Abstract
Anti-Hantaan virus monoclonal antibody (AHM) is a murine monoclonal antibody against Hantaan virus being developed for the treatment of hemorrhagic fever with renal syndrome. The purpose of the present study was to describe the tolerance and pharmacokinetics of an intravenously administered single ascending dose of AHM in Chinese healthy volunteers. Four cohorts of 22 healthy subjects received AHM at 2.5 to 20 mg, and the results indicated that AHM was well tolerated. We established a highly sensitive, rapid, and accurate immunoassay for the kinetic analysis of AHM in serum. Serial blood samples were obtained after intravenous administration for up to 17 days. A one-compartment model was determined to best describe the disposition of AHM. The maximal level in serum and the area under the serum concentration-time curve were proportional to the doses. The mean clearance, the half-life, and the volume of distribution were constant, irrespective of the dose. AHM was slowly cleared and had a half-life of approximately 110 h. These data support the use of a treatment regimen in which AHM is given only once intravenously.
Hemorrhagic fever with renal syndrome (HFRS) is caused by different strains of hantavirus and is a serious and often lethal disease. The syndrome is characterized by the systemic involvement of the capillaries and small vasculature, resulting in capillary leakage and hemorrhagic manifestations. At present, HFRS is endemic in 28 of 31 provinces of the People's Republic of China and is reported to have been responsible for 1.2 million symptomatic infections and a cumulative 44,300 deaths from 1950 to 1997 (14). In China, HFRS is mainly caused by two strains, i.e., Seoul virus and Hantaan virus (HTNV), with the latter being responsible for the majority cases of HFRS in Asia and Europe (5). Although more than a quarter century has elapsed since its discovery and despite persistent efforts to develop antiviral drugs and vaccines, safe and effective therapeutic agents for the treatment and/or prophylaxis of HFRS are not available (1, 4). The recent development of monoclonal antibodies (MAbs) for the prophylaxis and therapy of respiratory syncytial virus infection has been remarkable (6). We believe that similar to the anti-respiratory syncytial virus antibody, a murine MAb directed against HTNV could be developed for clinical use for the treatment of HTNV infections. Therefore, the development of effective therapeutic antibodies, such as neutralizing MAbs directed against HTNV is important, as such antibodies may prove to be a life-saving treatment for HFRS.
Wang et al. were the first to establish and characterize the hybridoma cells that secrete MAbs against HTNV (9). Wei et al. (11, 12) reported that the MAb passively protected suckling mice from HTNV infection by transplacental passage and provided protective effects against HTNV in HTNV-challenged suckling mice. Xu et al. (13) demonstrated that the MAb which recognizes the HTNV G2 envelope glycoprotein had in vivo hemagglutination inhibition activity. Moreover, virus-neutralizing MAb provided in vivo protection of animals from HTNV infection. The results indicate that the anti-HTNV MAb (AHM) has anti-HTNV activity in vitro and in vivo and could be an effective candidate for the treatment of patients infected by HTNV. Thus, on the basis of the promising preliminary data, we carried out a randomized phase I, dose-escalation clinical study of an AHM with healthy human volunteers. The purpose of this trial was to assess the tolerability and to characterize the pharmacokinetics of AHM after the intravenous (i.v.) administration of a single dose to healthy subjects.
(This paper was presented in part at the IXth World Conference on Clinical Pharmacology and Therapeutics, Quebec City, Quebec, Canada, 27 July to 1 August 2008.)
MATERIALS AND METHODS
Ethics.
The study was approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology and was conducted in accordance with guidelines for good clinical practice in China, the Declaration of Helsinki (2000), and all applicable national and local regulations. All subjects were informed of the investigational nature of this study and signed an informed consent statement prior to the initiation of the study.
Subjects.
All subjects (Han nationality) enrolled in this study were local university students who met the following criteria: (i) a body mass index (BMI) of 18 to 24; (ii) for female subjects, no pregnancy and not in the menstrual phase; (iii) no smoking, drinking, or recreational drug use before or during the study; (iv) normal vital signs and laboratory physical findings, including normal electrocardiography and chest fluoroscopy findings; (v) no history of allergy or nervous or psychological disease; (vi) no current use of prescription drugs or participation in another clinical trial 3 months before or during the study period; and (vii) voluntary signature of informed consent. Individuals with the following conditions were excluded from the study: systemic disease; addiction to alcohol, cigarettes, or psychic drugs; or laboratory test abnormalities, including positive test results for hepatitis B surface antigen, hepatitis A virus, and hepatitis C virus antibody.
Study drug.
AHM was prepared as a sterile lyophilized powder containing 5 mg murine MAb protein in each vial and was formulated and supplied by the Wuhan Institute of Biological Products.
Study design.
A randomized, open-label, dose-escalating study was designed for assessment of the safety and tolerability of AHM. For the safety study, 22 healthy adult volunteers, half male and half female, were randomized into groups (with 4 to 6 subjects in each group) that received doses of 2.5, 5, 10, and 20 mg. The trial was designed to begin testing with the lowest dose (2.5 mg) as the first dose and then to subsequently test the next higher dose, provided that the lower dose was well tolerated. Within each cohort, toxicity was considered unacceptable if any subject experienced a severe adverse event or half of the subjects in any dosage group experienced mild adverse events that were attributed to the study medication and that did not resolve within a reasonable time period. In a separate pharmacokinetic study, 30 healthy subjects (12 women and 18 men) were randomized into 5-, 10-, and 20-mg dose groups for determination of the pharmacokinetic profile of the study medication. The groups were balanced by gender and body weight. The study medication was diluted in 10% glucose solution (20 ml) and was administered by i.v. infusion over a 20-min period via a dedicated central line in the upper left arm.
Safety monitoring.
The variables used to assess safety included adverse events, clinical laboratory measurements (results of blood and urine tests), electrocardiography, vital signs, and the results of a physical examination. Safety and tolerability were assessed by measurement of the heart rate and blood pressure, by electrocardiographic monitoring, and from the results of laboratory safety tests. All subjects were kept in the study unit and were continually observed during the treatment period. Safety tests were conducted 24 to 48 h before and 24 h after drug administration. Signs of intolerance of AHM, such as asthenia, headache, dizziness, nausea, vomiting, diarrhea, and abdominal pain, were recorded by the study physician. The safety of AHM was assessed 24 h after dosing and at the end of the study period by an investigator blinded to the treatment assignment. Adverse events were evaluated for their intensity, seriousness, and relationship to the study medication. Adverse events were defined as mild (awareness of a sign or symptom but easily tolerated), moderate (discomfort sufficient to cause interference with normal activities), or severe (incapacitating, with an inability to perform normal activities). Causality between the study drug and an adverse event, described as “certainly,” “probably,” “possibly,” “suspected,” or “not related,” was defined by use of the criteria developed by the World Health Organization.
Sample collection.
Serial blood samples were collected prior to dosing (0 h) and then at 0.5, 1, 6, 12, 24, 72, 120, 192, 264, and 408 h postdosing. Serum samples were separated and stored frozen at −80°C until they were used for analysis.
Enzyme-linked immunosorbent assay (ELISA) for AHM concentration determination.
Microplates were coated with a rabbit anti-mouse immunoglobulin G (IgG) F(ab′)2 MAb (Pierce Chemical Co., Rockford, IL) and were subsequently blocked with phosphate-buffered saline (PBS)-3% bovine serum albumin. The second layer consisted of serial dilutions of human serum in PBS. Binding was detected with horseradish peroxidase-labeled goat antibody to human IgG Fc (Pierce Chemical Co.). The plates were developed with tetramethylbenzidine as a substrate and were read at an optical density of 450 nm on a microplate reader (Clinbio Co., Austin, TX). The procedures were repeated on different days with the same quality control samples to determine the interassay repeatability. All samples were run in duplicate.
Pharmacokinetic calculations.
The mean AHM concentration at each time point was determined by averaging the data, and the pharmacokinetic parameters were calculated by using the DAS (Drug and Statistics for Windows) software package (version 2.0 PK software; China). Single-dose pharmacokinetic parameters were calculated from serum concentration-time data by the compartmental method. The maximum concentration of drug in plasma (Cmax) and the time to Cmax (Tmax) were determined by visual inspection of the data. The parameters of the model were used to calculate values for clearance (CL), volume of distribution (V), elimination half-life (t1/2), the area under the curve (AUC) from time zero to infinity (AUC0-∞), and the AUC from time zero to the time of the last quantifiable concentration (AUC0-last).
RESULTS
Study population.
The characteristics of the study population are presented in Table 1. There were no major differences (P > 0.05, by analysis of variance) in age, weight, height, or BMI among the dose groups. All subjects completed the study as planned.
TABLE 1.
Demographic characteristics of study populationa
| Group | Safety study |
Pharmacokinetic study |
|||||
|---|---|---|---|---|---|---|---|
| 2.5 mg | 5 mg | 10 mg | 20 mg | 5 mg | 10 mg | 20 mg | |
| Gender (no. of F/no. of Ma) | 2/2 | 3/3 | 3/3 | 3/3 | 4/6 | 4/6 | 4/6 |
| Age (yr)b | 22.0 (19-24) | 23.0 (19-26) | 22.3 (20-26) | 22.8 (21-24) | 22.5 (20-24) | 21.6 (20-24) | 22.3 (20-24) |
| Ht (cm)b | 163 (160-168) | 166 (155-174) | 163 (152-173) | 163 (154-170) | 165 (155-175) | 167 (152-175) | 166 (154-182) |
| Wt (kg)b | 56.4 (52-60) | 60.8 (53-70) | 56.6 (45-70) | 56.1 (47.5-64) | 60.1 (53-67) | 58.6 (45-70) | 59.7 (47.5-74) |
| BMIb | 21.2 (19.6-23.4) | 22.0 (19.5-23.4) | 21.2 (19.0-23.4) | 21.0 (20.0-22.4) | 22.1 (19.5-23.9) | 21.1 (19.7-23.4) | 21.7 (19.3-23.6) |
F, female; M, male.
Values are means (ranges) and are considered significantly different when P was <0.05.
Safety.
A single dose of AHM was well tolerated at doses up to 20 mg, no severe adverse events occurred during the study, and all subjects were in good compliance with the sampling protocols. Overall, one female subject in the 2.5-mg dose group and two female subjects in the 20-mg dose group experienced mild dizziness, which continued for 6 to 12 h. One male in the 20-mg dose group presented with a mild injection site rash (pruritic urticaria) that disappeared after 48 h with no medical treatment. Assessments of blood pressure, pulse rate, body temperature, electrocardiographic findings, physical examination findings, hematology results, clinical chemistry results, and urinalysis results after dosing did not show any clinically relevant differences from the baseline values. The four aforementioned adverse events were considered to be possibly related to the study drug, but the subjects recovered without treatment. They were mild and tolerable and did not lead to study discontinuation.
ELISA for AHM concentration determination.
The standard curve of the AHM concentration (1 to 50 μg·liter−1) was linear with respect to the MAb concentration versus the optical densities at 450 nm, with a correlation coefficient of 0.989 to 0.999. The assay was further validated with human serum samples before use. The results revealed a high degree of reproducibility and accuracy for determination of the serum AHM concentrations.
For human serum AHM concentration determination, the percent intra-assay accuracy (relative error [RE]) ranged from 1.6 to 6.7%, and the precision (coefficient of variation [CV]) ranged from 5.5 to 6.5%. The resultant RE and CV were within ±12% at all concentrations studied. The interassay RE and CV ranged from −2.6 to 8.9% and 6.5 to 11.9%, respectively (Table 2). The accuracy of the assay (percent recovery; n = 5) ranged from 98.9 to 113.1%. After quality control samples were stored at −80°C for 30 days, the rates of recovery of the original concentrations were 101.7 to 103.8% (data not shown).
TABLE 2.
Accuracy and precision of ELISA method for detection of anti-HTNV MAb in human serum
| Parameter | Concn (μg·liter−1) |
RE (%) | CV (%) | |
|---|---|---|---|---|
| Prepared | Measureda | |||
| Intra-assay (n = 5) | 4 | 4.06 ± 0.24 | 1.6 | 5.87 |
| 20 | 21.3 ± 1.17 | 6.29 | 5.5 | |
| 40 | 42.7 ± 2.77 | 6.74 | 6.49 | |
| Interassay (n = 15) | 4 | 3.90 ± 0.46 | −2.55 | 11.9 |
| 20 | 21.8 ± 1.54 | 8.89 | 7.07 | |
| 40 | 39.5 ± 20.58 | −1.21 | 6.54 | |
Data represent the means±standard deviations.
Pharmacokinetics of AHM.
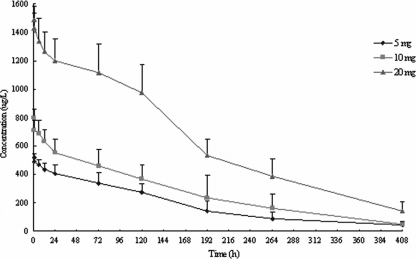
The mean concentration-versus-time profiles after the administration of a single dose of AHM are shown in Fig. 1. The serum AHM level decreased rapidly within 24 h after i.v. administration and then declined slowly and progressively. On day 5, the serum concentrations had declined by 50%. Low levels of AHM were still detectable in most samples after 408 h.
FIG. 1.
Serum concentration-time curves of AHM following i.v. administration of doses of 5, 10, or 20 mg. The data are the means ± standard deviations for 10 subjects in each group.
The serum concentration-time curve for AHM followed a monophasic decline and was well fitted by a one-compartment open model. The mean values for the pharmacokinetic parameters for AHM are summarized in Table 3. For doses of 5, 10, and 20 mg, the mean AUC0-last values were 71, 92, and 236 mg·h·liter−1, respectively, and the mean AUC0-∞ values were 78, 102, and 263 mg·h·liter−1, respectively. Cmax and AUC increased proportionally with AHM doses of 5, 10, and 20 mg. In addition, the mean CL was 0.069 to 0.12 liter·h−1, the mean V was 10.1 to 13.6 liters, and kel was 0.059 to 0.091 h−1; both CL and V were constant, irrespective of the dose. The mean t1/2 was between 92 and 124 h and did not show a tendency to change with an increase in the dose.
TABLE 3.
Pharmacokinetic parameters of AHM following single ascending i.v. infusions
| AHM dose (mg)a | AUC0- last (mg·h·liter−1) | AUC0-∞ (mg·h·liter−1) | Cmax (μg·liter−1) | Tmax (h) | t1/2 (h) | CL (liter·h−1) | V (liters) | Dose-normalizedb: |
||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC0-last | AUC0-∞ | Cmax | ||||||||
| 5 | 78.4 ± 22.3 | 70.5 ± 12.6 | 517 ± 23.8 | 0.50 ± 0.00 | 112 ± 42.0 | 0.069 ± 0.021 | 10.1 ± 1.22 | 14.1 ± 3.25 | 15.7 ± 4.47 | 103 ± 4.78 |
| 10 | 92.0 ± 33.0 | 101.6 ± 38.8 | 796 ± 66.5 | 0.50 ± 0.00 | 91.7 ± 34.9 | 0.12 ± 0.054 | 13.1 ± 2.71 | 9.20 ± 3.30 | 10.2 ± 3.88 | 79.6 ± 6.65 |
| 20 | 236 ± 29.8 | 263 ± 40.3 | 1,487 ± 91.0 | 0.50 ± 0.00 | 124 ± 21.9 | 0.078 ± 0.014 | 13.6 ± 1.61 | 11.8 ± 1.49 | 13.2 ± 2.02 | 74.3 ± 4.55 |
Values are means±standard deviations (n = 10 for each dose).
Regression analysis of dose-normalized parameters revealed no statistically significant difference across the dosage levels.
DISCUSSION
This study has shown that the i.v. administration of AHM at doses that range from 2.5 to 20 mg is well tolerated. Adverse events were generally mild in intensity and required no treatment intervention. They were not dose dependent and disappeared within several hours without clinical treatment.
Prior to the initiation of the pharmacokinetic study, the ELISA methodology for determination of the AHM concentration was validated. The sensitivity of detection was in the range of 1 to 50 μg·liter−1. The accuracy, precision, and stability results indicate that the method satisfied the general requirements for analysis in pharmacokinetic studies. Moreover, the lower limit of quantitation (1 μg·liter−1) indicates that the method is suitable for pharmacokinetic analysis of AHM for most human serum samples.
Following a single i.v. infusion of doses of 5, 10, and 20 mg, the mean Cmaxs of AHM were 517, 796, and 1,487 μg·liter−1, respectively. The Cmaxs of AHM in the healthy volunteers in the three dose groups appeared to be similar to those reported by Yang et al. for a large number of patients with HFRS in a phase II clinical trial (15). The results also demonstrated that over the dose range of 5 to 20 mg, AHM showed linear pharmacokinetics after the administration of a single i.v. dose. Dose-proportional increases in Cmax and AUC were observed following the administration of doses of 5, 10, and 20 mg; but CL, V, and t1/2 were similar across all doses. The results indicate that AHM exhibits linear pharmacokinetics over the dose range of 5 to 20 mg, thus greatly facilitating therapeutic monitoring for the future clinical development of AHM. A one-compartment open model allowed the adequate characterization of the disposition of AHM. This was similar to the disposition of murine MAbs in humans reported in the literature (7, 8). The observation that AHM fit into a one-compartment model may well reflect the fact that the rate of elimination (t1/2) was similar to the rate of equilibrium between the intravascular and the extravascular spaces, so that the curves for the two compartments do not show a difference. We should also take into account the fact that since the infusion time was 20 min and the first serum sample for AHM concentration determination was obtained 30 min after the infusion, it is possible that the early-distribution-phase kinetics may have been masked and compromised.
The results also showed that there is a large degree of intersubject variation in the t1/2s of AHM. For example, t1/2s varied approximately fourfold among individuals in the 5-mg-dose group (range, 54.9 to 193 h). It is known that many variables affect the elimination of IgG MAb. The rate of elimination of IgG is likely to be dominated by its affinity for the FcRn receptor and by the nature as well as the affinity of binding to the specific target. In addition, other factors may also attribute to the various rates of elimination of the MAb, including the immunogenicity, the degree and nature of glycosylation, and the susceptibility of the MAb to proteolysis (10). The reason for the variation could be due to individual differences in the variables mentioned above; human anti-mouse antibodies (HAMAs) may be one of the important ones. The formation of HAMAs can affect the safety, pharmacokinetics, and pharmacodynamics of therapeutic antibodies. The t1/2 of AHM could have been negatively affected by the HAMAs, particularly in subjects in whom the HAMA reaction to AHM had an early onset and was prominent, and thus could have attributed to the decreased individual t1/2s.
The development of HAMAs could also have complex therapeutic consequences. The development of HAMAs in patients could result in diminished therapeutic efficacy in some clinical trials but not in others (2). The reduced efficacy reported in patients who developed responses to HAMAs could be attributed to the rapid clearance because of complex formation or to the interference with MAb binding to the antigen by human anti-idiotypic antibody. In the case of the responses of individual patients, it is possible to correlate HAMAs with the potential antiviral efficacy of AHM when testing is advanced into phase II clinical development in patients with HFRS rather than in healthy volunteers. In the case of the potential formation of HAMAs to AHM, Yang et al. (15) reported that in an early phase II clinical study of AHM, 28.6% of the patients showed a HAMA response in 6 to 10 days and 78.0% of the patients had an HAMA response 12 days after treatment. On the basis of that information, the occurrence of HAMAs to AHM is a late-onset reaction, while the anti-HTNV neutralizing effects most likely took place in the first 2 or 3 days.
The mean t1/2 of AHM ranged from 92 to 124 h, longer than the reported t1/2 of 1 to 3 days for murine IgG MAbs (3). As we observed only a modest decrease in the serum t1/2, it is reasonable to assume that the HAMA response to AHM was light during the monitoring period. The relatively low rate of elimination suggests that approximately 30 days is needed for the vast majority of AHM to disappear from the systemic circulation, with approximately 10% of the drug still being detectable 17 days after the infusion. The results indicate that AHM is slowly cleared from serum, which supports the clinical use of AHM once only.
In conclusion, the results of the present study indicate that the established ELISA method can effectively be used for kinetic studies and for therapeutic drug monitoring with biological fluids. AHM was well tolerated, and none of the volunteers discontinued participation in the study due to adverse effects. No clear HAMA-related safety concerns or pharmacokinetic responses were detectable in the phase I study. AHM has predictable pharmacokinetics and displays linear pharmacokinetics over the dose range of 5 mg to 20 mg after the infusion of a single dose. AHM is slowly cleared from the blood and has an t1/2 of 110 h, which supports the use of a regimen of only one i.v. dose in future clinical studies.
Acknowledgments
We thank Zhikai Xu (Fourth Military Medical University of China) for comments and suggestions in performing the ELISA and the Wuhan Institute of Biological Products for providing AHM for clinical use.
Footnotes
Published ahead of print on 14 September 2009.
REFERENCES
- 1.De Clercq, E. 2005. Recent highlights in the development of new antiviral drugs. Curr. Opin. Microbiol. 8:552-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuus-Reichel, K., L. S. Grauer, L. M. Karavodin, C. Knott, M. Krusemeier, and N. E. Kay. 1994. Will immunogenicity limit the use, efficacy, and future development of therapeutic monoclonal antibodies? Clin. Diagn. Lab. Immunol. 1:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobo, E. D., R. J. Hansen, and J. P. Balthasar. 2004. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 93:2645-2668. [DOI] [PubMed] [Google Scholar]
- 4.Maes, P., J. Clement, I. Gavrilovskaya, and M. Van Ranst. 2004. Hantaviruses: immunology, treatment, and prevention. Viral Immunol. 17:481-497. [DOI] [PubMed] [Google Scholar]
- 5.Peters, C. J., G. L. Simpson, and H. Levy. 1999. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu. Rev. Med. 50:531-545. [DOI] [PubMed] [Google Scholar]
- 6.Reichert, J. M., and M. C. Dewitz. 2006. Anti-infective monoclonal antibodies: perils and promise of development. Nat. Rev. Drug Discov. 5:191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenblum, M. G., J. L. Murray, T. P. Haynie, H. J. Glenn, M. F. Jahns, R. S. Benjamin, J. M. Frincke, D. J. Carlo, and E. M. Hersh. 1985. Pharmacokinetics of 111In-labeled anti-p97 monoclonal antibody in patients with metastatic malignant melanoma. Cancer Res. 45:2382-2386. [PubMed] [Google Scholar]
- 8.Saleh, M. N., M. B. Khazaeli, W. E. Grizzle, R. H. Wheeler, S. Lawson, T. Liu, C. Russel, R. Meredith, J. Schlom, and A. F. LoBuglio. 1993. A phase I clinical trial of murine monoclonal antibody D612 in patients with metastatic gastrointestinal cancer. Cancer Res. 53:4555-4562. [PubMed] [Google Scholar]
- 9.Wang, L. Y., R. R. Zheng, Z. K. Xu, M. X. Wang, and S. C. Jiang. 1988. Establishment and identification of hybridoma cell lines secreting monoclonal antibodies against hemagglutinin of HFRS virus. J. Fourth. Mil. Med. Univ. 9:20-23. [Google Scholar]
- 10.Wang, W., E. Q. Wang, and J. P. Balthasar. 2008. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 84:548-558. [DOI] [PubMed] [Google Scholar]
- 11.Wei, L. X., M. X. Wang, Z. K. Xu, and L. Y. Wang. 1989. Study on protection effects of MAb on HFRS virus infected suckling mice. J. MAb 5:5-10. [Google Scholar]
- 12.Wei, L. X., M. X. Wang, H. Zhang, and L. Y. Wang. 1990. McAbs inoculated into pregnant mice passively protected filial suckling mice from HFRS virus by transplacental passage. J. MAb 6:16-18. [Google Scholar]
- 13.Xu, Z. K., L. X. Wei, L. Y. Wang, H. T. Wang, and S. B. Jiang. 2002. The in vitro and in vivo protective activity of monoclonal antibodies directed against Hantaan virus: potential application for immunotherapy and passive immunization. Biochem. Biophys. Res. Commun. 298:552-558. [DOI] [PubMed] [Google Scholar]
- 14.Yan, L., L. Q. Fang, H. G. Huang, L. Q. Zhang, D. Feng, W. J. Zhao, W. Y. Zhang, X. W. Li, and W. C. Cao. 2007. Landscape elements and Hantaan virus-related hemorrhagic fever with renal syndrome, People's Republic of China. Emerg. Infect. Dis. 13:1301-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang, W. T., Z. K. Xu, and H. T. Wang. 2004. Detection of metabolism of anti HFRS V antibody in HFRS patients. J. Fourth. Mil. Med. Univ. 25:1525-1527. [Google Scholar]