Abstract
A broth microdilution method was used to evaluate the in vitro activities of seven antifungal agents against 15 clinical strains of Rhizopus microsporus. Amphotericin B (AMB) and posaconazole (POS) were the most active drugs. In a model of disseminated R. microsporus infection in immunosuppressed mice, we studied the efficacy of POS administered once or twice daily against four of the strains previously tested in vitro and compared it with that of liposomal AMB (LAMB). LAMB was the most effective treatment for the two strains with intermediate susceptibility to POS. For the two POS-susceptible strains, LAMB and POS at 20 mg/kg of body weight twice a day orally showed similar efficacies. The in vivo efficacy of POS administered twice a day orally correlated with the in vitro susceptibility data and the serum drug concentrations.
Zygomycosis is a frequently lethal invasive infection that occurs predominantly in immunocompromised patients (4), a population with a very poor prognosis and a high mortality rate (8). The clinical manifestations include rhino-orbito-cerebral, cutaneous, pulmonary, gastrointestinal, and disseminated infections (4). In a recent study in which a large number of clinical isolates of zygomycetes from different regions of the United States were molecularly identified, it was demonstrated that Rhizopus oryzae and Rhizopus microsporus were the most common species (3). Traditionally, amphotericin B (AMB) and, more recently, its lipid formulations are the front-line agents for the treatment of zygomycosis (4). Specifically, liposomal amphotericin B (LAMB) is less nephrotoxic than AMB and has better central nervous system penetration than AMB and the other lipid formulations (21). Posaconazole (POS) is a broad-spectrum triazole antifungal with a large volume of distribution into tissues (12). This drug has shown good in vitro activity against zygomycetes (1, 2) and has been used successfully as salvage therapy in some case reports and clinical trials of disseminated zygomycosis (8, 22, 23). However, its effectiveness remains controversial, since in experimental studies it has shown poor activity against R. oryzae, the main species causing zygomycosis (6, 9, 17). Several in vitro studies have shown that POS also exhibits significant activity against R. microsporus, another relevant clinical species (1, 2, 11), and a few clinical (14) and experimental (6) studies seem to demonstrate in vivo efficacy as well.
In this study, after confirming the significant in vitro activity of POS and AMB, we evaluated the efficacy of POS against four strains of R. microsporus in a murine model of disseminated infection. Considering that antifungal susceptibility can differ substantially among different strains of a given species, which could explain the variable percentages of success demonstrated by POS and AMB in clinical trials (8, 18, 23), we tested multiple strains exhibiting various in vitro responses to obtain more-robust results.
MATERIALS AND METHODS
The in vitro antifungal susceptibilities of 15 clinical strains of R. microsporus to AMB, POS, voriconazole (VRC), fluconazole (FLC), itraconazole (ITC), micafungin (MFG), and flucytosine (5-FC) were determined by a broth microdilution method according to the CLSI guidelines for molds (13). Since AMB and POS were the most active drugs in vitro, we used these agents in the murine studies. Although there are no defined breakpoints with proven clinical efficacy, the working interpretive MIC breakpoints for POS and AMB against zygomycetes in vitro, described by the CLSI in document M38-A2, are as follows: susceptible (S), ≤1 μg/ml; intermediate (I), 2 μg/ml; resistant (R), ≥ 4 μg/ml (13).
For the murine studies, we chose four of the strains that had been tested in vitro. Two POS I strains (MIC, 2 μg/ml), FMR 3542 and IHEM 4770, and two POS S strains (MIC, 0.25 μg/ml), UTHSC 01-983 and UTHSC R-3466, were used to assess the possible correlation between in vitro and in vivo studies.
The isolates were stored at −80°C, and prior to testing they were subcultured on potato dextrose agar (PDA) at 35°C. On the day of infection, cultures on PDA were suspended in sterile saline and filtered through sterile gauze to remove clumps of spores or hyphae. The resulting suspensions were adjusted to the desired inoculum based on hemocytometer counts and plating on PDA to confirm viability.
Male OF1 mice weighing 30 g (Charles River, Criffa S.A., Barcelona, Spain) were used in this study. Animals were housed under standard conditions. All animal care procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare Committee. Animals were immunodepressed 1 day prior to infection by administering a single dose of 200 mg of cyclophosphamide per kg of body weight intraperitoneally (i.p.) plus a single dose of 150 mg of 5-fluorouracil per kg intravenously (i.v). Mice were challenged with 1 × 104 CFU of IHEM 4770 and with 2 × 103 CFU for the rest of the strains in 0.2 ml of sterile normal saline injected via a lateral tail vein. Preliminary experiments demonstrated that these concentrations were the optimal doses for producing an acute infection; 100% of the animals died within 6 days of infection (data not shown).
The efficacy of the drugs was evaluated in terms of prolongation of survival and reduction of the fungal burden in the tissues of infected mice. Groups of 10 mice were randomly established, one for each treatment and one as a control. To prevent bacterial infection, the mice received ceftazidime (5 mg/day subcutaneously) from days 1 to 7 after infection.
Previous experiments investigating survival and tissue burden with strains FMR 3542 and IHEM 4770 (data not shown) showed that LAMB at 10 mg/kg/day i.v. and POS at 40 mg/kg/day orally (p.o.) were significantly better than AMB at 0.8 mg/kg/day i.v. and POS at 20 mg/kg/day p.o., respectively. Thus, we tested only LAMB (Gilead Sciences S.A., Madrid, Spain), that was administered at 10 mg/kg of body weight/dose once daily i.v and POS (Noxafil; Schering Plough Ltd., Hertfordshire, United Kingdom), that was administered at 40 mg/kg of body weight/dose once daily p.o. (POS 40 QD) or at 20 mg/kg of body weight/dose twice a day p.o. (POS 20 BID). Control animals received no treatment.
All treatments began 24 h after challenge and lasted for 10 days. The survival of mice was evaluated daily for 20 days after challenge. For tissue burden studies, 10 mice from each group were sacrificed on day 4 postinfection. Kidneys and brains were removed aseptically and were homogenized in 1 ml of sterile saline; care was taken to minimize tissue trauma. Serial 10-fold dilutions of the homogenates were plated on PDA and incubated for 18 to 24 h at 35°C.
In order to perform a bioassay to determine the level of POS in serum, additional groups of five mice were similarly infected with strain FMR 3542 or UTHSC 01-983 and were treated with POS 40 QD or POS 20 BID for 5 days. Candida parapsilosis ATCC 22019 and yeast nitrogen broth were used for this purpose. POS standards (twofold dilutions ranging from 0.125 to 10 μg/ml) were prepared in methyl alcohol. Sera obtained from POS-treated mice at 3 and 24 h after the last dose were tested in duplicate on each plate. Zones of inhibition for each pair were determined in duplicate, and the mean of those four readings was compared to the standard curve in order to calculate serum drug concentrations. The correlation coefficient was ≥0.93 (5, 16).
Mean survival time was estimated by the Kaplan-Meier method and compared among groups using the log rank test. Colony counts in tissue burden studies were analyzed by the Kruskal-Wallis test. When the results of this test were significant, we used the Mann-Whitney U test to compare treatment pairs. Bonferroni's correction was used to avoid an increase in the type I error due to multiple comparisons. When P was <0.05, the differences observed were considered statistically significant.
RESULTS
Table 1 summarizes the in vitro activities of the seven antifungal agents tested against 15 strains of R. microsporus. AMB and POS were the most active drugs. ITC, with a MIC range of 1 to 4 μg/ml, was less active than AMB and POS. VRC showed poor activity, with a MIC range of 4 to 32 μg/ml. FLC, MFG, and 5-FC exhibited no significant activity.
TABLE 1.
In vitro activities of seven antifungal agents against 15 isolates of Rhizopus microsporus
| Strain | MIC (mg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| AMB | POS | VRC | FLC | ITC | MFG | 5-FC | |
| FMR 3542 | 0.5 | 2 | 4 | 32 | 1 | 64 | 128 |
| CBS 102277 | 0.5 | 0.5 | 4 | 128 | 1 | 128 | 128 |
| IHEM 18821 | 0.5 | 1 | 8 | 128 | 2 | 128 | 128 |
| IHEM 13267 | 0.5 | 0.5 | 8 | 128 | 1 | 128 | 128 |
| IHEM 9503 | 0.5 | 0.5 | 32 | 128 | 4 | 128 | 128 |
| IHEM 10123 | 0.5 | 1 | 8 | 128 | 2 | 128 | 128 |
| IHEM 4770 | 0.5 | 2 | 32 | 128 | 2 | 128 | 128 |
| IHEM 5234 | 0.5 | 0.25 | 4 | 128 | 1 | 128 | 128 |
| IHEM 13311 | 0.5 | 0.5 | 8 | 128 | 1 | 128 | 128 |
| IHEM 15210 | 0.5 | 0.5 | 8 | 128 | 1 | 128 | 128 |
| UTHSC R-3466 | 0.5 | 0.25 | 4 | 128 | 1 | 128 | 128 |
| UTHSC 03-1802 | 1 | 0.25 | 8 | 128 | 1 | 128 | 128 |
| UTHSC 04-3294 | 1 | 0.25 | 8 | 128 | 1 | 128 | 128 |
| UTHSC 07-371 | 1 | 0.25 | 8 | 128 | 1 | 128 | 128 |
| UTHSC 01-983 | 0.5 | 0.25 | 4 | 128 | 1 | 128 | 128 |
| GMa | 0.57 | 0.5 | 7.63 | 116.7 | 1.26 | 122.2 | 128 |
GM, geometric mean for the 15 strains studied.
The results of the bioassay revealed significantly higher serum POS levels when POS was administered twice daily than when it was given once daily (Table 2). The mean POS concentrations in serum were higher than the POS MICs for the two POS regimens, i.e., POS 40 QD and POS 20 BID, at 3 h after the last dose for both strains. At 24 h, the mean POS concentrations in the sera of animals that received POS 20 BID were similar to the highest POS MIC obtained (2 μg/ml).
TABLE 2.
Mean POS concentrations in sera of five animals infected with one of two R. microsporus strains at day 5 of treatment as determined by a bioassay
| Sampling time | Mean drug concn (μg/ml) in serum ± SD for animals infected with the following strain and receiving the indicated treatment: |
|||
|---|---|---|---|---|
| FMR 3542 |
UTHSC 01-983 |
|||
| POS 40 QD | POS 20 BID | POS 40 QD | POS 20 BID | |
| 3 h | 5.31 ± 0.97 | 7.60 ± 0.24 | 5.96 ± 0.55 | 8.04 ± 0.25 |
| 24 h | 0.95 ± 0.22 | 2.04 ± 0.42 | 1.05 ± 0.43 | 2.15 ± 1.35 |
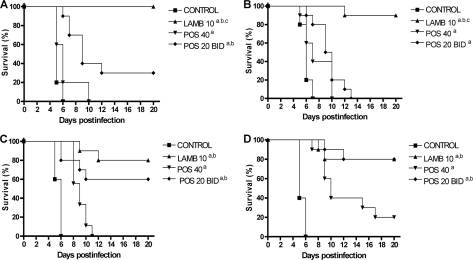
The results of survival studies are shown in Fig. 1. All the treatments were able to prolong survival significantly over that for the control group. LAMB was the most effective treatment against the two POS I strains. For the POS S strains, LAMB and POS 20 BID showed similar efficacies (60 to 80% survival), and both were significantly better than POS 40 QD.
FIG. 1.
Cumulative mortality of mice infected with Rhizopus microsporus FMR 3542 (A), IHEM 4770 (B), UTHSC 01-983 (C), or UTHSC R-3466 (D) and treated with LAMB or POS. a, P < 0.05 for comparison with the control; b, P < 0.05 for comparison with POS 40; c, P < 0.05 for comparison with POS 20 BID. LAMB 10, liposomal amphotericin B administered at 10 mg/kg i.v. once a day; POS 40, posaconazole administered at 40 mg/kg p.o. once a day; POS 20 BID, posaconazole administered at 20 mg/kg p.o. twice a day. Groups of 10 mice were randomly established, one for each treatment and one as a control.
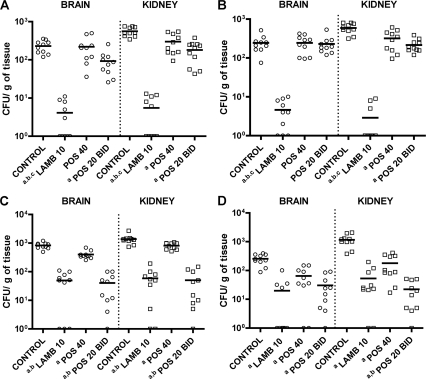
All the treatments significantly reduced the fungal loads in all the organs tested, except for POS 40 QD in the brain with strains FMR 3542 and IHEM 4770, and POS 20 BID in the brain with strain IHEM 4770 (Fig. 2). For the two strains that were POS I, LAMB was the most effective of the regimens, reducing the fungal burdens in both organs. For the POS S strain UTHSC 01-983, LAMB and POS 20 BID were the most effective treatments, reducing the fungal load with no statistical differences between the two. For the other POS S strain, UTHSC R-3466, POS 20 BID was even more effective than LAMB in the kidney; however, in the brain, there were no statistical differences among all treatments.
FIG. 2.
Effects of the antifungal treatment on colony counts of Rhizopus microsporus strains FMR 3542 (A), IHEM 4770 (B), UTHSC 01-983 (C), and UTHSC R-3466 (D) in the brains and kidneys of mice. a, P < 0.002 for comparison with the control; b, P < 0.002 for comparison with POS 40; c, P < 0.002 for comparison with POS 20 BID. Horizontal lines indicate mean values. LAMB 10, liposomal amphotericin B administered at 10 mg/kg i.v. once a day; POS 40, posaconazole administered at 40 mg/kg p.o. once a day; POS 20 BID, posaconazole administered at 20 mg/kg p.o. twice a day. Groups of 10 mice were randomly established, one for each treatment and one as a control.
DISCUSSION
While other studies have demonstrated no beneficial effects (6) or poor efficacy (17) of POS in the treatment of murine infection with R. oryzae, this study, utilizing four strains rather than one or two, demonstrated significant efficacy of POS against R. microsporus, an important agent of zygomycosis, in an experimental murine model. These results confirm the usefulness of in vitro POS susceptibility data for this organism (1, 2, 11) and support the efficacy noted in a few clinical studies where POS was able to resolve the infection (7, 14). In a previous experimental study where one strain of R. microsporus was tested, a POS dose-related response was demonstrated (6).
In most of the previous experimental studies with POS, this drug was administered once a day (6, 9, 20), but in human therapy this drug is administered twice a day to optimize its pharmacodynamics (10). In our model, the administration of POS twice a day proved to be more effective at prolonging survival and reducing the tissue burden than the once-a-day regimen and correlated with higher POS levels in serum.
Another interesting finding of the present study is the correlation between the in vitro and in vivo results with POS, since this drug was less effective against strains for which the POS MIC was higher (2 μg/ml) than against those for which the POS MIC was 0.25 μg/ml. Pharmacodynamic data revealed that POS induced optimal fungal killing when the peak drug concentrations achieved were 2 to 10 times the MIC (12). In our study, this pharmacodynamic parameter of POS efficacy was achieved for those strains against which POS MICs were low. Although further studies are necessary to confirm these data, our results seem to demonstrate that POS MICs against R. microsporus can be predictive of a more or less favorable outcome. In conclusion, POS could be a possible alternative to AMB or LAMB for the treatment of R. microsporus infections caused by strains for which POS MICs are low. In addition, the fact that POS achieves therapeutic concentrations in the central nervous system (15, 19) makes this drug useful against fungal pathogens that can cause cerebral infections, such as the zygomycetes.
Acknowledgments
This work was supported by a grant from the Fondo de Investigaciones Sanitarias from the Ministerio de Sanidad y Consumo of Spain (PI 050031).
Footnotes
Published ahead of print on 28 September 2009.
REFERENCES
- 1.Alastruey-Izquierdo, A., M. V. Castelli, I. Cuesta, A. Monzon, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2009. Activity of posaconazole and other antifungal agents against Mucorales strains identified by sequencing of internal transcribed spacers. Antimicrob. Agents Chemother. 53:1686-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almyroudis, N. G., D. A. Sutton, A. W. Fothergill, M. G. Rinaldi, and S. Kusne. 2007. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob. Agents Chemother. 51:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez, E., D. A. Sutton, J. Cano, A. W. Fothergill, A. Stchigel, M. G. Rinaldi, and J. Guarro. 2009. Spectrum of zygomycete species identified in clinically significant specimens in the United States. J. Clin. Microbiol. 47:1650-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chayakulkeeree, M., M. A. Ghannoum, and J. R. Perfect. 2006. Zygomycosis: the re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 25:215-229. [DOI] [PubMed] [Google Scholar]
- 5.Connolly, P., J. Wheat, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, J. Goldberg, E. Brizendine, and D. Loebenberg. 1999. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob. Agents Chemother. 43:322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dannaoui, E., J. F. G. M. Meis, D. Loebenberg, and P. E. Verweij. 2003. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 47:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Mol, P., and J. F. G. M. Meis. 2009. Disseminated Rhizopus microsporus infection in a patient on oral corticosteroid treatment: a case report. Neth. J. Med. 67:25-28. [PubMed] [Google Scholar]
- 8.Greenberg, R. N., K. Mullane, J. A. van Burik, I. Raad, M. J. Abzug, G. Anstead, R. Herbrecht, A. Langston, K. A. Marr, G. Schiller, M. Schuster, J. R. Wingard, C. E. Gonzalez, S. G. Revankar, G. Corcoran, R. J. Kryscio, and R. Hare. 2006. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 50:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim, A. S., T. Gebremariam, J. A. Schwartz, J. E. Edwards, Jr., and B. Spellberg. 2009. Posaconazole mono- or combination therapy for treatment of murine zygomycosis. Antimicrob. Agents Chemother. 53:772-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishna, G., A. Moton, L. Ma, M. M. Medlock, and J. McLeod. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53:958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messer, S. A., D. J. Diekema, R. J. Hollis, L. B. Boyken, S. Tendolkar, J. Kroeger, and M. A. Pfaller. 2007. Evaluation of disk diffusion and Etest compared to broth microdilution susceptibility testing of posaconazole against clinical isolates of filamentous fungi. J. Clin. Microbiol. 45:1322-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris, M. I. 2009. Posaconzaole: a new oral antifungal agent with an expanded spectrum of activity. Am. J. Health Syst. Pharm. 66:225-236. [DOI] [PubMed] [Google Scholar]
- 13.NCCLS/CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard, 2nd ed. Document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 14.Peel, T., J. Daffy, K. Thursky, P. Stanley, and K. Buising. 2008. Posaconazole as first line treatment for disseminated zygomycosis. Mycoses 51:542-545. [DOI] [PubMed] [Google Scholar]
- 15.Pitisuttithum, P., R. Negroni, J. R. Graybill, B. Bustamante, P. Pappas, S. Chapman, R. S. Hare, and C. J. Hardalo. 2005. Activity of posaconazole in the treatment of central nervous system fungal infections. J. Antimicrob. Chemother. 56:745-755. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez, M. M., E. Calvo, C. Serena, M. Mariné, F. J. Pastor, and J. Guarro. 2009. Effects of double and triple combinations of antifungal drugs in a murine model of disseminated infection by Scedosporium prolificans. Antimicrob. Agents Chemother. 53:2153-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez, M. M., C. Serena, M. Mariné, F. J. Pastor, and J. Guarro. 2008. Posaconazole combined with amphotericin B, an effective therapy for a murine disseminated infection caused by Rhizopus oryzae. Antimicrob. Agents Chemother. 52:3786-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers, T. R. 2008. Treatment of zygomycosis: current and new options. J. Antimicrob. Chemother. 61(Suppl. 1):i35-i40. [DOI] [PubMed] [Google Scholar]
- 19.Rüping, M. J. G. T., N. Albermann, F. Ebinger, I. Burckhardt, C. Beisel, C. Müller, J. J. Vehreschild, M. Kochanek, G. Fätkenheuer, C. Bangard, A. J. Ullmann, W. Herr, K. Kolbe, M. Hallek, and O. A. Cornely. 2008. Posaconazole concentrations in the central nervous system. J. Antimicrob. Chemother. 62:1468-1470. [DOI] [PubMed] [Google Scholar]
- 20.Simitsopoulou, M., C. Gil-Lamaignere, N. Avramidis, A. Maloukou, S. Lekkas, E. Havlova, L. Kaurounaki, D. Loebenberg, and E. Roilides. 2004. Antifungal activities of posaconazole and granulocyte-macrophage colony-stimulating factor ex vivo and in mice with disseminated infection due to Scedosporium prolificans. Antimicrob. Agents Chemother. 48:3801-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spellberg, B., T. J. Walsh, D. P. Kontoyiannis, J. E. Edwards, Jr., and A. S. Ibrahim. 2009. Recent advances in the management of mucormycosis: from bench to bedside. Clin. Infect. Dis. 48:1743-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobón, A. M., M. Arango, D. Fernández, and A. Restrepo. 2003. Mucormycosis (zygomycosis) in a heart-kidney transplant recipient: recovery after posaconazole therapy. Clin. Infect. Dis. 36:1488-1491. [DOI] [PubMed] [Google Scholar]
- 23.van Burik, J. A. H., R. S. Hare, H. F. Solomon, M. L. Corrado, and D. P. Kontoyiannis. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:e61-e65. [DOI] [PubMed] [Google Scholar]