Abstract
Orthopoxvirus infections, such as smallpox, can lead to severe systemic disease and result in considerable morbidity and mortality in immunologically naïve individuals. Treatment with ST-246, a small-molecule inhibitor of virus egress, has been shown to provide protection against severe disease and death induced by several members of the poxvirus family, including vaccinia, variola, and monkeypox viruses. Here, we show that ST-246 treatment not only results in the significant inhibition of vaccinia virus dissemination from the site of inoculation to distal organs, such as the spleen and liver, but also reduces the viral load in organs targeted by the dissemination. In mice intranasally infected with vaccinia virus, virus shedding from the nasal and lung mucosa was significantly lower (∼22- and 528-fold, respectively) upon ST-246 treatment. Consequently, virus dissemination from the nasal site of replication to the lung also was dramatically reduced, as evidenced by a 179-fold difference in virus levels in nasal versus bronchoalveolar lavage. Furthermore, in ACAM2000-immunized mice, vaccination site swabs showed that ST-246 treatment results in a major (∼3,900-fold by day 21) reduction in virus detected at the outside surfaces of lesions. Taken together, these data suggest that ST-246 would play a dual protective role if used during a smallpox bioterrorist attack. First, ST-246 would provide therapeutic benefit by reducing the disease burden and lethality in infected individuals. Second, by reducing virus shedding from those prophylactically immunized with a smallpox vaccine or harboring variola virus infection, ST-246 could reduce the risk of virus transmission to susceptible contacts.
Smallpox disease is marked by the dissemination of variola virus within the infected host and the appearance of skin lesions or pocks (reviewed in reference 3 and at http://whqlibdoc.who.int/smallpox/9241561106.pdf). The virus is transmitted between humans mainly by aerosol droplets; however, contact with variola virus-contaminated clothing or bedding may contribute to transmission. Once inhaled, variola virus appears to first infect the upper- or lower-respiratory-tract mucosa and spread to and replicate within the local lymph nodes. The virus then disseminates to the spleen and liver via a transient viremia and replicates in reticuloendothelial cells through an average incubation period of 10 to 12 days. During the high-fever prodrome period that follows, a second wave of viremia occurs and results in the dissemination of the virus to mucous membranes of the mouth and pharynx and to the dermal epithelium of the skin. This dissemination leads to the eruption of lesions over the tongue, mouth, and oropharynx and of rashes that start at the face and extremities and may eventually envelope the whole body. Studies performed for 2 to 3 weeks after the onset of fever indicated that infectious virus is excreted in the throat, urine, and conjunctiva of smallpox patients (20). Additionally, the level and duration of secretion was higher in clinically severe (those with hemorrhagic and confluent lesions) cases than less severe (those with discrete lesions) cases (20). Evidence from epidemiological studies further suggests that transmission from smallpox patients to their contacts occurs only from the time of the earliest appearance of rash, and that excretions from the mouth and nose are the most important source of infectious virus for transmission (8). The transmission rate of smallpox to unvaccinated contacts is estimated to be in the range of 37 to 88% (3). Smallpox was declared eradicated from the natural environment in 1980 as a result of a worldwide vaccination campaign conducted by the World Health Organization. Despite the potential threat of the intentional release of variola virus as a biological weapon in the future, routine mass vaccinations are not implemented due to adverse events associated with live vaccinia virus (VV) immunization (6). The occurrence of vaccine-associated adverse events, such as progressive vaccinia, eczema vaccinatum, generalized vaccinia, and postvaccinial encephalitis, suggests that uncontrolled VV dissemination in the vicinity of the vaccination site or to distal sites elsewhere on the body may have serious consequences in vaccinees, especially those that are immunocompromised. In addition, virus shed from the vaccination site and inadvertently transferred to a household contact with a history of atopic dermatitis may lead to a life-threatening case of eczema vaccinatum (26).
VV, the prototypic member of the Orthopoxvirus genus, is used to study virus infection, replication, morphogenesis, and dissemination (reviewed in reference 19). After DNA replication in the cytoplasm, two infectious but structurally and functionally different forms of the virus are formed: intracellular mature virus (IMV or MV) and extracellular enveloped virus (EEV or EV). MVs remain confined to the cytoplasm and do not disseminate from the cell until cell lysis occurs. However, MVs that acquire a double membrane from early endosomes or the trans-Golgi network form intracellular enveloped virus (IEV) and get transported to the cell surface. After the fusion of its outer membrane with the plasma membrane, IEV may be either retained as cell-associated enveloped virus (CEV) or released from the cell surface as EV. In vitro, CEV is involved in the cell-to-cell spreading of VV, whereas EV is involved in virus dissemination to nonadjoining nearby or distal cells (2, 15). The 37-kDa protein p37, encoded by the VV F13L gene, plays a critical role in this process, since the deletion of the gene results in the blockade of MV envelopment and the inhibition of plaque formation (2). In vivo, EV is implicated in virus dissemination within the host and has been shown to be released from squamous epithelial cells of the nasal cavity in mice intranasally (i.n.) infected with the IHD-J strain of VV (15, 16). Despite the abundance of MVs in the cytoplasm of nasal squamous epithelial cells, no MVs are detected extracellularly and almost all free extracellular virions are EV, suggesting that the mechanism of virus release in vivo is by the exocytosis of EV, not by cell disruption (16). Although the original form of infectious VV released from infected hosts appears to be EV (not MV), due to the fragility of the EV membrane to withstand environmental factors, it is thought that the more physically stable MV released by the disruption of the EV outer membrane is responsible for the infection of susceptible hosts (22). As such, reducing the amount of EV released from epithelial cells of the respiratory tract might lower the risk of person-to-person transmission of VV by limiting the amount of shed EV available for conversion to MV, thus resulting in the infection of contacts.
ST-246 is an orally bioavailable low-molecular-size (376 g/mol) compound that is selectively active against several orthopoxviruses, including VV and ectromelia, cowpox, camelpox, monkeypox, and variola viruses (4, 23, 28). In vitro, ST-246 treatment resulted in a 10-fold reduction of EV formation (without affecting MV production) and in the inhibition of plaque formation and virus-induced cytopathic effect (28). The analysis of ST-246-resistant cowpox virus variants in vitro has revealed that the antiviral activity of ST-246 is targeted against a product of the V061 gene, which is homologous to the VV F13L gene required for MV envelopment (28). In vivo, ST-246 treatment has been shown to protect mice (ectromelia, vaccinia, or cowpox viruses), rabbits (rabbitpox), and ground squirrels (monkeypox) from lethal orthopoxvirus infections (14, 17, 21, 28). ST-246 treatment additionally reduced virus replication in organs such as spleen and liver (17, 28). In nonhuman primates, ST-246 fully protected cynomolgus monkeys from lethal intravenous (i.v.) challenges with monkeypox and variola virus and significantly reduced viral load and lesion formation (9, 10). In this study, we extended on these previous reports and examined the impact of ST-246 treatment on the in vivo dissemination of virus from the site of inoculation to various distal organs in BALB/c mice infected with the Western Reserve strain of VV (VV-WR) by i.n., percutaneous, i.v., subcutaneous (s.c.), and intraperitoneal (i.p.) routes. We also investigated the effect of ST-246 treatment on the level of virus shedding from the nasal and lung cavities of mice challenged i.n. and from vaccine-induced lesions of ACAM2000-vaccinated mice.
MATERIALS AND METHODS
Viruses and cell lines.
BSC-40 cells (ATCC CRL-2761) and VV-WR (ATCC VR-1354) were obtained from the American Type Culture Collection (Manassas, VA). BSC-40 cells were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS), 4 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 1 mM sodium pyruvate (DMEM-10; all reagents were from Invitrogen, Carlsbad, CA). VV-WR was propagated in BSC-40 cells and purified by sucrose gradient centrifugation, and the titer was determined by plaque assay in BSC-40 cells as described previously (5). ACAM2000 vaccine was kindly provided by Acambis Inc. (Cambridge, MA). The lyophilized live virus was reconstituted in 0.3 ml of diluent (50% [vol/vol] glycerin USP and 0.25% [vol/vol] phenol USP in water) provided with the vaccine, and the titer was determined to be 1 × 108 PFU/ml by plaque assay in BSC-40 cells.
VV challenges and vaccinations.
All mouse studies were conducted in accordance with public health service policy and were approved by the Institutional Animal Care and Use Committee of Oregon State University (Corvallis, OR). To assess the in vivo dissemination and target organ replication of VV from a site of inoculation, 7-week-old female BALB/c mice (Charles River, Wilmington, MA) were challenged on day 0 with 1.2 × 106 PFU (equivalent to 10 50% lethal doses [LD50] for the i.n. route) of VV-WR by five different routes: i.n., percutaneous or dermal scarification (DS), i.v., s.c., and i.p. For i.n. challenge, mice were lightly anesthetized using 3% isoflurane, and the virus was administered in 10 μl of phosphate-buffered saline (PBS) applied equally between the two nares. DS was performed by applying virus resuspended in 10 μl PBS at the base of the tail and scratching the droplet into the tail using a 25-gauge needle. For i.v. challenge, mice were held in a restrainer that allows the tail to protrude, and the virus was injected into the tail vein in 100 μl of PBS. The s.c. and i.p. VV-WR challenges were carried out in 100 μl of PBS by injecting s.c. into the right abdominal area or into the peritoneal cavity, respectively, with a 25-gauge needle. To vaccinate mice with ACAM2000, 2.5 × 105 PFU, which represents the standard human vaccine dose, of virus was administered by DS as described above. For the examination of extracellular virus shedding into the nasal or lung cavity, mice were challenged (i.n.) with a low dose, 5 × 103 PFU, of VV-WR in 10 μl of PBS. For studies involving the determination of the minimum number of ST-246 doses required for therapeutic efficacy and how long ST-246 treatment can be delayed, the mice were challenged i.n. with 1.2 × 106 PFU in 10 μl.
ST-246 treatment.
ST-246 was prepared as a 20 mg/ml suspension in an aqueous solution containing 0.75% methylcellulose (Sigma, St. Louis, MO) and 1% Tween 80 (Sigma). Mice were treated once daily with 100 mg ST-246/kg of body weight by oral gavage immediately before or 24, 48, 72, or 96 h after virus challenge or vaccination. There is no overt toxicity (i.e., clinical signs or body weight changes) associated with mice given repeated oral doses of 100 mg/kg/day ST-246 (28 and unpublished observations). Mice treated similarly with an equal volume of vehicle (aqueous solution to which ST-246 was not added) were used as controls. Depending on the aim of the experiment, mice were administered up to 14 doses of vehicle or ST-246.
Disease state and survival monitoring.
After virus challenge and treatment with vehicle or ST-246, the mice were monitored daily for signs of illness and survival and scored as 0 (normal), 1 (slightly ruffled), 2 (significantly ruffled), 3 (hunched posture and/or conjunctivitis in addition to significant ruffling), 4 (score of 3 combined with difficulty in breathing/moving/socializing), and 5 (death). Additionally, the weight and body temperature (infrared thermometer; Mikron Infrared, Inc., Oakland, NJ) of each mouse was taken immediately before challenge and on alternate days postchallenge. Those with serious breathing difficulty, inability to stand or move, or greater than 30% loss of starting body weight were humanely euthanized. The systemic manifestation of VV-WR-induced disease also was documented by capturing pictures of the body (for signs of ruffling and hunching), tail (for primary and/or satellite tail lesion formation), and the eye (for signs of conjunctivitis) using a Nikon D40 digital camera (Nikon, Tokyo, Japan) and processed by Adobe Photoshop CS3 software (Adobe Systems Incorporated, San Jose, CA). Pictures were taken on day 6 (i.n.) or day 9 (DS, i.v., s.c., and i.p.) postinfection, when disease symptoms were more pronounced. In ACAM2000-vaccinated mice, pictures of tails were taken on days 5, 7, 14, and 21 to document the different stages of lesion formation and healing.
Tissue collection, processing, and viral load quantitation.
On days 3, 5, 8, and 14 after VV-WR challenge, three mice/group were euthanized with CO2 and blood, spleen, liver, lung, and brain were collected into preweighed, impact-resistant 2.0-ml tubes containing 1.0 mm silica spheres and one 1/4-inch ceramic sphere (MP Biomedicals, Solon, OH). The tubes then were reweighed and kept frozen at −80°C. On the day of processing, the tissues were thawed out, and 0.5 ml (blood and brain) or 1.0 ml (spleen, liver, and lung) of DMEM containing 2.5% of FBS (DMEM-2.5) was added to the tubes and homogenized at a speed of 4.0 m/s for 60 s in a high-speed benchtop homogenizer (FASTPREP-24; MP Biomedicals). Qiagen's DNeasy blood and tissue kit then was used to purify total DNA from 75 μl of blood; 20 mg of liver, lung, and brain; or 10 mg of spleen homogenates as recommended by the manufacturer (Qiagen Inc., Valencia, CA). The quantitation of VV genome copies was performed by real-time PCR (RT-PCR) on a 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA) using ribonucleotide reductase (I4L)-specific primers and a protocol kindly provided by Eva-Jasmin Freyschmidt (Harvard Medical School, Boston, MA). The PCRs were carried out in MicroAmp optical 96-well plates (Applied Biosystems) in a 20-μl volume containing (i) 2 μl of eluted DNA; (ii) 500 nm each of forward (5′-GAC ACT CTG GCA GCC GAA AT-3′) and reverse (5′-CTG GCG GCT AGA ATG GCA TA-3′) primers (Integrated DNA Technologies, San Diego, California); (iii) 150 nM of a TaqMan probe labeled at the 5′ end with the reporter fluorescence dye 6-carboxyfluorescein (FAM) and at the 3′ end with the quencher dye 6-carboxytetramethylrhodamine (TAMRA) (FAM-5′-AGC AGC CAC TTG TAC TAC ACA ACA TCC GGA-3′-TAMRA; Applied Biosystems); and (iv) 2× TaqMan universal PCR master mix (Applied Biosystems). The amplification conditions were 50°C for 2 min, 95°C for 10 min for one cycle, and 40 cycles of 95°C for 15 s and 60°C for 1 min following the melting curve analysis. A standard curve generated using known concentrations of VV-WR genomic DNA extracted from highly purified VV-WR was used to quantitate virus genome copies in tissue samples. Animals were not perfused prior to harvesting organs. Thus, it is possible that blood circulating in tissues contributed to the overall virus levels detected in tissues. Tail swab samples from ACAM2000-vaccinated mice were collected on days 5, 7, 14, and 21 by swabbing the surface of the lesions by the rolling of sterile Q-Tips (Fisher Scientific, Pittsburgh, PA) and cutting the cotton tip into 1.5-ml Eppendorf tubes containing 0.5 ml of PBS. The tubes were vortexed, and 200 μl of sample was used without additional processing to extract total DNA. After the last tail swab on day 21, the tail section encompassing a scab or a scar remaining from the vaccination was excised by using surgical scissors and processed in the same manner as that for other tissues as discussed above. Nasal wash and bronchoalveolar lavage (BAL) samples were obtained using PBS on day 7 postinfection and were centrifuged at 400 × g for 5 min to pellet washed-off cells and collect the cell-free supernatant. The amount of infectious virus shed into the extracellular space and thus present in these samples was determined by plaque assay on BSC-40 cells. Tenfold serial dilutions of the samples (starting from a 1:3 dilution) were prepared in 0.2 ml of DMEM-2.5, added to confluent monolayers of BSC-40 cells in 24-well plates, and allowed to incubate for 1.5 h at 37°C and 5% CO2. Subsequently, 800 μl of 1% methylcellulose overlay was added to the wells, incubated for 48 h, and stained for 10 min with 0.1% crystal violet in 5% methanol, and the number of plaques was enumerated manually.
Statistical analyses.
An unpaired Student t test was used to analyze vehicle- or ST-246-treated mice for differences in viral load within blood, lung, spleen, liver, or brain. The Mann-Whitney rank sum test was used for the comparison of tail, tail swabs, nasal wash, and BAL values between vehicle- and ST-246-treated mice. In all tests, raw titer values were used for analysis, and a P value of <0.05 was considered statistically significant. SigmaStat 3.1 software (San Jose, CA) was used to perform all statistical analyses.
RESULTS
VV-infected mice are protected from virus-induced systemic disease by treatment with ST-246.
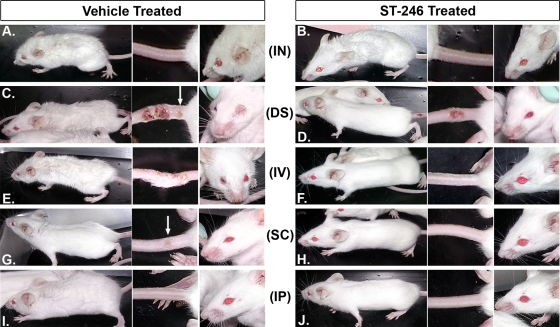
Although BALB/c mice infected with VV do not exhibit the extensive rash observed in smallpox patients as an indicator of disease progression, they can become extensively ruffled and emaciated and develop hunched posture and conjunctivitis in their eyes as well as satellite lesions on their tails, depending on the dose and route of virus administration (1, 7). We assessed the effect of ST-246 treatment on the appearance of clinical signs of viral disease after challenging BALB/c mice with 1.2 × 106 PFU of VV-WR by the i.n., DS, i.v., s.c., and i.p. routes. This dose of virus is equal to 10 50% lethal doses (LD50) for 7-week-old BALB/c mice challenged by the i.n. route. Starting immediately before virus challenge, the mice were treated orally with vehicle or 100 mg/kg of ST-246 once daily and monitored for external signs of disease. Mice challenged i.n. and treated with vehicle started to appear sick by day 3, and they developed severe disease and had to be euthanized for humane reasons by day 6. At the height of their disease symptoms on day 6, these mice exhibited extensive ruffling of their face and body fur, hunching, wasting, and conjunctivitis (Fig. 1A). Additionally, they were very limited in their mobility and showed significant respiratory distress. On the contrary, ST-246-treated mice appeared significantly healthier, although they exhibited some level of fur ruffling, hunching, and weight loss (Fig. 1B). These mice were very active, sociable, and did not develop conjunctivitis, respiratory distress, or severe lethal disease. Regardless of their treatment, none of the mice challenged i.n. developed satellite lesions on their tails. DS with VV-WR was not lethal, but vehicle-treated mice appeared ruffled by day 9 and conjunctivitis was evident in one of three mice (Fig. 1C). Even though they eventually heal, the primary tail lesions were severe and expansive, and the virus disseminated to a distal area of the tail and formed a secondary lesion in one of three mice. However, there was no ruffling, conjunctivitis, or any other disease symptom in mice that were treated with ST-246 (Fig. 1D). The inhibition of virus dissemination to adjoining areas of the site of scarification or to a distal site was evident in ST-246-treated mice by the reduced severity and confinement of the tail lesions and the lack of secondary, satellite lesions. By day 9, fur ruffling, conjunctivitis, and one death out of three were observed in mice challenged i.v. and treated with vehicle (Fig. 1E). Most significantly, multiple disseminated lesions or confluent lesions formed at or near the virus injection site on the tail. In contrast, ST-246-treated mice were fully protected from all external signs of disease observed in the vehicle-treated mice, including death (Fig. 1F). There were no significant clinical signs of disease in the majority of the mice that were challenged with VV-WR s.c. or i.p. and treated with vehicle (Fig. 1G and I). However, virus dissemination to the tail in a form of a single secondary lesion was observed in one of the three mice challenged s.c. In mice infected i.p., there was very slight ruffling of the fur on days 5 to 9 and death in one of three mice on day 8. There were no physical signs of disease or the occurrence of death in any of the mice challenged s.c. or i.p. and treated with ST-246 (Fig. 1H and J). Taken together, the data presented in Fig. 1 demonstrate that ST-246 treatment contributes significantly to the reduction of virus dissemination and the pathogenesis of systemic disease in routes of VV challenge that result in overt signs of clinical disease, especially in mice challenged i.n.
FIG. 1.
Systemic manifestation of virus-induced disease in VV-WR-infected mice is significantly inhibited by treatment with ST-246. Seven-week-old BALB/c mice were challenged with a high dose (1.2 × 106 PFU) of VV-WR by the i.n. (A and B), DS (C and D), i.v. (E and F), s.c. (G and H), or i.p. (I and J) route and treated by oral gavage with vehicle (A, C, E, G, and I) or 100 mg/kg of ST-246 (B, D, F, H, and J) once daily. Pictures were taken on day 6 (i.n.) or day 9 (DS, i.v., s.c., and i.p.) postinfection, at which time disease symptoms were more pronounced. Systemic disease symptoms are clearly evident by signs of fur ruffling, weight loss, and/or hunching (first panel), satellite lesion formation on tails (indicated by arrows in the second panel), and conjunctivitis in the eye (third panel). The pictures shown represent one of two to three mice/group that were alive at the time of photography.
Treatment with ST-246 has a significant impact on VV multiorgan dissemination and target organ replication.
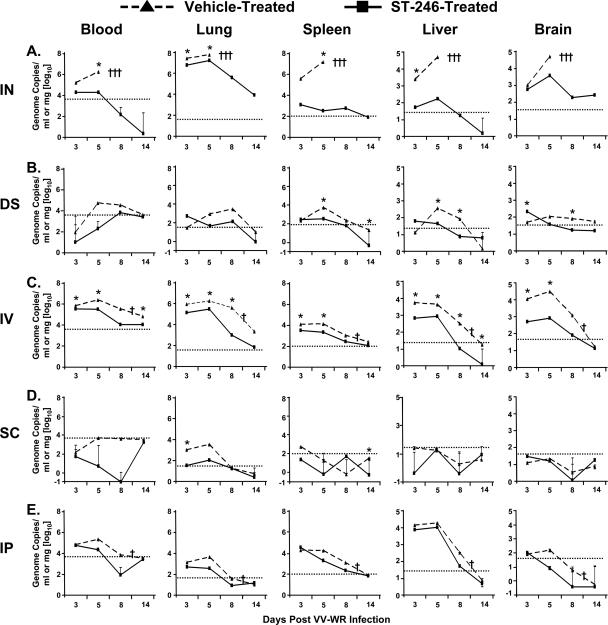
The clinical manifestation of smallpox disease and the appearance of rashes is preceded by the extensive seeding of the virus throughout the body, presumably due to viremic dissemination (3 and http://whqlibdoc.who.int/smallpox/9241561106.pdf). Initial high-level replication within the lungs and then the dissemination of virus to spleen, liver, and brain also has been documented in mice challenged i.n. with VV-WR, a route that closely resembles the natural mode of infection of smallpox (25, 27). Similarly, in mice infected by a percutaneous or s.c. route, the dissemination of VV to systemic organs would be expected to occur after a period of time following virus replication in the local site of inoculation. Therefore, in the three routes described above, virus detected early (by days 3 to 5 postinfection) in a target tissue distal to an inoculation site would be attributable to infection resulting from systemic virus dissemination. At later time points, however, both the active production of virus within the infected tissue and additional systemic virus dissemination may contribute to virus levels detected within a distal tissue. In contrast, after VV infection via i.v. and i.p. routes, virus infection and replication would be detected very early following inoculation, since these routes lead to the immediate and systemic distribution of VV to several distal tissues. To assess the impact of ST-246 treatment on virus dissemination from the site of inoculation and on virus replication within targeted organs, BALB/c mice were challenged with 1.2 × 106 PFU of VV-WR by the five different routes mentioned above and treated daily for 14 days with vehicle or 100 mg/kg of ST-246. On days 3, 5, 8, or 14 after infection and treatment, three mice per group were sacrificed, and blood, lung, spleen, liver, and brain were obtained for the measurement of virus load by real-time PCR. In mice challenged i.n. and treated with vehicle, VV-WR replicated to a high level in the lung, was detected in the blood, and disseminated to the spleen, liver, and brain by day 3 (Fig. 2A). Virus replication within target tissues seemed unhindered in these mice, since the virus load was, on average, 2-, 10-, 40-, 20-, and 50-fold higher in the lung, blood, spleen, liver, and brain, respectively, on day 5 than on day 3. Virus replication also was very high in the lungs of ST-246-treated mice (Fig. 2A), although compared to that of vehicle-treated mice, ∼4- and ∼3.5-fold less virus was detected on days 3 and 5, respectively, and these differences were statistically significant (P = 0.041 and P = 0.018, respectively). On day 3, the virus level was ∼8-fold lower in the blood of these mice, and the significant inhibition of virus dissemination to the spleen and liver was evident. The virus load on this day was ∼300-fold lower in the spleen (P = 0.072) and ∼45-fold lower in the liver (P = 0.008) of ST-246-treated mice. ST-246 treatment appears to have minimal impact on the dissemination of VV-WR, a strain adapted to be neurotropic, to the brain, since the reduction in viral load (n-fold) was only ∼1.78 and was not statistically significant (P = 0.432). However, by day 5, the level of virus detected in the blood (∼90-fold), spleen (∼44,000-fold), liver (∼300-fold), and brain (∼13-fold) all were markedly lower in mice treated with ST-246 than in mice treated with vehicle. By day 7, vehicle-treated mice exhibited severe disease burden (Fig. 1A) and had to be euthanized. On the contrary, ST-246-treated mice appeared much healthier (Fig. 1B), and continued treatment until day 13 resulted in drastic declines in virus levels in all tissues.
FIG. 2.
ST-246 treatment results in greatly reduced dissemination of VV-WR from the local site of inoculation and replication within distal target organs. Twelve mice/group were challenged with VV-WR by the i.n. (A), DS (B), i.v. (C), s.c. (D), or i.p. (E) route and treated with vehicle or ST-246 as described in the legend to Fig. 1. On days 3, 5, 8, and 14 postchallenge, three mice/group were sacrificed and blood, lung, spleen, and liver samples were collected and processed individually from each mouse. Total DNA was isolated from homogenized tissues, and virus genome copy quantitation was carried out by real-time PCR using VV ribonucleotide reductase (I4L)-specific primers. The data shown are geometric means ± geometric standard deviations of genome copies per milliliter (blood) or milligram (lung, spleen, liver, and brain) of tissue from two to three mice/group. Error bars may be obscured by lines or symbols when geometric standard deviations are very small. †, Death or humane euthanasia of vehicle-treated mice due to severe viral disease; *, P < 0.05. The horizontal dotted line indicates the limits of detection.
Though not to the extent seen in mice challenged i.n., low levels of virus dissemination to the blood, lung, spleen, liver, and brain were observed in mice dermally scarified with VV-WR (Fig. 2B). Virus levels were highest on day 5 in most tissues (except on day 7 in lungs) and seemed to coincide with the appearance of fur ruffling in vehicle-treated mice. At this peak day of viremia in vehicle-treated mice, viral loads still were reduced ∼260-fold (blood), ∼18-fold (lung), ∼16-fold (spleen), ∼8-fold (liver), and ∼3-fold (brain) in ST-246-treated mice. Regardless of the type of treatment administered, virus levels were significantly reduced to the limits of detection in all mice by day 14. In mice infected i.v., virus was detected in all assayed tissues on day 3 (Fig. 2C), and the difference in viral load between vehicle- and ST-246-treated mice was statistically significant (P < 0.05). On this day, the differences were highest in the liver and brain, where ∼8- and ∼23-fold reductions in virus levels were observed in ST-246-treated mice. In all of the days that follow, the viral load in all tissues also was consistently lower in ST-246-treated mice. Regardless of treatment with vehicle or ST-246, virus dissemination from the site of inoculation to internal organs seemed to be very limited in mice challenged s.c. (Fig. 2D). In most cases, the viral loads in the organs of these mice were near or below the limits of detection, with the only exceptions being days 3 and 5 in the lungs of vehicle-treated mice. Still, on both days, viral loads in the lungs of ST-246-treated mice were 30-fold lower than in vehicle-treated mice. In mice infected i.p., VV-WR seeded the bloodstream, lung, spleen, liver, and, to a clearly reduced extent, the brain (Fig. 2E). On day 3, there was no major difference in virus levels between the two treatment groups in any of the tissues. However, starting on day 5, the viral loads were consistently lower in most tissues of ST-246-treated mice, although the treatment effect in the liver was very modest.
In the data discussed above, the viral load was presented as determined by the quantitation of VV-WR genome copies, since real-time PCR provided consistent and reproducible results. However, virus levels in all of the organs also were measured by the plaque titration of tissue homogenates on BSC-40 cells (see Fig. S1 in the supplemental material). Despite the lower sensitivity of plaque titration for viral load quantitation in tissue homogenates, especially in the blood and liver, the results of this method paralleled those obtained by real-time PCR (Fig. 2; also see Fig. S1 in the supplemental material). The results of the real-time PCR and plaque titration were strikingly similar in all tissues of mice infected i.n. (Fig. 2A; also see Fig. S1A in the supplemental material). Collectively, the data presented above show that depending on the route of virus inoculation, ST-246 treatment may reduce not only virus dissemination from the site of inoculation but also virus replication within infected targeted organs, with both effects being particularly evident in mice infected i.n.
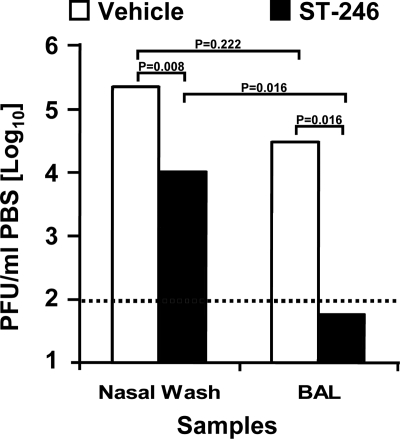
ST-246 treatment results in significant reductions in extracellular virus shedding occurring within the nasal and lung cavities after VV-WR i.n. challenge and at the site of lesion formation after ACAM2000 vaccination.
Bioluminescence imaging over the course of nonlethal i.n. virus infection in mice has shown that a high level of VV replication occurs at the local site of viral inoculation in the nose, even before a slight level of infection is detected in the trachea, bronchioles, and adjacent lung tissues by day 3 (13). This observation suggested that initial virus replication in the nasal mucosa followed by dissemination to the lung mucosa appears to be critical to the establishment of infection in the lung and then to the eventual spread of virus to other visceral organs. Of course, virus released in the respiratory tract is also important in host-to-host transmission (8). To assess the effect of ST-246 on the level of virus released into the nasal and lung cavities, mice were infected i.n. with low doses of VV-WR (5 × 103 PFU) and treated daily with vehicle or ST-246. On day 7 postinfection mice were sacrificed, and nasal and BAL samples were obtained for viral load assessment by plaque titration on BSC-40 cells (Fig. 3). We found that infectious viral titers in nasal wash and BAL samples were ∼22- and 528-fold reduced, respectively, in mice treated with ST-246, and these differences were statistically significant (P = 0.008 and P = 0.016, respectively). In addition, the amount of virus in the nasal washes compared to that in BAL also was significantly higher in vehicle-treated mice. In these mice, the difference in virus levels in nasal washes and BAL was only ∼7-fold and was not statistically significant (P = 0.222). In contrast, this difference was 179-fold and was statistically significant (P = 0.016) in ST-246-treated mice samples. These data demonstrate a clear effect of ST-246 on virus shedding in the nasal and lung mucosa and also suggest a potential role in the inhibition of virus dissemination from the nasopharynx and sinuses to the lung.
FIG. 3.
After i.n. infection, the dissemination of VV-WR to the lung and the level of virus shedding within the nasal and lung extracellular milieu are significantly lower in ST-246-treated mice. Five mice/group were challenged i.n. with low doses (5 × 103 PFU) of VV-WR in 10 μl of PBS and treated as described in the legend to Fig. 1. On day 7 the mice were sacrificed, and nasal and BAL samples were collected. The amount of cell-free virus present in these samples was determined by plaque titration on BSC-40 cells. The bar graphs represent geometric means ± geometric standard deviations in PFU/milliliter from five mice/group. P < 0.05 is considered statistically significant.
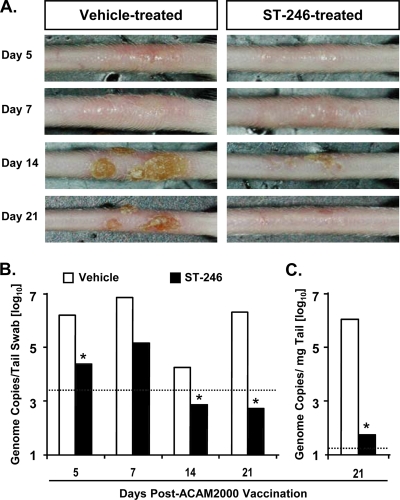
Due to the concern of inadvertent VV autoinoculation and transmission to contacts of smallpox vaccine recipients, covering the site of inoculation with semipermeable dressings and paying careful attention to dressing care and hand hygiene is recommended until scabs separate (24). The significant impact of ST-246 treatment on virus shedding in the respiratory tract (Fig. 3) suggested that it may also reduce the amount of virus released to the surface of vaccine lesions. To test this hypothesis, mice were vaccinated with 2.5 × 105 PFU (the standard human vaccine dose) by DS and treated with vehicle or ST-246 for 14 consecutive days. On days 5, 7, 14, and 21 postvaccination, lesion sites were photographed and surface swabbed by gentle rollings of dry cotton Q-Tips. The total amount of virus recovered from the vaccination site then was quantitated by real-time PCR. ST-246 treatment did not appear to inhibit the formation of vaccine “take” (primary vesicle), since all of the mice developed one by day 7 (Fig. 4A). However, it was clearly evident that the severity of the lesions that formed and the time until full resolution was significantly reduced in all mice by ST-246 treatment, although there was some level of variability among the five mice in the group. By day 21, vehicle-treated mice still had crusted lesions visible on their tails, whereas scabs had fallen off of ST-246-treated mice, leaving behind visible scars. At all four sample days, the amount of virus recovered from the surface of vaccine lesions in ST-246-treated mice was considerably lower than that from vehicle-treated mice (Fig. 4B). The differences were approximately 71-, 53-, 25-, and 3,900-fold in swab virus titers on days 5, 7, 14, and 21, respectively, and the differences were statistically significant (P < 0.05), except on day 7 (P = 0.151). The difference between vehicle- and ST-246-treated mice was most striking when virus replication levels within the tails were analyzed on day 21 postvaccination (Fig. 4C). The amount of virus in the tails of vehicle-treated mice was, on average, ∼19,400-fold higher than that in ST-246-treated mice and was statistically significant (P = 0.008). Collectively, the data shown in Fig. 4 demonstrate that both the magnitude and temporal progression of virus replication and shedding at the vaccination site are better controlled when the smallpox vaccine is administered concomitantly with ST-246.
FIG. 4.
ST-246 treatment of ACAM2000-vaccinated mice does not affect lesion formation but considerably reduces not only lesion severity and time to resolution (A) but also virus load and shedding (B). Five mice/group were vaccinated with 2.5 × 105 PFU of ACAM2000 by DS and treated with vehicle or ST-246 for 14 consecutive days. Photographs and lesion swabs of the vaccination site were taken on days 5, 7, 14, and 21. Representative tail lesions are shown (n = 5/group). On the last day of the experiment (day 21), mice were sacrificed and the section of the tail containing vaccine lesion/scab was collected and homogenized. After purifying total DNA, the amount of virus genomes in all samples was determined by real-time PCR. Geometric means ± geometric standard deviations of genome copies for five mice/group are shown. *, P < 0.05. The horizontal dotted line indicates the limits of detection.
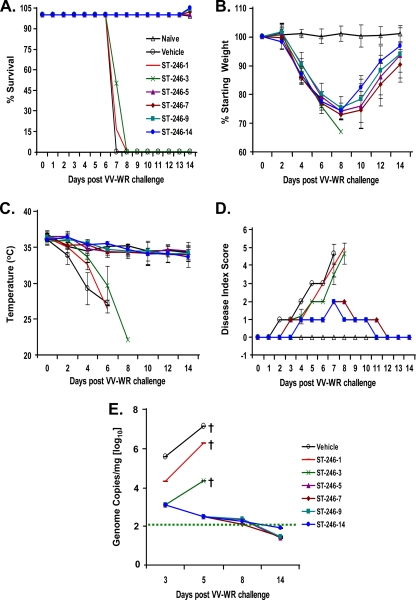
A minimum of five days of treatment with ST-246 is required to prevent death resulting from lethal VV disease.
Uninterrupted daily treatment for 14 days with ST-246 not only affords protection from lethal VV-WR challenge by the i.n. route but also inhibits virus dissemination to and replication within visceral organs (Fig. 2A). We therefore were interested in finding out what effect the interruption of ST-246 treatment at different days postinfection would have on survival and in vivo virus dissemination and replication. To do this, mice were challenged i.n. with 10 LD50 (1.2 × 106 PFU) of VV-WR and received once-a-day treatment with 100 mg/kg ST-246 for 1, 3, 5, 7, 9, or 14 consecutive days. Mice treated every day with vehicle alone served as controls. Changes in weight, temperature, and physical signs of disease were monitored to compare disease progression and survival. As shown in Fig. 5A, all of the mice that received five or more days of ST-246 treatment were protected from death, while mice that received vehicle or 1 or 3 days of ST-246 treatment developed severe disease and succumbed by days 6 to 8. Compared to their starting weight, all of the virus-challenged mice, regardless of the duration of ST-246 treatment, exhibited continuous weight loss beginning on day 2 postinfection (Fig. 5B). However, mice treated with ST-246 for 5 or more days maintained >70% of their starting weight and began to regain their weight dramatically beginning on day 8. Another marker of disease severity and impending death in mice infected i.n. is the inability to maintain body temperature, i.e., hypothermia. Starting on day 2, a continuous decline in body temperature was observed in mice treated with vehicle or with 1 or 3 days of ST-246 and reached an average temperature of less than 30°C by day 6 postchallenge (Fig. 5C). On the contrary, mice that received 5 or more days of ST-246 treatment maintained average body temperatures of greater than 34°C throughout the infection period. Signs of illness monitored daily (Fig. 5D) were assigned numerical scores ranging in severity from 0 (normal) to 5 (death) as described in Materials and Methods. After appearing slightly ruffled (score of 1) between days 2 and 3 postchallenge, mice that received vehicle or one or three ST-246 treatments became gravely ill by day 7 and exhibited significant respiratory distress, hunching, and inactivity (score of 4). For humane reasons, mice that received a disease score of 4 were euthanized immediately. On the contrary, mice that received five or more ST-246 treatments appeared slightly ruffled between days 3 and 6 (score of 1), became more ruffled between days 7 and 8 (score of 2), rebounded to a score of 1 between days 9 and 11, and appeared completely healthy by day 12 (score of 0). The only noticeable difference in these mice was that those receiving 9 or 14 days of treatment recovered to normal health 1 day earlier than those receiving 5 or 7 days of treatment. Collectively, these data suggest that a minimum of five days of ST-246 treatment is necessary for the prevention of severe disease and death after lethal VV-WR challenge. Consistent with our finding, previous studies of mice have reported considerable (80% [28] to 93% [17]) survival in those treated with ST-246 for 5 days.
FIG. 5.
Minimum of 5 days of ST-246 treatment is necessary for full therapeutic efficacy against lethal VV challenge. Six mice/group were challenged i.n. with 10 LD50 of VV-WR (1.2 × 106 PFU) and treated once a day with 100 mg/kg of ST-246 for 1, 3, 5, 7, 9, or 14 days. Mice were monitored for survival (A), weight loss (B), hypothermia (C), and degree of illness (D) (scored as described in Materials and Methods). Group averages ± standard deviations are shown. (E) In addition, the impact of the six different durations of ST-246 treatments on virus dissemination to a distal site was assessed by quantitating virus genome copies in the spleen of mice sacrificed on days 3, 5, 8, or 14. Geometric means ± geometric standard deviations for three mice/group are shown. †, Death or humane euthanasia of mice due to severe viral disease. The horizontal dotted line indicates the limits of detection.
To determine if the protection afforded by five or more days of ST-246 treatment correlated with a reduction in virus dissemination and replication in vivo, virus levels were measured by real-time PCR in the spleen, blood, lung, liver, and brain on days 3, 5, 8, or 14 after i.n. infection. Interestingly, even 1 day of ST-246 treatment had a significant impact on the initial dissemination of virus to the spleen (Fig. 5E). Compared to vehicle-treated mice spleens on day 3, the amount of virus in the spleens of those treated with ST-246 for 1 and 3 days was ∼18- and ∼300-fold lower, respectively, and was statistically significant (P = 0.030 and P = 0.025, respectively). Three days of treatment with ST-246 indeed was more effective than 1 day of treatment in inhibiting virus dissemination to the spleen (∼17-fold reduction on day 3; P = 0.004) and replication within the spleen (∼85-fold reduction on day 5; P = 0.018). However, the effective control of virus replication within the spleen appears to require more than 3 consecutive days of ST-246 treatment, since virus levels continue to rise if treatment is interrupted after one or three doses but fall if continued for 5 or more days. A similar effect was observed in the blood, liver, and brain, except that virus levels in mice treated for 1 day with ST-246 were consistently higher than those of vehicle-treated mice (see Fig. S2A to D in the supplemental material). There was no clear correlation between the duration of ST-246 treatment and virus replication in the lung, although virus titers in vehicle-treated mice were always higher than those of mice receiving any ST-246 dose (see Fig. S2B in the supplemental material).
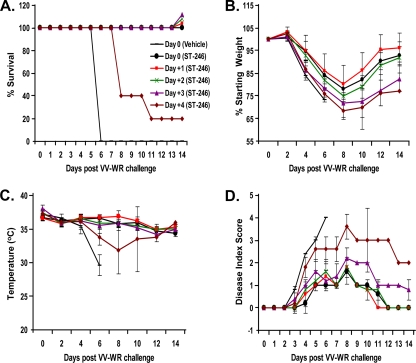
Initiation of ST-246 treatment as late as 3 days postinfection is effective in providing protection from VV-induced death.
The appearance of early physical signs of disease in mice infected i.n. on day 3 is preceded by high levels of virus replication in the lung and dissemination to the blood, spleen, liver, and brain tissues (Fig. 2A and 5D). Hence, we were interested in testing the therapeutic efficacy of starting ST-246 administration before, at, or after significant virus replication and the appearance of clinical signs of disease. For this purpose, survival and disease progression was monitored in mice that were administered ST-246 treatment immediately before i.n. infection with 1.2 × 106 PFU of VV-WR (day 0) or 1, 2, 3, or 4 days postinfection. ST-246 administered as late as 3 days postinfection protected 100% of the mice from death, while all vehicle-treated mice died by day 6 (Fig. 6A). Compared to vehicle-treated mice, ST-246 treatment that was started 4 days postinfection delayed death by 2 to 4 days, but ultimately only one of five mice survived. Mice in all six treatment groups exhibited continuous weight loss after day 2, but survivors started to regain their weight after day 8. Interestingly, the average weight loss was hierarchical, in that the later the ST-246 treatment was started, the greater the weight loss. Furthermore, as a sign of impending death, all mice that succumbed to infection eventually developed hypothermia, with body temperatures ranging from 28 to 31°C (Fig. 6C). In sharp contrast, all of the mice that survived maintained body temperatures of ≥34°C at all times postinfection. Signs of disease were limited to scores of 2 or below if ST-246 treatment was started no later than 72 h postinfection (Fig. 6D). However, treatment delay for 72 h resulted in slow recovery to normal appearance compared to results after delays of 24 or 48 h. Disease symptoms were more pronounced (score of 3) and recovery was even more gradual for the only surviving member of the group whose treatment was delayed for 96 h. For nonsurviving members of that group, disease progression was slightly delayed compared to that of vehicle-treated mice, but they developed severe disease (score of 4) between days 8 and 10. The data presented above show that ST-246 treatment is effective even when it is delayed up to 3 days postexposure. Our finding in this mouse model of VV-WR infection is consistent with a previous report of a ground squirrel monkeypox infection model (21).
FIG. 6.
Despite high-level viral dissemination and replication in the first 3 days after i.n. challenge, ST-246 treatment initiated as late as 3 days postinfection provides 100% survival. Five mice/group received a lethal dose of VV-WR as described in the legend to Fig. 5. ST-246 treatment was started immediately before the i.n. infection or 1, 2, 3, or 4 days postinfection and continued for 14 consecutive days. Mice were monitored for survival (A), weight loss (B), hypothermia (C), and degree of illness (D). Group averages ± standard deviations are shown.
DISCUSSION
Data presented in this study clearly demonstrate that VV dissemination from the local site of inoculation and its replication in targeted organs is significantly inhibited by ST-246 treatment. This ST-246-mediated effect on VV pathogenesis in vivo translated to a clinically relevant outcome, since ST-246-treated mice not only survived lethal challenges but also exhibited very limited physical signs of generalized infection. In vitro, the kinetics of distal virus spread from cell monolayers has been shown to be strongly related to the magnitude of EV released from primary infected cells, but not to the quantity of MV produced (15). ST-246 treatment in vitro significantly reduces the amount of EV released from infected cells, although MV production is not affected (28). In vivo, it is believed that EV is responsible for the long-range dissemination of VV in infected hosts. To this effect, Payne found that the amount of EV released by VV strains (with the exception of the neurotropic Western Reserve strain) in vitro correlated well with their ability to spread from the respiratory tract to the livers and brains of mice and to cause death (15). Although small amounts of EV are released by VV-WR in vitro, it produces significant amounts of CEV and thus is able to disseminate well and cause a high rate of mortality (15). In addition, Payne and Kristensson observed that the only form of virus released from infected mouse nasal epithelial cells in vivo is EV, and this process occurs by budding at the plasma membrane and not by cell disruption (16).
Given the in vitro-demonstrated effect of ST-246 on EV production and plaque formation (28), our data suggest that the same mechanism of ST-246 action is at play in vivo to inhibit the widespread dissemination of virus from the initially targeted cells of the inoculation site. Indeed, our finding that the amount of VV-WR released into the nasal and lung cavities of mice infected i.n. is greatly diminished in those treated with ST-246 lends strong support to this view. Since inhaled virus replicates in the respiratory tract before viremic spread to other internal organs, a reduction of virus release from the respiratory mucosa is expected to have a profound effect on viral titers at distal sites. In direct correlation to the lower level of extracellular free virus detected in BAL samples of ST-246-treated mice (Fig. 3), the amount of virus that spread to and infected the spleen and liver, which served as indicators of disseminated disease, was markedly diminished (Fig. 2A). Interestingly, ST-246 had little effect on VV-WR dissemination to the brain, as similar levels of virus were detected in the brain of both vehicle- and ST-246-treated mice on day 3 after i.n. infection. This could be due to the inherent neurotropic nature of VV-WR as a result of its passage in mouse brain, and/or virus spread to the brain may occur directly via the nasal mucosa and cribriform plate rather than hematogenous spread, as proposed by Turner previously (25). However, similarly to its effect in the liver and spleen, ST-246 treatment significantly limited VV-WR replication in the infected brain, as evidenced by a 1-log reduction in virus titers by day 5.
CEV has been shown to be responsible for the cell-to-cell spread of VV in vitro (2, 15). The effect of ST-246 in the reduction of virus replication in infected organs most likely is due to a combinatorial effect on the inhibition of the CEV-mediated cell-to-cell spread of virus among neighboring cells and the EV-mediated spread of virus among nonadjoining cells. If so, it would be consistent with the in vitro-observed inhibition of MV envelopment by ST-246 that affects not only EV production but also CEV formation. However, we cannot rule out the potential involvement of ST-246 treatment induced early and/or more effective anti-VV innate or adaptive immune responses in suppressing virus replication or contributing to the clearance of virus-infected cells. Immune-mediated viral clearance may play a major role during the sharp decline in viral titers 5 days postinfection, since the number of lymphocytes infiltrating the lung has been shown to rise after day 3 and peak at day 10 during the course of i.n. VV infection (18).
Since i.n. VV infection mimics the natural route of smallpox infection (25, 27), our data suggest that ST-246 treatment may have a twofold benefit if administered to those with suspected or confirmed exposure to variola virus or other pathogenic orthopoxviruses in the event of a bioterroristic attack. One benefit of ST-246 treatment would be the prevention or reduction of severe clinical disease by reducing variola virus replication and dissemination during the first and/or second wave of viremia. Even in patients that have disseminated virus and have developed skin rashes, there might be a significant therapeutic window for ST-246 efficacy, since treatment delayed for up to 3 days (day of initial appearance of physical signs of disease) postinfection in mice results in 100% survival (Fig. 5). However, several consecutive days of treatment would be an absolute requirement for full therapeutic efficacy, since the interruption of ST-246 treatment in mice after three doses resulted in the rebound of virus replication and in high lethality (Fig. 5). This might be because until the time that the antiviral immune response is activated and starts to play a role in virus clearance, continued treatment with ST-246 is necessary to keep virus replication in check. A second potential benefit of ST-246 treatment might be a reduction in the risk of virus transmission from infected individuals to their naïve contacts. Since smallpox is spread by the inhalation of aerosolized virus (3 and http://whqlibdoc.who.int/smallpox/9241561106.pdf), if the significant reduction in free virus released from mouse nasal and lung mucosa (Fig. 3) also occurs in humans during variola virus infection, ST-246 treatment could substantially cut the rate of secondary transmissions. To test the effect of ST-246 on secondary transmission rates, attempts were made to establish a mouse-to-mouse aerosol transmission model of i.n. VV-WR infection by cohousing infected mice with naïve mice. However, we observed that VV transmission (as assessed by seroconversion and/or the resistance of contact mice to lethal challenge) was low (<25% to immunocompetent BALB/c contacts or 0% to SCID contacts) even in vehicle-treated mice (data not shown). Further, even in cases where transmission was evident, we could not reliably attribute transmission to aerosol droplet acquisition, since some of the infected mice, especially those treated with vehicle, exhibited tail-biting aggression. Via tail-biting, the infected mice appear to directly inoculate virus into the tail of naïve contacts, since they end up developing a few to several VV lesions at sites of bite marks.
Recently, we have demonstrated that the induction of protective immunity is not affected by the administration of ST-246 in combination with the previously approved smallpox vaccine Dryvax (Wyeth Ayerst Laboratories, Marietta, PA) (7). It was suggested that the use of ST-246 in combination with smallpox vaccine may play a role in the prevention or treatment of adverse events due to vaccination (7). The data presented in Fig. 4A performed using the recently approved smallpox vaccine ACAM2000 (Acambis, Inc., Cambridge, MA) provides positive support for that view. The development of ACAM2000 vaccine lesion or take typically observed by day 7 was not affected by ST-246 treatment. However, the severity of the lesions and the time to full resolution was substantially reduced. Most importantly, the vaccination of mice with Dryvax or ACAM2000 along with ST-246 treatment does not interfere with the induction of short-term (30 days postvaccination) or long-term (6 months postvaccination) protective immunity from lethal respiratory challenge with VV-WR (7 and unpublished observations). Although not observed in ACAM2000-vaccinated mice, secondary lesion formation away from the initial lesion site was documented in VV-WR-scarified mice (Fig. 1C and D). Taken together, these observations suggest that ST-246 may be beneficial in lowering the risks of adverse events associated with vaccinations. These events could include those associated with the dissemination of virus to adjoining areas of the lesion's perimeters or to distal areas away from the vaccination site, such as progressive or generalized vaccinia. The amount of virus shed at the surface of the lesion site was significantly lower in mice treated with ST-246 (Fig. 4B). Hence, in addition to benefiting the vaccinee, ST-246 may also help reduce the risk of autoinoculation or virus transmission to susceptible contacts, such as atopic dermatitis or immunosuppressed individuals. The recent case of life-threatening eczema vaccinatum developed by a child in Chicago, after being in contact with his military personnel father who was vaccinated 21 days earlier (26), highlights the importance of reducing virus shedding and transmission. In this case, shed virus also was detected from the child's skin and personal belongings and may have contributed to tertiary transmission to the mother and his two siblings (12, 26). Fortunately, the child recovered after receiving intensive care and treatment consisting of ST-246, vaccinia immune globulin, and cidofovir. Although it may not be due to a singular effect of ST-246, the rapid decline in viremia and improvements in the child's clinical signs after ST-246 treatment was started mirrors our observations shown in Fig. 1 and 2 (also see Fig. S2 in the supplemental material). Although the use of bandages to cover vaccine lesions may help decrease virus transmission, additionally limiting virus shedding by ST-246 treatment may markedly cut transmission rates.
Taken together, the data presented in this report provide additional support for the ongoing clinical development of ST-246 as a biodefense agent (11). The antiviral action of ST-246 would be beneficial for the prevention or treatment of not only smallpox and other orthopoxvirus diseases but also of adverse events associated with vaccination. Its antiviral role also might broaden to include the prevention of secondary and tertiary transmissions.
Supplementary Material
Acknowledgments
This work was supported by Small Business Innovation Research grant R43AI075747 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 14 September 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Berhanu, A., R. L. Wilson, D. L. Kirkwood-Watts, D. S. King, T. K. Warren, S. A. Lund, L. L. Brown, A. K. Krupkin, E. Vandermay, W. Weimers, K. M. Honeychurch, D. W. Grosenbach, K. F. Jones, and D. E. Hruby. 2008. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J. Virol. 82:3517-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasco, R., and B. Moss. 1992. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 66:4170-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breman, J. G., and D. A. Henderson. 2002. Diagnosis and management of smallpox. N. Engl. J. Med. 346:1300-1308. [DOI] [PubMed] [Google Scholar]
- 4.Duraffour, S., R. Snoeck, R. de Vos, J. J. van Den Oord, J. M. Crance, D. Garin, D. E. Hruby, R. Jordan, E. De Clercq, and G. Andrei. 2007. Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures. Antivir. Ther. 12:1205-1216. [PubMed] [Google Scholar]
- 5.Earl, P. L., N. Cooper, S. Wyatt, B. Moss, and M. W. Carroll. 1998. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16.13. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. John Wiley and Sons, Inc., New York, NY. [Google Scholar]
- 6.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251-271. [DOI] [PubMed] [Google Scholar]
- 7.Grosenbach, D. W., R. Jordan, D. S. King, A. Berhanu, T. K. Warren, D. L. Kirkwood-Watts, S. Tyavanagimatt, Y. Tan, R. L. Wilson, K. F. Jones, and D. E. Hruby. 2008. Immune responses to the smallpox vaccine given in combination with ST-246, a small-molecule inhibitor of poxvirus dissemination. Vaccine 26:933-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson, D. A. 1999. Smallpox: clinical and epidemiologic features. Emerg. Infect. Dis. 5:537-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huggins, J., A. Goff, L. Hensley, E. Mucker, J. Shamblin, C. Wlazlowski, W. Johnson, J. Chapman, T. Larsen, N. Twenhafel, K. Karem, I. K. Damon, C. M. Byrd, T. C. Bolken, R. Jordan, and D. Hruby. 2009. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob. Agents Chemother. 53:2620-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan, R., A. Goff, A. Frimm, M. L. Corrado, L. E. Hensley, C. M. Byrd, E. Mucker, J. Shamblin, T. C. Bolken, C. Wlazlowski, W. Johnson, J. Chapman, N. Twenhafel, S. Tyavanagimatt, A. Amantana, J. Chinsangaram, D. E. Hruby, and J. W. Huggins. 2009. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob. Agents Chemother. 53:1817-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan, R., D. Tien, T. C. Bolken, K. F. Jones, S. R. Tyavanagimatt, J. Strasser, A. Frimm, M. L. Corrado, P. G. Strome, and D. E. Hruby. 2008. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob. Agents Chemother. 52:1721-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lederman, E., R. Miramontes, J. Openshaw, V. A. Olson, K. L. Karem, J. Marcinak, R. Panares, W. Staggs, D. Allen, S. G. Weber, S. Vora, S. I. Gerber, C. M. Hughes, R. Regnery, L. Collins, P. S. Diaz, M. G. Reynolds, and I. Damon. 2009. Eczema vaccinatum resulting from the transmission of vaccinia virus from a smallpox vaccinee: an investigation of potential fomites in the home environment. Vaccine 27:375-377. [DOI] [PubMed] [Google Scholar]
- 13.Luker, K. E., M. Hutchens, T. Schultz, A. Pekosz, and G. D. Luker. 2005. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology 341:284-300. [DOI] [PubMed] [Google Scholar]
- 14.Nalca, A., J. M. Hatkin, N. L. Garza, D. K. Nichols, S. W. Norris, D. E. Hruby, and R. Jordan. 2008. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antivir. Res. 79:121-127. [DOI] [PubMed] [Google Scholar]
- 15.Payne, L. G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 50:89-100. [DOI] [PubMed] [Google Scholar]
- 16.Payne, L. G., and K. Kristensson. 1985. Extracellular release of enveloped vaccinia virus from mouse nasal epithelial cells in vivo. J. Gen. Virol. 66:643-646. [DOI] [PubMed] [Google Scholar]
- 17.Quenelle, D. C., R. M. Buller, S. Parker, K. A. Keith, D. E. Hruby, R. Jordan, and E. R. Kern. 2007. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 51:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reading, P. C., and G. L. Smith. 2003. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J. Gen. Virol. 84:1973-1983. [DOI] [PubMed] [Google Scholar]
- 19.Roberts, K. L., and G. L. Smith. 2008. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 16:472-479. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar, J. K., A. C. Mitra, M. K. Mukherjee, S. K. De, and D. G. Mazumdar. 1973. Virus excretion in smallpox. 1. Excretion in the throat, urine, and conjunctiva of patients. Bull. W. H. O. 48:517-522. [PMC free article] [PubMed] [Google Scholar]
- 21.Sbrana, E., R. Jordan, D. E. Hruby, R. I. Mateo, S. Y. Xiao, M. Siirin, P. C. Newman, A. P. Da Rosa, and R. B. Tesh. 2007. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 76:768-773. [PubMed] [Google Scholar]
- 22.Smith, G. L., B. J. Murphy, and M. Law. 2003. Vaccinia virus motility. Annu. Rev. Microbiol. 57:323-342. [DOI] [PubMed] [Google Scholar]
- 23.Smith, S. K., V. A. Olson, K. L. Karem, R. Jordan, D. E. Hruby, and I. K. Damon. 2009. ST-246 in vitro efficacy against smallpox and monkeypox. Antimicrob. Agents Chemother. 53:1007-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talbot, T. R., E. Ziel, J. K. Doersam, B. LaFleur, S. Tollefson, and K. M. Edwards. 2004. Risk of vaccinia transfer to the hands of vaccinated persons after smallpox immunization. Clin. Infect. Dis. 38:536-541. [DOI] [PubMed] [Google Scholar]
- 25.Turner, G. S. 1967. Respiratory infection of mice with vaccinia virus. J. Gen. Virol. 1:399-402. [DOI] [PubMed] [Google Scholar]
- 26.Vora, S., I. Damon, V. Fulginiti, S. G. Weber, M. Kahana, S. L. Stein, S. I. Gerber, S. Garcia-Houchins, E. Lederman, D. Hruby, L. Collins, D. Scott, K. Thompson, J. V. Barson, R. Regnery, C. Hughes, R. S. Daum, Y. Li, H. Zhao, S. Smith, Z. Braden, K. Karem, V. Olson, W. Davidson, G. Trindade, T. Bolken, R. Jordan, D. Tien, and J. Marcinak. 2008. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 46:1555-1561. [DOI] [PubMed] [Google Scholar]
- 27.Williamson, J. D., R. W. Reith, L. J. Jeffrey, J. R. Arrand, and M. Mackett. 1990. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J. Gen. Virol. 71:2761-2767. [DOI] [PubMed] [Google Scholar]
- 28.Yang, G., D. C. Pevear, M. H. Davies, M. S. Collett, T. Bailey, S. Rippen, L. Barone, C. Burns, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, R. L. Buller, E. Touchette, K. Waller, J. Schriewer, J. Neyts, E. DeClercq, K. Jones, D. Hruby, and R. Jordan. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J. Virol. 79:13139-13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.