Abstract
Serum bactericidal antibodies are important for protection against systemic Neisseria meningitidis infections. Consequently, identifying the specific targets of bactericidal antibodies is important for understanding protective immunity to meningococcal disease and for vaccine development and evaluation. We have developed a new assay that can be used to investigate the specificity of serum bactericidal antibodies. Prior to testing for bactericidal activity, antibodies specific for a given antigen or group of antigens are depleted from a serum sample by incubation with the antigen(s) bound to the wells of a 96-well microplate. A dilution series of the antigen is bound to the plate to assess the effectiveness of the antigen in removing the bactericidal antibodies. Removal of antibodies with solid-phase antigen prior to bactericidal testing avoids depletion of complement by soluble immune complexes that can form when soluble antigen is present in the bactericidal test mixture (direct inhibition). The parameters associated with this assay are investigated and compared with those associated with a direct-inhibition assay. The bactericidal depletion assay can be an effective tool for studying the specificity of serum bactericidal antibodies.
The presence of serum bactericidal antibodies is generally accepted as the best available correlate of immunity to meningococcal disease (1, 7). Bactericidal antibodies may be directed against any of a relatively large number of surface antigens, including capsular polysaccharide, lipooligosaccharide (LOS), and a relatively large number of outer membrane proteins (7, 8, 13, 14). In some cases, antibodies to minor antigens may cooperate to initiate a bactericidal event (23). Analysis of bactericidal antibody responses to capsular polysaccharide-based vaccines has been straightforward because the vaccines contain a single purified antigen. However, candidate vaccines for group B meningococcal disease typically contain multiple antigens, particularly those based on outer membrane vesicles. Similarly, analysis of bactericidal antibodies in normal or convalescent-phase human sera is also complex, because the immunizing agent is the whole organism. In addition, the bactericidal antibody responses of different individuals to vaccination or natural infection would be expected to differ because of immune polymorphisms or prior carriage in the case of human subjects.
For analysis of bactericidal antibody responses to complex group B vaccines and to natural infections, it is desirable to know which surface antigen(s) is the target of the bactericidal antibodies present in a serum sample. Various approaches have been used to obtain specificity information, including the use of different bactericidal test strains that differ in known ways from each other, the use of genetically engineered test strains (3, 19), correlation of results of Western blotting with bactericidal activity (11, 21), and inhibition of bactericidal activity by addition of soluble antigen at various concentrations to the bactericidal assay mixture (12, 15). All of these approaches have limitations and potential problems.
In this report, we describe a new assay that can be an effective tool for investigation of the major targets of serum bactericidal antibodies. This assay has proven to be particularly effective when used in conjunction with purified antigens, knockout mutants, or specific phase variants of the test strain.
MATERIALS AND METHODS
Bacterial strains and sera.
Strains of Neisseria meningitidis used as test strains were characterized by colony blotting with monoclonal antibodies to verify the expression of particular antigens, including the PorB serotype, the PorA serosubtype, and the LOS immunotype. The characterized strains were frozen in aliquots as a cell bank. Strains 9162(B:15:P1.7-2,3:L3,7) and 8532(B:15:P1.7-2,3:L3,7) were case isolates from Iquique, Chile. A phase variant of strain 8532 that expressed L8 rather than L3,7 was obtained by colony blotting with an L8-specific monoclonal antibody. Strain H44/76(B:15:P1.7,16: L3,7) is an isolate from Norway that was obtained from Oddvar Frøholm. Strain 8570(B:4:P1.19,15:L3-5,7-5) is an isolate from Miami, FL, and was obtained from Carl Frasch. The immunotype L3-5,7-5 is used to specify a LOS with an L3,7 alpha chain and a Hep II configuration, like the L5 immunotype. Additional strains used for purification of antigens were 126E(C:8,19:P1.5,2:L1), 89I(C:11:P1.16:L4), 6505(Y:2c:P1.5,2:L3-5,7-5), and B16B6(B:2a:P1.5,2:L2), which were isolates from U.S. military personnel.
Human sera used in this study, including those used for a source of complement, were obtained and used under an institutional review board-approved human use protocol. Prior to the use of the sera in the depletion assay, the titers of the sera were determined in a conventional bactericidal assay using the same conditions and reagents as those used in the bactericidal assay part of the depletion assay. For these studies, sera were not heat inactivated to destroy intrinsic complement. Heat inactivation of the sera or other variations in the bactericidal assay would not be expected to affect the outcome of the depletion assay as long as the sera are treated the same and the bactericidal assay conditions are the same during measurement of the serum titer and during the depletion test. Human complement was used for all assays in this study. Each test strain was pretested with a panel of normal human serum pools to identify a suitable complement source for that strain.
Purification of antigens.
LOS was purified by the hot-phenol-water method of Westphal et al. (22). Native outer membrane vesicles (NOMV) were prepared as described previously (17) from pelleted cells grown in liquid medium (modified Catlin's medium [6]). The purified LOS used as an antigen in the assay was noncovalently complexed to an equal weight of fatty acid-free bovine serum albumin (4). This provided a more consistent presentation of the LOS for binding to antibody.
Direct-bactericidal-inhibition assay.
The test antigen was diluted in a twofold dilution series in sterile Gey's balanced salt solution with 0.2% gelatin (GBSS/G; GibcoBRL, Gaithersburg, MD [Bacto gelatin; Difco Laboratories]), beginning at 100 μg/ml and leaving 50 μl of diluted antigen per well. To each well, 50 μl of serum dilution was added, and the plate was allowed to incubate at 37°C for 1 h with shaking. A volume of 50 μl from each well was transferred to a new bactericidal test plate, and the assay mixture was completed by addition of bacteria (25 μl) and complement (25 μl). The bactericidal assay was then completed as described below.
Bactericidal antibody depletion assay.
A brief description of the assay has previously been published (4). Two 96-well plates were used for the test, one for absorbing antibodies from the serum (high-level-binding enzyme immunoassay plate; Corning/Costar) and one for measuring the residual bactericidal activity in the serum (a covered cell culture plate). The test antigen was diluted to 100 μg/ml in Dulbecco's phosphate-buffered saline with 0.1% magnesium chloride (GibcoBRL, Gaithersburg, MD).
A twofold dilution series was prepared using sterile Dulbecco's phosphate-buffered saline with 0.1% magnesium chloride in a 96-well enzyme immunoassay plate (Corning/Costar, Cambridge, MA), beginning at 100 μg/ml and extending through 11 wells of each row. A volume of 100 μl remained in each well after the dilutions were made. The last well in each row was left without antigen and contained only diluent to serve as an antigen-free control. In some experiments, every other dilution (fourfold dilutions) was used in the assay. The plate was placed without a cover at 37°C overnight, allowing the antigen to dry down in the wells of the plate. The plate(s) was washed three times with 150 μl of sterile GBSS/G. The solution for each wash remained in the wells for 1 h, while the plate was placed on a microplate shaker (Labline Instruments, Melrose Park, IL) at 37°C with a shaker setting of 2.0. After the three washes, the wells were filled with 150 μl of GBSS/G blocking buffer and the plates maintained at 4°C in a covered, high-humidity container until being used for serum absorption (often overnight). When the blocking step was completed, the blocking buffer was removed and the wells were washed one time with sterile GBSS/G. The extended blocking time was used to minimize the amount of antigen released from the plate during incubation with the serum. After the wells were washed and emptied, any residual buffer was removed from the wells by tapping the plates upside down on clean, dry paper towels.
The serum to be tested was diluted with sterile GBSS/G to a concentration that was expected to result in 40 to 60% killing of the organism, as predetermined by a previous bactericidal assay of activity against the relevant test strain. A volume of 60 μl of the diluted serum was placed in each well of the plate. The plate was covered with a plate sealer (Nunc/Nalgene, Milwaukee, WI) and incubated for 3 to 4 h at 37°C with shaking on a microplate shaker.
After the absorption was complete, 50 μl of the diluted serum solution was transferred from each well of the plate to the corresponding well of a fresh bactericidal assay plate. The bactericidal assay was then performed as described below to determine the residual bactericidal activity in the sera.
Bactericidal assay.
The bactericidal assay was similar to that previously described (10). The test strain was streaked for isolation from a stock culture and incubated overnight at 37°C with 5% CO2. Isolated colonies from the plate were used to inoculate 25 ml of Mueller-Hinton broth in a Nephelo culture flask to an initial optical density at 650 nm of 0.1 ± 0.02. The liquid culture was grown in a shaking water bath to an optical density of 0.28 to 0.30. The cells were pelleted, washed once with cold sterile GBSS/G, resuspended in the same buffer, and diluted to give approximately 3 × 104 cells per milliliter. A volume of 25 μl of the bacterial suspension was added to the 50 μl of absorbed serum in each well of the 96-well assay plate, followed by addition of 25 μl of complement (normal human serum without bactericidal activity for the test strain). The reaction mixture was mixed by gentle tapping, and the number of CFU at time zero was determined by plating duplicate 20-μl samples of the reaction mixture from one set of control wells (control for growth during incubation, control for killing by complement alone, and control for killing by serum alone) on GC/DS agar (GC agar with defined supplement) (4). The bacteria were spread by the tilt method and the plates incubated overnight at 37°C in a 5% CO2 atmosphere. After the zero time controls were plated, the assay plate was incubated at 37°C for 60 min with shaking. The plate was removed from the incubator and placed on crushed ice, and duplicate 20-μl samples from each well (including the other set of control wells) were plated on GC/DS agar plates by using the tilt method. Colonies were counted after overnight incubation at 37°C with 5% CO2. The percent depletion was calculated as the percent decrease in killing compared to the zero-antigen control. In the assays reported here, all controls performed as expected. No growth or death of cells was observed in the control wells during the 1-hour incubation period.
RESULTS
Comparison of direct-inhibition and solid-phase depletion assays.
In attempts to analyze the specificity of bactericidal antibodies by directly inhibiting the bactericidal activity in selected sera by addition of defined antigens at different concentrations to the bactericidal assay mixture, we identified several potential problems, which led to the development of the bactericidal depletion assay, which we believe is more reliable and informative.
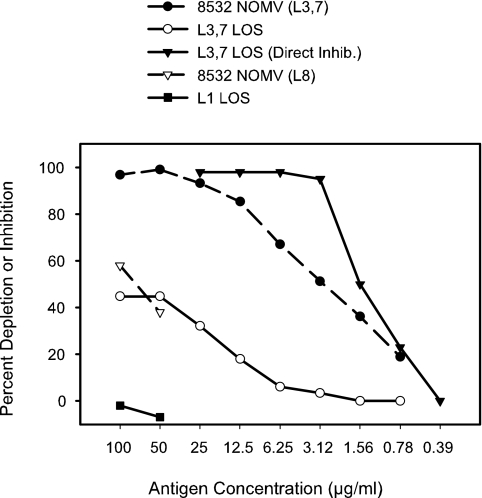
In the context of examining the bactericidal antibody responses of children in Chile to vaccination with a candidate meningococcal group B vaccine (2), we observed that an number of individuals had relatively high prevaccination titers of bactericidal antibodies toward the test strain 8532 (a representative isolate from the epidemic outbreak in Iquique, Chile). We undertook some experiments to attempt to determine the specificity of these naturally occurring bactericidal antibodies. Initially, we attempted to inhibit the bactericidal antibodies with different antigens by using a direct-fluid-phase-inhibition assay but obtained inconsistent and sometimes confusing results. For example, two different antigens would sometimes both inhibit nearly 100% of the bactericidal activity. A comparison of the levels of removal of bactericidal activity from one of the sera from Chile by use of the direct-inhibition assay and the depletion assay is shown in Fig. 1.
FIG. 1.
Comparison of fluid-phase direct inhibition and solid-phase depletion of L3,7 LOS-specific bactericidal antibodies. Antibodies in normal human serum (no. 1225-2) with bactericidal activity toward test strain 8532(L3,7,8) were depleted by different antigens at a series of different concentrations. One antigen, L3,7 LOS, was used both for antibody depletion and for direct inhibition of specific antibodies. The antigen concentration for the NOMV was based on protein content, and the LOS concentration was based on dry weight. The LOS content of the NOMV was approximately 25% relative to the protein content.
Direct inhibition in the fluid phase with purified L3,7 LOS resulted in strong, complete inhibition of the bactericidal activity. When the same serum was examined using the depletion assay, a maximum of about 45% of the bactericidal antibodies were removed. We reasoned that with the direct-inhibition assay we may not simply be setting up a competition between the soluble antigen and the organisms for binding the bactericidal antibody, but we may be also binding complement to soluble immune complexes, thereby reducing the complement available to bind to antibodies on the bacterial surface.
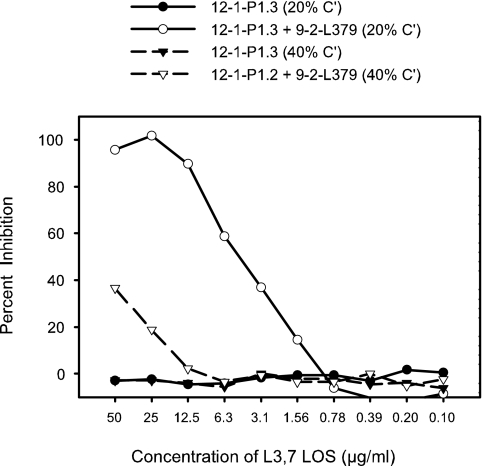
An inhibition experiment was set up using monoclonal antibodies to test this hypothesis. The test strain was 8532(B:15:P1.7-2,3:L8). Two murine monoclonal antibodies with demonstrated bactericidal activity were used. The first was 12-1-P1.3, which is specific for the PorA subtype P1.3 (used at a titer of 1:160,000), and the second was 9-2-L379, which is specific for L3,7 LOS (not expressed by the test strain). Consequently, 9-2-L379 had no bactericidal activity toward the L8-expressing test strain but killed a variant of the test strain, which expressed L3,7 LOS, with a titer of 1:3,500 (data not shown). Purified L3,7 LOS was used as the inhibitor even though it would not be expected to inhibit killing of the test strain. The test was set up using two different concentrations of complement (normal human serum lacking bactericidal activity toward the test strain), 20% of the reaction mixture and 40% of the reaction mixture. The results of the experiment (Fig. 2) showed that when only the monoclonal antibody 12-1-P1.3 was present in the assay mixture, the L3,7 LOS inhibitor had no effect on the bactericidal activity of the serum, as expected. If, however, the 9-2-L379 monoclonal antibody was present in addition to 12-1-P1.3, the L3,7 LOS inhibitor was able to inhibit up to 100% of the bactericidal activity in the presence of 20% complement. When the complement concentration was 40%, however, only a small amount of inhibition was observed. This result indicated that binding of complement components by soluble antigen-antibody complexes was depleting active complement from the assay mixture and thereby leading indirectly to a pronounced decrease in bactericidal activity.
FIG. 2.
The killing of strain 8532(B:15:P1.7-2,3:L8) by monoclonal antibody 12-1-P1.3, which is specific for the outer membrane protein PorA, was inhibited (direct-inhibition method) by purified L3,7 LOS in the presence or absence of monoclonal antibody 9-2-L379, which is specific for L3,7 LOS. The test strain did not express L3,7 LOS and was not killed by the monoclonal antibody 9-2-L379 used alone. The same experiment was done with two different concentrations of complement.
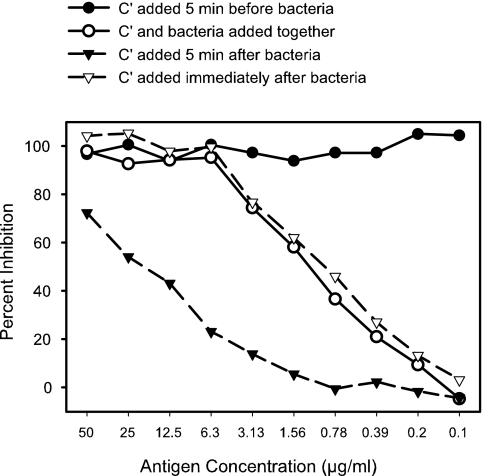
The results of direct-inhibition experiments were also strongly dependent on the order and timing of the addition of the components of the test. The bactericidal activity of a human postvaccination serum sample, NM44-B7 (4), for test strain 8532(B:15:P1.7-2,3:L8) was inhibited by addition of homologous NOMV prepared from the test strain. The inhibitor was diluted out in a twofold dilution series with GBSS/G, leaving 25 μl of antigen solution in each well. Diluted serum (25 μl) was then added to each well, followed by the addition of 25 μl each of complement and bacteria, changing the sequence and timing for each row. The results (Fig. 3) revealed that when complement was added 5 min before the bacteria, there appeared to be 100% inhibition at all concentrations of antigen tested. This was likely the result of depletion of all the complement by binding to soluble immune complexes. If complement and bacteria were added at the same time or the complement was added immediately after the bacteria, similar inhibition curves were obtained, showing 100% inhibition only at the higher inhibitor concentrations. When complement was added 5 min after the bacteria, much less inhibition was observed.
FIG. 3.
The sequence and timing of the addition of complement and bacteria were examined for impact on the results of a direct-inhibition assay. The bactericidal activity of a human postvaccination serum sample, NM44-B7, against test strain 8532(L8) was inhibited by different concentrations of NOMV prepared from the test strain.
The results obtained with the direct-inhibition assay (Fig. 1 to 3) emphasized the potential problems associated with the presence of soluble antigen in the bactericidal assay mixture. The bactericidal depletion assay was developed to avoid these problems. The depletion assay greatly reduced the potential for indirectly inhibiting bactericidal activity by fixing complement to soluble immune complexes.
Optimization of test parameters.
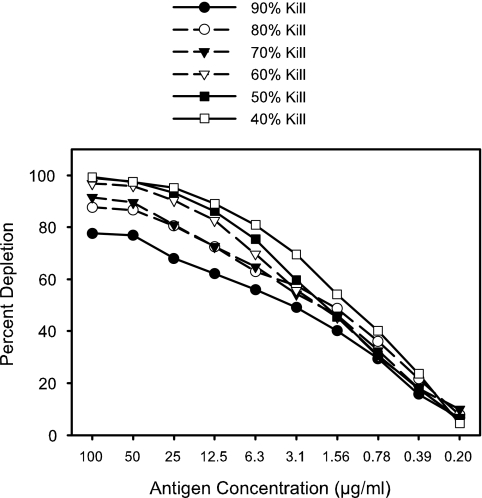
Two parameters of the depletion test were investigated to optimize the assay conditions. The optimal dilution of serum for use in the test was investigated by finding the dilutions required to give 40%, 50%, 60%, 70%, 80%, and 90% killing in the bactericidal assay and setting up an identical depletion test with each different dilution of serum (Fig. 4). Purified LOS homologous to the test strain and noncovalently complexed to fatty acid-free bovine serum albumin was used for the plate-bound antigen in each test. The test strain was H44/76(B:15:P1.7,16:L3,7), and the serum sample, HCC007, was a convalescent-phase serum sample from a patient with systemic meningococcal disease caused by a group C strain, 9495(C:2a:P1.5,2:L2,3,7). In this serum, the cross-reactive bactericidal antibodies against the group B test strain had previously been shown to be almost all directed against the LOS (data not shown). The remaining assay conditions were as described in Materials and Methods. The results are presented in Fig. 4 and show that less than 100% of the bactericidal antibody was removed when serum dilutions corresponding to killing percentages of 70% or higher were used. Thus, a serum concentration that corresponded to approximately 40% to 60% killing in the bactericidal assay was used for the depletion test. It appears that at higher serum concentrations the plate-bound antigen does not have the capacity to remove all the bactericidal antibodies.
FIG. 4.
The effect of the dilution of the serum (concentration of specific antibodies) on the bactericidal depletion test was evaluated over the range of serum dilutions corresponding to 40% to 90% killing. The test strain was H44/76(B:15:P1.7,16:L3,7), and the plate-bound antigen was L3,7 LOS complexed to fatty acid-free bovine serum albumin.
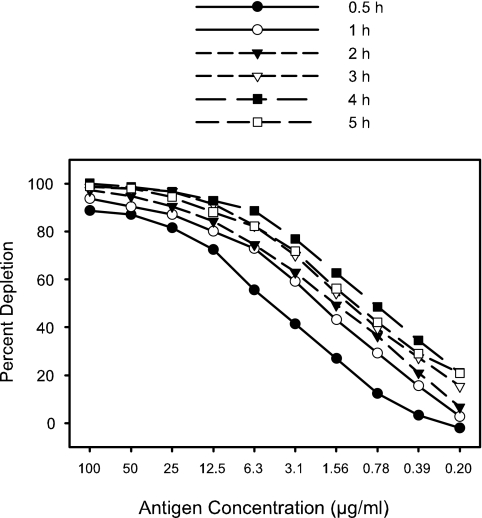
A second experiment was done to investigate the time of incubation of the serum with the plate-bound antigen. The same test strain and serum were used as in the previous experiment (Fig. 4). Incubation times ranging from 0.5 to 5.0 h with shaking at 37°C were compared. The results (Fig. 5) revealed that an incubation time of 4 h was optimal.
FIG. 5.
The effect of incubation time on the depletion of bactericidal antibodies from human convalescent-phase serum sample HCC007 was evaluated. Bactericidal antibodies against strain H44/76 were depleted by purified L3,7 LOS bound to the wells of a 96-well plate. Serum diluted to give 50% killing was incubated in wells coated with L3,7 LOS complexed to fatty acid-free bovine serum albumin.
Effect of antigen-antibody avidity.
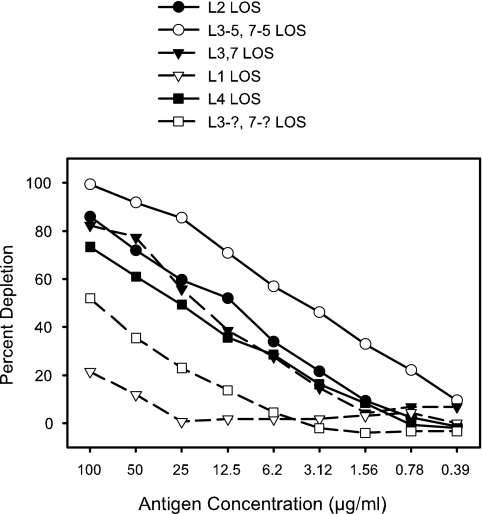
The concentration of antigen required to remove all the bactericidal antibody of a given specificity from a particular serum sample might be expected to depend on the avidity of the antibody for the antigen bound to the plate. This was observed upon comparison of the capacities of LOSs of different immunotypes to remove the LOS-specific bactericidal antibodies from a human convalescent-phase serum sample. The population of antibodies in a particular serum sample that is responsible for the bactericidal activity toward a given test strain is determined by the antigens expressed on the test strain. A serum sample may contain, for example, a wide spectrum of antibody specificities for LOS, but only those that bind to the test strain with sufficient affinity will be involved in the bactericidal activity toward that strain. LOSs with similar but different structures may cross-react with the bactericidal antibodies, but with lower avidity than the homologous LOS. The results for an experiment illustrating the relative levels of effectiveness of different immunotypes of LOS in removing from a human convalescent-phase serum sample a particular population of LOS antibodies that were bactericidal for strain 8750(B:4:P1.19,15:L3-5,7-5) is shown in Fig. 6. By using an entire dilution series of antigen for depletion and thereby generating a depletion curve of antigen concentration versus percent depletion, one can obtain some information about the relative avidities of different forms of an antigen for a particular population of antibodies. However, the actual amount of antigen that becomes bound to the solid phase for each concentration of antigen placed in the wells is not known. Thus, quantitative estimates of avidity are not possible with the assay in the present form.
FIG. 6.
The levels of effectiveness of purified LOSs of different immunotypes in depleting the bactericidal antibodies in a human convalescent-phase serum sample were evaluated. Strain 8750(B:4:P1.19,15:L3-5,7-5) was used as the test strain, and the human convalescent-phase serum was obtained from a patient that was infected with a group C strain (C:2a:P1.5,2:L3). LOS from strain 6505 was the same immunotype as that expressed by the test strain.
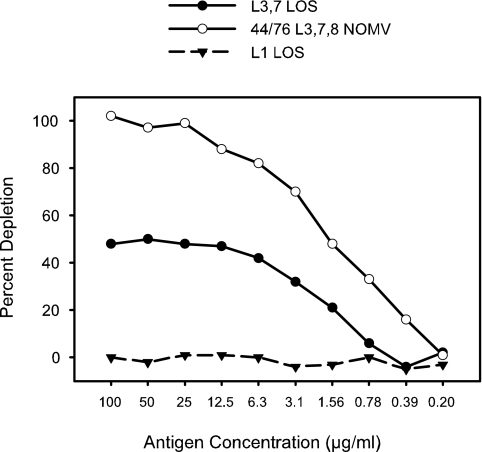
The removal of all the antibodies with specificity for a given antigen could be distinguished by the shape of the curve from a situation where partial removal of antibodies is effected by an antigen that has low-avidity binding to the antibody (most of the curves in Fig. 6). In a serum sample where part of the bactericidal activity was due to LOS, the anti-LOS antibodies were efficiently removed by the homologous LOS bound to the plate (Fig. 7). The binding curve reached a maximum of 45% to 50% depletion at about 25 to 50 μg/ml of antigen and remained at the same level with 100 μg/ml. Thus, when the binding curve shows a plateau, it is most likely that all of the antibodies specific for that particular antigen have been removed.
FIG. 7.
Depletion of bactericidal antibodies against strain 8532(L3,7,8) from a normal human serum sample by three different antigens.
Assay reproducibility.
The overall reproducibility of the assay was examined by repeating the same assay seven times in four tests run on four different days. The bactericidal activity of a human postvaccination serum sample, HOPS 038-98, toward the test strain, 9162(B:15:P1.7-2,3:L3,7), was examined to determine the amount of the bactericidal activity that could be removed by purified LOS. The homologous NOMV was used as a positive control and a heterologous L1 LOS as a negative control. The mean percent depletion at each antigen concentration, along with the standard deviation for each point, is given in Table 1. Generally, the standard deviations are in the range of 1 to 6 percentage points. The smoothness of the depletion curves can also be used as a qualitative measure of the accuracy of the data points on a depletion curve.
TABLE 1.
Reproducibility of the depletion assay performed seven times on four different daysa
| Antigen dilution (μg/ml) | Mean (SD) for indicated test antigen |
||
|---|---|---|---|
| 9162 NOMV | 9162 L3,7 LOS | 126E L1 LOS | |
| 100 | 97 (0.83) | 83 (1.76) | 2 (0.75) |
| 25 | 82 (2.23) | 70 (2.12) | 0 (1.28) |
| 6.25 | 58 (4.71) | 47 (3.41) | 2 (1.40) |
| 1.56 | 35 (6.11) | 28 (2.38) | 2 (1.87) |
| 0.39 | 20 (5.28) | 11 (2.59) | 3 (1.91) |
| 0.098 | 7 (4.06) | 3 (2.13) | 1 (1.32) |
Data given are the mean percentages of depletion of bactericidal activity at different concentrations of three different antigens. SD, standard deviation (n = 7).
DISCUSSION
The bactericidal antibody depletion assay has potential to be a useful tool for analyzing the specificity of bactericidal antibodies induced by various candidate meningococcal group B vaccines as well as the specificity of antibodies induced by natural infections. The depletion assay has some advantages over alternative approaches. The assay does not require the use of different test strains that may have known antigenic differences (3, 19) but may have unknown differences in sensitivity to complement-dependent killing as a result of other factors, such as capsule density, LOS sialylation, and level of factor H binding protein expression (5, 9, 18, 20). Furthermore, the depletion assay does not require laborious affinity purification of antibodies specific for each antigen, as required for direct determination of bactericidal activity. While direct testing of affinity-purified antibodies can provide strong evidence of bactericidal activity, it does not provide an indication of the relative proportion of the total bactericidal activity for which the purified antibody is responsible. In addition, a relatively large amount of serum is required, and the chemical bonding of the antigen to the matrix may alter important antigen epitopes. The depletion assay does not require denaturation of the antigen by hot detergent prior to resolution on a polyacrylamide gel, as required for Western blot analysis (11, 21). Since bactericidal antibodies may bind preferentially to conformational determinants on outer membrane proteins, some bactericidal antibodies may not bind on a Western blot. Most importantly, the present assay minimizes the presence of soluble antigen in the bactericidal assay mixture, which is required for a direct-inhibition assay (12, 15). As demonstrated in this paper, the direct-inhibition method has the problem of creating soluble antigen-antibody complexes that can fix complement and thereby reduce or exhaust the complement needed for bactericidal activity. Thus, the direct-inhibition method was shown to involve both a competition for complement and a competition for antibody.
A variety of antigens, ranging from whole cells to purified proteins or LOS, can be compared in the same depletion assay, and the binding of the antigens to the plate is accomplished without modification or denaturation. However, the process of drying the antigen on the plate may cause some conformational changes. In situations where that may be a concern, the antigen can be allowed to bind directly from the fluid phase under the same conditions as those used to coat a plate for an enzyme-linked immunosorbent assay. In most cases, we have used the dry-down method of binding because of the potential for increasing the amount of applied antigen that binds to the plate. The percentage of the total antigen placed in the wells that binds to the plate and remains bound after blocking and washing of the plate has not been determined. However, it is clearly important to minimize antigen coming off the plate during the incubation with serum, so the antigen plate was blocked and washed thoroughly following antigen binding. Development of methods for quantitative, covalent binding of antigen to the solid phase may improve the assay for some applications.
A dilution series of the antigen bound to the plate was used in the assay rather than a single concentration of antigen. This was done to generate a binding curve that could give additional information about the relative levels of effectiveness of different antigens in depleting the bactericidal antibodies. In the instance where the curve reaches a plateau at higher antigen concentrations, one can infer that all the antibodies capable of binding to the antigen on the plate have been removed from the serum (Fig. 7). In instances where the percent depletion continues to increase through the highest concentration of antigen tested, one can infer that maximum removal of antibody was not achieved and that additional bactericidal activity would potentially be removed with higher concentrations of antigen.
A result of using serum that is diluted to near the bactericidal endpoint is that the test is most sensitive to bactericidal antibodies with the highest titer. Bactericidal antibodies present at much lower concentrations may be difficult to detect. However, the assay can often be set up to focus on antibodies present at lower concentrations by choosing a bactericidal test strain that does not express the antigen(s) to which the dominant bactericidal antibodies bind. In addition, the avidity of the antibodies for the antigen affects the antigen concentration required to obtain maximal removal of bactericidal antibodies. If the avidity of serum antibodies is relatively low, a higher concentration of the antibodies is required to produce a lytic event. Consequently, a higher concentration of plate-bound antigen is required to deplete the antibody. In some tests, maximal removal of antibody was not achieved in the antigen concentration range used. However, that pattern is undoubtedly dependent on the dilution of serum used, which depends on the test strain used in the assay. Therefore, more information about the specificity of the bactericidal antibodies in a particular serum sample can be extracted by careful selection of test strains and antigens.
In some cases where the antigen density on the bacterial cell surface is low, the bactericidal event may require that antibodies specific for two different antigens be present. In this case, the removal of either antibody could deplete the bactericidal activity. In some instances, it may be desirable to deplete bactericidal antibodies with more than one specific antigen. We tested the option of mixing two purified proteins to coat the antigen plate or doing sequential incubations of serum in wells coated with a single antigen. Most often, we obtained the best depletion of antibodies with the sequential-depletion option (data not shown).
In those instances where the assay is used to analyze the specificity of bactericidal antibodies in human sera, it is important to use human complement. Antibodies that are bactericidal with rabbit complement, for example, may not be bactericidal with human complement or may have much less activity with human complement than with rabbit complement (16, 24).
The assay offers a practical new approach to the analysis of the specificity of bactericidal antibodies in animal or human serum. It avoids the complication of the depletion of complement from the bactericidal reaction mixture by fixation of complement to soluble antigen-antibody complexes that may form when soluble antigen is present in the reaction mixture (direct inhibition), allows for the use of nondenatured antigens, and gives an estimate of the proportion of bactericidal antibodies that are specific for a particular antigen. This method is also very economical in terms of amount of serum required; many tests can be run with less than a milliliter of high-titer serum. However, the depletion assay also has limitations and may give an incomplete picture of specificity in cases where antibodies to multiple antigens act synergistically in killing the organism.
Acknowledgments
The material in the manuscript has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the true views of the Department of the Army or the Department of Defense.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection-serum bactericidal antibody activity. Vaccine 23:2222-2227. [DOI] [PubMed] [Google Scholar]
- 2.Boslego, J. B., J. Garcia, C. Cruz, W. D. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, W. Silva, E. Moran, W. Hankins, J. Gilly, J. Mays, and the Chilean National Committee for Meningococcal Disease. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 3.Boutriau, D., J. Poolman, R. Borrow, J. Findlow, J. D. Domingo, J. Puig-Barbera, J. M. Baldó, V. Planelles, A. Jubert, J. Colomer, A. Gil, K. Levie, A. D. Kervyn, V. Weynants, F. Dominguez, R. Barberá, and F. Sotolongo. 2007. Immunogenicity and safety of three doses of a bivalent (B:4:1.19,15 and B:4:1.7-2,4) meningococcal outer membrane vesicle vaccine in healthy adolescents. Clin. Vaccine Immunol. 14:65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drabick, J. J., B. L. Brandt, E. E. Moran, N. B. Saunders, D. R. Shoemaker, and W. D. Zollinger. 1999. Safety and Immunogenicity testing of an intranasal group B meningococcal native outer membrane vesicle vaccine in healthy volunteers. Vaccine 18:160-172. [DOI] [PubMed] [Google Scholar]
- 5.Findlow, J., A. Holland, N. Andrews, V. Weynants, F. Sotolongo, P. Balmer, J. Poolman, and R. Borrow. 2007. Comparison of Phenotypically Indistinguishable but geographically distinct Neisseria meningitidis group B isolates in a serum bactericidal antibody assay. Clin. Vaccine Immunol. 14:1451-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisseha, M., P. Chen, B. L. Brandt, T. Kijek, E. E. Moran, and W. D. Zollinger. 2005. Characterization of native outer membrane vesicles from LpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect. Immun. 73:4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jódar, L., I. M. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 359:1499-1508. [DOI] [PubMed] [Google Scholar]
- 9.Kahler, C. M., L. E. Martin, G. C. Shih, M. M. Rahman, R. W. Carlson, and D. S. Stephens. 1998. The (alpha2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect. Immun. 66:5939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran, E. E., B. L. Brandt, and W. D. Zollinger. 1994. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infect. Immun. 62:5290-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norheim, G., A. Aseffa, M. A. Yassin, G. Mengistu, A. Kassu, D. Fikremariam, W. Tamire, Y. Merid, E. A. Høiby, D. A. Caugant, E. Fritzsønn, T. Tangen, B. Melak, D. Berhanu, M. Harboe, J. Kolberg, and E. Rosenqvist. 2008. Specificity of subcapsular antibody responses in Ethiopian patients following disease caused by serogroup A meningococci. Clin. Vaccine Immunol. 15:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peeters, C. C., H. C. Rümke, L. C. Sundermann, E. M. Rouppe van der Voort, J. Meulenbelt, M. Schuller, A. J. Kuipers, P. van der Ley, and J. T. Poolman. 1996. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine 14:1009-1015. [DOI] [PubMed] [Google Scholar]
- 13.Perrett, K. P., and A. J. Pollard. 2005. Towards an improved serogroup B Neisseria meningitidis vaccine. Expert Opin. Biol. Ther. 5:1611-1625. [DOI] [PubMed] [Google Scholar]
- 14.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Aricò, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J., Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 15.Rouppe van der Voort, E. M., B. Kuipers, H. F. Brugghe, L. M. van Unen, H. A. Timmermans, P. Hoogerhout, and J. T. Poolman. 1997. Epitope specificity of murine and human bactericidal antibodies against PorA P1.7,16 induced with experimental meningococcal group B vaccines. FEMS Immunol. Med. Microbiol. 17:139-148. [DOI] [PubMed] [Google Scholar]
- 16.Santos, F. G., R. R. Deck, J. Donnelly, W. Blackwelder, and D. M. Granoff. 2001. Importance of complement source in measuring meningococcal bactericidal titers. Clin. Diagn. Lab. Immunol. 8:616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders, N. B., D. R. Shoemaker, B. L. Brandt, E. E. Moran, T. Larsen, and W. D. Zollinger. 1999. Immunogenicity of intranasally administered meningococcal native outer membrane vesicles in mice. Infect. Immun. 67:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uria, M. J., Q. Zhang, Y. Li, A. Chan, R. M. Exley, B. Gollan, H. Chan, I. Feavers, A. Yarwood, R. Abad, R. Borrow, R. A. Fleck, B. Mulloy, J. A. Vazquez, and C. M. Tang. 2008. A generic mechanism in Neisseria meningitidis for enhanced resistance against bactericidal antibodies. J. Exp. Med. 205:1423-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Voort, E. R., P. van der Ley, J. van der Biezen, S. George, O. Tunnela, H. van Dijken, B. Kuipers, and J. Poolman. 1996. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect. Immun. 64:2745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel, U., A. Weinberger, R. Frank, A. Müller, J. Köhl, J. P. Atkinson, and M. Frosch. 1997. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect. Immun. 65:4022-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wedege, E., E. A. Høiby, E. Rosenqvist, and G. Bjune. 1998. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect. Immun. 66:3223-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westphal, O., O. Luderitz, and F. Bister. 1952. Uber die Extraktion von bakterien mit Phenol/Wasser. Z. Naturforsch. 7b:148-155. [Google Scholar]
- 23.Weynants, V. E., C. M. Feron, K. K. Goraj, M. P. Bos, P. A. Denoël, V. G. Verlant, J. Tommassen, I. R. A. Peak, R. C. Judd, M. P. Jennings, and J. T. Poolman. 2007. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. Immun. 75:5434-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zollinger, W. D., and R. E. Mandrell. 1983. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococci. Infect. Immun. 40:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]