Abstract
Antileishmanial therapy is suboptimal due to toxicity, high cost, and development of resistance to available drugs. Pyrazinamide (PZA) is a constituent of short-course tuberculosis chemotherapy. We investigated the effect of PZA on Leishmania major promastigote and amastigote survival. Promastigotes were more sensitive to the drug than amastigotes, with concentrations at which 50% of parasites were inhibited (MIC50) of 16.1 and 8.2 μM, respectively (48 h posttreatment). Moreover, 90% of amastigotes were eliminated at 120 h posttreatment, indicating that longer treatments will result in parasite elimination. Most strikingly, PZA treatment of infected C57BL/6 mice resulted in protection against disease and in a 100-fold reduction in the parasite burden. PZA treatment of J774 cells and bone marrow-derived dendritic cells and macrophages increased interleukin 12, tumor necrosis factor alpha, and activation marker expression, as well as nitric oxide production, suggesting that PZA enhances effective immune responses against the parasite. PZA treatment also activates dendritic cells deficient in Toll-like receptor 2 and 4 expression to initiate a proinflammatory response, confirming that the immunostimulatory effect of PZA is directly caused by the drug and is independent of Toll-like receptor stimulation. These results not only are strongly indicative of the promise of PZA as an alternative antileishmanial chemotherapy but also suggest that PZA causes collateral immunostimulation, a phenomenon that has never been reported for this drug.
The leishmaniases are a group of insect-transmitted parasitic diseases prevalent worldwide, endemic in 88 countries; 350 million people are at risk, and 12 million people are affected. Two million new cases are estimated to occur annually, although only 600,000 are officially reported (10). During the last two decades, it has become increasingly apparent that the leishmaniases are much more prevalent than had been previously suspected. With human migration and vector expansion dramatically affecting the spread of disease, dramatic outbreaks have occurred in locations with previously low levels of infection (e.g., Kabul, Afghanistan, with more than 200,000 infected [22]). Immunologically naive individuals from the developed world traveling to areas of endemicity are particularly prone to infection. Leishmaniasis has been found among American soldiers deployed to the Middle East during both Gulf wars, current conflicts in Afghanistan, and Central America (3, 7, 12, 14, 18, 20, 24, 29). Civilians traveling into these areas are also at risk (2).
Currently, there are nearly 25 licensed compounds with antileishmanial effects, but only a few are used in humans. Drawbacks associated with conventional treatment with antimonials and amphotericin B include high toxicity, differences in strain sensitivity, and resistance. Moreover, the expense of these drugs often precludes their use. As recently as 2004, liposomal amphotericin B, miltefosine, and paromomycin were identified by WHO/TDR as the three most promising drugs in the market. These drugs are not new: amphotericin B has been used extensively for decades as a second-line drug for treatment of leishmaniasis (in addition to its antifungal activity), miltefosine was developed long ago as an anticancer agent, and paromomycin is more than 50 years old. To date, these three agents, together with antimonials and nonliposomal amphotericin B, are the reference chemotherapeutic agents for the leishmaniases. Oral miltefosine has been shown to be as efficacious against leishmaniasis as the standard amphotericin B treatment in India; however, important side effects, such as teratogenicity, are associated with this drug (28). With this limitation, the need for safer, inexpensive, and widely available treatments continues to be one of the top research priorities for disease control (5).
In contrast to other possible strategies (“orphan drugs,” combinatory chemistry, or rational design), we seek new indications for existing drugs, which can be a very fruitful route for drug discovery and development (6). Pyrazinamide (PZA), an essential constituent of short-course tuberculosis chemotherapy (11), was developed as an analog of nicotinamide and used in the late 20th century in the treatment of Mycobacterium tuberculosis. PZA and related analogs have also demonstrated activity against other Mycobacterium spp. (9, 21, 27). In mycobacteria, we found that PZA inhibits the enzyme fatty acid synthase I (FASI) (33) by competitive inhibition of a NADPH binding site (25). By analogy, it could be proposed that PZA interferes with fatty acid synthesis in trypanosomatids. Although no genes homologous to the FASI gene have been identified in the Leishmania genome, this parasite employs microsomal elongases in an iterative manner to synthesize fatty acids (17). This suggests that inhibition of fatty acid synthesis might be an attractive chemotherapeutic target in Leishmania (16, 23).
In this article, we demonstrate that PZA has antileishmanial effect in vitro on both promastigotes and amastigotes. More importantly, PZA dramatically decreases lesion development and the parasite burden in C57BL/6 mice infected with Leishmania major. Finally, we show that PZA increases activation of infected macrophages and dendritic cells by increasing expression of costimulatory molecules and secretion of proinflammatory cytokines and nitric oxide. These results not only show that PZA constitutes a very promising alternative therapy for leishmaniasis but also suggest that the drug causes collateral immunostimulation.
MATERIALS AND METHODS
Mice.
C57BL/6 mice (5 to 6 weeks of age) were purchased from Taconic (Germantown, NY). Toll-like receptor 2 (TLR-2)- and TLR-4-deficient mice were kindly provided by David Russell at Cornell University. All mice were maintained in the Baker Institute Animal Care Facility under pathogen-free conditions.
Parasite and cell culture.
L. major clone V1 (MHOM/IL/80/Friedlin) promastigotes were grown at 26°C in medium 199 supplemented with 20% heat-inactivated fetal calf serum (FCS) (Gemini, Sacramento, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 40 mM HEPES, 0.1 mM adenine (in 50 mM HEPES), and 5 mg/ml hemin (in 50% triethanolamine).
The macrophage murine cell line J774 (catalog no. TIB-67) was cultured in Dulbecco's modified Eagle medium (DMEM) (Sigma-Aldrich, St. Louis, MO) with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine (Sigma-Aldrich) at 37°C under a 5%-CO2 atmosphere. Culture medium was changed twice per week. Subcultures were performed when monolayers covered 90% of the bottom of culture flasks. For experiments involving macrophages and dendritic cells, bone marrow was obtained from C57BL/6 mouse femurs and grown for 6 to 8 days in RPMI 1640 supplemented as described above in the presence of 10% L929 conditioned medium (to generate macrophages [13]) or 20 ng/ml granulocyte-macrophage colony-stimulating factor (CSF) (to generate dendritic cells [31]).
Drugs.
PZA was provided by Cynamon and Welch and dissolved in dimethyl sulfoxide with subsequent dilutions in water (in vivo assays) or culture medium (in vitro assays).
Promastigote and amastigote drug treatment.
Mid-log-phase (day 3 of culture) L. major promastigotes were employed. Parasite concentration was adjusted to 106 promastigotes/ml, and parasites were seeded into 96-well plates in a volume of 100 μl (final concentration, 105 promastigotes/well). PZA was tested in triplicate in a concentration gradient from 1,000 to 0.5 μg/ml and added to the wells containing the parasite in a volume of 100 μl. A negative control was included with three wells containing only parasites and medium. The positive control consisted of amphotericin B (0.1 and 1 μg/ml; Sigma), as previously tested by us (19). After 48 h of incubation at 26°C, 10 μl of each well was diluted in 90 μl of the vital colorant (trypan blue in phosphate-buffered saline), and the parasites were quantified in a Neubauer chamber. Data were normalized as percentages of survival compared to data for untreated controls. The 50% lethal dose was extrapolated from the graph as the concentration of the products that inhibited the parasitic growth at 50% of the values of the negative control.
Amastigotes were generated by infecting either J774 murine macrophages or bone marrow-derived macrophages or dendritic cells from C57BL/6 mice. Infections were carried out after cells were seeded in eight-well Labtek chambers (Thermo Physic Scientific, Rochester, NY) at a concentration of 5 × 104 cells/well. To avoid multiplication, cells were incubated with mitomycin C at a concentration of 0.8 μg/ml for 16 h. Infective-stage promastigotes of L. major were isolated from stationary cultures (4 to 5 days old) by Ficoll enrichment (26), added to macrophage cultures (five promastigotes to one cell), and kept overnight at 37°C in the presence of 5% CO2 and DMEM with 10% FCS. Sixteen hours later, noninternalized promastigotes were washed and replaced by culture medium containing the drug. Forty-eight hours later, wells were detached from the slides, stained with Diff-Quick (Dade Behring, Newark, DE), and counted under a light microscope. The parasite burden was determined by observation by light microscopy (magnification, ×1,000) as the number of amastigotes per 100 cells. The percentage of survival of amastigotes and concentration at which 50% of parasites were inhibited (MIC50) were calculated as described above.
J774 cell viability following incubation with PZA was also determined. Cells were seeded on eight-well Labtek chambers as above and incubated with different concentrations of PZA (up to 1 mg/ml) for 48 h, stained with Diff-Quick, and counted under a light microscope. The percentage of viable cells was determined after quantifying the number of cells present per field (in 25 fields, ca. 500 cells). The percentage of survival and MIC50 were calculated as above.
In vivo infection and treatment studies.
Mice (n = 6) were anesthetized with isoflurane (Abbott Laboratories, Chicago, IL) and inoculated intradermally in both ears with 5 × 105 L. major promastigotes in a volume of 10 μl using a 27-gauge needle. PZA was diluted in water and administered by oral gavage in a 0.2-ml volume. A control group was treated with water containing dimethyl sulfoxide (3.8%).
The timetable for the experiment was as follows: day 0, infection; days 1 to 5, 8 to 12, 15 to 19, and 22 to 26, drug administration; and day 70, sacrifice. Lesion size was monitored one to two times per week by measuring the lesion diameter with a vernier caliper. Mice were euthanized by CO2 inhalation.
Parasite quantitation.
Parasite loads in the ears were determined as described previously (4). Briefly, the ventral and dorsal sheets of the infected ears were separated and deposited in RPMI containing 100 U/ml penicillin, 100 μg/ml streptomycin, and Liberase CI enzyme blend (0.5 mg/ml; Boehringer Mannheim). Ears were incubated for 60 min at 37°C. The sheets were dissociated using a handheld tissue homogenizer. The homogenates were filtered using a 70-μm cell strainer (BD Falcon, San Jose, CA) and serially diluted in a 96-well flat-bottom microtiter plate containing biphasic medium prepared using 50 μl NNN medium (25 g Bacto beef, 10 g neopeptone, 10 g Bacto agar, 2.5 g Nacl) containing 20% defibrinated rabbit blood overlaid with 100 μl medium 199. The number of viable parasites in each ear was estimated from the highest dilution at which promastigotes could be grown out after 7 days of incubation at 26°C.
Analysis of cell activation following treatment.
J774 cells or bone marrow-derived cells were seeded on 24-well plates at a concentration of 5 × 105 cells/ml. Twenty-four hours later, PZA was added to the wells at different concentrations in the absence or presence of L. major (1:5 parasite ratio). In some experiments, J774 cells were treated with amphotericin B (0.1, 0.5, or 1 μg/ml) (Sigma). Two control groups were also included in this experiment: a positive control for activation, consisting of a group of uninfected cells treated with 100 ng/ml lipopolysaccharide (LPS) and 10 IU gamma interferon (IFN-γ), and a negative control for activation, consisting of uninfected, untreated cells. Cell cultures were maintained overnight and then cultured for an additional 6 h with brefeldin A (10 μg/ml), harvested by scraping, and fixed in 2% paraformaldehyde. In some experiments, flow cytometry was employed. Prior to staining of cells with fluorescent antibodies, they were incubated with an anti-Fcγ III/II receptor and 10% normal mouse serum in phosphate-buffered saline containing 0.1% bovine serum albumin-0.01% NaN3. Cells were permeabilized with saponin and stained for the expression of surface markers CD80 (clone 16-10A1), CD86 (clone GL1), major histocompatibility complex (MHC) class I (clone 28-14-8), and MHC class II (clone M5/114.15.2) and for the cytokines interleukin 12p40/p70 (IL-12p40/p70) (clone C17.8), IL-10 (clone JES5-16E3), and tumor necrosis factor alpha (TNF-α) (clone 1D9) and inducible nitric oxide synthase (iNOS) (clone NOS-IN). Incubations were carried out for 30 min on ice. All antibodies were purchased from BD Biosciences or eBioscience. For each sample, at least 50,000 cells were analyzed. The data were collected and analyzed using the CELLQuest software program and a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA). In other experiments, supernatants from treated cells were collected and assayed by enzyme-linked immunosorbent assay (ELISA) for the secretion of IL-12, IL-10, or TNF-α using commercial kits (BD Biosciences). The levels of nitric oxide in treated and untreated cultures was also measured in supernatants using the Griess reagent (Invitrogen).
Statistical analysis.
Statistical analysis of the in vivo data used a one-way analysis of variance followed by Bonferroni's post hoc test using the GraphPad Prism software program (San Diego, CA) (n = 7). Results were considered significant when P values were ≤0.05. MIC50s were interpolated from curves generated by nonlineal regression analysis using GraphPad Prism.
RESULTS
PZA has antileishmanial effect in vitro.
The effect of PZA was first determined with promastigotes of L. major after 48 h. L. major promastigotes treated with PZA experienced a decrease in cell proliferation. The MIC50 was established as 16.2 μg/ml (16.1 μM) (Table 1). Intracellular amastigotes appeared to be slightly more sensitive to the effect of PZA than the extracellular forms (MIC50 = 10.2 μg/ml or 8.2 μM). Incubation of L. major with 1 μg/ml amphotericin B (as a positive control) caused 100% mortality of promastigotes and 90% of amastigotes.
TABLE 1.
Effect of PZA on L. major and J774 cell survivala
| Group assessed | % Survival at PZA concn (μg/ml) of: |
MIC50 of PZA (μg/ml and μM) | % Survival with amphotericin B treatment | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 12.5 | 50 | 100 | 200 | 1,000 | |||
| Promastigotes | 88 ± 18 | 50 ± 18 | 38 ± 14 | — | 20 ± 10 | 10 ± 12 | 16.2 and 16.1 | 0 |
| Amastigotes | 89 ± 12 | 47 ± 12 | —b | 23 ± 10 | — | 20 ± 15 | 10.2 and 8.2 | 10 ± 5 |
| Uninfected J774 cells | 100 ± 11 | 100 ± 10 | 100 ± 12 | 100 ± 15 | 85 ± 12 | 0 | 524.8 and 425.6 | 95 ± 16 |
Amphotericin B (1 μg/ml) was included in the experiment as a control. After 48 h, parasite and cell survival was determined. Data are expressed as percentages of survival compared to results for the untreated control (100% survival). Values are means ± standard deviations of at least three independent determinations.
—, not determined.
To determine the potential cytotoxicity of PZA toward the mammalian cell line, coincubation of PZA with uninfected J774 cells was also carried out for 48 h. Results showed that the drug was cytotoxic at 200 μg/ml, resulting in mortality of 25% of the cell monolayer. The MIC50 for mammalian cells was established as 425.6 μg/ml. The control drug amphotericin B caused only 5% mortality of the cell monolayer at the concentration tested.
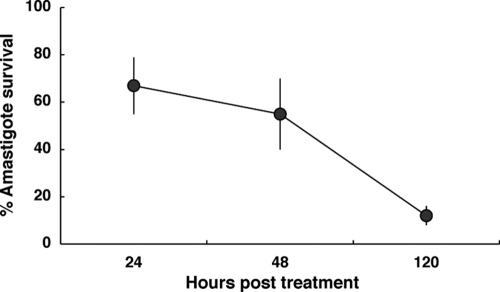
To distinguish between leishmaniostatic and leishmanicidal effects, a time course survival curve was generated for J774 cultures infected with L. major. Figure 1 shows parasite loads in macrophages at 24, 48, and 120 h posttreatment with 100 μM PZA. Interestingly, about 90% of L. major parasites were efficiently eliminated from the cells after 120 h, indicating that PZA is a leishmanicidal drug.
FIG. 1.
Leishmanicidal effect of PZA. Effects of PZA (100 μM) on the survival of L. major amastigotes within J774 cells at different time points after infection are shown. Data are expressed as percentages of survival compared to that of the untreated control (100% survival). Values are means ± standard deviations of at least three independent determinations.
PZA significantly reduces clinical disease and parasite burdens in infected mice.
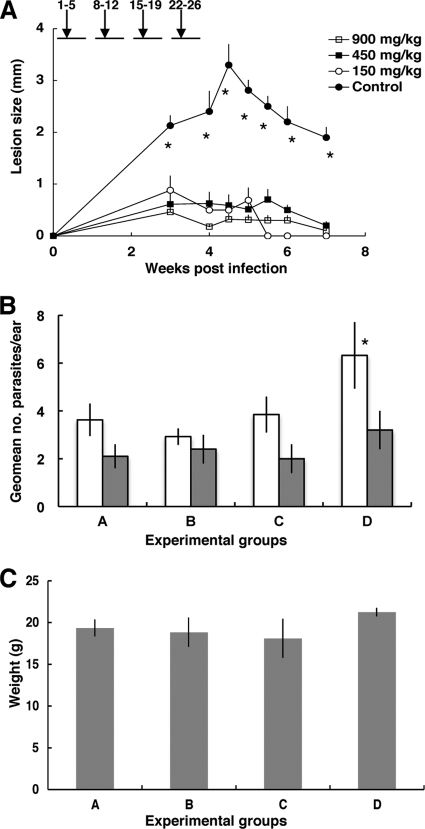
To assess the efficacy of PZA in vivo, C57BL/6 mice were intradermally infected with 5 × 105 L. major parasites in each ear (n = 6 mice; 12 ears) and treated orally with PZA at several concentrations (900 to 150 mg/kg of body weight). As shown in Fig. 2A, the oral administration of PZA produced a significant (P = 0.0001) reduction of the average lesion size in all treated groups compared with results for untreated mice at all time points. However, there were no statistical differences among the three experimental groups treated with different doses of PZA. Parasite burdens in the ears were determined at 5 weeks postinfection in the experimental groups. Figure 2B shows that treatment dramatically decreased the parasite burden in infected ears at week 6 (100-fold; P = 0.008) compared to results for the control. As before, no statistical differences were detected among the PZA-treated groups. Parasite burdens were also comparable among all experimental groups when determined after healing, at 12 weeks postinfection. Finally, PZA treatment was not toxic and did not affect the growth of the experimental animals since no significant differences in body weight were found at week seven postinfection (Fig. 2C).
FIG. 2.
(A) PZA modifies the course of L. major infection. Mean lesion diameter in C57BL/6 mouse ears infected with L. major and treated with PZA versus that for controls (n = 6 to 12 ears ± standard error of the mean [SEM]). *, statistically significant; P = 0.0001 compared with results for the PZA-treated groups. Numbers above the graph represent treatment periods in days postinfection. (B) Ear parasite burden per ear (n = 4 to 6 ± SEM) for C57BL/6 mouse ears infected with L. major and treated with PZA (A, 900 mg/kg; B, 450 mg/kg; C, 150 mg/kg; D, control). Parasite burden was estimated by limiting dilution at 6 weeks postinfection (open bars) or 12 weeks postinfection (gray bars). *, statistically significant; P = 0.008 compared with results for the PZA-treated groups. (C) Body weights of experimental mice (n = 4) 7 weeks postinfection.
PZA increases J774 cell activation as well as release of proinflammatory cytokines and nitric oxide.
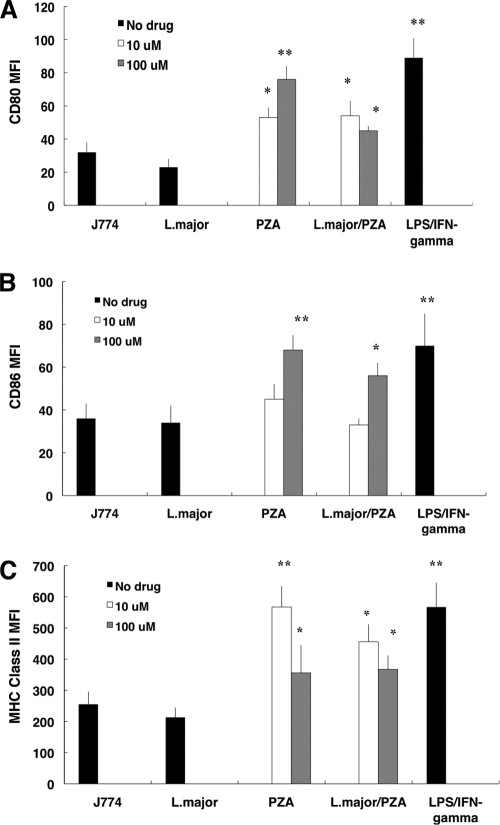
Because of the striking in vivo effect of PZA, we studied the effect of treatment on macrophages. First, we looked at activation markers and cytokine secretion by J774 cells (a murine macrophage cell line) following drug treatment (10 and 100 μM) in the presence or absence of L. major infection. As a positive control, cells were exposed to a mixture of IFN-γ and LPS. Figure 3 shows that drug treatment increased expression of the costimulatory molecules CD80, CD86, and MHC class II, suggesting that treatment alone increases the ability of the macrophage to present antigen. L. major-infected J774 cells downregulated the expression of costimulatory molecules and class II MHC molecules compared to that for infected, untreated controls, a phenomenon typically associated with L. major infection. Interestingly, treatment of L. major-infected cells with PZA rescued the ability of the cell line to upregulate all surface markers studied at the same level as that for cells treated with the drug alone.
FIG. 3.
PZA treatment of J774 cells increased expression of surface markers. Mean fluorescence intensity (MFI) for CD80 (A), CD86 (B), or MHC class II molecules (C), determined by flow cytometry, in J774 cells infected with L. major and treated with 10 and 100 μM PZA. A group of uninfected cells was treated with 100 ng/ml LPS and 10 IU IFN-γ as a positive control of activation. The level of marker expression in untreated, L. major-infected cells was also determined. Unstimulated cell expression fluorescence is also shown. Data are expressed as average MFI ± standard deviation from three independent experiments. *, statistically significant [P ≤ 0.001]; **, statistically significant [P ≤ 0.0001] compared with result for untreated, L. major-infected control group.
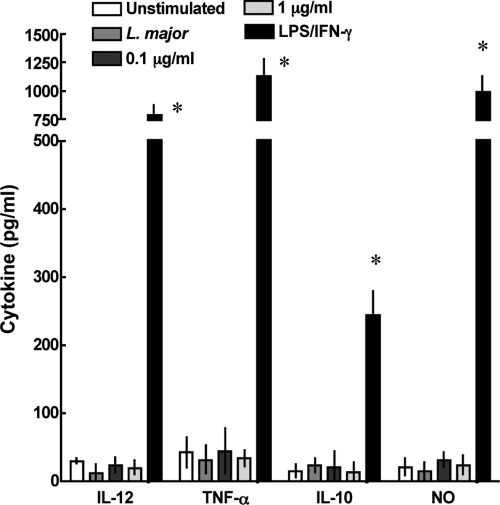
We also determined the ability of J774 cells to produce cytokines in response to infection and/or treatment. Cells were infected, treated, or activated as described above. The amounts of the proinflammatory cytokines IL-12 and TNF-α (implicated in Th1 response and parasite killing) and the repressive cytokine IL-10 in the culture supernatants were determined by ELISA. The level of nitric oxide in the same supernatants was also determined. PZA treatment alone increased cytokine production, especially that of IL-12 and TNF-α, and NO release. Cytokine production was also increased in the wells treated with PZA and infected with L. major compared with that for infection with L. major alone, suggesting again that the immune response is enhanced by PZA in infected cells (Table 2). Treatment also slightly increased IL-10 production, although the response of the cell line to the drug was dominated by the release of proinflammatory factors. Finally, to test the specificity of the immune activation by PZA, we determined the effect that amphotericin B treatment had on activation and cytokine expression by J774 cells. Treatment of J774 cells with the drug did not result in activation at the doses tested (Fig. 4).
TABLE 2.
IL-12, TNF-α, IL-10, and nitric oxide production determined by ELISA (cytokines) or Griess test (nitric oxide) in J774 cells infected with L. major and treated with 10 or 100 μM PZAa
| Cytokine or substance | Drug concn (μM) | Level of cytokine or nitric oxide (pg/ml) with indicated treatment |
||||
|---|---|---|---|---|---|---|
| None | L. major | PZA | L. major, PZA | LPS, IFN-γ | ||
| IL-12 | 0 | 34 ± 12 | 44 ± 24 | 678 ± 125* | ||
| 10 | 178 ± 38* | 253 ± 68* | ||||
| 100 | 359 ± 65* | 299 ± 55* | ||||
| TNF-α | 0 | 135 ± 21 | 105 ± 19 | 1,256 ± 132* | ||
| 10 | 245 ± 159* | 259 ± 163* | ||||
| 100 | 377 ± 105* | 489 ± 154* | ||||
| IL-10 | 0 | 14 ± 39 | 54 ± 8 | 236 ± 22* | ||
| 10 | 112 ± 45* | 109 ± 39* | ||||
| 100 | 145 ± 56* | 112 ± 44* | ||||
| Nitric oxide | 0 | 37 ± 11 | 24 ± 18 | 921 ± 223* | ||
| 10 | 254 ± 44* | 289 ± 55* | ||||
| 100 | 476 ± 52* | 498 ± 74* | ||||
A group of uninfected cells was treated with 100 ng/ml LPS and 10 IU IFN-γ as a positive control of activation. Cytokine levels (± standard deviations) in L. major-infected macrophages and in untreated cells were also determined. Data were obtained from three independent experiments. *, statistically significant when compared with result for untreated, L. major-infected control group (P < 0.05).
FIG. 4.
PZA increases proinflammatory cytokine production in J774 cells. IL-10, IL-12, TNF-α, and nitric oxide production were determined by ELISA (cytokines) or the Griess test (nitric oxide) in J774 cells infected or not with L. major and treated with 0.1 or 1 μg/ml amphotericin B. Uninfected cells were treated with 10 U IFN-γ and 100 ng/ml LPS as a positive control of activation. Unstimulated cell cytokine levels are shown. Data are expressed as means ± standard deviations for three determinations. *, statistically significant; P = 0.0001 compared with results for the group infected with L. major that did not receive the drug.
Bone marrow-derived macrophages and dendritic cells from C57BL/6 mice also release proinflammatory factors in response to PZA.
The background of J774 cells is the susceptible BALB/c mouse strain. Because BALB/c susceptibility to L. major is mediated by its inability to initiate Th1 responses, we tested the effect of the drugs on primary cells isolated from the resistant mouse strain C57BL/6.
We obtained bone marrow cells and grew them in the presence of the cytokines macrophage CSF or granulocyte-macrophage CSF to induce differentiation of macrophages or dendritic cells, respectively. In this experiment, we included the study of the immune response of dendritic cells because they are essential for the initiation the immune response against L. major. Table 3 shows that, as before, parasite infection inhibits the initiation of inflammatory responses by macrophages, as evidenced by their inability to produce cytokines or release nitric oxide. Again, this effect was rescued by the treatment of macrophages with PZA.
TABLE 3.
IL-12, TNF-α, IL-10, and nitric oxide production in bone marrow-derived macrophages or dendritic cells infected with L. major and treated with 10 or 100 μM PZA, determined by ELISA (cytokines) or Griess test (nitric oxide)a
| Cell group and cytokine or substance | Concn (pg/ml) with treatment |
||||
|---|---|---|---|---|---|
| Unstimulated | L. major | PZA (100 μM) | L. major, PZA (100 μM) | LPS, IFN-γ | |
| Macrophages | |||||
| IL-12 | 56 ± 11 | 34 ± 6 | 867 ± 546* | 921 ± 445* | 3,678 ± 456* |
| IL-10 | 15 ± 13 | 30 ± 22 | 155 ± 21* | 199 ± 120* | 321 ± 156* |
| TNF-α | 35 ± 22 | 104 ± 89 | 758 ± 246* | 921 ± 345* | 2,678 ± 625* |
| Nitric oxide | 30 ± 3 | 12 ± 21 | 543 ± 221* | 699 ± 112* | 1,240 ± 516 |
| Dendritic cells | |||||
| IL-12 | 46 ± 31 | 114 ± 26 | 956 ± 145* | 1,035 ± 785* | 3,365 ± 789* |
| IL-10 | 35 ± 33 | 160 ± 52 | 185 ± 63* | 203 ± 60* | 621 ± 102* |
| TNF-α | 102 ± 45 | 637 ± 67 | 1,654 ± 546* | 1,856 ± 125* | 3,456 ± 768* |
| Nitric oxide | 156 ± 39 | 243 ± 71 | 545 ± 221* | 699 ± 212* | 806 ± 506* |
A group of uninfected cells was treated with 100 ng/ml LPS and 10 IU IFN-γ as a positive control of activation. Cytokine levels (± standard deviations) in L. major-infected cells and in untreated cells were also determined. Data are expressed as pg/ml and were obtained in three independent experiments. *, statistically significant when compared with results for untreated, L. major-infected control group (P < 0.05).
Dendritic cells infected with L. major were able to release IL-12, TNF-α, and nitric oxide following infection. However, this effect was greatly enhanced (10-fold) if PZA was added to the infected cells. Both dendritic cells and macrophages also increased the expression of costimulatory molecules (not shown). Together, these results suggest that PZA has immunostimulatory properties that may contribute to parasite killing beyond the leishmanicidal effect of the compounds.
The stimulatory effect of PZA is independent of TLR engagement.
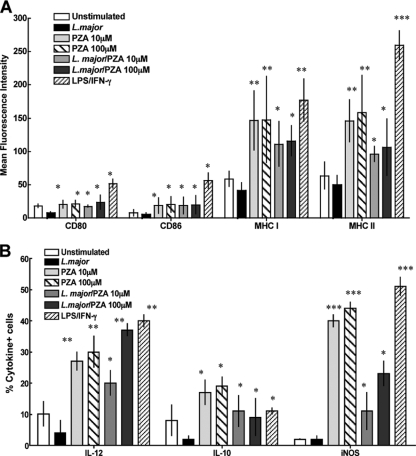
To confirm that the immunostimulatory effect of PZA is directly caused by the drug and is not due to contaminants (e.g., endotoxin) or other ligands that could cause cell activation via TLRs, we studied the effect of drug treatment on bone marrow-derived dendritic cells isolated from TLR-2/TLR-4 double knockout mice. In this experiment, we treated the dendritic cells as described above in the presence or absence of L. major infection. Again, as a positive control, cells were primed with IFN-γ and LPS. Figure 5A shows that drug treatment increased expression of activation markers and MHC molecules in a dose-dependent manner in dendritic cells lacking TLR-2 and -4, suggesting that PZA treatment and not a contaminant was responsible for cell activation. As before, infected cells treated with PZA showed an increase in upregulation of activation markers compared to results for cells treated with L. major alone. In the same way, the expression of IL-12, IL-10, and iNOS was increased in treated cells, irrespective of infection (Fig. 5B). These data further confirmed that PZA activates dendritic cells to initiate an inflammatory response and that this is a direct effect caused by the compound.
FIG. 5.
The immunostimulatory effect of PZA is independent of TLR engagement. Mean fluorescence values for the expression of CD80, CD86, MHC class I, and MHC class II molecules (A) or IL-12, IL-10, and iNOS expression determined by flow cytometry (B) in bone marrow-derived dendritic cells from TLR-2/-4 double knockout mice infected or not with L. major and treated with 10 or100 μM PZA are shown. Uninfected cells were treated with 10 U IFN-γ/100 ng/ml LPS as a positive control of activation. Results for unstimulated, untreated cells are also shown. Data are expressed as means ± standard deviations of three determinations. *, statistically significant (P ≤ 0.03); **, statistically significant (P ≤ 0.005); ***, statistically significant (P ≤ 0.0001) compared to results for the group infected with L. major and not treated.
DISCUSSION
The emergence of the leishmaniases and the lack of affordable therapy have necessitated the development of novel antileishmanial therapies. In this report, it has been shown that the clinical antituberculous drug PZA has activity against L. major both in vivo and in vitro. PZA is a drug that has been employed extensively, first having been used in the treatment of pulmonary tuberculosis in humans in 1949 (30). The use of a licensed, well-known drug for indications other than the treatment of tuberculosis could eliminate hurdles associated with the development of new antileishmanial antigens and provide therapeutic alternatives for a disease for which chemotherapy is suboptimal. Moreover, PZA is an orally administered drug, therefore obviating the need for parenteral injections.
Our data show that PZA is efficient at controlling the growth of L. major in vitro. The MIC50 is estimated to be 10 μM for promastigotes and 100.1 μM for amastigotes. These MIC50s are comparable to what was obtained by Klemens et al. (15) with a murine model of tuberculosis. Although intracellular amastigotes appeared to be less sensitive than promastigotes to the effect of the PZA at 48 h postinfection (10-fold increase in MIC50), an extended kinetic analysis revealed that PZA, employed at 100 μM, eliminated 90% of the parasites in cultured cells after 120 h of culture. This concentration is equivalent to what was found by Zhu et al. (32) in pharmacokinetic studies of PZA-treated children (serum concentration was 41 μg/ml), indicating that the standard antituberculous treatment regimen will be appropriate for the control of L. major infections. This is clearly supported by our striking in vivo data, which demonstrate that PZA treatment significantly decreased lesion development in mice infected with L. major at all concentrations tested (900, 450, and 150 mg/ml). PZA treatment also significantly decreased the parasite burden in the infection site without compromising the overall health of the infected mice. Now that it has been established that PZA is effective, and not toxic, at the maximum dose proposed for humans, these initial studies will be followed by others to determine the ideal dose/regimen in models of cutaneous and visceral disease.
The mechanism of action of PZA is just beginning to be unraveled. We have previously reported that PZA inhibits the enzyme FASI (33) by competitive inhibition of a NADPH binding site (25). L. major lacks FASI but possesses microsomal elongases (17) that effect many of the same chemical transformations as FAS. Our in vitro, and especially our in vivo, results are consistent with our hypothesis that this pathway is indeed an optimal target for the development of antileishmanial drugs.
It has been proposed, however, that Leishmania parasites can survive with greater altered lipid profiles than trypanosomes and even acquire lipids from the hosting macrophage (23). Our results demonstrate that long-term treatment of leishmanial cultures with PZA resulted in almost complete elimination of parasites from macrophages. If fatty acid synthesis is nonessential in Leishmania or these organisms are highly resistant to lipid depletion, it is possible that parasite killing is not mediated exclusively by the direct effect of the drug on parasite survival and replication. Thus, the antileishmanial effect of PZA may be caused (or enhanced) by chemotherapeutic interaction with the macrophage. Our data show that J774 cells, as well as primary cells from C57BL/6 mice, upregulate activation markers and release cytokines following treatment with PZA, demonstrating that the drug enhances the immune response to L. major infection. This immunoenhancing effect was not replicated in cells treated with amphotericin B, despite publications reporting the immunostimulatory effect of the drug (8), or in cells deficient in TRL-2 and -4 receptors, confirming that immunostimulation is a PZA-specific event. This phenomenon would be especially desirable in situations where patients are immunocompromised. Because Leishmania/HIV coinfections have been extensively documented (1), the development of drugs that boost the immune system of the host may be extremely useful.
The data presented here together provide the grounds for further testing of PZA (and PZA analogs) as an antileishmanial drug, for the determination of the immune status of the L. major-infected mice following PZA treatment, and for extension of these screens to other models of Leishmania infection. This pioneering alternative may work as a novel chemotherapeutic approach to treating leishmaniasis, in particular visceral leishmaniasis, the most severe leishmanial disease.
Acknowledgments
This work was funded by the Baker Institute for Animal Health.
Footnotes
Published ahead of print on 21 September 2009.
REFERENCES
- 1.Alvar, J., P. Aparicio, A. Aseffa, M. Den Boer, C. Canavate, J. P. Dedet, L. Gradoni, R. Ter Horst, R. Lopez-Velez, and J. Moreno. 2008. The relationship between leishmaniasis and AIDS: the second 10 years. Clin. Microbiol. Rev. 21:334-359, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antinori, S., E. Gianelli, S. Calattini, E. Longhi, M. Gramiccia, and M. Corbellino. 2005. Cutaneous leishmaniasis: an increasing threat for travellers. Clin. Microbiol. Infect. 11:343-346. [DOI] [PubMed] [Google Scholar]
- 3.Aronson, S. 2007. The Bagdad boil deploys to the United States. Med. Health R. I. 90:231. [PubMed] [Google Scholar]
- 4.Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappuis, F., S. Sundar, A. Hailu, H. Ghalib, S. Rijal, R. W. Peeling, J. Alvar, and M. Boelaert. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5:873-882. [DOI] [PubMed] [Google Scholar]
- 6.Chong, C. R., and D. J. Sullivan, Jr. 2007. New uses for old drugs. Nature 448:645-646. [DOI] [PubMed] [Google Scholar]
- 7.Coleman, R. E., D. A. Burkett, V. Sherwood, J. Caci, S. Spradling, B. T. Jennings, E. Rowton, W. Gilmore, K. Blount, C. E. White, and J. L. Putnam. 2007. Impact of phlebotomine sand flies on U.S. military operations at Tallil Air Base, Iraq. 2. Temporal and geographic distribution of sand flies. J. Med. Entomol. 44:29-41. [DOI] [PubMed] [Google Scholar]
- 8.Cuna, W. R., R. Velasquez, J. Riva, I. Guachalla, and C. Rodriguez. 2007. Enhancement of a t(h)1 immune response in amphotericin B-treated mucocutaneous leishmaniasis. J. Biomed. Biotechnol. 2007:96410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cynamon, M. H., S. P. Klemens, T. S. Chou, R. H. Gimi, and J. T. Welch. 1992. Antimycobacterial activity of a series of pyrazinoic acid esters. J. Med. Chem. 35:1212-1215. [DOI] [PubMed] [Google Scholar]
- 10.Desjeux, P. 2004. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 27:305-318. [DOI] [PubMed] [Google Scholar]
- 11.Doggrell, S. A. 2005. New drugs being developed for the treatment of tuberculosis. Expert Opin. Investig. Drugs 14:917-920. [DOI] [PubMed] [Google Scholar]
- 12.Halsey, E. S., L. M. Bryce, G. W. Wortmann, P. J. Weina, J. R. Ryan, and C. C. DeWitt. 2004. Visceral leishmaniasis in a soldier returning from Operation Enduring Freedom. Mil. Med. 169:699-701. [DOI] [PubMed] [Google Scholar]
- 13.Harding, C. V. 2001. Choosing and preparing antigen-presenting cells. Curr. Protoc. Immunol., chapter 16, unit 16.1. [DOI] [PubMed]
- 14.Kern, F., and J. K. Pedersen. 1973. Leishmaniasis in the United States. A report of ten cases in military personnel. JAMA 226:872-874. [PubMed] [Google Scholar]
- 15.Klemens, S. P., C. A. Sharpe, and M. H. Cynamon. 1996. Activity of pyrazinamide in a murine model against Mycobacterium tuberculosis isolates with various levels of in vitro susceptibility. Antimicrob. Agents Chemother. 40:14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, S. H., J. L. Stephens, K. S. Paul, and P. T. Englund. 2006. Fatty acid synthesis by elongases in trypanosomes. Cell 126:691-699. [DOI] [PubMed] [Google Scholar]
- 17.Livore, V. I., K. E. Tripodi, and A. D. Uttaro. 2007. Elongation of polyunsaturated fatty acids in trypanosomatids. FEBS J. 274:264-274. [DOI] [PubMed] [Google Scholar]
- 18.Magill, A. J., M. Grogl, R. A. Gasser, Jr., W. Sun, and C. N. Oster. 1993. Visceral infection caused by Leishmania tropica in veterans of Operation Desert Storm. N. Engl. J. Med. 328:1383-1387. [DOI] [PubMed] [Google Scholar]
- 19.Mendez, S., M. Nell, F. J. Fernandez-Perez, and J. M. Alunda. 1999. Sensitivity of Leishmania infantum amastigotes to fluorinated L-ornithine analogues. Med. Sci. Res. 1999:87-89. [Google Scholar]
- 20.Morris-Jones, S. D., and A. D. Bryceson. 1990. Cutaneous leishmaniasis after expedition to Panama. Lancet 336:691-692. [DOI] [PubMed] [Google Scholar]
- 21.Ngo, S. C., O. Zimhony, W. J. Chung, H. Sayahi, W. R. Jacobs, Jr., and J. T. Welch. 2007. Inhibition of isolated Mycobacterium tuberculosis fatty acid synthase I by pyrazinamide analogs. Antimicrob. Agents Chemother. 51:2430-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reithinger, R., and P. G. Coleman. 2007. Treating cutaneous leishmaniasis patients in Kabul, Afghanistan: cost-effectiveness of an operational program in a complex emergency setting. BMC Infect. Dis. 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts, C. W., R. McLeod, D. W. Rice, M. Ginger, M. L. Chance, and L. J. Goad. 2003. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol. Biochem. Parasitol. 126:129-142. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez, J. L., B. M. Diniega, J. W. Small, R. N. Miller, J. M. Andujar, P. J. Weina, P. G. Lawyer, W. R. Ballou, and J. K. Lovelace. 1992. Epidemiologic investigation of an outbreak of cutaneous leishmaniasis in a defined geographic focus of transmission. Am. J. Trop. Med. Hyg. 47:47-54. [DOI] [PubMed] [Google Scholar]
- 25.Sayahi, H., S. Putamadappa, S. C. Ngo, W. R. Jacobs, A. Shekhtman, J. T. Welch, and O. Zimhony. 2007. Saturation transfer difference NMR studies on the binding of the antitubercular agent pyrazinamide to Mycobacterium tuberculosis fatty acid synthase I, abstr. C1-1495. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 26.Spath, G. F., and S. M. Beverley. 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol. 99:97-103. [DOI] [PubMed] [Google Scholar]
- 27.Speirs, R. J., J. T. Welch, and M. H. Cynamon. 1995. Activity of n-propyl pyrazinoate against pyrazinamide-resistant Mycobacterium tuberculosis: investigations into mechanism of action of and mechanism of resistance to pyrazinamide. Antimicrob. Agents Chemother. 39:1269-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundar, S., and M. Chatterjee. 2006. Visceral leishmaniasis—current therapeutic modalities. Indian J. Med. Res. 123:345-352. [PubMed] [Google Scholar]
- 29.Woodrow, J. P., J. D. Hartzell, J. Czarnik, D. M. Brett-Major, and G. Wortmann. 2006. Cutaneous and presumed visceral leishmaniasis in a soldier deployed to Afghanistan. MedGenMed 8:43. [PMC free article] [PubMed] [Google Scholar]
- 30.Yeager, R. L., W. G. Munroe, and F. I. Dessau. 1952. Pyrazinamide (aldinamide*) in the treatment of pulmonary tuberculosis. Trans. Annu. Meet. Natl. Tuberc. Assoc. 48:178-201. [PubMed] [Google Scholar]
- 31.Zanoni, I., R. Ostuni, G. Capuano, M. Collini, M. Caccia, A. E. Ronchi, M. Rocchetti, F. Mingozzi, M. Foti, G. Chirico, B. Costa, A. Zaza, P. Ricciardi-Castagnoli, and F. Granucci. 2009. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460:264-268. [DOI] [PubMed] [Google Scholar]
- 32.Zhu, M., J. R. Starke, W. J. Burman, P. Steiner, J. J. Stambaugh, D. Ashkin, A. E. Bulpitt, S. E. Berning, and C. A. Peloquin. 2002. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy 22:686-695. [DOI] [PubMed] [Google Scholar]
- 33.Zimhony, O., J. S. Cox, J. T. Welch, C. Vilcheze, and W. R. Jacobs, Jr. 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 6:1043-1047. [DOI] [PubMed] [Google Scholar]