Abstract
Neisseria meningitidis is a leading cause of bacterial meningitis in the United States, with the highest case fatality rates reported for individuals ≥15 years of age. This study compares the safety and immunogenicity of the Novartis Vaccines investigational quadrivalent meningococcal CRM197 conjugate vaccine, MenACWY-CRM, to those of the licensed meningococcal conjugate vaccine, Menactra, when administered to healthy adults. In this phase III multicenter study, 1,359 adults 19 to 55 years of age were randomly assigned to one of four groups (1:1:1:1 ratio) to receive a single dose of one of three lots of MenACWY-CRM or a single dose of Menactra. Serum samples obtained at baseline and 1 month postvaccination were tested for serogroup-specific serum bactericidal activity using human complement (hSBA). The hSBA titers following vaccination with MenACWY-CRM and Menactra were compared in noninferiority and prespecified superiority analyses. Reactogenicity was similar in the MenACWY-CRM and Menactra groups, and neither vaccine was associated with a serious adverse event. When compared with Menactra, MenACWY-CRM met the superiority criteria for the proportions of recipients achieving a seroresponse against serogroups C, W-135, and Y and the proportion of subjects achieving postvaccination titers of ≥1:8 for serogroups C and Y. MenACWY-CRM's immunogenicity was statistically noninferior (the lower limit of the two-sided 95% confidence interval was more than −10%) to that of Menactra for all four serogroups, with the postvaccination hSBA geometric mean titers being consistently higher for MenACWY-CRM than for Menactra. MenACWY-CRM is well tolerated in adults 19 to 55 years of age, with immune responses to each of the serogroups noninferior and, in some cases, statistically superior to those to Menactra.
Neisseria meningitidis is a leading cause of bacterial meningitis in the United States (11). While the incidence of meningococcal disease is highest in infants, the highest case fatality rates are observed among older subjects: 4.6% in children <15 years of age, 22.5% in individuals 15 to 24 years of age, and 16.5% in adults >25 years of age (5). In 2007, approximately 72% of all cases of meningococcal disease in the United States were reported in individuals ≥18 years of age (4a).
Two quadrivalent meningococcal vaccines, for prevention of meningococcal disease caused by serogroups A, C, W-135, and Y, are available in the United States: an unconjugated polysaccharide vaccine (MPSV4 [Menomune]; Sanofi Pasteur, Inc., Swiftwater, PA) and a diphtheria toxoid protein conjugated vaccine (Menactra; Sanofi Pasteur, Inc., Swiftwater, PA). In the United States, the quadrivalent meningococcal polysaccharide protein conjugate vaccine is currently recommended for all persons 11 to 18 years of age and for those persons 2 to 55 years of age who are at increased risk of meningococcal disease (4). This vaccine is only available in the United States and Canada. Outside North America, polysaccharide vaccines are the only quadrivalent meningococcal vaccines available.
To expand the options for prevention of meningococcal disease, an investigational quadrivalent meningococcal CRM197 conjugate vaccine (MenACWY-CRM; Novartis Vaccines, Siena, Italy) was recently developed. Studies have shown that MenACWY-CRM is well tolerated and elicits robust immunogenicity when administered to infants as young as 2 months of age (10, 12), as well as children (2a) and adolescents (9). A large, randomized, controlled, direct comparative phase III study was recently completed, examining the safety and immunogenicity of MenACWY-CRM versus those of Menactra when administered to healthy subjects 11 to 55 years of age. Due to differences in expected immune responses between adolescents and adults, the predefined analyses were powered and analyzed separately for the 11- to 18-year-old and 19- to 55-year-old groups. The larger adolescent age stratum included in the study was powered to demonstrate the consistency of the immune response to three lots of MenACWY-CRM, and the results showed that MenACWY-CRM generated a significantly higher immune response to all four serogroups than Menactra (8). Here, we present the analysis of the safety and immunogenicity of MenACWY-CRM versus those of Menactra among the adult subjects 19 to 55 years of age.
MATERIALS AND METHODS
A phase III multicenter, randomized, controlled study was conducted in the United States to evaluate the safety and immunogenicity of MenACWY-CRM compared with those of Menactra in healthy subjects 11 to 55 years of age. Institutional Review Board approval of the protocol was obtained prior to enrollment, and written informed consent was obtained from each subject.
Subjects.
Healthy subjects 11 to 55 years of age were eligible for inclusion in the study; data from those subjects 19 to 55 years of age are the focus of the current analysis. Subject exclusion criteria included household contact with or intimate exposure to an individual with N. meningitidis infection in the 60 days pre-enrollment, previous vaccination with a meningococcal vaccine, receipt of any vaccine within a period of 1 month prior to enrollment, serious acute or chronic illness, or history of hypersensitivity to any vaccine component. Data were collected on the history of previous diphtheria-tetanus-containing vaccinations within the previous 5 years.
Subjects were randomly allocated 1:1:1:1 to one of four groups (MenACWY-CRM lot 1, lot 2, or lot 3 or Menactra). Randomization was implemented using four-subject blocks and stratified by center via an interactive voice response system (IVRS) provided by an external vendor. An unblinded member of the study site staff called the IVRS, and based on the age of the subject (adult or adolescent) and on the site, the IVRS assigned a subject number and treatment group.
Study vaccines.
Each dose of the study vaccine (MenACWY-CRM) consisted of two components: one component contained 10 μg of lyophilized meningococcal serogroup A capsular polysaccharide conjugated to CRM197 (MenA), and the other contained 5 μg each of capsular polysaccharide of serogroups C, W-135, and Y, conjugated to CRM197 in 0.5 ml of phosphate-buffered saline, which was used to reconstitute the lyophilized MenA component before injection. The lots differed by batch number only. The comparator vaccine (Menactra) contained 4 μg of capsular polysaccharide from each serogroup (A, C, Y, and W-135) covalently bound to diphtheria toxoid protein in each 0.5-ml dose. All subjects received a single dose (0.5 ml) of vaccine administered intramuscularly in the left deltoid area.
Safety monitoring.
Subjects were observed for 30 min postvaccination for any local or systemic reactions or for hypersensitivity reactions. The oral temperature was recorded, and the subjects were given diary cards to record any local (pain, erythema, and induration) or systemic (chills, nausea, malaise, myalgia, arthralgia, headache, and rash) reactions that occurred between days 1 and 7. Any adverse events (AEs) requiring medical attention were recorded for 1 month postvaccination, and any medically significant and serious AEs (SAEs) were recorded for 6 months postvaccination.
Serology.
Blood samples (20 ml) were obtained at baseline immediately prior to vaccination and 1 month postvaccination. The immunogenicity of study vaccines was evaluated using serum bactericidal activity using human complement (hSBA) for meningococcal serogroups A, C, W-135, and Y at the laboratories of Novartis Vaccines, Marburg, Germany, according to methods described previously (12).
Statistical methods and analyses.
The larger, adolescent arm of this study, which was specifically powered to demonstrate the consistency of the immune response to three lots of MenACWY-CRM, confirmed that these lots had similar reactogenicity and immunogenicity profiles. Therefore, data from these three lots in the adult arm of this study were pooled according to the protocol to determine the following prespecified end points: the proportion of seroresponders, the proportion of subjects with a postvaccination hSBA titer of ≥1:8, and the postvaccination hSBA geometric mean titer (GMT). Seroresponse was a composite end point defined by increases in the hSBA titer from pre- to postvaccination. If the prevaccination titer was below the limit of detection (<1:4), seroresponse was defined by seroconversion to a postvaccination titer of ≥1:8. If the prevaccination titer was ≥1:4, seroresponse was defined by a fourfold or greater increase in titer from pre- to postvaccination.
The statistical criteria defining noninferiority and statistical superiority for each end point were prespecified in the study protocol. For the proportion of subjects achieving a seroresponse and those with a postvaccination hSBA titer of ≥1:8, the two-sided 95% confidence interval (CI) for the difference in proportions (MenACWY-CRM minus Menactra) was determined first; noninferiority was defined if the lower limit (LL) of the two-sided 95% CI was more than −10%, and superiority was defined if the LL was >0%. For the GMT end point, the immunogenicity of MenACWY-CRM was considered noninferior to the immunogenicity of Menactra if the LL of the two-sided 95% CI around the ratio of the GMTs (MenACWY-CRM:Menactra) was >0.5; superiority was defined as an LL of >1.0. All statistical analyses were performed using SAS software, version 9.1 or higher (SAS Institute, Cary, NC).
RESULTS
Subject characteristics.
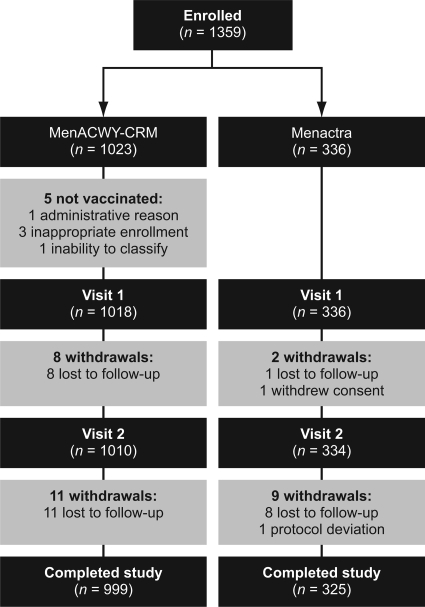
A total of 1,359 subjects (19 to 55 years of age) were enrolled at 44 sites in the United States between March and July 2007. Subject characteristics were similar between the study groups (Table 1). Of the 1,359 subjects enrolled, 1,324 completed the study according to protocol (Fig. 1). Five subjects from the MenACWY-CRM group were not vaccinated due to inappropriate enrollment (n = 3), administrative reasons (n = 1), or an inability to classify (an adult enrolled into a pediatric practice was refused a physical examination) (n = 1) (Fig. 1). Of the subjects who were vaccinated, 30 subjects withdrew from the study: 28 were lost to follow-up, 1 withdrew consent, and 1 withdrew following a protocol deviation (Fig. 1).
TABLE 1.
Subject characteristics
| Characteristic | No. of subjects vaccinated with: |
|
|---|---|---|
| MenACWY-CRM (n = 1,023) | Menactra (n = 336) | |
| Mean age [yr (SD)] | 39.0 (9.6) | 38.7 (9.9) |
| Male [no. (%)] | 249 (24) | 84 (25) |
| Race/ethnicity [no. (%)] | ||
| Asian | 37 (4) | 8 (2) |
| Black | 85 (8) | 34 (10) |
| Caucasian | 828 (81) | 261 (78) |
| Hispanic | 60 (6) | 29 (9) |
| Other | 13 (1) | 4 (1) |
FIG. 1.
Subject disposition flowchart.
Safety and tolerability.
Pain was the most commonly reported solicited local reaction and was reported by 38.4% and 40.8% of MenACWY-CRM and Menactra recipients, respectively (Table 2). All but three cases (all in the MenACWY-CRM group) were reported as mild or moderate. Erythema and induration were reported by ≤16.4% of subjects in each group, and the majority of instances of erythema (72.6 to 85.2%) or induration (71.4 to 86.0%) were classed as mild (0 to 25 mm in diameter). The most commonly reported solicited systemic reactions were headache, myalgia, and malaise, which were reported in similar proportions of subjects in each group (Table 2). Other indicators of reactogenicity, including the use of analgesic/antipyretic medication (MenACWY-CRM, 23.6%; Menactra, 22.0%) and staying at home due to a reaction (MenACWY-CRM, 1.7%; Menactra, 0.9%), were similar between vaccine groups (Table 2). Fever was uncommon, being reported by <1% of recipients in each group (Table 2).
TABLE 2.
Solicited local and systemic reactions following vaccination with MenACWY-CRM or Menactra
| Type of reaction | Severity | % of subjects with reaction following vaccination with: |
|
|---|---|---|---|
| MenACWY-CRM (n = 1,018) | Menactra (n = 336) | ||
| Local | |||
| Pain | Any | 38.4 | 40.8 |
| Severe | 0.3 | 0 | |
| Erythema | Any | 16.4 | 12.2 |
| >50 mm in diameter | 2.8 | 0.9 | |
| Induration | Any | 12.6 | 8.6 |
| >50 mm in diameter | 1.4 | 0.3 | |
| Systemic | |||
| Chills | Any | 4.0 | 3.6 |
| Severe | 0.1 | 0 | |
| Nausea | Any | 7.2 | 5.4 |
| Severe | 0.4 | 0.3 | |
| Malaise | Any | 10.0 | 10.1 |
| Severe | 0.9 | 1.5 | |
| Myalgia | Any | 14.0 | 14.9 |
| Severe | 0.5 | 1.5 | |
| Arthralgia | Any | 5.9 | 6.0 |
| Severe | 0.4 | 0.9 | |
| Headache | Any | 24.9 | 25.0 |
| Severe | 1.6 | 1.5 | |
| Fever (≥38°C) | Any | 0.9 | 0.6 |
| Other | |||
| Analgesic/antipyretic used | 23.6 | 22.0 | |
| Stayed at home due to reaction | 1.7 | 0.9 | |
SAEs were reported by 0.7% and 0.9% of MenACWY-CRM and Menactra recipients, respectively. No SAEs occurred that were considered to be possibly or probably related to the study vaccines, and no deaths occurred.
Immunogenicity.
The percentages of seroresponders were consistently higher in the MenACWY-CRM group than in the Menactra group for serogroups C (67% versus 58%), W-135 (50% versus 41%), and Y (56% versus 40%), while the percentages were similar in the two vaccine groups for serogroup A (67% versus 68%) (Table 3). Noninferiority of the proportion of seroresponders to MenACWY-CRM compared with that to Menactra was demonstrated for all four serogroups, and statistical superiority criteria were met for serogroups C, W-135, and Y (Table 3). In post hoc analyses restricted to those subjects who were seronegative (hSBA titer of <1:4) at baseline, a higher proportion of subjects achieved an hSBA titer of ≥1:8 for serogroups C, W-135, and Y following vaccination with MenACWY-CRM than with Menactra (Table 3).
TABLE 3.
Immunogenicity against Neisseria meningitidis serogroups A, C, W-135, and Y 1 month postvaccination with MenACWY-CRM or Menactraa
| Serogroup | Prevaccination status (hSBA titer) | Seroresponse or seroconversion |
hSBA titer ≥ 1:8 |
GMT |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of subjects with indicated response (95% CI); total no. |
Group differencec (95% CI) | No. of subjects with indicated response (95% CI); total no. |
Group difference (95% CI) | No. of subjects with indicated response (95% CI); total no. |
MenACWY-CRM/ Menactra vaccine group ratio (95% CI) | |||||
| MenACWY-CRM | Menactra | MenACWY-CRM | Menactra | MenACWY-CRM | Menactra | |||||
| A | Seronegative (<1:4) | 67 (63-70); 875 | 67 (62-73); 283 | NA | 69 (66-72); 963 | 71 (65-76); 321 | −2 (−7-4)b | 31 (27-36); 963 | 30 (24-37); 321 | 1.06 (0.82-1.37)d |
| Seropositive (≥1:4) | 70 (60-80); 88 | 71 (54-85); 38 | NA | |||||||
| Overall | 67 (64-70); 963 | 68 (63-73); 321 | −1 (−7-5)b | |||||||
| C | Seronegative (<1:4) | 71 (67-75); 596 | 62 (55-69); 214 | NA | 80 (77-83); 961 | 72 (67-77); 318 | 8 (3-14)e | 52 (44-60); 961 | 32 (25-40); 318 | 1.63 (1.24-2.13)f |
| Seropositive (≥1:4) | 61 (56-66); 365 | 51 (41-61); 104 | NA | |||||||
| Overall | 67 (64-70); 961 | 58 (53-64); 318 | 9 (3-15)e | |||||||
| W-135 | Seronegative (<1:4) | 82 (75-88); 160 | 71 (61-80); 94 | NA | 94 (91-96); 484 | 90 (86-93); 292 | 4 (0-9)b | 111 (93-132); 484 | 69 (55-85); 292 | 1.61 (1.24-2.1)f |
| Seropositive (≥1:4) | 35 (29-40); 324 | 26 (20-33); 198 | NA | |||||||
| Overall | 50 (46-55); 484 | 41 (35-47); 292 | 9 (2-17)e | |||||||
| Y | Seronegative (<1:4) | 66 (60-71); 263 | 52 (44-60); 160 | NA | 79 (76-83); 503 | 70 (65-75); 306 | 9 (3-15)e | 44 (37-52); 503 | 21 (17-26); 306 | 2.1 (1.6-2.75)f |
| Seropositive (≥1:4) | 45 (39-52); 240 | 27 (20-35); 146 | NA | |||||||
| Overall | 56 (51-60); 503 | 40 (34-46); 306 | 16 (9-23)e | |||||||
Noninferiority/superiority analyses as predefined in the study protocol were only performed for the overall population.
Noninferiority criterion met (LL of the two-sided 95% CI was more than −10%).
NA, not applicable.
Noninferiority criterion met (LL of the two-sided 95% CI was >0.5%).
Superiority criterion met (LL of the two-sided 95% CI was >0%).
Superiority criterion met (LL of the two-sided 95% CI was >1.0%).
The proportions of subjects with an hSBA titer of ≥1:8 1 month postvaccination were 69 to 94% following vaccination with MenACWY-CRM and 70 to 90% following vaccination with Menactra (Table 3). Using the proportion of subjects with an hSBA titer of ≥1:8 as the end point, the response to MenACWY-CRM was noninferior compared with that to Menactra for all four serogroups and met the criteria for statistical superiority for serogroups C and Y (Table 3).
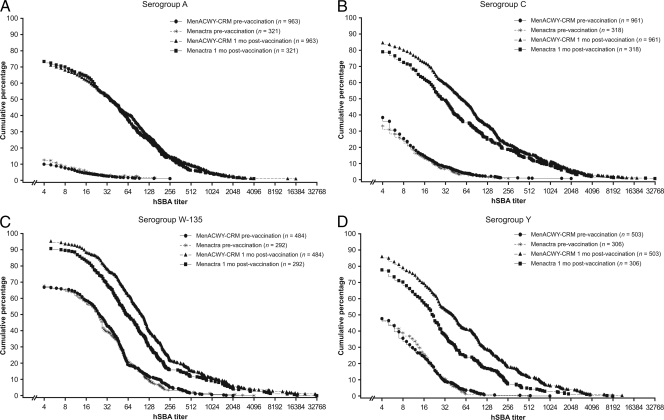
Prevaccination GMTs were similar for all four serogroups across vaccine groups. At 1 month postvaccination, the GMTs showed a large increase for all four serogroups across both vaccine groups. The GMTs to serogroup A with MenACWY-CRM were noninferior to those with Menactra (31 versus 30), but the GMTs with MenACWY-CRM were consistently statistically superior to those with Menactra for serogroups C (52 versus 32), W-135 (111 versus 69), and Y (44 versus 21) (Table 3 and Fig. 2).
FIG. 2.
Reverse cumulative distribution of hSBA titers for serogroups A (A), C (B), W-135 (C), and Y (D) prior to and 1 month after vaccination with MenACWY-CRM or Menactra.
DISCUSSION
These results confirm that MenACWY-CRM is well tolerated and immunogenic when administered to adults 19 to 55 years of age. The primary end point in the larger adolescent arm of this study was a lot-to-lot comparison, which confirmed that there were no differences in immunogenicity or reactogenicity profiles between lots (8). This result permitted the comparison of the combined MenACWY-CRM lots with Menactra in both age groups. The rates of local reactions were slightly higher in the MenACWY-CRM group, and in contrast, the rates of systemic reactions were slightly higher in the Menactra group. Overall, both MenACWY-CRM and Menactra were well tolerated, with similar reactogenicity profiles, and no unexpected or otherwise clinically significant AEs related to the vaccines administered were reported in this study.
Following vaccination with MenACWY-CRM, the immune response, measured by hSBA GMT and seroresponse and highlighted in the reverse cumulative distribution curves (Fig. 2), was statistically superior for serogroups C, W-135, and Y in comparison to the immune response of Menactra. Using the proportion of subjects with a postvaccination hSBA titer of ≥1:8, noninferiority of MenACWY-CRM to Menactra was demonstrated for all four serogroups, and statistical superiority criteria were met for serogroups C and Y. These results are similar to those reported for adolescents in this study (9), in which the immune response to MenACWY-CRM, measured by hSBA GMT, was greater for all serogroups. However, in this study, subtle differences were noted in the immune response to serogroups A and C in the two age groups. In the adolescent group, statistical superiority criteria were met for all serogroups when using GMTs as the end point and for serogroups C, W-135, and Y when seroresponse and the proportion of subjects with a postvaccination hSBA titer of ≥1:8 were used as the end points. In comparison, statistical superiority criteria were not met for serogroup A using any end point. The reason for this difference between the two age groups is not clear.
While the clinical relevance of these higher hSBA GMT immune responses is difficult to assess, these results nonetheless suggest that the efficacy of MenACWY-CRM should be at least comparable to that of Menactra. Furthermore, the long-term persistence of the hSBA titers to the four vaccine serogroups following MenACWY-CRM vaccination is currently unknown, although studies to assess the persistence of the immune response following vaccination with MenACWY-CRM in various age groups are either ongoing or planned.
Immune response differences between MenACWY-CRM and Menactra may be related to differences in the carrier proteins used in each vaccine, namely, CRM197 and diphtheria toxoid, respectively. As CRM197 is a nontoxic mutant of the diphtheria toxin, it does not require detoxification using formaldehyde or glutaraldehyde, a process employed in the preparation of diphtheria toxoids that can cause extensive cross-linking of the carrier protein to accessory antigens, with significant epitope modification (3). Additional features of the vaccine-manufacturing process for MenACWY-CRM that may contribute to these differences in immune responses include the techniques for producing oligosaccharides within a prespecified size range, the chemical linker used in the conjugation process, and the selective conjugation chemistry. These enable the generation of a consistent, reproducible, and well-characterized vaccine product with MenACWY-CRM (1).
Due to the dynamic and varied nature of global meningococcal disease epidemiology, protection against as many serogroups as possible is desirable, particularly for high-risk groups, such as infants and adolescents. Vaccination is also important in populations such as young adults (notably, matriculating college students), military personnel, people with asplenia or terminal complement deficiencies, and travelers to or people residing in areas where meningococcal disease is endemic. Currently, the U.S. Advisory Committee on Immunization Practices (ACIP) recommends vaccination with a quadrivalent meningococcal polysaccharide protein conjugate vaccine for these groups (2). However, there are no general recommendations for children and adolescents in most European countries, due to the relatively low incidence of vaccine-preventable meningococcal disease, apart from that caused by serogroup C. Broad meningococcal protection has particular relevance for adults, given that the serogroup distribution of meningococcal disease shifts with age, with serogroup Y contributing disproportionately in older age groups (6). Recent data show serogroup Y to be responsible for approximately 44% of cases of meningococcal disease in individuals ≥18 years of age in the United States, compared with 18% in individuals ≤18 years of age (http://www.cdc.gov/ncidod/dbmd/abcs/survreports/mening07.pdf), and the incidence of serogroup Y is also increasing in countries such as Colombia (7).
In summary, the results of this study show that MenACWY-CRM is well tolerated and highly immunogenic, with a robust immunogenicity profile that compares favorably with that of the currently licensed diphtheria toxoid protein conjugate vaccine. These results build upon previous studies demonstrating the safety and immunogenicity of MenACWY-CRM across a full spectrum of age ranges (9, 10, 12; Black et al., submitted) and suggest that this vaccine has the potential to be a highly effective tool to help prevent invasive meningococcal disease caused by serogroups A, C, W-135, and Y in populations at risk.
Acknowledgments
This work was supported by Novartis Vaccines & Diagnostics, Inc., Cambridge, MA.
We thank Marti Spalding (Kentucky Pediatric and Adult Research) and Carol L. Miller (Primary Physicians Research).
Medical writing support during the preparation of this paper was provided by Sarah Angus at Alpharmaxim Healthcare Communications, supported by Novartis Vaccines.
Footnotes
Published ahead of print on 7 October 2009.
REFERENCES
- 1.Bardotti, A., G. Averani, F. Berti, S. Berti, V. Carinci, S. D'Ascenzi, B. Fabbri, S. Giannini, A. Giannozzi, C. Magagnoli, D. Proietti, F. Norelli, R. Rappuoli, S. Ricci, and P. Costantino. 2008. Physicochemical characterisation of glycoconjugate vaccines for prevention of meningococcal diseases. Vaccine 26:2284-2296. [DOI] [PubMed] [Google Scholar]
- 2.Bilukha, O. O., and N. Rosenstein. 2005. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 54(RR-7):1-21. [PubMed] [Google Scholar]
- 2a.Black, S., N. P. Klein, J. Shah, L. Bedell, A. Karsten, and P. M. Dull. Immunogenicity and tolerability of a quadrivalent meningococcal glycoconjugate vaccine in 2- to 10-year-old children. Vaccine, in press. [DOI] [PubMed]
- 3.Bröker, M., P. M. Dull, R. Rappuoli, and P. Costantino. 2009. Chemistry of a new investigational quadrivalent meningococcal conjugate vaccine that is immunogenic at all ages. Vaccine 27:5574-5580. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2007. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb. Mortal. Wkly. Rep. 56:794-795. [PubMed] [Google Scholar]
- 4a.Centers for Disease Control and Prevention. 2008. Active bacterial core surveillance report, emerging infections program network, Neisseria meningitidis, 2007. http://www.cdc.gov/abcs/survreports/mening07.pdf.
- 5.Harrison, L. H., M. A. Pass, A. B. Mendelsohn, M. Egri, N. E. Rosenstein, A. Bustamante, J. Razeq, and J. C. Roche. 2001. Invasive meningococcal disease in adolescents and young adults. JAMA 286:694-699. [DOI] [PubMed] [Google Scholar]
- 6.Harrison, L. H. 2006. Prospects for vaccine prevention of meningococcal infection. Clin. Microbiol. Rev. 19:142-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inés Agudelo, C., O. M. Sanabria, and M. V. Ovalle. 2008. Serogroup Y meningococcal disease, Colombia. Emerg. Infect. Dis. 14:990-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson, L. A., R. Baxter, K. S. Reisinger, A. Karsten, J. Shah, L. Bedell, P. M. Dull, and V59P13 study group. 2009. Phase III, comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin. Infect. Dis. 49:e1-e10. [DOI] [PubMed]
- 9.Jackson, L. A., R. M. Jacobson, K. S. Reisinger, A. Anemona, L. E. Danzig, and P. M. Dull. 2009. A randomized trial to determine the tolerability and immunogenicity of a quadrivalent meningococcal glycoconjugate vaccine in healthy adolescents. Pediatr. Infect. Dis. J. 28:86-91. [DOI] [PubMed] [Google Scholar]
- 10.Perrett, K. P., M. D. Snape, K. J. Ford, T. M. John, L. M. Yu, J. M. Langley, S. McNeil, P. M. Dull, F. Ceddia, A. Anemona, S. A. Halperin, S. Dobson, and A. J. Pollard. 2009. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr. Infect. Dis. J. 28:186-193. [DOI] [PubMed] [Google Scholar]
- 11.Shepard, C. W., I. R. Ortega-Sanchez, R. D. Scott II, N. E. Rosenstein, and ABCs Team. 2005. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 115:1220-1232. [DOI] [PubMed] [Google Scholar]
- 12.Snape, M. D., K. P. Perrett, K. J. Ford, T. M. John, D. Pace, L. M. Yu, J. M. Langley, S. McNeil, P. M. Dull, F. Ceddia, A. Anemona, S. A. Halperin, S. Dobson, and A. J. Pollard. 2008. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA 299:173-184. [DOI] [PubMed] [Google Scholar]