Abstract
The aim of the current study was to evaluate viral suppression following combined treatment with an S/pre-S1/pre-S2 vaccine and lamivudine in patients with chronic hepatitis B. We established a randomized, controlled clinical trial to compare the responses of three different treatment groups: those receiving vaccine monotherapy, lamivudine monotherapy, or combination treatment. Viral response was evaluated via hepatitis B virus (HBV) DNA suppression using different levels of classification. Seroconversion was evaluated via HBeAg loss, HBeAg seroconversion, HBsAg loss, and anti-HBs response. We found that the group receiving combination treatment demonstrated a significant increase in viral suppression over that for the lamivudine or vaccine monotherapy group, although the HBeAg seroconversion rate was not different. This enhanced suppression effect in the combination group was reversed after the discontinuation of vaccine treatment, suggesting that booster doses are required for a sustained viral response. Anti-HBs was detected in 55/120 vaccine recipients, but only 3 patients demonstrated HBsAg loss, indicating that the vaccine-induced anti-HBs was unable to completely neutralize HBsAg in the serum. At the study end point, anti-HBs responders showed significantly higher HBeAg seroconversion rates, greater suppression of HBV DNA levels, and a lower median reduction in HBV DNA levels than those of anti-HBs nonresponders. Our results suggest that combined treatment with the vaccine and lamivudine was significantly more effective than lamivudine monotherapy in the short term and was especially successful in producing viral suppression and an enhanced anti-HBs antibody response.
Chronic hepatitis B virus infection (CHB) is well established as a major health problem worldwide. Each year, more than 500,000 deaths are reported from an estimated 350 million individual sufferers, due to complications of CHB-related chronic liver disease (20, 23, 30). CHB is characterized by periodic activation of the host immune system against infected hepatocytes. This activation is often unable to eradicate the virus, and the infection usually progresses to liver fibrosis, cirrhosis, and even hepatocellular carcinoma (3, 32). Therefore, antiviral or immune therapy is generally administered with the aim of suppressing viral replication, eradicating the virus, and normalizing alanine aminotransferase (ALT) levels to prevent hepatocyte necrosis and cirrhosis. This is especially important for CHB patients exhibiting high viremia and high ALT levels (usually higher than two times the upper limit of normal [>2 ULN]) (8, 28).
Interferon (IFN) and nucleoside analogues are the main therapies currently used in the treatment of CHB (28). However, treatment with IFN is costly and has limited positive effects and numerous important side effects (28). Antiviral nucleoside analogues have rapid virological suppression effects, but patients are often unable to sustain the response when therapy is discontinued. In addition, the selection of hepatitis B virus (HBV)-resistant strains after long-term therapy with these agents has also been reported (26, 34) and has led to virological and hepatitis breakthrough (24, 40). Furthermore, adefovir, the antiviral nucleoside analogue used to treat CHB, has also been associated with nephrotoxicity following long-term therapy (11). These issues have promoted the development of novel therapeutic approaches in the treatment of CHB patients, including immunomodulatory drugs, adoptive transfer of immunity, and therapeutic vaccination (17).
Differences in antigenic structure have been investigated in experimental vaccine therapy studies in order to enhance both the humoral and the cytotoxic T-cell response during the eradication of infected liver cells. The recombinant triple hepatitis B vaccine, which contains pre-S1, pre-S2, and S antigenic components derived from mammalian cells, has shown significantly enhanced immunogenicity relative to that of conventional yeast-derived vaccines and has been used in immunotherapy (43, 45). It has been reported that therapeutic vaccination with GenHevacB (Aventis Pasteur, France) or Recombivax (containing S and pre-S2 antigens; Merck Sharp Dohme-Chibret, France) significantly reduces HBV DNA levels and leads to more-rapid HBeAg seroconversion in chronic adult HBV carriers (38), a response that likely occurs via induction of a CD4+ T-lymphocyte response to envelope antigens (6). In addition, vaccine monotherapy studies using various antigenic structures have also been conducted with different study populations and have shown a range of effects on HBeAg seroconversion and viral inhibition. However, only modest HBsAg seroconversion was observed in CHB patients (6, 37, 42).
Studies investigating combined vaccine and lamivudine (LAM) treatment have also been reported (12, 18, 36, 41). The effects of combination treatment compared to those of LAM monotherapy appear to be quite variable, a result that is thought to be due to differences in the timing of the vaccination, the type and dose of vaccine, the duration of vaccine intervention, and even the selection criteria for the study population. Therefore, we performed a randomized, controlled trial to evaluate the effects of combination treatment with a double dose of a triple hepatitis B vaccine (Sci-B-Vac; BTGC USA, SciGen, Ltd.) and LAM on CHB patients during the active-disease stage. The effects of treatment intervention were based on viral suppression and seroconversion, evaluated at different time points. The association between the viral response and the serological anti-HBs response was also analyzed. At the commencement of our study, LAM was considered the standard initial treatment for CHB patients (29, 39), and it continues to be one of the treatments of choice for naïve CHB patients in Vietnam (14).
MATERIALS AND METHODS
Patient selection.
The prospective, open-label study was carried out in the outpatient department of the Hospital for Tropical Diseases of Ho Chi Minh City, Vietnam. A total of 180 patients exhibiting CHB were enrolled in the study from 2003 to 2006. These patients met the following entry criteria: (i) they were adult males or females between the ages of 16 and 70 years; (ii) they demonstrated positive HBsAg in two separate samples assayed during the 6 months prior to the beginning of the study; (iii) they demonstrated positive HBeAg and no detectable anti-HBs, anti-HBc immunoglobulin M, or anti-HBe antibodies; and (iv) they exhibited elevated serum ALT levels that were >2 ULN but <10 ULN in at least two consecutive tests during the 3 months before enrollment in the study. Patients positive for HCV, HDV, or human immunodeficiency virus and those found to have a history of other liver diseases, including autoimmune diseases, alcoholic liver disease, and metabolic diseases, or kidney disease were excluded from the current study.
The study protocol was approved in advance by the Ethical and Scientific Committee of the Hospital for Tropical Diseases of Ho Chi Minh City and was performed according to the Declaration of Helsinki. All patients signed an informed-consent form prior to enrollment in this study. Clinical examination and laboratory tests, including tests for serum ALT and blood creatinine levels and complete blood counts, were performed every month during the first 9 months and every 3 months thereafter. HBsAg, anti-HBs, HBeAg, anti-HBe, and HBV DNA levels were measured every 3 months, until 18 months after inclusion in the study.
Study design.
Patients were randomly assigned at a 1:1:1 ratio to one of the following groups: (i) vaccine monotherapy (VAC group), (ii) combination LAM and vaccine therapy (V+L group), or (iii) LAM monotherapy (LAM group) (Fig. 1). The patients in the V+L and LAM groups were treated orally with 100 mg LAM (Stada) once daily. LAM treatment was discontinued for patients who exhibited HBeAg loss for 6 months. Patients in the VAC and V+L groups received a 20-μg intramuscular injection (a doubled adult dose of the commercial triple vaccine Sci-B-Vac [BTGC USA, SciGen, Ltd.]) in the deltoid muscle each month for 8 consecutive months.
FIG. 1.
Outline of the study design.
Evaluation.
Following the initiation of the study (baseline), patients were subjected to routine laboratory tests (ALT) and observed for side effects. Efficacy analyses were calculated for all patients who received at least one dose of drug or vaccine, in accordance with the intention-to-treat principle (33). Patients who were not available for testing at any time point were classified as exhibiting no response.
A reduction of more than 1 or 2 log copies/ml from baseline in the HBV DNA level after the first 3 months was considered to be a rapid virological response, termed the primary response. A biochemical response was defined as a normalization of serum ALT levels during the study period (<1.5 ULN in two consecutive tests). HBeAg loss was indicated by a disappearance of HBeAg, while HBeAg seroconversion was defined by a loss of HBeAg in conjunction with the appearance of anti-HBe antibody. Anti-HBs antibody levels of ≥10 mIU/ml were considered to represent an anti-HBs response. HBsAg seroconversion was indicated by a loss of HBsAg in conjunction with the appearance of anti-HBs antibody.
To analyze for a sustained response (off-vaccine treatment response), we classified the response after 18 months, compared with that after 9 months, into four categories: sustained response (sustained HBeAg loss or HBV DNA suppression from 9 to 18 months), additional response (no response after 9 months, but response after 18 months), no response (no response observed during the study), and HBeAg reversion (HBeAg loss after 9 months but positivity after 18 months) or HBV DNA breakthrough (HBV DNA level suppressed to <4 log copies/ml after 9 months but elevated to >4 log copies/ml after 18 months).
Serum HBV DNA levels were quantified by a commercial quantitative real-time PCR assay using TaqMan probes with a detection limit of ≥500 copies/ml. HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HCV, anti-HDV, and anti-human immunodeficiency virus antibodies were detected using the Abbott (Wiesbaden, Germany) microparticle enzyme immunoassay.
Statistical analysis.
The data for HBV DNA levels in each group before and after treatment are presented as medians and were compared using the median test. The rates of response in the groups were reported as percentages and were analyzed using the chi-square test, or Fisher's exact test for subgroups with small numbers of responses. P values of <0.05 were considered statistically significant. Missing values were not included when the mean and median values were calculated and compared, and the number of patients per group was also adjusted before the analysis. All statistical analyses were performed using the SPSS program, version 10.0.
RESULTS
Baseline characteristics.
At baseline, we observed similar biochemical and virological characteristics in the three treatment groups with respect to both age and sex (P > 0.05) (Table 1). Although the median HBV DNA level for the combination group (6.1 log copies/ml) was slightly lower than those for the other groups (6.8 and 6.5 log copies/ml for the VAC and LAM monotherapy groups, respectively), these differences were not statistically significant when they were assessed using the median test (P = 0.058). The majority of the study population was found to be male (63.3%), and 15% had been treated with LAM for hepatitis B previously. These subjects had ceased LAM treatment more than 6 months prior to the onset of the study. No patients had received prior IFN treatment. Of the 180 subjects initially enrolled, only 14 patients discontinued the study prior to completion. Three patients exited the vaccine group after 12 months; two left the combination group after 14 months; and nine left the LAM group after at least 14 months of treatment.
TABLE 1.
Baseline characteristics of CHB patients
| Characteristic | No. (%) of all patients (n = 180) | % of patients in the following treatment group: |
Pa | ||
|---|---|---|---|---|---|
| VAC (n = 60) | V+L (n = 60) | LAM (n = 60) | |||
| Male gender | 114 (63.3) | 65.0 | 65.0 | 60.0 | NS |
| Age, <30 yr | 109 (60.6) | 63.3 | 55.0 | 63.3 | NS |
| BMI,b <24.5 | 161 (89.4) | 85.0 | 90.0 | 93.3 | NS |
| Previous LAM therapy | 27 (15.0) | 15.0 | 20.0 | 10.0 | NS |
| Baseline HBV DNA level (log copies/ml)c | |||||
| >6 | 107 (59.4) | 68.3 | 48.3 | 61.7 | NS |
| >5 | 149 (82.8) | 83.3 | 78.3 | 86.7 | NS |
| >4 | 169 (93.9) | 95 | 90 | 96.7 | NS |
| Baseline ALT level, ≤5 ULN | 133 (73.9) | 68.3 | 75.0 | 78.3 | NS |
For the group comparison. NS, P > 0.05.
BMI, body mass index.
The median baseline HBV DNA level for all patients was 6.5 log copies/ml; for the VAC, V+L, and LAM treatment groups, these levels were 6.8 (range, 3.1 to 9.1), 6.1 (range, 3.5 to 8.9), and 6.5 (range, 3.9 to 9.5) log copies/ml, respectively. The P value for the group comparison was 0.058 by the median test.
We did not observe any serious side effects, intense biochemical flare, or hepatic decompensation due to immune induction in any patient during the treatment and follow-up periods.
HBeAg loss and HBeAg seroconversion.
Our results showed that HBeAg seroconversion rates for the three groups were approximately 7 to 20%, 18 to 27%, and 21 to 33% after 3, 12, and 18 months of treatment, respectively. The rate of HBeAg seroconversion increased gradually over the study period in the two vaccine groups but remained unchanged in the LAM monotherapy group (Table 2). We did not observe any significant differences among the three treatment groups in terms of HBeAg loss and HBeAg seroconversion over the course of the study.
TABLE 2.
Serological and virological responses of the three groups
| Time of evaluation and response | No. (%) of patients in the following treatment group: |
Pa | ||
|---|---|---|---|---|
| VAC | V+L | LAM | ||
| After 3 mo | ||||
| HBeAg loss | 6 (10.0) | 10 (16.7) | 11 (18.3) | NS |
| HBeAg seroconversion | 4 (6.7) | 7 (11.7) | 11 (18.3) | NS |
| HBV DNA reduction (log copies/ml)b | ||||
| >1 | 11 (18.3) | 39 (65.0) | 33 (55.0) | <0.001 |
| >2 | 5 (8.3) | 32 (53.3) | 20 (33.3) | <0.001 (0.027) |
| HBV DNA level, ≤4 log copies/ml | 5 (8.3) | 33 (55.0) | 17 (28.3) | <0.001 (0.044) |
| After 12 mo | ||||
| HBeAg loss | 18 (30.0) | 14 (23.3) | 15 (25.0) | NS |
| HBeAg seroconversion | 16 (26.7) | 14 (23.3) | 11 (18.3) | NS |
| HBV DNA level (log copies/ml) | ||||
| ≤4 | 9 (15.0) | 39 (65.0) | 26 (43.3) | <0.001 (0.017) |
| ≤5 | 19 (31.7) | 44 (73.3) | 39 (66.1) | <0.001 |
| After 18 mo | ||||
| HBeAg loss | 21 (35.0) | 18 (30.0) | 17 (28.3) | NS |
| HBeAg seroconversion | 20 (33.3) | 15 (25.0) | 13 (21.7) | NS |
| HBV DNA level (log copies/ml) | ||||
| ≤4 | 10 (16.7) | 32 (53.3) | 27 (45.0) | 0.001 |
| ≤5 | 20 (33.3) | 35 (58.3) | 35 (58.3) | 0.02 |
P values for the overall test of treatment effect are given. P values for differences between the V+L and LAM groups are given in parentheses only when the differences are significant. NS, not significant (P > 0.05).
The median reductions in HBV DNA levels were 0.0 (range, −3.16 to 3.75) log copies/ml for the VAC treatment group (n = 56), −2.60 (range, −7.06 to 4.38) log copies/ml for the V+L group (n = 54), and −1.9 (range, −7.09 to 2.23) log copies/ml for the LAM group (n = 52). The P value was <0.001 by the median test.
Reduction in HBV DNA levels.
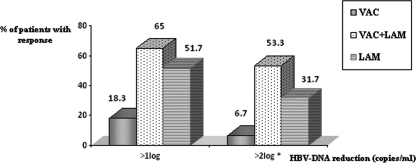
At the end of the third treatment month, the rates of HBV DNA reduction (by >1 log copy/ml) from baseline were found to be significantly higher for the V+L and LAM groups (65% and 55%) than for the VAC monotherapy group (18.3%) (P < 0.001). The median reductions in HBV DNA levels after 3 months were also significantly higher for the V+L group (−2.6 log copies/ml) and the LAM group (−1.9 log copies/ml) than for the VAC monotherapy group (0.0 log copies/ml), suggesting an early virological response related to LAM treatment (Table 2) (P < 0.001). Furthermore, the rate of virological responses with HBV DNA reductions of >2 log copies/ml from baseline (53.3% versus 31.7% [Fig. 2]) (P = 0.016) and the rate of HBV DNA suppression (<4 log copies/ml) (55% versus 28.3% [Table 2]) (P < 0.001) were also significantly higher for the V+L group than for the LAM group. The frequency of viral suppression (HBV DNA levels, <4 log copies/ml) after 12 months was also significantly higher for the combination group than for the LAM group (P < 0.05) (Table 2). However, the enhanced suppression effect in the combination group compared to the LAM monotherapy group was lost during follow-up (after 18 months).
FIG. 2.
HBV DNA reductions after 3 months of treatment. The percentage of patients with early HBV DNA reduction (after 3 months) was expressed using two cutoff levels (reductions of >1 log copy/ml and >2 log copies/ml). The asterisk indicates a significant difference between the V+L and LAM groups (P < 0.05).
Biochemical response.
In the early stages of the current study (3 to 12 months), a significantly higher number of patients receiving treatment with LAM (in either the V+L or the LAM group) than of patients receiving VAC alone exhibited normal ALT levels (P < 0.05) (Table 3). However, at the end of the study (18 months), the rates of biochemical response were similar for all three treatment groups.
TABLE 3.
Biochemical responses of the three treatment groups
| Time of evaluation | No. (%) of patients with biochemical responses (ALT level, <1.5 ULN) to: |
Pa | ||
|---|---|---|---|---|
| VAC | V+L | LAM | ||
| 3 mo | 13 (21.7) | 23 (38.3) | 33 (55.0) | <0.001 |
| 12 mo | 31 (50.0) | 43 (71.7) | 44 (73.3) | 0.011 |
| 18 mo | 38 (63.3) | 40 (66.7) | 47 (78.3) | NS |
For the overall treatment effect. NS, not significant (P > 0.05).
HBsAg loss, appearance of anti-HBs, and HBsAg seroconversion.
Four patients experienced HBsAg loss during the follow-up period: one patient in the vaccine monotherapy group after 12 months, two combination group patients after 14 and 18 months, and one LAM monotherapy patient after 18 months. Anti-HBs responses were detected in 50 of the 120 vaccine recipients (27.8%) but were not detected in patients in the LAM group. HBsAg seroconversion (that is, HBsAg loss with anti-HBs detection) was observed for two patients in the VAC monotherapy group (one after 15 months and one after 18 months). However, anti-HBs was not detected in two patients of the V+L group and the LAM group who experienced HBsAg loss. The rates of anti-HBs responses (≥10 mIU/ml) in both the VAC and V+L groups increased in a time-dependent manner during the study period (Table 4). Additionally, the proportion of anti-HBs responders was statistically higher in the V+L therapy group than in the VAC monotherapy group. Furthermore, 49% of patients with anti-HBs detection (27/55 cases) also exhibited high levels of anti-HBs (between 100 and 1,000 mIU/ml) after 12 months.
TABLE 4.
Vaccine-induced anti-HBs responses after 18 months for the three treatment groups
| Mo | No. (%) of anti-HBs responders in the following treatment group: |
Pa | ||
|---|---|---|---|---|
| VAC | V+L | LAM | ||
| 3 | 2 (3.3) | 3 (5.0) | 0 | NS |
| 9 | 13 (21.7) | 21 (35.0) | 0 | <0.001 |
| 12 | 17 (28.3)b | 27 (45.0)b | 0 | <0.001 |
| 15 | 17 (28.3)b | 28 (46.7)b | 0 | <0.001 |
| 18 | 19 (31.7)b | 31 (51.7)b | 0 | <0.001 |
For the overall treatment effect.
The difference between the VAC and V+L groups was significant (P < 0.05).
At the end point of this study (month 18), anti-HBs responders demonstrated a significantly higher frequency of HBeAg seroconversion (40% versus 21.4%) (P = 0.027), a higher rate of HBV DNA suppression (<4 log copies/ml) (P < 0.001), and a lower median reduction in HBV DNA levels (P = 0.027) than anti-HBs nonresponders (Table 5).
TABLE 5.
Association between vaccine-induced anti-HBs and serological or virological response
| Response and group | No. (%) of patients: |
P | |
|---|---|---|---|
| With vaccine-induced anti-HBs after 18 mo (n = 50) | Without vaccine-induced anti-HBs after 18 mo (n = 70) | ||
| HBeAg seroconversion | |||
| Both vaccine groups | 20 (40.0) | 15 (21.4) | 0.027 |
| VAC | 11 (57.9) | 9 (22.0) | 0.006 |
| V+L | 9 (29.0) | 6 (20.7) | 0.45 |
| HBV DNA suppression (<4 log copies/ml)a | |||
| Both vaccine groups | 28 (56.0) | 14 (20.0) | <0.001 |
| VAC | 7 (36.8) | 4 (9.8) | 0.027b |
| V+L | 21 (67.7) | 10 (34.5) | 0.01 |
Median HBV DNA reductions were 4.13 ± 2.9 log copies/ml for patients with vaccine-induced anti-HBs and 5.52 ± 2.7 log copies/ml for patients without vaccine-induced anti-HBs (P = 0.027).
By Fisher's exact test.
Off-treatment response.
The off-treatment serological and virological responses for the three treatment groups are presented in Tables 6 and 7, respectively. The results revealed that HBeAg reversion was observed following LAM monotherapy and V+L combination therapy. In addition, patients in the V+L combination group demonstrated a lower rate of virological nonresponse (28.3% versus 48.3%) and a higher rate of sustained or additional HBV DNA response (51.7% versus 43.3%) than those in the LAM monotherapy group. However, the combination group also demonstrated a higher rate of viral breakthrough than the LAM monotherapy group (20.0% versus 8.3%; P = 0.04 [Table 7]).
TABLE 6.
HBeAg seroconversion at month 18 compared to that at month 9
| HBeAg seroconversion status after 18 mo | No. (%) of patients in the following treatment groupa: |
||
|---|---|---|---|
| VAC | V+L | LAM | |
| Sustained or additional seroconversion | 20 (33.3) | 15 (25.0) | 13 (21.7) |
| No response | 40 (66.7) | 41 (68.3) | 44 (73.3) |
| HBeAg reversion | 0 (0.0) | 4 (6.7) | 3 (5.0) |
Differences between treatment groups were not significant (P > 0.05 by Fisher's exact test).
TABLE 7.
Virological responsea at month 18 compared to that at month 9 (off-treatment response)
| Virological response status at mo 18 | No. (%) of patients in the following treatment groupb: |
||
|---|---|---|---|
| VAC | V+L | LAM | |
| Sustained or additional HBV DNA suppression | 11 (18.3) | 31 (51.7) | 26 (43.4) |
| No virological response | 46 (76.7) | 17 (28.3) | 29 (48.3) |
| HBV DNA breakthrough | 3 (5.0) | 12 (20.0)c | 5 (8.3)c |
Defined as an HBV DNA level of <4 log copies/ml.
P < 0.001 for comparison among the three groups.
P = 0.04 for comparison between the V+L and LAM groups.
DISCUSSION
The practical aim of CHB therapy is to suppress HBV replication, normalize ALT levels, and induce HBeAg seroconversion in order to prevent disease progression to cirrhosis and hepatocellular carcinoma. Treatment options for CHB patients are limited to two approved therapeutic approaches including treatment with IFN and nucleoside analogues. Our novel approach using therapeutic vaccination combined with LAM was thought to be effective in generating potential synergistic or additive effects. LAM is known to directly reduce the viral load, while cytokines released following vaccine therapy may enhance the cellular immune response to suppress viral replication or to clear the virus. The limited combination therapy studies of CHB patients using HBV vaccine and LAM conducted to date have shown differing results for HBeAg seroconversion and viral suppression (12, 18, 41). Very few reports have noted any anti-HBs response after vaccine therapy or its effect on viral clearance.
In the natural course of HBV infection, HBeAg seroconversion has been recognized as an important event. Early HBeAg seroconversion suggests a better clinical outcome, while an absent or late HBeAg seroconversion after multiple hepatitis flares enhances progression to cirrhosis (5, 16). For HBeAg-positive CHB patients, the current aims of treatment are to obtain a biochemical response, HBeAg seroconversion, and suppression of viral replication to less than 104 to 105 copies of HBV DNA/ml (7, 25, 28). Sustaining virological and biochemical responses has also been suggested as an additional treatment target (9, 35).
In the current study, the rates of HBeAg seroconversion were not significantly different among the three groups at any time point. However, the HBeAg seroconversion rate did increase with time during the first 12 months in the VAC and V+L groups. This result differed from that for the LAM monotherapy group, where a rapid increase in HBeAg seroconversion was observed, suggesting that the vaccine slowly but steadily enhanced the immune response, thus controlling the clearance of HBV. Higher HBeAg seroconversion rates for the combination group than for the LAM monotherapy group after 12 months of treatment have also been reported (12), further supporting our hypothesis. Additional studies using a greater number of participants are required to further support this hypothesis.
The current randomized study resulted in an enhanced virological response in the combination group compared to the vaccine and LAM monotherapy groups. The combination of the vaccine and LAM was also found to be superior to vaccine monotherapy or LAM monotherapy in terms of early suppression (months 3 to 12), the continuous control of HBV replication to HBV DNA levels of <4 log copies/ml or below the limit of detection, and the promotion of a sustained virological response. However, the results obtained in our study differ from those reported previously. Using 12 fortnightly doses of pre-S2/S vaccine, Horiike et al. reported that 9 (100%) HBeAg-positive patients receiving combination treatment demonstrated undetectable HBV DNA levels (<3.7 log copies/ml) after treatment but that only 15 of 31 patients (48%) in the LAM monotherapy group showed undetectable HBV DNA levels (12). In contrast, using 12 doses of HBsAg/AS02 vaccine, Vandepapelière et al. showed that rates of viral suppression, as defined by HBV DNA levels of <5 log copies/ml, were not different between the combination and LAM groups during the study period (41). These variations in response are likely due to differences between the antigen compositions of the vaccines, the vaccination schedules, or the evaluation criteria for response in the two studies.
The mechanisms underlying viral clearance following therapeutic vaccination have been investigated in numerous studies. It is thought that specific exogenous antigens present in the vaccine may enhance the uptake and processing of HBsAg, recruit dendritic cells for antigen presentation around the injection site, upregulate major histocompatibility complex class II and CD86 on dendritic cells (1, 13), or upregulate the production of interleukin-2, IFN-γ, and tumor necrosis factor alpha from antigen-stimulated T cells and differentiated lymphocyte T helper cells toward Th1 (19). The production of cytokines by HBV-specific T lymphocytes may also reduce serum HBV DNA levels via cytopathogenic and noncytopathogenic pathways. These actions may be sufficient to suppress HBV DNA levels but not to eradicate HBeAg. These findings suggest that the combination of vaccine and LAM may have more of an effect on the viral load than on the HBeAg seroconversion rate. Additionally, combination treatment with the vaccine may delay the appearance of LAM-resistant mutations and may help explain the steady increase in the rate of treatment response in the V+L group but not in the LAM monotherapy group. Further studies are required to investigate this hypothesis.
On the other hand, our results also demonstrate that the suppression effects were not well sustained after the discontinuation of treatment with the HBV vaccine, a finding in agreement with previous reports (18). The fact that all patients in the combination group in our study continued to receive LAM but not the vaccine after 8 months indicated that the efficacy of viral suppression by the vaccine in the schedule was reduced when treatment with the vaccine was discontinued. Despite the rate of sustained HBV DNA suppression after 9 months, and the higher rate of additional HBV DNA suppression in the combination group than in the LAM monotherapy group, the rate of HBV DNA breakthrough after 9 months was also found to be higher in the combination group, suggesting that the vaccine therapy schedule of eight injections was not sufficient to produce a sustained response. Further studies may be required to optimize the immunization protocols for effective combination treatment of CHB patients.
Consistent with previous observations, we found that the number of patients exhibiting ALT normalization in the LAM monotherapy group was approximately 60 to 80% at the end of the study (10, 22, 27). Similar numbers of patients with biochemical responses have also been reported in the three treatment groups at the end of the study, suggesting that a biochemical response is achieved by LAM monotherapy, vaccine monotherapy, or the V+L combination. At 18 months, a biochemical response appears to be achieved without HBeAg seroconversion or HBV DNA suppression. However, patients in the vaccine monotherapy group normalized their ALT levels more slowly than those in the LAM monotherapy group (21% versus 55% after 3 months), suggesting that the immune response to HBV after vaccine intervention contributed to the immune-induced pathogenic clearance of HBV during the first 12 months of treatment.
The HBsAg gene contains a neutralizing epitope termed α, which is located at codon positions 124 to 147. Anti-HBs antibodies classically indicate viral clearance and lifelong immunity after recovery from natural infection. These antibodies have been considered to be a key factor in the clearance of virions and HBsAg-containing particles from the circulation (2), and they are able to blockade viral particle receptors on new target cells (4). Hence, coexistence of HBsAg and anti-HBs is generally not detected in natural infection. In the current study, anti-HBs antibodies were detected in 55/120 vaccine recipients (51.7% of patients in the combination group and 31.7% in the monotherapy group at the end of the study); however, only 4 patients also lost HBsAg during the study period. The remainder of the vaccine recipients demonstrated coexistence of HBsAg and anti-HBs. Hence, the anti-HBs demonstrated in the study should be considered to be vaccine-induced anti-HBs. Coexistence of HBsAg and anti-HBs was also observed in patients treated with a combination of an HBV vaccine and LAM in the study of Vandepapelière et al. (41), indicating that either anti-HBs induced by therapeutic vaccination in CHB patients is not able to completely neutralize HBsAg in the serum or a longer period is required for the complete removal of existing HBV particles from the circulation. Additional potential reasons for the ineffectiveness of anti-HBs in the clearance of HBsAg may be the presence of nonneutralizing epitopes, low sensitivity of HBsAg to anti-HBs, or the emergence of HBsAg mutants (21, 31). Mutations in the α region are thought to affect the neutralization HBsAg by its corresponding anti-HBs, aiding the escape of virus from the host immune system (44). In support of this hypothesis, a small number of HBV escape mutants have been reported in children following HBV immunization (15); however, further analysis is required to confirm this possibility.
Interestingly, the current study also shows higher rates of anti-HBs response in the LAM and vaccine combination groups than in the vaccine monotherapy groups, suggesting an interesting role for LAM in fostering responses following vaccine treatment. In contrast, vaccine recipients exhibiting a positive anti-HBs response demonstrate a greater probability of HBeAg seroconversion and higher rate and degree of HBV DNA suppression, suggesting that anti-HBs may play an important role in enhancing HBV DNA suppression in vaccine monotherapy and combination therapy.
In conclusion, our study suggests that combination treatment with the vaccine and LAM is more beneficial than LAM monotherapy in the short term, especially in terms of enhanced viral suppression and the anti-HBs antibody response to the vaccine.
Acknowledgments
This work was supported in part by the Department of Science and Technology (DOST) of Ho Chi Minh City, Vietnam, and was managed by the University of Pharmacy and Medicine (Ho Chi Minh City, Vietnam). It also received support from a Grant-in-Aid for Young Scientists (17301870, 2008 to 2010; to N.T.H.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT; Japan), a Grant-in-Aid for Scientific Research from Nagasaki University (2007 to 2009; to N.T.H.), and the Global COE Program (2008 to 2012; to K.H.).
We thank the staff of the Hospital for Tropical Diseases (Ho Chi Minh City, Vietnam) for cooperation in the study process and Nam Khoa Biotech Company (Ho Chi Minh City, Vietnam) for assistance with the HBV DNA analysis.
The authors declare no competing interests due to commercial or other affiliations.
Footnotes
Published ahead of print on 21 September 2009.
REFERENCES
- 1.Akbar, S. M., M. Abe, T. Masumoto, N. Horiike, and M. Onji. 1999. Mechanism of action of vaccine therapy in murine hepatitis B virus carriers: vaccine-induced activation of antigen presenting dendritic cells. J. Hepatol. 30:755-764. [DOI] [PubMed] [Google Scholar]
- 2.Böcher, W. O., S. Herzog-Hauff, W. Herr, K. Heermann, G. Gerken, K. H. Meyer zum Buschenfelde, and H. F. Lohr. 1996. Regulation of the neutralizing anti-hepatitis B surface (HBs) antibody response in vitro in HBs vaccine recipients and patients with acute or chronic hepatitis B virus (HBV) infection. Clin. Exp. Immunol. 105:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch, F. X., J. Ribes, R. Cleries, and M. Diaz. 2005. Epidemiology of hepatocellular carcinoma. Clin. Liver Dis. 9:191-211. [DOI] [PubMed] [Google Scholar]
- 4.Brown, J. L., W. F. Carman, and H. C. Thomas. 1990. The hepatitis B virus. Baillieres Clin. Gastroenterol. 4:721-747. [DOI] [PubMed] [Google Scholar]
- 5.Chu, C. J., M. Hussain, and A. S. Lok. 2002. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology 122:1756-1762. [DOI] [PubMed] [Google Scholar]
- 6.Couillin, I., S. Pol, M. Mancini, F. Driss, C. Brechot, P. Tiollais, and M. L. Michel. 1999. Specific vaccine therapy in chronic hepatitis B: induction of T cell proliferative responses specific for envelope antigens. J. Infect. Dis. 180:15-26. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis, R., A. Hadengue, G. Lau, D. Lavanchy, A. Lok, N. McIntyre, A. Mele, G. Paumgartner, A. Pietrangelo, J. Rodes, W. Rosenberg, and D. Valla. 2003. EASL International Consensus Conference on Hepatitis B. 13-14 September, 2002, Geneva, Switzerland. Consensus statement (long version). J. Hepatol. 39(Suppl. 1):S3-S25. [PubMed] [Google Scholar]
- 8.Dusheiko, G., and N. Antonakopoulos. 2008. Current treatment of hepatitis B. Gut 57:105-124. [DOI] [PubMed] [Google Scholar]
- 9.Fattovich, G., F. Bortolotti, and F. Donato. 2008. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J. Hepatol. 48:335-352. [DOI] [PubMed] [Google Scholar]
- 10.Gish, R. G. 2007. Improving outcomes for patients with chronic hepatitis B. Curr. Gastroenterol. Rep. 9:14-22. [DOI] [PubMed] [Google Scholar]
- 11.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T. T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 348:800-807. [DOI] [PubMed] [Google Scholar]
- 12.Horiike, N., S. M. Fazle Akbar, K. Michitaka, K. Joukou, K. Yamamoto, N. Kojima, Y. Hiasa, M. Abe, and M. Onji. 2005. In vivo immunization by vaccine therapy following virus suppression by lamivudine: a novel approach for treating patients with chronic hepatitis B. J. Clin. Virol. 32:156-161. [DOI] [PubMed] [Google Scholar]
- 13.Horiike, N., S. M. Fazle Akbar, T. Ninomiya, M. Abe, K. Michitaka, and M. Onji. 2002. Activation and maturation of antigen-presenting dendritic cells during vaccine therapy in patients with chronic hepatitis due to hepatitis B virus. Hepatol. Res. 23:38-47. [DOI] [PubMed] [Google Scholar]
- 14.Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam. 2006. Guidance for diagnosis and treatment of common infectious diseases, p. 54-59. Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam.
- 15.Hsu, H. Y., M. H. Chang, S. H. Liaw, Y. H. Ni, and H. L. Chen. 1999. Changes of hepatitis B surface antigen variants in carrier children before and after universal vaccination in Taiwan. Hepatology 30:1312-1317. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, Y. S., R. N. Chien, C. T. Yeh, I. S. Sheen, H. Y. Chiou, C. M. Chu, and Y. F. Liaw. 2002. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 35:1522-1527. [DOI] [PubMed] [Google Scholar]
- 17.Hui, C. K., and G. K. Lau. 2005. Advances in immunomodulating therapy of HBV infection. Int. J. Med. Sci. 2:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa, T., and S. Kakumu. 2007. Combination therapy with lamivudine and HB vaccine on chronic hepatitis B. Hepatol. Res. 37:S62-S66. [DOI] [PubMed] [Google Scholar]
- 19.Jung, M. C., U. Spengler, W. Schraut, R. Hoffmann, R. Zachoval, J. Eisenburg, D. Eichenlaub, G. Riethmuller, G. Paumgartner, H. W. Ziegler-Heitbrock, et al. 1991. Hepatitis B virus antigen-specific T-cell activation in patients with acute and chronic hepatitis B. J. Hepatol. 13:310-317. [DOI] [PubMed] [Google Scholar]
- 20.Kane, M. 1995. Global programme for control of hepatitis B infection. Vaccine 13(Suppl. 1):S47-S49. [DOI] [PubMed] [Google Scholar]
- 21.Lada, O., Y. Benhamou, T. Poynard, and V. Thibault. 2006. Coexistence of hepatitis B surface antigen (HBs Ag) and anti-HBs antibodies in chronic hepatitis B virus carriers: influence of “a” determinant variants. J. Virol. 80:2968-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau, G. K., T. Piratvisuth, K. X. Luo, P. Marcellin, S. Thongsawat, G. Cooksley, E. Gane, M. W. Fried, W. C. Chow, S. W. Paik, W. Y. Chang, T. Berg, R. Flisiak, P. McCloud, and N. Pluck. 2005. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 352:2682-2695. [DOI] [PubMed] [Google Scholar]
- 23.Lavanchy, D. 2004. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J. Viral Hepat. 11:97-107. [DOI] [PubMed] [Google Scholar]
- 24.Lee, Y. S., D. J. Suh, Y. S. Lim, S. W. Jung, K. M. Kim, H. C. Lee, Y. H. Chung, Y. S. Lee, W. Yoo, and S. O. Kim. 2006. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 43:1385-1391. [DOI] [PubMed] [Google Scholar]
- 25.Liaw, Y. F., N. Leung, R. Guan, G. K. Lau, I. Merican, G. McCaughan, E. Gane, J. H. Kao, and M. Omata. 2005. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 25:472-489. [DOI] [PubMed] [Google Scholar]
- 26.Locarnini, S. 2005. Molecular virology and the development of resistant mutants: implications for therapy. Semin. Liver Dis. 25(Suppl. 1):9-19. [DOI] [PubMed] [Google Scholar]
- 27.Lok, A. S. 2005. The maze of treatments for hepatitis B. N. Engl. J. Med. 352:2743-2746. [DOI] [PubMed] [Google Scholar]
- 28.Lok, A. S., and B. J. McMahon. 2007. Chronic hepatitis B. Hepatology 45:507-539. [DOI] [PubMed] [Google Scholar]
- 29.Lok, A. S. F., and B. J. McMahon. 2001. Chronic hepatitis B. Hepatology 34:1225-1241. [DOI] [PubMed] [Google Scholar]
- 30.Maddrey, W. C. 2001. Hepatitis B—an important public health issue. Clin. Lab. 47:51-55. [PubMed] [Google Scholar]
- 31.Margeridon, S., A. Lachaux, C. Trepo, F. Zoulim, and A. Kay. 2005. A quasi-monoclonal anti-HBs response can lead to immune escape of ‘wild-type’ hepatitis B virus. J. Gen. Virol. 86:1687-1693. [DOI] [PubMed] [Google Scholar]
- 32.McMahon, B. J. 2004. The natural history of chronic hepatitis B virus infection. Semin. Liver Dis. 24(Suppl. 1):17-21. [DOI] [PubMed] [Google Scholar]
- 33.Montori, V. M., and G. H. Guyatt. 2001. Intention-to-treat principle. CMAJ 165:1339-1341. [PMC free article] [PubMed] [Google Scholar]
- 34.Papatheodoridis, G. V., E. Dimou, A. Laras, V. Papadimitropoulos, and S. J. Hadziyannis. 2002. Course of virologic breakthroughs under long-term lamivudine in HBeAg-negative precore mutant HBV liver disease. Hepatology 36:219-226. [DOI] [PubMed] [Google Scholar]
- 35.Papatheodoridis, G. V., S. Manolakopoulos, G. Dusheiko, and A. J. Archimandritis. 2008. Therapeutic strategies in the management of patients with chronic hepatitis B virus infection. Lancet Infect. Dis. 8:167-178. [DOI] [PubMed] [Google Scholar]
- 36.Phuong, N. T. M., B. Dai, and N. T. Chinh. 2004. Efficacy of lamivudine monotherapy and lamivudine combination with Hepa-B-vac for active chronic hepatitis B. Tap Chi Thong Tin Y Duoc (The national seminar on hepatobiliary diseases in HCM city) 2004:57-61.
- 37.Pol, S., F. Driss, M. L. Michel, B. Nalpas, P. Berthelot, and C. Brechot. 1994. Specific vaccine therapy in chronic hepatitis B infection. Lancet 344:342. [DOI] [PubMed] [Google Scholar]
- 38.Pol, S., B. Nalpas, F. Driss, M. L. Michel, P. Tiollais, J. Denis, and C. Brecho. 2001. Efficacy and limitations of a specific immunotherapy in chronic hepatitis B. J. Hepatol. 34:917-921. [DOI] [PubMed] [Google Scholar]
- 39.Sherman, M., V. Bain, J. P. Villeneuve, R. P. Myers, C. Cooper, S. Martin, and C. Lowe. 2004. The management of chronic viral hepatitis: a Canadian consensus conference 2004. Can. J. Gastroenterol. 18:715-728. [DOI] [PubMed] [Google Scholar]
- 40.Stuyver, L. J., S. A. Locarnini, A. Lok, D. D. Richman, W. F. Carman, J. L. Dienstag, and R. F. Schinazi. 2001. Nomenclature for antiviral-resistant human hepatitis B virus mutations in the polymerase region. Hepatology 33:751-757. [DOI] [PubMed] [Google Scholar]
- 41.Vandepapelière, P., G. K. Lau, G. Leroux-Roels, Y. Horsmans, E. Gane, T. Tawandee, M. I. Merican, K. M. Win, C. Trepo, G. Cooksley, M. Wettendorff, and C. Ferrari. 2007. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 25:8585-8597. [DOI] [PubMed] [Google Scholar]
- 42.Wen, Y. M., X. H. Wu, D. C. Hu, Q. P. Zhang, and S. Q. Guo. 1995. Hepatitis B vaccine and anti-HBs complex as approach for vaccine therapy. Lancet 345:1575-1576. [DOI] [PubMed] [Google Scholar]
- 43.Yap, I., R. Guan, and S. H. Chan. 1995. Study on the comparative immunogenicity of a recombinant DNA hepatitis B vaccine containing pre-S components of the HBV coat protein with non pre-S containing vaccines. J. Gastroenterol. Hepatol. 10:51-55. [DOI] [PubMed] [Google Scholar]
- 44.Zheng, X., K. M. Weinberger, R. Gehrke, M. Isogawa, G. Hilken, T. Kemper, Y. Xu, D. Yang, W. Jilg, M. Roggendorf, and M. Lu. 2004. Mutant hepatitis B virus surface antigens (HBsAg) are immunogenic but may have a changed specificity. Virology 329:454-464. [DOI] [PubMed] [Google Scholar]
- 45.Zuckerman, J. N., C. Sabin, F. M. Craig, A. Williams, and A. J. Zuckerman. 1997. Immune response to a new hepatitis B vaccine in healthcare workers who had not responded to standard vaccine: randomised double blind dose-response study. BMJ 314:329-333. [DOI] [PMC free article] [PubMed] [Google Scholar]