Abstract
After ertapenem was added to the formulary of a 344-bed community teaching hospital, we retrospectively studied its effect on antimicrobial utilization and on the in vitro susceptibility of various antimicrobial agents against Pseudomonas aeruginosa. Three study periods were defined as preintroduction (months 1 to 9), postintroduction but before the autosubstitution of ertapenem for ampicillin-sulbactam (months 10 to 18), and after the policy of autosubstitution (months 19 to 48) was initiated. Ertapenem usage rose slowly from introduction to a range of 36 to 48 defined daily doses/1,000 patient days (DDD) with a resultant decrease in ampicillin-sulbactam usage due to autosubstitution. Imipenem usage peaked 6 months after the introduction of ertapenem and started to decline coincidently with the increased use of ertapenem. During the second period, imipenem usage decreased (slope = −1.28; P = 0.002). Prior to the introduction of ertapenem, the susceptibility of P. aeruginosa to imipenem increased from 61 to 81% at month 7 but then decreased slightly to 67% at month 9. After the introduction of ertapenem, susceptibility continued to increase; the increasing trend was significant (slope = 1.74; P < 0.001). In the third period, the median susceptibility (interquartile range) was 88% (82 to 95%). This change appeared related to decreased imipenem usage. For every unit decrease in the monthly DDD of imipenem, there was an increase of 0.38% (P = 0.008) in the susceptibility of P. aeruginosa to imipenem in the same month. Ertapenem was effective in our antimicrobial stewardship program and may have helped improve the P. aeruginosa antimicrobial susceptibility to imipenem by decreasing the unnecessary usage and selective pressure of antipseudomonal agents.
The development of multidrug-resistant gram-negative pathogens driven by antimicrobial selective pressures has become an increasing concern in hospitalized patients (1, 14, 16). For Enterobacteriaceae, β-lactamases such as the extended-spectrum β-lactamases (ESBLs) and plasmid and chromosomal AmpCs are probably the most important resistance mechanisms, although other extended-spectrum class A enzymes, such as KPC, also are emerging (1). For P. aeruginosa, chromosomal AmpC, permeability changes (OprD), and the multidrug efflux pumps are probably the determining factors. Metallo-β-lactamases are distinctly unusual in Enterobacteriaceae and P. aeruginosa in the United States but are a more important problem in other geographic areas (10). The development, spread, and persistence of these resistance mechanisms complicates the selection of antimicrobial therapy when trying to avoid the increased selective pressures caused by the utilization of any one class of antimicrobial agents. Consequently, the Infectious Diseases Society of America has issued guidelines to enhance antimicrobial stewardship programs (4), with goals that include minimizing the emergence of resistance by the appropriate use of antimicrobials.
The increased importance of ESBL resistance in Escherichia coli and Klebsiella species has necessitated the use of ESBL-stable β-lactams like ertapenem (15, 18). The FDA approved ertapenem, a carbapenem (19) without Pseudomonas aeruginosa activity, for use against community-acquired pneumonia, complicated intraabdominal infections, pelvic infections, complicated urinary tract infections, and skin and soft-tissue infections, including diabetic foot infections. However, concern as to whether ertapenem might adversely affect the activity of the carbapenems imipenem and meropenem against P. aeruginosa has been a barrier for its use.
Studies performed prior to ertapenem's approval attempted to define risk factors for Pseudomonas resistance (6, 11). Lautenbach et al. (11) found not only a higher mortality rate (31.1%) for patients infected with an imipenem-resistant P. aeruginosa than for those infected with imipenem-susceptible strains (16.7%) but also concluded that the only independent risk factor for imipenem resistance was fluoroquinolone use. Fortaleza et al. (6) evaluated the problem of P. aeruginosa resistance and concluded that imipenem, amikacin, and vancomycin use were associated with imipenem-resistant P. aeruginosa. Patterson (16) has reviewed the various conflicting studies of this topic and concludes that “the lack of alternative agents on the horizon that are active against gram-negative bacteria makes our efforts at controlling emergence of resistance all the more imperative.” Because we felt the addition of ertapenem to our formulary would have cost savings and clinical utility but were concerned about collateral damage, we retrospectively studied the effect of the addition of ertapenem on antimicrobial utilization and on the in vitro activity of imipenem, ertapenem, levofloxacin, cefepime, tobramycin, and piperacillin-tazobactam on P. aeruginosa.
(Parts of this study were presented previously at the 44th Annual Meeting of the Infectious Diseases Society of America, Toronto, Canada, 2006 [3].)
MATERIALS AND METHODS
Study design.
We retrospectively collected and analyzed data on the antimicrobial usage (defined daily dose/1,000 patient days [DDD]) and antimicrobial susceptibility for P. aeruginosa in a 344-bed community teaching hospital from January 2002 to December 2005. The hospital has a 51-bed oncology unit that is involved in research programs, a 32-bed intensive care unit, and a 32-bed stepdown unit. The average daily census for the study period was approximately 200; the average length of stay varied from 4.6 to 9.1 days, with an average stay of 5 days, excluding newborns and outliers. Ertapenem was added to the formulary in September 2002, and in July 2003 a policy of autosubstituting ertapenem for ampicillin-sulbactam was instituted because of increased (40%) E. coli resistance to ampicillin-sulbactam and because of its relatively high cost. (Ampicillin-sulbactam was ∼$45/day, not including nursing and pharmacy time or the bag and intravenous administration setup materials for three to four daily administrations, while ertapenem was ∼$45/day with one administration per day). The policy of reminding physicians to voluntarily substitute cefotaxime for cefepime if no P. aeruginosa was isolated from cultures after 72 h of empirical therapy was continued. No other antimicrobial-prescribing restrictions were instituted, and no other new agents were added to the formulary during the study period. No unusual infection control measures were instituted during this period.
Statistical analysis.
To investigate the trends in antibiotic usage in the susceptibility of P. aeruginosa, we first defined three periods in the 48-month study. The first period, months 1 to 9, was prior to the introduction of ertapenem to the formulary. During the second period, months 10 to 19, ertapenem was in the formulary but there was no required substitution for ampicillin-sulbactam. The last period, months 20 to 48, was after the autosubstitution policy was implemented.
The impact of introducing ertapenem to the hospital formulary on the usage of imipenem and on the susceptibility of P. aeruginosa to imipenem was evaluated by the use of time-series analyses. Time-series analysis estimates regression models while relaxing the assumption that observations are independent by estimating the serial correlation or autocorrelation among observations collected over time (e.g., antibiotic usage and susceptibility across different periods) (21). An autoregressive error model that corrects for serial correlation was built using PROC AUTOREG in SAS (version 8) for Windows (20). The Yule-Walker method proposed by Gallant and Goebel (7) was used as the estimation method.
A segmented model was used to assess the changes in trends (i.e., slopes) of the usage of imipenem and the susceptibility of P. aeruginosa to imipenem in each of the three periods. The trend is defined as the slope of the response (i.e., antimicrobial usage or susceptibility) over time (x unit change for every 1 month change of time). Similar methods were applied to evaluate changes in the trends of the usage of levofloxacin, cefepime, and piperacillin-tazobactam and the susceptibility of P. aeruginosa to these agents, except that data for the second and third periods were combined. To model the susceptibility of P. aeruginosa to imipenem, initially we included the current and historical (last 13 months) usage of imipenem and ertapenem and the time in the model. Only statistically significant (α = 0.05) predictors of the outcome variable were kept in the final model; thus, the variables in the model were the current use of imipenem and the current month.
RESULTS
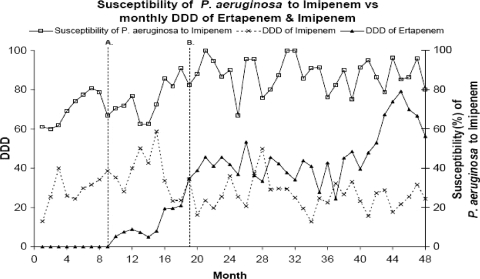
Table 1 and Fig. 1 show the monthly usage (DDD) of imipenem and ertapenem and the susceptibility of P. aeruginosa to imipenem during the 48 months of observation. The usage of ertapenem increased slowly from its introduction to the formulary in month 10 through month 15 (DDD = 8), and then usage increased more sharply. Prior to the implementation of the policy of autosubstituting ertapenem for ampicillin-sulbactam, the DDD was 35. Subsequently through month 42, usage was relatively consistent, generally in the range of 36 to 48 DDD. Toward the end of the observation period (months 43 to 48), ertapenem usage increased and then began to decrease again.
TABLE 1.
Usage of ertapenem and imipenem and susceptibility of P. aeruginosa to imipenem
| Period | Months | Ertapenem usage (DDD) |
Imipenem usage (DDD) |
Susceptibility (%) of P. aeruginosa to imipenem |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentilea |
Range | Percentilea |
Range | Percentilea |
Range | ||||||||
| 25 | 50 | 75 | 25 | 50 | 75 | 25 | 50 | 75 | |||||
| Prior to ertapenem usage | 0-9 | 0 | 0 | 0 | 0 | 25 | 30 | 34 | 13-40 | 62 | 69 | 77 | 60-81 |
| After addition of ertapenem to formulary | 10-19 | 7 | 8 | 20 | 5-35 | 28 | 35 | 43 | 23-59 | 71 | 75 | 82 | 63-91 |
| After policy substitution | 20-48 | 39 | 44 | 53 | 24-79 | 22 | 25 | 29 | 13-50 | 82 | 88 | 95 | 67-100 |
50th percentile is the median; 25th and 75th percentiles are the first and third quartiles (interquartile range), respectively.
FIG. 1.
Usage (DDD) of imipenem and ertapenem and susceptibility of P. aeruginosa to imipenem. Line A indicates the introduction of ertapenem to the formulary. Line B indicates the implementation of the policy of substituting ertapenem for ampicillin-sulbactam.
In the 9 months of observation prior to the introduction of ertapenem, the usage of imipenem increased significantly (13 to 39 DDD; slope = 3.18; P < 0.001). Imipenem usage peaked in month 15 (DDD = 59) and then started to decline (the slope during the second period was −1.28; P = 0.002), which is coincident in time with the sharper increase in the use of ertapenem. Overall, usage declined significantly during the second period (the change of slope was −4.46; P < 0.001). During the third period, imipenem usage was relatively constant, generally between 22 and 29 DDD. Contrary to the trend of increasing the use of imipenem prior to the introduction of ertapenem, there was a significant decrease in the use of imipenem when ertapenem became available.
Prior to the introduction of ertapenem, the susceptibility of P. aeruginosa to imipenem increased from 61 to 81% at month 7 but then decreased slightly to 67% at month 9 (slope = 0.60; P = 0.51). After the introduction of ertapenem (months 10 to 19), susceptibility continued to increase and was above 80% for the last 4 months; the increasing trend was significant (slope = 1.74; P < 0.001); however, the change in the rate of increase (the change in the slope was 1.14) was not statistically significant (P = 0.36). In the third period (months 20 to 48), there was no consistent increasing or decreasing trend (slope = 0.02; P = 0.85) in susceptibility. The median susceptibility (interquartile range) was 88% (82 to 95%). For 2006 to 2008, ∼87% of our P. aeruginosa isolates (∼300/year) remained susceptible to imipenem with continued ertapenem usage (data not shown).
During the study periods, there was a decreased use of imipenem and an increased use of ertapenem (Table 1). This resultantly decreased use of imipenem was statistically significantly related to the improved susceptibility of P. aeruginosa to imipenem (Table 1). For every unit decrease in the monthly DDD of imipenem, there was an increase of 0.38% (P = 0.008) in the susceptibility of P. aeruginosa to imipenem in the same month.
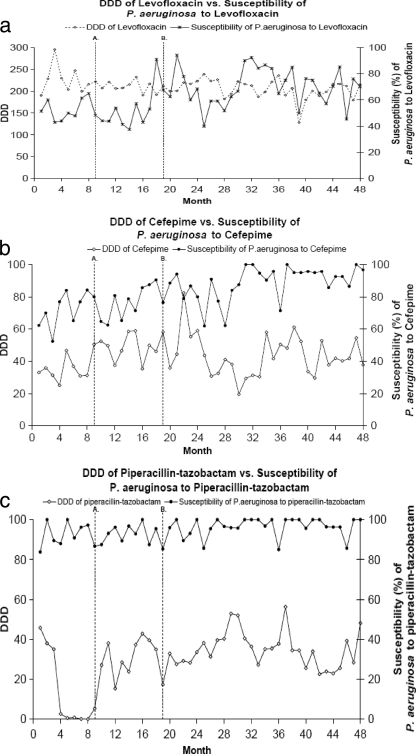
The use of levofloxacin generally was constant throughout the study period; the susceptibility of P. aeruginosa to levofloxacin tended to increase (slope = 0.53; P = 0.021) after the introduction of ertapenem (Fig. 2a). The use of cefepime generally was constant throughout the study period using the three-period model; cefepime use was increasing during the first period (before ertapenem was added, slope = 2.70 and P = 0.006), while use during the second period was flat. The susceptibility of P. aeruginosa to cefepime tended to increase (slope = 0.54; P < 0.0001) after the introduction of ertapenem (Fig. 2b). Cefoxitin and piperacillin-tazobactam usage were affected by distributor/manufacturer supply shortages for brief periods; otherwise, their usage was constant. The susceptibility of P. aeruginosa to piperacillin-tazobactam became more stable and tended to increase slightly after ertapenem was added (slope = 0.14; P = 0.040) due to less variability (Fig. 2c). While the susceptibility of P. aeruginosa to these agents tended to increase slightly during the study period, the increasing trends began before the introduction of ertapenem.
FIG. 2.
Usage (DDD) and susceptibility of P. aeruginosa to levofloxacin (a), cefepime (b), and piperacillin-tazobactam (c). Line A indicates the introduction of ertapenem to the formulary. Line B indicates the implementation of the policy of substituting ertapenem for ampicillin-sulbactam.
Against E. coli, P. mirabilis, Klebsiella species, and Enterobacter cloacae, there was either no change or minor changes in susceptibilities to the various agents, including imipenem, subsequently to the inclusion of ertapenem in the formulary (data not shown). All of the aforementioned species remained 100% susceptible to ertapenem. E. coli susceptibility to levofloxacin declined from 90 to 83%. ESBL enzymes (data not shown) were present in 3% of Klebsiella species isolates in 2002 prior to the introduction of ertapenem, but the level fell to 2% in 2005. ESBL enzymes were present in 1% of E. coli isolates without significant change. These rates remained constant during 2006 and 2007 (data not shown).
DISCUSSION
While studies often yield diverse and conflicting results when attempting to define specific antimicrobial resistance risk factors (16, 17), selective antibiotic pressure generally is accepted as an important causal reason. For ESBL-producing strains and other multiresistant gram-negative bacteria, carbapenems are the current antimicrobial therapy of choice (9, 15). There is also a need for a nonpseudomonal carbapenem to diminish the selective pressure exerted by carbapenem use on P. aeruginosa.
The Infectious Diseases Society of America stewardship guidelines (4) have noted that “education alone, without incorporation of active intervention, is only marginally effective in changing antimicrobial prescribing practices and has not demonstrated a sustained effect (evidence grade B-II).” Our study suggests that adding ertapenem to our formulary was an effective antimicrobial management program tool. The increased ertapenem usage per se did not show a statistically significant impact on the imipenem susceptibility of P. aeruginosa; however, the increased ertapenem use was simultaneous with a decline in imipenem usage, and this decreased imipenem use paralleled the improved imipenem susceptibility of P. aeruginosa.
There are several limitations to our study, which include the limitation of group-level studies, the issue of using proportion versus incidence as the main outcome, and the limitation of a single-institution study. Time effects versus exposure effects, which are a potential problem, were adjusted for by our statistical methods.
Several other studies, most presented in abstract form, also have noted that the addition of ertapenem to a hospital formulary did not adversely affect the in vitro activity of imipenem and/or meropenem against P. aeruginosa. Crank et al. (3) reported that 2 years after the addition of ertapenem to the Rush University Medical Center in Chicago formulary, there was no effect on carbapenem resistance to P. aeruginosa. Goff et al. (8) also reported that P. aeruginosa susceptibility to imipenem remained at approximately 72% during the 4 years after the addition of ertapenem to the formulary at Ohio State University. Similarly, Carmeli et al. (2) retrospectively studied the effect of ertapenem on their formulary and found by multivariate analysis that ertapenem was not associated with a high incidence (P = 0.88) or increased proportion (P = 0.66) of imipenem-resistant P. aeruginosa, but imipenem and meropenem usage were associated with both a high incidence (P = 0.0014) and an increased proportion (P = 0.036) of imipenem-resistant P. aeruginosa strains. Our findings and those of the aforementioned studies are in accord with those of Livermore et al. (13), who reported that the selectivity of imipenem-resistant P. aeruginosa strains by ertapenem usage “should be minimal under clinical conditions.”
Recently, Lima et al. (12) evaluated the impact of ertapenem use for ESBL-Enterobacteriaceae infections at a tertiary-care university hospital in Brazil from March 2006 to February 2007. The use of ertapenem was mandated and substituted for imipenem for the treatment of these infections, unless there was a coinfection with a nonfermenting gram-negative aerobic bacillus. Imipenem use decreased 64.5% (from 46.3 to 16.1 DDD) during the study period, and ertapenem use rose to 42.57 DDD. During the study period, 1 of the 18 P. aeruginosa strains isolated was imipenem resistant, whereas 4/20 (20%) were resistant during the study of the prior year, which showed a trend but was not statistically significant. They speculated that increased ertapenem use “may have had a positive effect on the hospital ecology, with no evidence of resistance development associated with its use.”
Other potential benefits of ertapenem use may be on limiting collateral damage to the fecal flora, which can act as a reservoir of resistance. DiNubile et al. (5) reviewed the experience with ertapenem in two large multicenter, comparative trials (OASIS I and OASIS II) of complicated, community-acquired intraabdominal infections. Resistant Enterobacteriaceae organisms were significantly (P < 0.001) less likely to emerge in patients treated with ertapenem than in those treated with the comparator agents piperacillin-tazobactam or ceftriaxone plus metronidazole. No ertapenem-treated patient became fecally colonized with an ESBL-producing organism at the end of therapy, while this occurred in 2.1% of piperacillin-tazobactam- and 9.3% of ceftriaxone plus metronidazole-treated patients. These data are in accord with our study (data are not presented on ESBL-stable rates of incidence) and provide assurance that increased resistance, including that to ESBLs, in gram-negative rods is unlikely when ertapenem is used clinically in the hospital setting and when coupled with standard infection control practices.
Our study suggests that when coverage for P. aeruginosa is not required, as in most community-acquired infections, the use of Pseudomonas-sparing agents, such as ertapenem, reduces antibiotic pressure. It is possible that additional restrictions of other antipseudomonal agents, such as ceftazidime, cefepime, piperacillin-tazobactam, and fluoroquinolones, also help with decreasing Pseudomonas resistance. In our study, the addition of ertapenem to our formulary was an important component of our antimicrobial stewardship program and was cost-effective and helped improve our P. aeruginosa susceptibilities.
Acknowledgments
We thank Judee Knight and Alice E. Goldstein for various forms of assistance.
This study was supported in part by a grant from Merck & Co., Inc.
Ellie J. C. Goldstein is on the advisory boards of Merck, Bayer, Schering-Plough, GlaxoSmithKline, Optimer, and Theravance, and he is in the Speakers Bureau of Merck, Schering-Plough, GlaxoSmithKline, OrthoMcNiel, and Aventis. He received research support from Merck, Schering-Plough, GlaxoSmithKline, Optimer, Theravance, Aventis, OrthoMcNiel, Wyeth, and Pfizer. Shuang Lu is employed by Merck Research Laboratories and may own stock or stock options. Anne R. Meibohm formerly was employed by Merck Research Laboratories and may own stock or stock options.
Footnotes
Published ahead of print on 28 September 2009.
REFERENCES
- 1.Bratu, E. P., D. Landman, R. Haag, R. Recco, A. Eramo, M. Alam, and J. Quale. 2005. Rapid spread of single ribotype of KPC-producing, carbapenems-resistant Klebsiella pneumoniae in New York City. Ann. Intern. Med. 165:1430-1435. [DOI] [PubMed] [Google Scholar]
- 2.Carmeli, Y., S. Lidji, E. Shsbsti, S. Navon-Venezia, and M. J. Schwaber. 2007. The effect of group 1 versus group 2 carbapenems on imipenem resistant P. aeruginosa: ecological study, abstr. K-396, p. 315. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 3.Crank, C. W., B. Hota, and J. Segreti. 2006. Effect of ertapenem utilization on Pseudomonas aeruginosa susceptibility to imipenem, abstr. 285, p. 99. Abstr. 44th Ann. Meet. Inf. Dis. Soc. Am.
- 4.Dellit, T. H, R. C. Owens, J. E. McGowan, Jr., D. N. Gerding, R. A. Weinstein, J. Burke, W. C. Huskins, D. L. Paterson, N. O. Fishman, C. F. Carpenter, P. J. Brennan, M. Billeter, and T. M. Hooton. 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 44:159-177. [DOI] [PubMed] [Google Scholar]
- 5.DiNubile, M. J., I. Friedland, C. Y. Chan, M. R. Motyl, H. Giezek, M. Shivaprakash, R. A. Weinstein, and J. P. Quinn. 2005. Bowel colonization with resistant gram-negative bacilli after antimicrobial therapy of intra-abdominal infections: observations from two randomized comparative clinical trials of ertapenem therapy. Eur. J. Clin. Microbiol. Infect. Dis. 24:443-449. [DOI] [PubMed] [Google Scholar]
- 6.Fortaleza, C. M., M. P. Freire, D. de C. Filho, and M. de Caravalho Ramos. 2006. Risk factors for the recovery of imipenem- or ceftazidime-resistant Pseudomonas aeruginosa among patients admitted to a teaching hospital in Brazil. Infect. Control Hosp. Epidemiol. 27:901-906. [DOI] [PubMed] [Google Scholar]
- 7.Gallant, A. R., and J. J. Goebel. 1976. Nonlinear regression with autoregressive errors. J. Am. Stat. Assoc. 71:961-967. [Google Scholar]
- 8.Goff, D. A., and J. E. Mangino. 2008. Ertapenem: no effect on aerobic gram-negative susceptibilities to imipenem. J. Infect. 57:123-127. [DOI] [PubMed] [Google Scholar]
- 9.Hyle, E. P., A. D. Lipworth, T. E. Zaoutis, I. Nachamkin, W. B. Bilker, and E. Lautenbach. 2005. Impact of inadequate initial antimicrobial therapy on mortality in infections sue to extended-spectrum B-lactamase-producing Enterobacteriaceae. Ann. Intern. Med. 165:1375-1380. [DOI] [PubMed] [Google Scholar]
- 10.Jones, R. N., D. J. Biedenbach, and A. C. Gales. 2003. Sustained activity and spectrum of selected extended-spectrum B-lactams (carbapenems and cefepime) against Enterobacter spp. and ESBL-producing Klebsiella spp: report from the SENTRY antimicrobial surveillance program (USA, 1997-2000). Int. J. Antimicrob. Agents 21:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Lautenbach, E., M. G. Weine, I. Nachamkin, W. B. Bilker, A. Sheridan, and N. O. Fishman. 2006. Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect. Control Hosp. Epidemiol. 27:893-900. [DOI] [PubMed] [Google Scholar]
- 12.Lima, A. L. L., P. R. Olivera, A. P. Paula, K. Dal-Paz, F. Rossi, and A. V. Zumiotti. 2009. The impact of ertapenem use on the susceptibility of Pseudomonas aeruginosa to imipenem: a hospital case study. Infect. Control Hosp. Epidemiol. 30:487-490. [DOI] [PubMed] [Google Scholar]
- 13.Livermore, D. M., S. Mushtaq, and M. Warner. 2005. Selectivity of ertapenem for Pseudomonas aeruginosa mutants cross-resistant to other carbapenems. J. Antimicrob. Chemother. 55:306-311. [DOI] [PubMed] [Google Scholar]
- 14.Neuhauser, M. M., R. A. Weinstein, R. Rydman, L. H. Danziger, G. Karam, and J. P. Quinn. 2003. Antibiotic resistance among gram-negative bacilli in US intensive care units. JAMA 289:885-888. [DOI] [PubMed] [Google Scholar]
- 15.Paterson, D. L. 2006. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control 34(Suppl. I):S20-S28. [DOI] [PubMed] [Google Scholar]
- 16.Patterson, J. E. 2006. Multidrug-resistant gram-negative pathogens: multiple approaches and measures for prevention. Infect. Control Hosp. Epidemiol. 27:889-892. [DOI] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., and J. Segreti. 2006. Overview of the epidemiological profile and laboratory detection of extended-spectrum β-lactamases. Clin. Infect. Dis. 42(Suppl. 4):S153-S163. [DOI] [PubMed] [Google Scholar]
- 18.Ramphal, R., and P. C. Ambrose. 2006. Extended-spectrum β-lactamases and clinical outcomes: current data. Clin. Infect. Dis. 42(Suppl. 4):S164-S172. [DOI] [PubMed] [Google Scholar]
- 19.Shah, P., and R. D. Isaacs. 2003. Ertapenem, the first of a new group of carbapenems. J. Antimicrob. Chemother. 52:538-542. [DOI] [PubMed] [Google Scholar]
- 20.SAS Institute. 1999. SAS/ETS 8 user's guide. SAS Institute, Cary, NC.
- 21.Shardell, M., A. D. Harris, S. S. El-Kamary, J. P. Furuno, R. R. Miller, and E. N. Perencevich. 2007. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin. Infect. Dis. 45:901-907. [DOI] [PubMed] [Google Scholar]