the S. mansoni worm killing assay, HDP-(S)-HPMPA and ODE-(S)-HPMPA were marginally more active than unmodified (S)-HPMPA, but the increase in activity was only two- to fourfold instead of the multiple log10 increases in antiviral activity noted against human immunodeficiency virus type 1, vaccinia virus, cowpox virus, and human cytomegalovirus (1, 2, 16, 17, 32). The order of antischistosomal activity appears to be related to the negative charges on the phosphonate moiety (Table 2). (S)-HPMPA (two negative charges) and HDP-(S)-HPMPA (one negative charge) have EC50s of >100, while HDP-cyclic-(S)-HPMPA (neutral) has an EC50 of 5 μM. If one compares the percent mortality at 100 μM drug, the values are as follows: (S)-HPMPA, 13.3%; HDP-(S)-HPMPA, 30.8%; HDP-cyclic-(S)-HPMPA, 93.3% (Table 2). This is in contrast with the antiviral activity of this type of analog where the open form, i.e., HDP-CDV, is more active than the cyclic compound (1, 32).
In conclusion, HDP-cyclic-(S)-HPMPA and HDP-cyclic-CDV exhibit substantial antischistosomal activities as judged by in vitro worm killing. It would be of interest to examine the in vivo effects of these compounds in S. mansoni-infected animals, because a previous study with (S)-HPMPA found promising in vivo activity (4).
Acknowledgments
These studies were supported in part by the Theodor Bilharz Research Institute (to S. Botros) and by the National Institute of Allergy and Infectious Disease, AI-071803 and AI-074057, and the San Diego Veterans Medical Research Foundation (to K. Y. Hostetler).
Footnotes
Published ahead of print on 24 August 2009.
REFERENCES
- 1.Beadle, J. R., N. Rodriquez, K. A. Aldern, C. Hartline, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication, in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beadle, J. R., W. B. Wan, S. L. Ciesla, K. A. Keith, C. Hartline, E. R. Kern, and K. Y. Hostetler. 2006. Synthesis and antiviral evaluation of alkoxyalkyl derivatives of 9-(S)-(3-hydroxy-2-phosphono-methoxypropyl)adenine against cytomegalovirus and orthpoxviruses. J. Med. Chem. 49:2010-2015. [DOI] [PubMed] [Google Scholar]
- 3.Blas, B. L., M. I. Rosales, I. L. Lipayon, K. Yasuraoka, H. Matsuda, and M. Hiyashi. 2004. The schistosomiasis problem in the Philippines: a review. Parasitol. Int. 53:127-134. [DOI] [PubMed] [Google Scholar]
- 4.Botros, S., S. William, O. Hammam, Z. Zidek, and A. Holý. 2003. Activity of 9-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine against Schistosomiasis mansoni in mice. Antimicrob. Agents Chemother. 47:3853-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botros, S., and J. Bennett. 2007. Praziquantel resistance. Expert Opin. Drug Discov. 2(Suppl. 1):535-540. [DOI] [PubMed] [Google Scholar]
- 6.Buller, R. M., G. Owens, J. Schriewer, L. Melman, J. R. Beadle, and K. Y. Hostetler. 2004. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology 318:474-481. [DOI] [PubMed] [Google Scholar]
- 7.Ciesla, S. L., J. Trahan, K. L. Winegarden, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 59:163-171. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq, E. 1991. Broad-spectrum anti-DNA virus and anti-retrovirus activity of phosphonomethoxyalkylpurines and -pyrimidines. Biochem. Pharmacol. 42:963-972. [DOI] [PubMed] [Google Scholar]
- 9.de Vries, E., J. G. Stam, F. F. Franssen, H. Niewenhuijs, P. Chavalitshewinkoon, E. De Clercq, J. P. Overdulve, and P. C. van der Vliet. 1991. Inhibition of the growth of Plasmodium falciparum and Plasmodium berghei by the DNA polymerase inhibitor HPMPA. Mol. Biochem. Parasitol. 47:43-50. [DOI] [PubMed] [Google Scholar]
- 10.Doenhoff, M. J., and L. Pica-Mattoccia. 2006. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev. Antiinfect. Ther. 4:199-210. [DOI] [PubMed] [Google Scholar]
- 11.Duvall, R. H., and W. B. DeWitt. 1967. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am. J. Trop. Med. Hyg. 16:483-486. [DOI] [PubMed] [Google Scholar]
- 12.Fenwick, A., and J. P. Webster. 2006. Schistosomiasis: challenges for control, treatment and drug resistance. Curr. Opin. Infect. Dis. 19:577-582. [DOI] [PubMed] [Google Scholar]
- 13.Gonnert, R., and P. Andrews. 1977. Praziquantel, a new broad spectrum antischistosomal agent. Z. Parasitenkd. 52:129-150. [DOI] [PubMed] [Google Scholar]
- 14.Hartline, C. B., K. M. Gustin, W. B. Wan, S. L. Ciesla, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2005. Activity of ether lipid ester prodrugs of acyclic nucleoside phosphonates against adenovirus replication in vitro. J. Infect. Dis. 191:396-399. [DOI] [PubMed] [Google Scholar]
- 15.Holý, A., I. Votruba, A. Merta, J. Cerný, J. Veselý, J. Vlach, K. Sedivá, I. Rosenberg, M. Otmar, and H. Hrebabecký. 1990. Acyclic nucleotide analogues: synthesis, antiviral activity and inhibitory effects on some cellular and virus-encoded enzymes in vitro. Antivir. Res. 13:295-311. [DOI] [PubMed] [Google Scholar]
- 16.Hostetler, K. Y., K. A. Aldern, W. B. Wan, S. L. Ciesla, and J. R. Beadle. 2006. Alkoxyalkyl esters of (S)-9-[3-hydroxy-3-(phosphonomethoxy)propyl]adenine are potent inhibitors of the replication of wild-type and drug-resistant human immunodeficiency virus type 1 in vitro. Antimicrob. Agents Chemother. 50:2857-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hostetler, K. Y. 2009. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antivir. Res. 82:A84-A98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keiser, J., and J. Utzinger. 2007. Advances in the discovery and development of trematocidal drugs. Expert Opin. 2(1):S9-S13. [DOI] [PubMed] [Google Scholar]
- 19.Keith, K. A., W. B. Wan, S. L. Ciesla, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2004. Inhibitory activity of alkoxyalkyl and alkyl esters of cidofovir and cyclic cidofovir against orthopoxvirus replication in vitro. Antimicrob. Agents Chemother. 48:1869-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang, Y. S., J. I. Bruce, and D. A. Boy. 1987. Laboratory cultivation of schistosome vector snails and maintenance of schistosome life cycle. Proc. First Sino-American Symp. 1:34-48. [Google Scholar]
- 22.Morrey, J. D., B. E. Korba, J. R. Beadle, D. L. Wyles, and K. Y. Hostetler. 2009. Alkoxyalkyl esters of 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl) adenine are potent and selective inhibitors of hepatitis B virus (HBV) replication in vitro and in HBV transgenic mice in vivo. Antimicrob. Agents Chemother. 53:2865-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Painter, G. R., and K. Y. Hostetler. 2004. Design and development of oral drugs for the prophylaxis and treatment of smallpox infection. Trends Biotechnol. 22:423-427. [DOI] [PubMed] [Google Scholar]
- 24.Quenelle, D. C., D. J. Collins, B. P. Herrod, K. A. Keith, J. Trahan, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2007. Effect of oral treatment with hexadecyloxypropyl-[(S)-9-(3-hydroxy-2-phosphonylmethoxy-propyl)adenine] [(S)-HPMPA] or octadecyloxy-ethyl-(S)-HPMPA on cowpox or vaccinia virus infections in mice. Antimicrob. Agents Chemother. 51:3940-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quenelle, D. C., D. J. Collins, K. Y. Hostetler, J. R. Beadle, W. B. Wan, and E. R. Kern. 2004. Oral treatment of cowpox and vaccinia infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quenelle, D. C., D. J. Collins, L. R. Pettway, C. B. Hartline, J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2008. Effect of oral treatment with (S)-HPMPA, HDP-(S)-HPMPA or ODE-(S)-HPMPA on replication of murine cytomegalovirus or human cytomegalovirus in animal models. Antivir. Res. 79:133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smeijsters, L. J., F. F. Franssen, L. Naesens, E. de Vries, A. Holý, J. Balzarini, E. De Clercq, and J. P. Overdulve. 1999. Inhibition of the in vitro growth of Plasmodium falciparum by acyclic nucleoside phosphonates. Int. J. Antimicrob. Agents 12:53-61. [DOI] [PubMed] [Google Scholar]
- 28.Southgate, V. R., D. Rollinson, L. A. Tchuem Tchuente, and P. Hagan. 2005. Towards control of schistosomiasis in sub-Saharan Africa. J. Helminthol. 79:181-185. [DOI] [PubMed] [Google Scholar]
- 29.Steinmann, P., J. Keiser, R. Bos, M. Tanner, and J. Utzinger. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis and estimates of people at risk. Lancet Infect. Dis. 6:411-425. [DOI] [PubMed] [Google Scholar]
- 30.Utzinger, J., and J. Keiser. 2004. Schistosomiasis and soil-transmitted helminthiasis: common drugs for treatment and control. Expert Opin. Pharmacother. 5:263-285. [DOI] [PubMed] [Google Scholar]
- 30a.Valiaeva, N., M. N. Prichard, R. M. Buller, J. R. Beadle, C. B. Hartline, J. Schriewer, J. Trahan, and K. Y. Hostetler. Antiviral evaluation of octadecyloxyethyl esters of (S)-3-hydroxy-2-(phosphonomethoxy)propyl nucleosides against herpesviruses and orthopoxviruses. Antivir. Res., in press. [DOI] [PMC free article] [PubMed]
- 31.Van der Werf, M. J., S. J. de Vlas, S. Brooker, C. W. Looman, N. J. Nagelkerke, J. D. Habbema, and D. Engels. 2003. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 86:125-139. [DOI] [PubMed] [Google Scholar]
- 32.Wan, W. B., J. R. Beadle, C. B. Hartline, E. R. Kern, S. L. Ciesla, N. Valiaeva, and K. Y. Hostetler. 2005. Comparison of the antiviral activity of alkoxyalkyl and alkyl esters of cidofovir against human and murine cytomegalovirus replication in vitro. Antimicrob. Agents Chemother. 49:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. 1993. The control of schistosomiasis. Second report of the W.H.O. Expert Committee. World Health Organization, Geneva, Switzerland. [PubMed]
- 34.World Health Organization. 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a W.H.O. expert committee. WHO Tech. Rep. Ser. 912. World Health Organization, Geneva, Switzerland. [PubMed]
- 35.Wyles, D. L., K. A. Kaihara, B. E. Korba, R. T. Schooley, J. R. Beadle, and K. Y. Hostetler. 2009. The octadecyloxyethyl ester of (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine is a potent and selective inhibitor of hepatitis C virus replication in genotype 1A, 1B and 2A replicons. Antimicrob. Agents Chemother. 53:2660-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]