Abstract
We compared a lateral flow device to galactomannan and (1→3)-β-d-glucan assays to detect invasive aspergillosis in an established guinea pig model of pulmonary disease. The lateral flow device became positive earlier (day 3) than the (1→3)-β-d-glucan and galactomannan assays (day 5), with all samples positive by each assay on day 7.
Early diagnosis of invasive aspergillosis is critical for the initiation of appropriate antifungal therapy and may improve outcomes in high-risk patients (2). The use of sensitive biomarkers, including the noninvasive assays for galactomannan and (1→3)-β-d-glucan, also reduces the use of unnecessary antifungal agents (3, 5, 6). Despite their advantages, the galactomannan and the (1→3)-β-d-glucan assays are confined to laboratories equipped for these tests or require samples be sent to reference laboratories. Lateral flow technology incorporates immunochromatographic assays into simple devices for point-of-care diagnosis. When coupled to a monoclonal antibody specific to an extracellular glycoprotein of Aspergillus spp., this technology is a sensitive and specific biomarker (8). Our objective was to evaluate the time to positivity and sensitivity of a lateral flow device in an established guinea pig model of invasive pulmonary aspergillosis (9) and directly compare these results to those obtained using the galactomannan and (1→3)-β-d-glucan assays.
Immunosuppressed male Hartley guinea pigs (Charles River Laboratories) were exposed to conidia for 1 h in an aerosol chamber (9). Serum samples were collected on days 3, 5, and 7 postinoculation. A previously described lateral flow device was used for the serodiagnosis of invasive aspergillosis (8). Briefly, an immunoglobulin G (IgG) monoclonal antibody (JF5) to an epitope on an extracellular antigen secreted constitutively during active growth of Aspergillus was immobilized to a capture zone on a porous nitrocellulose membrane. JF5 IgG was also conjugated to colloidal gold particles to serve as the detection reagent. Serum was added to a release pad containing the antibody-gold conjugate, which bound the target antigen, and then passed along the porous membrane and bound to JF5 IgG monoclonal antibody immobilized in the capture zone. Test results were available within 10 to 15 min after loading the sample. Bound antigen-antibody-gold complexes were observed as a red line with an intensity proportional to the antigen concentration and were classified as negative, weakly positive, moderately positive, or strongly positive (Fig. 1A, B, C, and D). Anti-mouse immunoglobulin immobilized to the membrane in a separate zone served as an internal control.
FIG. 1.
Examples of results from negative (A), weakly positive (B), moderately positive (C), and strongly positive (D) lateral flow device assays. In the absence of the Aspergillus antigen, no complex was formed in the zone containing solid-phase JF5 antibody, and a single internal control line was observed (A).
The (1→3)-β-d-glucan assay was performed using a commercially available kit (Fungitell; Associates of Cape Cod) according to the manufacturer's instructions. The mean rate of change in optical density (OD) at 405 nm over time was measured using a microplate spectrophotometer (Synergy HT; Biotek Instruments). Serum galactomannan was measured using a commercially available kit (Platelia Aspergillus enzyme immunoassay; Bio-Rad Laboratories) according to the manufacturer's instructions. The OD values of each sample, positive control, negative control, and cutoff control were measured using a microplate spectrophotometer at 450 and 630 nm, and the galactomannan index was calculated as the OD of each sample divided by the mean cutoff of the control. The lateral flow assay and the (1→3)-β-d-glucan and galactomannan assays were performed in separate laboratories by different investigators blinded to the results of the other.
For each biomarker, the time to positivity was defined as the first time point at which 20% of samples became positive. Time to positivity was plotted by Kaplan-Meier analysis, and differences in median time at which the assays became positive were analyzed by the log-rank test. Differences in the number of positive samples per time point between the assays were determined by Fisher's exact test. The overall specificity of each assay was also measured in uninfected controls. All statistical tests were performed using Prism 5.0 (GraphPad Software, Inc.).
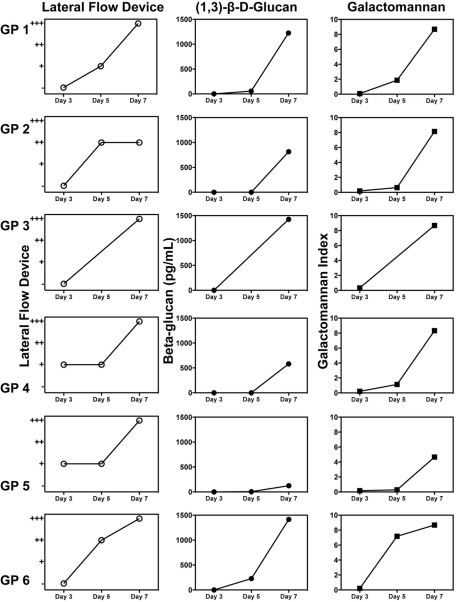
The assays were negative 1 h postinoculation, prior to the onset of invasive disease, with the exception of a galactomannan test result (Table 1), which likely represented a false-positive result, as invasive disease was not yet established (9). Each biomarker became positive early, with more than three samples positive for each assay by day 5 postinoculation. In serial samples from the same animals, each biomarker continued to increase throughout the study (Fig. 2A, B, and C). When the weakly positive lateral flow device results were considered positive, the time to positivity for this assay occurred on day 3, which was significantly earlier than with the galactomannan (day 5; P = 0.03) and (1→3)-β-d-glucan (day 7; P < 0.001) assays. When the weakly positive lateral flow results were considered negative and only the moderately and strongly positive results as positive, the time to positivity for each biomarker assay occurred at the day 5 time point.
TABLE 1.
Comparison of the lateral flow device and galactomannan and (1→3)-β-d-glucan assays
| Assay and result | No. of positive results/no. tested |
||||
|---|---|---|---|---|---|
| 1 h | Day 3 (sensitivity) | Day 5 (sensitivity) | Day 7 (sensitivity) | Uninfected (specificity) | |
| Lateral flow device positive | 0/5 | 12/25 (48%) | 14/17 (82%) | 6/6 (100%) | 0/10 (100%) |
| β-Glucan of >80 pg/ml | 0/5 | 0/25 (0%) | 4/17 (23%) | 6/6 (100%) | 2/10 (80%) |
| Galactomannan index of >0.5 | 1/5 | 1/25 (4%) | 10/17 (59%) | 6/6 (100%) | 0/10 (100%) |
FIG. 2.
Results from serial serum samples collected over time from the same guinea pigs with invasive aspergillosis as measured by lateral flow technology (○), (1→3)-β-d-glucan assay (•), and galactomannan assay (▪). Each line represents the biomarker results from one animal at multiple time points. Serial samples were available for measurement of each biomarker at the multiple time points for six guinea pigs (GP 1 to 6). Symbols for the y axis of the lateral flow device graphs: +, weakly positive results; ++, moderately positive results; +++, strongly positive results.
The sensitivity of each biomarker increased throughout the study period (Table 1). Similar to the time-to-positivity results, when the weakly positive results were considered positive, the sensitivity of the lateral flow device on day 3 (48%) was greater than the galactomannan (4%; P < 0.001) and (1→3)-β-d-glucan (0%; P < 0.001) assays. The sensitivity of the lateral flow device also remained higher than the (1→3)-β-d-glucan assay on day 5 (82% versus 23%, respectively; P < 0.001) but was not significantly different than the galactomannan assay (59%). When the weakly positive lateral flow device results were considered negative and only the moderately to strongly positive results as positive, the sensitivity of this biomarker was similar to that of the galactomannan and (1→3)-β-d-glucan assays (35%, 59%, and 23%, respectively; P > 0.05). Each biomarker was 100% sensitive at the day 7 time point. Excellent specificity was also observed for each biomarker, with only two false positives observed in uninfected animals with the (1→3)-β-d-glucan assay (Table 1).
Certain limitations of this study must be considered. We did not evaluate the assays in the presence of antifungal therapy, which can reduce the sensitivity of both the galactomannan and (1→3)-β-d-glucan assays (4, 7). Furthermore, we did not assess the utility of this device using other biological fluids, such as urine or bronchial alveolar lavage fluid, against pulmonary aspergillosis caused by other Aspergillus isolates, or in a nonneutropenic model, where the pathogenesis of invasive aspergillosis differs from that observed in neutropenic hosts (1). Although previous work using the lateral flow device in samples from patients with invasive aspergillosis demonstrated good sensitivity and specificity (8), further work is needed to establish the clinical utility of this assay. Despite these limitations, this study demonstrates the utility of lateral flow technology as a rapid diagnostic tool for invasive aspergillosis and warrants further study.
Acknowledgments
We thank Destiny Molina and Marcos Olivo for their assistance in the animal studies.
This project was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract no. N01-AI-30041. Fungitell kits were provided by Associates of Cape Cod.
N.P.W. has received research support from CyDex Pharmaceuticals, Pfizer, and Schering-Plough. T.F.P. has received research support from Basilea, Merck, Pfizer, and Schering-Plough, has received speaker fees from Merck and Pfizer, and has been a consultant for Basilea, Merck, Pfizer, and Toyoma. L.K.N., W.R.K., and R.B. have no such disclosures to report.
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Balloy, V., M. Huerre, J. P. Latge, and M. Chignard. 2005. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect. Immun. 73:494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caillot, D., O. Casasnovas, A. Bernard, J. F. Couaillier, C. Durand, B. Cuisenier, E. Solary, F. Piard, T. Petrella, A. Bonnin, G. Couillault, M. Dumas, and H. Guy. 1997. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J. Clin. Oncol. 15:139-147. [DOI] [PubMed] [Google Scholar]
- 3.Maertens, J., K. Theunissen, G. Verhoef, J. Verschakelen, K. Lagrou, E. Verbeken, A. Wilmer, J. Verhaegen, M. Boogaerts, and J. Van Eldere. 2005. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin. Infect. Dis. 41:1242-1250. [DOI] [PubMed] [Google Scholar]
- 4.Marr, K. A., M. Laverdiere, A. Gugel, and W. Leisenring. 2005. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin. Infect. Dis. 40:1762-1769. [DOI] [PubMed] [Google Scholar]
- 5.Odabasi, Z., G. Mattiuzzi, E. Estey, H. Kantarjian, F. Saeki, R. J. Ridge, P. A. Ketchum, M. A. Finkelman, J. H. Rex, and L. Ostrosky-Zeichner. 2004. Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199-205. [DOI] [PubMed] [Google Scholar]
- 6.Pfeiffer, C. D., J. P. Fine, and N. Safdar. 2006. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin. Infect. Dis. 42:1417-1427. [DOI] [PubMed] [Google Scholar]
- 7.Senn, L., J. O. Robinson, S. Schmidt, M. Knaup, N. Asahi, S. Satomura, S. Matsuura, B. Duvoisin, J. Bille, T. Calandra, and O. Marchetti. 2008. 1,3-Beta-d-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin. Infect. Dis. 46:878-885. [DOI] [PubMed] [Google Scholar]
- 8.Thornton, C. R. 2008. Development of an immunochromatographic lateral flow device for rapid serodiagnosis of invasive aspergillosis. Clin. Vaccine Immunol. 15:1095-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallor, A. C., W. R. Kirkpatrick, L. K. Najvar, R. Bocanegra, M. C. Kinney, A. W. Fothergill, M. L. Herrera, B. L. Wickes, J. R. Graybill, and T. F. Patterson. 2008. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob. Agents Chemother. 52:2593-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]