Abstract
In the present work, we evaluated the neutralizing capacity of the antibodies induced by dengue virus type 1 and 2 envelope domain III recombinant proteins in monkeys against strains of different dengue virus type 1 and 2 genotypes. Here we demonstrated that dengue virus type 1 and 2 recombinant proteins induced high titers of neutralizing antibodies against different genotype strains.
An effective humoral immune response is critical for protecting against dengue viruses (DEN) (12-14) and is the essential goal of recombinant subunit vaccines based on envelope (E) domain III. Domain III is thought to mediate interactions between the virus and cellular receptors involved in virus attachment (6, 21). In addition, many of the most potent anti-DEN neutralizing monoclonal antibodies characterized to date recognize this domain (8, 9, 24).
There is some evidence that the antibody response to a DEN genotype does not necessarily neutralize homogenotypic DEN strains. In fact, sera from patients infected with DEN type 2 (DEN 2) or DEN 3 show variations in the neutralizing antibody responses against strains isolated early and late during the same epidemic (1, 26). Preclinical studies have exposed that the primary immune responses induced after infection of mice and monkeys with DEN 2 strains belonging to both Asian and American genotypes show differences in the responses of neutralizing antibodies against the same and different strains of infection (4, 5). Based on monoclonal antibody data, changes of specific amino acids in domain III result in the loss of binding of neutralizing monoclonal antibodies (11, 19, 24). A recent study has also demonstrated that monoclonal antibodies show differentiated neutralizing activities depending on the virulence of the strain (7). Based on the previously reported evidence (1, 4, 5, 7, 11, 19, 24, 26), the humoral immune response induced by a vaccine candidate should be evaluated against strains of different genotypes of each serotype.
Previously, we have reported that recombinant proteins containing domain III of DEN 1 or DEN 2 E proteins fused to the P64k protein from Neisseria meningitidis (PD10 and PD5, respectively) induce neutralizing antibodies and partial protection in immunized monkeys (3, 10). In the context of P64k, amino acid changes in E domain III included in the fusion protein have been involved in the antigenicity and immunogenicity of the resultant molecules in the mouse model (28). In the present work, we evaluate the neutralizing antibody activity in sera collected from Macaca fascicularis monkeys immunized with such recombinant proteins against strains of different genotypes.
Sera from Macaca fascicularis monkeys previously immunized with DEN 1 or DEN 2 recombinant fusion proteins were evaluated by a plaque reduction neutralization test (PRNT) against DEN 1 or DEN 2 strains belonging to different genotypes of the corresponding serotype (Table 1) (17, 23). The E domain III used for the PD10 or PD5 genetic construction belongs to strain DEN 1 Jamaica or DEN 2 Jamaica, respectively (27). In brief, monkeys were immunized subcutaneously with four doses of PD10 (two animals, 100 μg/dose) or PD5 (three animals, 50 μg/dose) in Freund solution as an adjuvant. Sera were collected 15 days after the last immunization dose (3, 10). Sera collected 60 days after DEN 1 (two monkeys, 106 PFU of the Jamaica strain) or DEN 2 (five monkeys, 104 to 105 PFU of the A15 strain) virus inoculation were also evaluated (3, 4, 10). PRNT was performed with BHK-21 cells as described by Morens et al. with some modifications (2, 20). The serum dilution that resulted in a 50% reduction in the plaque count, as determined by probit analysis, was considered the neutralizing antibody titer. Monkeys were maintained in accordance with Cuban guidelines for the care and use of laboratory animals.
TABLE 1.
Characteristic of the DEN strains used in this study
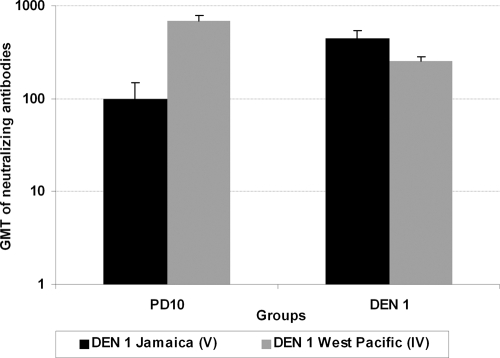
Sera from monkeys immunized with PD10 showed high geometric mean titers (GMT) of neutralizing antibodies against DEN 1 strains of the genotypes studied (IV and V) (Fig. 1). Similarly, the antibodies induced in monkeys infected with DEN 1 neutralized strains of both DEN 1 genotypes. The GMT of neutralizing antibodies in sera from DEN 1-infected monkeys was slightly higher to the DEN 1 Jamaica strain (genotype V), which was the infecting strain, than to the genotype IV strain. However, sera from animals immunized with PD10 showed GMT of neutralizing antibodies to strain DEN 1 West Pacific (genotype IV) sevenfold higher than those to strain DEN 1 Jamaica (genotype V), even when E domain III of PD10 construction belonged to the latter. The DEN 1 E domains III of genotypes IV and V differ by a substitution of Thr for Ile at position 359 in the genotype V strain (17). Thr-359 is a conserved residue among serotypes 1, 2, and 3. DEN 2 Thr-359-Ile mutants have resulted in marked reductions in the binding affinity of monoclonal antibodies (24). It has also been postulated that the specificity of the E domain III epitopes is associated with variations in charge or hydrophobicity at the surface of this domain (25). The substitution of Thr-359 in DEN 1 genotype IV for Ile-359 in genotype V represents a change from a polar amino acid to a nonpolar one that decreases the binding affinity of neutralizing antibodies directed to epitopes containing this residue.
FIG. 1.
Titers of neutralizing antibody against DEN 1 strains in sera collected from monkeys immunized with PD10 or inoculated with DEN 1. Monkeys immunized with PD10 (n = 2) received four doses of 100 μg of the recombinant protein, and sera were collected 15 days after the last immunization. Monkeys inoculated with DEN 1 (n = 2) received one dose of 106 PFU of the strain Jamaica, and sera were collected 60 days later. Titers of neutralizing antibody against DEN 1 strains West Pacific (genotype IV) and Jamaica (genotype V) were evaluated by PRNT in BHK-21 cells and estimated as the higher serum dilution that reduced the number of plaques by 50%. Data represent the reciprocals of the GMT of neutralizing antibodies ± standard deviations per group.
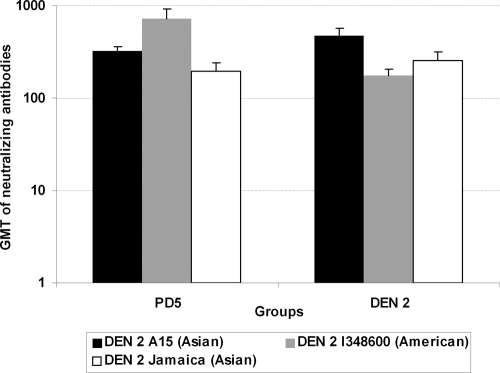
On the other hand, sera from monkeys immunized with PD5 recombinant protein neutralized the strains of Asian and American DEN 2 genotypes (Fig. 2). Monkeys inoculated with DEN 2 also showed neutralizing antibodies to both genotypes. In this case, the titers of neutralizing antibody to strain DEN 2 A15, which was used for inoculating the animals, were higher than those to the Jamaica and I348600 strains. However, the GMT of neutralizing antibodies in sera from monkeys immunized with PD5 protein were fourfold higher to the American-genotype strain than to the Asian strain DEN 2 Jamaica, which was used for the genetic construction of PD5.
FIG. 2.
Titers of neutralizing antibody against DEN 2 strains in sera collected from monkeys immunized with PD5 or inoculated with DEN 2 virus. Monkeys immunized with PD5 (n = 3) received four doses of 50 μg of this recombinant protein, and sera were collected 15 days after the last immunization. Monkeys inoculated with DEN 2 virus (n = 5) received one dose of 104 to 105 PFU of strain A15, and sera were collected 60 days later. Titers of neutralizing antibody against strains DEN 2 A15 (Asian genotype), Jamaica (Asian genotype), and I348600 (American genotype) were evaluated by PRNT in BHK-21 cells and estimated as the higher serum dilution that reduced the number of plaques by 50%. Data represent the reciprocals of the GMT of neutralizing antibodies ± standard deviations per group.
Previous studies of humans and monkeys have shown that DEN 2 American-genotype strains are neutralized more effectively than Asian strains by homologous and heterologous immunization, which has been associated with the lower pathogenicity of the American strains during secondary heterotypic infections (15, 16). There is an amino acid change at position 390 of envelope domain III from Asp in the Asian genotype to Asn in the American genotype. The E-390 residue has a major importance since it is located in a highly hydrophilic region at the outer lateral surface of the E dimer, which has been suggested to contain residues that determine virulence in different flaviviruses (18, 22). It is known that the charge of the residues is significant in the interaction of antigenic sites with antibodies (25). Even when Asp and Asn amino acids have the same hydrophilicity value, perhaps a change from neutral Asp (Asian genotype) to acidic Asn (American genotype) increases antibody-mediated virus neutralization.
In spite of the fact that some strains appeared to be better neutralized than the vaccine-homologous strain, the results indicated that neutralizing antibodies were elicited against different genotypes. With regard to the crucial role of neutralizing antibodies as the primary correlate of protection for DEN viruses, a broad neutralizing response is desirable for any vaccine candidate. The present results consistently demonstrate the capacity of PD5 and PD10 recombinant proteins to induce such a response for serotypes 1 and 2.
Acknowledgments
We thank Luis Morier (Pedro Kourí Tropical Medicine Institute) for providing cell cultures.
This investigation received financial support from the Cuban Program for Dengue Vaccine Development.
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Alvarez, M., A. Pavon-Oro, R. Rodriguez-Roche, L. Bernardo, L. Morier, L. Sanchez, A. M. Alvarez, and M. G. Guzman. 2008. Neutralizing antibody response variation against dengue 3 strains. J. Med. Virol. 80:1783-1789. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, M., R. Rodriguez-Roche, L. Bernardo, L. Morier, and G. Guzman. 2005. Improved dengue virus plaque formation on BHK21 and LLCMK2 cells: evaluation of some factors. Dengue Bull. 29:1-9. [Google Scholar]
- 3.Bernardo, L., A. Izquierdo, M. Alvarez, D. Rosario, I. Prado, C. López, R. Martínez, J. Castro, E. Santana, L. Hermida, G. Guillen, and M. Guzmán. 2008. Immunogenicity and protective efficacy of a recombinant fusion protein containing the domain III of the dengue 1 envelope protein in non-human primates. Antivir. Res. 80:194-199. [DOI] [PubMed] [Google Scholar]
- 4.Bernardo, L., A. Izquierdo, I. Prado, D. Rosario, M. Alvarez, E. Santana, J. Castro, R. Martinez, R. Rodriguez, L. Morier, G. Guillen, and M. G. Guzman. 2008. Primary and secondary infections of Macaca fascicularis monkeys with Asian and American genotypes of dengue virus 2. Clin. Vaccine Immunol. 15:439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardo, L., A. Yndart, S. Vazquez, L. Morier, and M. G. Guzman. 2005. Antibody responses to Asian and American genotypes of dengue 2 virus in immunized mice. Clin. Diagn. Lab. Immunol. 12:361-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu, J. J., R. Rajamanonmani, J. Li, R. Bhuvanakantham, J. Lescar, and M. L. Ng. 2005. Inhibition of West Nile virus entry by using a recombinant domain III from the envelope glycoprotein. J. Gen. Virol. 86:405-412. [DOI] [PubMed] [Google Scholar]
- 7.Falconar, A. K. 2008. Monoclonal antibodies that bind to common epitopes on the dengue virus type 2 nonstructural-1 and envelope glycoproteins display weak neutralizing activity and differentiated responses to virulent strains: implications for pathogenesis and vaccines. Clin. Vaccine Immunol. 15:549-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gromowski, G. D., and A. D. Barrett. 2007. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 366:349-360. [DOI] [PubMed] [Google Scholar]
- 9.Gromowski, G. D., N. D. Barrett, and A. D. Barrett. 2008. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J. Virol. 82:8828-8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermida, L., L. Bernardo, J. Martin, M. Alvarez, I. Prado, C. Lopez, L. Sierra Bde, R. Martinez, R. Rodriguez, A. Zulueta, A. B. Perez, L. Lazo, D. Rosario, G. Guillen, and M. G. Guzman. 2006. A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine 24:3165-3171. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu, K., M. Tadano, R. Men, and C. J. Lai. 1996. Mutational analysis of a neutralization epitope on the dengue type 2 virus (DEN2) envelope protein: monoclonal antibody resistant DEN2/DEN4 chimeras exhibit reduced mouse neurovirulence. Virology 224:437-445. [DOI] [PubMed] [Google Scholar]
- 12.Hombach, J., M. J. Cardosa, A. Sabchareon, D. W. Vaughn, and A. D. Barrett. 2007. Scientific consultation on immunological correlates of protection induced by dengue vaccines report from a meeting held at the World Health Organization 17-18 November 2005. Vaccine 25:4130-4139. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman, B. M., P. L. Summers, D. R. Dubois, and K. H. Eckels. 1987. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 36:427-434. [DOI] [PubMed] [Google Scholar]
- 14.Kliks, S. C., S. Nimmanitya, A. Nisalak, and D. S. Burke. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 38:411-419. [DOI] [PubMed] [Google Scholar]
- 15.Kochel, T. J., D. M. Watts, A. S. Gozalo, D. F. Ewing, K. R. Porter, and K. L. Russell. 2005. Cross-serotype neutralization of dengue virus in Aotus nancymae monkeys. J. Infect. Dis. 191:1000-1004. [DOI] [PubMed] [Google Scholar]
- 16.Kochel, T. J., D. M. Watts, S. B. Halstead, C. G. Hayes, A. Espinoza, V. Felices, R. Caceda, C. T. Bautista, Y. Montoya, S. Douglas, and K. L. Russell. 2002. Effect of dengue-1 antibodies on American dengue-2 viral infection and dengue haemorrhagic fever. Lancet 360:310-312. [DOI] [PubMed] [Google Scholar]
- 17.Laille, M., and C. Roche. 2004. Comparison of dengue-1 virus envelope glycoprotein gene sequences from French Polynesia. Am. J. Trop. Med. Hyg. 71:478-484. [PubMed] [Google Scholar]
- 18.Leitmeyer, K. C., D. W. Vaughn, D. M. Watts, R. Salas, I. Villalobos, de Chacon, C. Ramos, and R. Rico-Hesse. 1999. Dengue virus structural differences that correlate with pathogenesis. J. Virol. 73:4738-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin, B., C. R. Parrish, J. M. Murray, and P. J. Wright. 1994. Localization of a neutralizing epitope on the envelope protein of dengue virus type 2. Virology 202:885-890. [DOI] [PubMed] [Google Scholar]
- 20.Morens, D. M., S. B. Halstead, P. M. Repik, R. Putvatana, and N. Raybourne. 1985. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J. Clin. Microbiol. 22:250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rey, F. A. 2003. Dengue virus envelope glycoprotein structure: new insight into its interactions during viral entry. Proc. Natl. Acad. Sci. USA 100:6899-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 23.Rico-Hesse, R. 2007. Dengue virus evolution and virulence models. Clin. Infect. Dis. 44:1462-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukupolvi-Petty, S., S. K. Austin, W. E. Purtha, T. Oliphant, G. E. Nybakken, J. J. Schlesinger, J. T. Roehrig, G. D. Gromowski, A. D. Barrett, D. H. Fremont, and M. S. Diamond. 2007. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J. Virol. 81:12816-12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volk, D. E., Y. C. Lee, X. Li, V. Thiviyanathan, G. D. Gromowski, L. Li, A. R. Lamb, D. W. Beasley, A. D. Barrett, and D. G. Gorenstein. 2007. Solution structure of the envelope protein domain III of dengue-4 virus. Virology 364:147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong, S. S., J. Abd-Jamil, and S. Abubakar. 2007. Antibody neutralization and viral virulence in recurring dengue virus type 2 outbreaks. Viral Immunol. 20:359-368. [DOI] [PubMed] [Google Scholar]
- 27.Zulueta, A., L. Hermida, L. Lazo, I. Valdes, R. Rodriguez, C. Lopez, R. Silva, D. Rosario, J. Martin, M. G. Guzman, and G. Guillen. 2003. The fusion site of envelope fragments from each serotype of Dengue virus in the P64k protein influence some parameters of the resulting chimeric constructs. Biochem. Biophys. Res. Commun. 308:619-626. [DOI] [PubMed] [Google Scholar]
- 28.Zulueta, A., J. Martin, L. Hermida, M. Alvarez, I. Valdes, I. Prado, G. Chinea, D. Rosario, G. Guillen, and M. G. Guzman. 2006. Amino acid changes in the recombinant dengue 3 envelope domain III determine its antigenicity and immunogenicity in mice. Virus Res. 121:65-73. [DOI] [PubMed] [Google Scholar]