Abstract
Vaccine development, which began with Edward Jenner's observations in the late 18th century, has entered its 4th century. From its beginnings, with the use of whole organisms that had been weakened or inactivated, to the modern-day use of genetic engineering, it has taken advantage of the tools discovered in other branches of microbiology. Numerous successful vaccines are in use, but the list of diseases for which vaccines do not exist is long. However, the multiplicity of strategies now available, discussed in this article, portends even more successful development of vaccines.
That vaccines have had a greater effect on mortality than any measure besides clean water has become a cliché (93). Nevertheless, there are no vaccines for many important diseases. Although in some cases, commercial priorities have been inhibitory, for many of the lacking vaccines, the problems are the complexity of correlates of protection and the difficulty in constructing the right presentation of antigens. Most of our current vaccines have been created by adaptation of living organisms to growth in conditions that attenuate their virulence, by preparation of suspensions of killed microbes or through the concentration and purification of proteins or polysaccharides from pathogens. Fortunately, the advent of molecular biology has given us multiple new tools with which vaccines can be developed. These tools may enable us to make more use of cellular immune responses in addition to the antibody responses that are key to the success of almost all of the vaccines now in use (92).
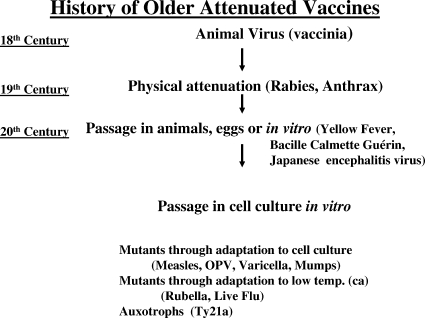
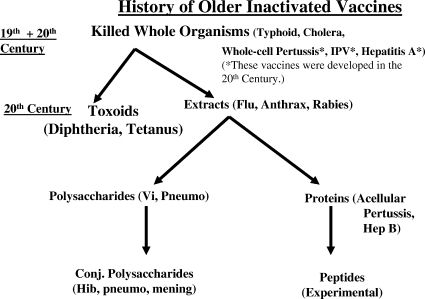
Figures 1 and 2 outline the strategies used until recently to develop attenuated and inactivated vaccines, respectively. Development of the former began with the poxvirus related to smallpox used by Jenner, the physical attenuation of organisms by Louis Pasteur, and the use of passage in vitro or in vivo by workers early in the 20th century. The discovery in midcentury of the technique of cell culture for viruses enabled the development of many live vaccines. For inactivated vaccines, the story began in the late 19th century with killed whole bacteria or viruses, a technique which continues to yield important biologicals. Later development depended on taking apart the microbes and using extracts, purified proteins, purified polysaccharides, or detoxified extracellular products (toxoids). The chemical linkage of proteins to polysaccharides dramatically increased the immunogenicity of vaccines based on bacterial capsules. Table 1 lists the vaccines currently licensed in the United States.
FIG. 1.
An outline of the history of live, attenuated vaccines. OPV, oral polio vaccine; ca, cold adapted.
FIG. 2.
An outline of the history of inactivated vaccines. IPV, inactivated polio; Vi, a capsular polysaccharide of typhoid bacillus; Pneumo, pneumococcus; Hep B, hepatitis B; Conj., conjugated; Hib, Haemophilus influenzae type B; mening, meningococcus.
TABLE 1.
Diseases and disease-causing organisms for which there are vaccines licensed in the United States
| Disease or disease-causing organism |
|---|
| Anthrax |
| Diphtheria |
| Haemophilus influenzae b |
| Hepatitis A |
| Hepatitis B |
| Human papillomavirus |
| Influenza virus |
| Japanese encephalitis |
| Measles |
| Meningococcus |
| Mumps |
| Pertussis |
| Pneumococcus |
| Polio |
| Rabies |
| Rotavirus |
| Rubella |
| Smallpox |
| Tetanus |
| Tuberculosis |
| Typhoid |
| Varicella |
| Yellow fever |
| Herpes zoster |
Newer strategies for vaccine development and new domains in vaccinology are listed in Table 2. The list is inevitably incomplete, as new strategies are proposed almost daily, and this space is insufficient to do more than describe the most promising.
TABLE 2.
Some newer tools and strategies for vaccine development in the 21st century
| Vaccine tool(s) or strategy |
|---|
| Reverse genetics and temperature-sensitive mutations |
| Reassortment |
| Viral recombinants and deletion mutants |
| Codon deoptimization |
| Increased replication fidelity |
| Replicating vectors recombined with genes from pathogens |
| Replication-defective VLPs |
| DNA plasmids |
| Reverse vaccinology |
| Prime-boost |
| Fusion proteins |
| Immune refocusing |
| Gene delivery by invasive bacteria |
| Transcriptomics |
| Proteomics |
| DNA shuffling |
| Killed but metabolically active Listeria bacteria for |
| dendritic cell targeting |
| Transcutaneous vaccination |
| Adjuvants, including cytokines |
| Nanoparticles |
| Therapeutic vaccines |
| Vaccines for noninfectious diseases |
NEW STRATEGIES FOR LIVE VACCINES
Live attenuated vaccines have been among the most powerful for the purpose of disease control and even eradication, owing to the strong antibody and cellular responses elicited by them. However, they have also been associated with genetic instability and residual virulence (31). A number of strategies are now available for dealing with those issues, of which we will mention six: reassortment, reverse genetics, recombination, deletion mutants, codon deoptimization, and control of replication fidelity.
Reassortment is not really a new strategy but has been used frequently for the creation of influenza vaccine strains that bear new hemagglutinins (HAs) and also grow well in vitro and, more recently, for the development of a vaccine against the human rotaviruses that cause infantile gastroenteritis (19). In the development of this vaccine, a bovine rotavirus naturally attenuated for humans served as a donor for 10 of 11 double-stranded RNA (dsRNA) fragments that code for the proteins of rotaviruses. This virus was coinfected in cell cultures with human rotaviruses of the four predominant G serotypes that are defined by the presence of the vp7 protein on the surface of the virus. Vp7 and another surface protein, vp4, contain neutralizing epitopes, and reassortants were selected in which the 10 dsRNA segments from the bovine virus were supplemented by a vp7 from G serotypes 1 to 4. A fifth reassortant was made from the 10 dsRNAs of the bovine virus and the dsRNA of the most common vp4 genotype, called P[8], to yield a pentavalent vaccine that, when given orally, conferred broad properties protective against rotavirus diarrhea (119).
Reverse genetics involves the alteration of DNA complementary to viral negative RNA strands and reconstitution of the viruses through coinfection of cells with plasmids containing those cDNAs. The phenotypic characteristics of the resulting viruses can then be correlated with the genetic changes made to the cDNAs. In this way, new properties can be induced in many different negative-stranded RNA viruses, including that of attenuation (84).
Recombination allows the insertion of desirable genes of one microbe into the genome of another. One example is the parainfluenza 3/respiratory syncytial virus (RSV) recombinant developed at the NIH (103). A parainfluenza virus was attenuated by reverse genetics and recombination with a naturally attenuated bovine parainfluenza 3 virus. The HA-neuraminidase gene of the parainfluenza virus was then excised, and the gene for the RSV fusion protein was inserted in its place. The resulting virus was then given intratracheally to monkeys, which were subsequently challenged with wild RSV, also given intratracheally. Whereas control monkeys were easily infected in both the upper and lower respiratory tracts, vaccinated monkeys were completely protected (112).
A similar strategy has been employed to insert the Ebola virus glycoprotein into the parainfluenza 3 virus in order to protect animals against Ebola virus (12), and Sendai virus has been recombined with the HA-neuraminidase genes of human parainfluenza viruses 2 and 3 to give protection in animals against parainfluenza types 1 to 3 (59).
Numerous molecular strategies have been proposed to effect attenuation more rapidly than by passage in unnatural conditions in tissue culture or in animals that are not normal hosts. The simplest strategy employs deletion mutants, such as those used to attenuate dengue viruses (79) or polioviruses through excision of portions of 5′ noncoding regions (72). Other strategies include codon deoptimization (13, 20, 21, 23, 83, 99), which attenuates by changing the original nucleotide triplets preferred by the virus to code for amino acids to triplets less often used to code for the same amino acids; enhancement of replication fidelity of error-prone RNA polymerases through mutation, thus reducing the likelihood of reversion from attenuation to virulence (120); and insertion of RNA into vaccine strains that are blocked by complementary micro-RNA in some host cells but not in others (2, 61). These strategies can create new attenuated viruses for use in live vaccines.
One example of the possibilities offered by deletion is the deletion of open reading frames (ORFs) 10 to 12 from the varicella virus. Those ORFs are essential for the replication of varicella virus in the skin, and such a deletion mutant might induce fewer vesicular lesions than the current vaccine (17).
VECTORS
The strategy that is now used more than any other in experimental vaccinology is that of the use of vectors. Vectors can be defined as nonpathogenic vehicles into which genes from pathogens are inserted and from which those genes are expressed. The range of vectors now includes a huge variety of viruses and bacteria, and only a flavor can be given of the richness of this strategy. Table 3 lists some, but not all, of the vectors being explored.
TABLE 3.
Examples of vectors under consideration for use in vaccines
| Vector(s) |
|---|
| Poxviruses |
| Adenoviruses |
| Vesicular stomatitis virus |
| Adenovirus-associated virus |
| Alphaviruses |
| Cytomegalovirus |
| Listeria bacteria |
| Lentiviruses |
| Venezuelan equine encephalitis |
| BCG |
| Salmonella bacteria |
An example of a vectored vaccine that is in advanced clinical development is a vaccine for dengue fever. This vaccine is based on the use of the attenuated 17D yellow fever virus as a vector (16). As indicated by the cartoon shown in Fig. 3, the main surface antigens of this virus are coded by the PrM and E genes. These two genes can be removed and replaced by the corresponding genes of other flaviviruses, such as dengue virus serotypes 1 to 4. Thus, to vaccinate against dengue virus, a quadrivalent vaccine has been created, each component of which is a 17D virus vector expressing the genes of a single dengue serotype. The results of early clinical trials showed that neutralizing antibodies are uniformly elicited against all four dengue serotypes after two subcutaneous injections of the quadrivalent vaccine (28, 43, 44). This is a requirement, as dengue hemorrhagic fever is probably caused by a heterotypic enhancing antibody produced in response to a prior dengue virus infection. The same 17D vector has been used to create a new Japanese encephalitis virus vaccine (80).
FIG. 3.
The strategy used to develop a candidate dengue vaccine based on the yellow fever attenuated 17D strain as the vector. C, core; E, envelope. (Courtesy of Jean Lang, Sanofi Pasteur, reproduced with permission).
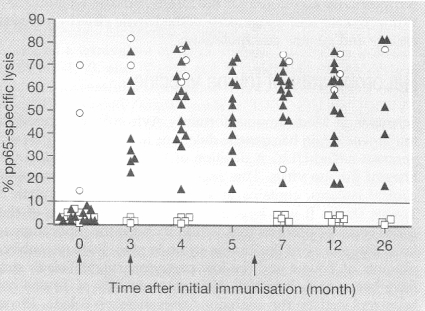
Another example of the utility of vectors for a different approach, that of eliciting cellular immunity, derives from efforts to develop a vaccine against the human cytomegalovirus (HCMV), which is now the most common congenital infection (15, 22, 62). Fetuses infected during pregnancy may later exhibit mental retardation, deafness, and many other anomalies. In addition, HCMV is frequently responsible for complications of solid-organ or bone marrow transplantation (11, 41, 123). Both antibody and cellular immune responses may be needed to control the virus (25). The gene coding for pp65, the lower matrix phosphoprotein of HCMV, was inserted into a canarypox virus vector, which was then injected twice into seronegative volunteers. As shown in Fig. 4, all of the volunteers developed cytotoxic T cells directed against the virus (8, 42).
FIG. 4.
Induction of specific cytotoxic T lymphocytes against pp65 CMV matrix protein in volunteers given doses of a canarypox virus vector containing the gene for pp65 at 0, 3, and 6 months (arrows). Black triangles indicate previously seronegative volunteers, open circles previously seropositive volunteers, and open squares seronegative volunteers who received a placebo. Arrows signify vaccinations. (Reprinted from reference 42 with permission of the publisher).
Vectors containing human immunodeficiency virus (HIV) genes are now the most frequently used experimental vaccines against AIDS. Vectors for adenovirus (both nonreplicating and replicating), poxviruses, vesicular stomatitis virus, and many others are being tested (37).
REPLICATION-DEFECTIVE VLPs
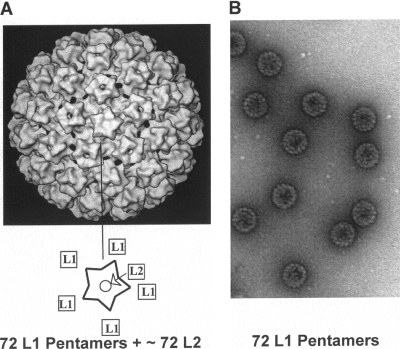
Some viral proteins are capable of self-assembly, even in the absence of other viral proteins. So we now have two powerful vaccines against human papillomavirus (HPV) serotypes that cause cervical and other cancers, and one of those vaccines protects against papillomavirus serotypes that cause genital warts. In these vaccines, the L1 proteins of the viruses are produced by insertion of the genes into Saccharomyces cerevisiae or into baculoviruses (36, 87). This yields virus-like particles (VLPs) consisting of only a single protein, as shown in Fig. 5. Unfortunately, these vaccines are expensive to make, and their use in developing countries may therefore be delayed. Recently, an approach to making an oral papillomavirus vaccine was proposed. A vaccine against typhoid already exists that uses an auxotrophic variant of Salmonella enterica serovar Typhi called Ty21a. The gene for the L-1 protein of HPV serotype 16 (HPV16), an oncogenic serotype, was inserted into Salmonella bacteria. In mice, oral administration of the modified Ty21a resulted in invasion of intestinal cells and local production of VLPs. The mice also developed serum and vaginal antibodies against HPV16 (34).
FIG. 5.
A diagram of the HPV virion showing the location of the L1 protein (A) and an electron micrograph of HPV16 VLPs composed of L1 made using yeast (B). (Reprinted from reference 102 with permission of the publisher, copyright Elsevier [2008]).
The strategy employing VLP production has become widespread, as immunogenicity to proteins presented in a structure rather than in solution is increased (57, 102). Thus, VLPs against viruses as diverse as influenza virus and Ebola virus are viable candidates for vaccine development (12, 38). A special case that combines vectors with VLPs is the replicon strategy based on alphaviruses and flaviviruses (1, 122). These viruses can be deprived of a portion of the genome that allows replication; the excised portion is then placed in a producer cell. As depicted in Fig. 6, transfection of the producer cell with the part of the genome that codes for the structural and nonstructural proteins of the viruses, together with a foreign gene of interest, results in the synthesis of a particle containing viral and foreign proteins. However, whereas such a particle can enter the cells of a vaccinated individual, it can replicate only in a single cycle, because it lacks the gene permitting replication. Nevertheless, during that single cycle, it produces VLPs containing both vector and foreign proteins that stimulate the immune system. Replicons can also be made by the use of DNA plasmids; plasmids containing the genetic material for replicons are also immunogenic but exhibit lesser induction of antivector immunity (68).
FIG. 6.
Single-component RepliVax (single-round vaccine). The strategy of replicon formation based on flavivirus genomes by which single-cycle viruses are produced that can induce only noninfectious but immunogenic particles in the injected host is shown. C, core; E, envelope. (Reprinted from reference 122 with permission of the publisher, copyright Elsevier [2009]).
It is noteworthy that the technologies of VLPs and vectors overlap: some vectors can be used to produce VLPs (48).
DNA PLASMIDS
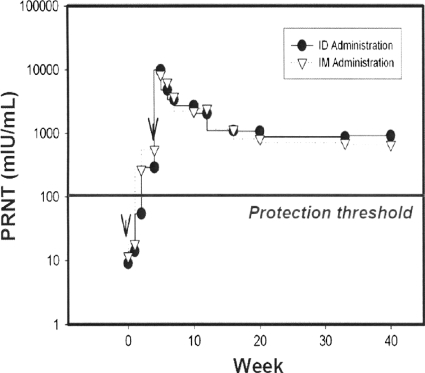
The serendipitous discovery that injection of DNA plasmids containing gene inserts of interest is followed by transcription and translation to proteins that are carried to antigen-presenting cells in the bone marrow was followed by optimism concerning the value of this strategy in vaccinology (115, 124). That optimism has not been justified heretofore by the results of immunization of humans, principally because the induction of antibodies has been poor (29). However, recent results have indicated that this defect appears to be ripe for correction through the use of either of two modalities: electroporation or adjuvantation. Electroporation consists of the simultaneous application of injected DNA together with a small electric current that enhances the uptake of the DNA by antigen-presenting cells (51, 64, 71). Adjuvants that also enhance immunogenicity of DNA plasmids consist of poloxamers and cationic lipids (88; L. Smith, M. Wioch, M. Ye, et al., unpublished data). Through the use of these two modalities, protective levels of antibody against pathogens such as avian influenza virus and measles virus are induced. The results of an experiment performed using monkeys and measles virus cDNA in which protective levels of antibody against measles virus HA and fusion proteins were obtained are shown in Fig. 7.
FIG. 7.
Cationic lipid-based adjuvant formulated with measles Ha+F DNA Vaccine protects nonhuman primates. Neutralizing antibody responses in monkeys given measles virus hemagglutinogen and fusion genes in the form of DNA plasmids together with a cationic lipid-based adjuvant are shown. F, fusion; ID, intradermal; IM, intramuscular; PRNT, plaque reduction neutralization test. (Reprinted from reference 88 with permission).
Thus, the problems involving induction of B-cell-based immunity conferred by DNA plasmids may be solved, but in any case, DNA plasmids have the virtue of priming T-cell immunity and T-cell memory responses, even in the presence of passively transmitted maternal antibodies (47, 97). The latter property makes DNA potentially interesting for immunization of newborns. Of more interest, DNA is now clearly one of the best priming agents in prime-boost regimens (see below).
REVERSE VACCINOLOGY
Reverse vaccinology is a term coined by Rino Rappuoli for the mining of microbial genomes, usually bacterial, to find proteins of vaccine interest (81, 96). The process begins with the sequencing of a genome followed by computer analysis of DNA ORFs to predict which will produce surface or secreted antigens. The candidate ORFs are then inserted into Escherichia coli bacteria for expression of the corresponding proteins. The expressed proteins are used to immunize the mice from which serum samples are collected to test for bactericidal activity and surface localization. If the serum samples show that the protein is conserved in multiple bacterial strains, it can then be included in vaccine development.
Reverse vaccinology was used to identify five proteins from group B Neisseria meningitidis that were combined into a vaccine now in clinical trial (4, 39, 91). A group B vaccine has not been available owing to cross-reaction between capsular antigen and neural cell adhesion molecules in the developing brain. Development of many other bacterial vaccines, including experimental vaccines against group B streptococci, pneumococci, Bacillus anthracis, Chlamydia pneumoniae, and Porphyromonas gingivalis, the principal agent of periodontitis, is profiting from the strategy of reverse vaccinology.
Newer variations on reverse vaccinology include pan-genome reverse vaccinology and comparative genome analysis (104). The former consists of genomic sequencing of numerous strains of a particular organism in order to identify antigens present in common. These then become components of a vaccine that should cover all or almost all strains. This approach has been fruitful for group B streptococci (73). In comparative genome analysis, the objective is to identify antigens produced only in pathogenic strains, which is important for organisms that exist in both pathogenic and nonpathogenic forms.
PRIME-BOOST
A principle has emerged from recent experimental vaccine development, namely, that priming with an antigen presented one way, followed by a boost with the same antigen but presented in a different way, leads to an augmented response to the antigen. This principle is called “prime-boost.” It has been used primarily for AIDS vaccines but is being applied in many other domains (33, 95). It appears that a heterologous boost induces more effector T cells (53).
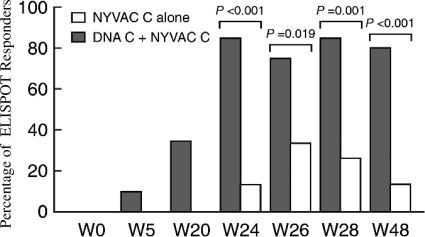
In AIDS vaccine development, DNA plasmids coding for HIV proteins have often been used as the prime, whereas poxviruses containing the genes for the same proteins have often been used as the boost (37). An example is shown in Fig. 8, in which it is shown that the prime-boost regimen was much more immunogenic than administration of the poxvirus alone (46).
FIG. 8.
Percent cytotoxic T lymphocyte responses in volunteers given a vaccinia virus mutant (NYVAC) containing HIV clade C genes alone or preceded by priming with a DNA plasmid containing the same genes, showing the enhancement by the prime-boost strategy. ELISPOT, enzyme-linked immunospot. (Reprinted from reference 46 [originally published in J. Exp. Med. doi:10.1084/jem.20071331] with permission of the publisher.)
MISCELLANEOUS STRATEGIES
Examples of a host of new leads that may prove to be fruitful deserve to be mentioned. Gene delivery by invasive bacteria in relation to the use of Ty21a to deliver HPV genes into intestinal cells is described above, and other uses are under exploration (106). Adenovirus-associated viruses can be vectors for antibody molecules that are continuously produced in host cells because of the integration of the virus into the host genome. For example, this might allow the induction of HIV-neutralizing antibodies without active immunization (58). Although the use of peptide epitopes as vaccines has thus far not yielded a licensed product, several strategies are promising, aside from adjuvantation. Multiple peptides may be linked to provide immunity to multiple strains, as in the case of group A streptococci (26). Protein conjugation increases the immunogenicity of peptides as well as of carbohydrates. Fusion proteins that string together epitopes important for immunity avoid responses to unimportant epitopes (110). Immune refocusing by excision of immunodominant but unprotective epitopes has the same goal (114). So does DNA shuffling, a process in which recombination of genes that induce cross-reactive proteins results in broader immunogenicity (69). Lastly, mimotopes may be identified by screening of peptides with antibodies, enabling immunization with epitopes that are difficult to create artificially, which is particularly important for vaccination against cancer (125). A special case of vectors is represented by killed but metabolically active Listeria bacteria (10, 107). These bacteria can still be taken up by dendritic cells, carrying with them foreign genes that express proteins in those professional antigen-processing cells.
The success of genomics may be carried further by transcriptomics and proteomics (56, 67). In the former case, mRNA transcripts that are active in vivo rather than in vitro may identify proteins that are virulence factors, whereas in the case of proteomics, all of the proteins and their epitopes that are produced by an organism are analyzed to determine structure and function. The application of systems biology to vaccinology is simply the search for gene expression signatures that associate with protective immune responses (35, 94).
ADJUVANTS
For many years, aluminum salts were the only acceptable adjuvants for vaccines. However, the need to induce immune responses to poorly immunogenic proteins, combined with newer knowledge of innate immunity and its effect on adaptive immunity, has encouraged the search for and use of other adjuvants (7, 74, 77, 98). This review is too short to mention all of the adjuvants that are under investigation. Table 4 lists some of the new types of adjuvants and gives examples of each. Already, new oil-in-water adjuvants such as MF-59 have been licensed. Toll-like receptor (TLR) ligands for each receptor are under investigation (27, 45); the first one to be licensed is a monophosphoryl lipid A that stimulates TLR-4 and has been incorporated into an HPV vaccine (87). Safety data on this adjuvant are excellent (118). Other promising TLR ligand adjuvants include dsRNA (TLR-3) (108), flagellin (TLR-5) (54), and CpG oligodinucleotides (TLR-9) (3).
TABLE 4.
New adjuvants (selected)
| Adjuvant type(s) | Example(s)a |
|---|---|
| Oil in water | MF-59 |
| Water in oil | Montanide |
| Saponins | QS-21 |
| Liposomes | ASO1 |
| Bacterial toxins | CT, LT |
| Cytokines | Interleukin-12, granulocyte-macrophage colony-stimulating factor |
| TLR dsRNA ligands | |
| (TRL-3) | MPL (TLR-4) |
| Flagellin (TLR-5) | |
| CpG (TLR-9) |
ASO1, adjuvant system O1; CT, cholera toxin; LT, labile toxin; MPL, monophosphoryl lipid A.
The incorporation of antigens and adjuvants into nanoparticles and nanoemulsions is another way of increasing immune responses, as they can be taken up by antigen-presenting cells, even on a mucosal surface (63, 89, 100).
ROUTE OF VACCINATION
It is obvious that there is a limit to the number of simultaneous injections that can be given, particularly for infants. Efforts to solve that problem have centered on the combination of vaccines into one injection and on alternate routes of vaccination. Although up to six vaccines have been combined into one product, unanticipated problems of interference have demonstrated the limitations of that approach.
Thus, strenuous efforts are being made to develop other routes of vaccine administration: intranasal, aerosol, oral, transcutaneous, and even rectal. Intranasal administration is already used for live influenza vaccine (5) and is potentially interesting for the treatment of other respiratory infections and sexually transmitted infections, as genital mucosal responses may be elicited by antigens placed in the nose (9, 86). Aerosol administration of measles and rubella vaccines has already demonstrated experimental success and is being actively explored by the WHO (70). Many investigators are looking for ways to overcome intestinal tolerance to foreign antigens to facilitate oral administration of vaccines (75, 121).
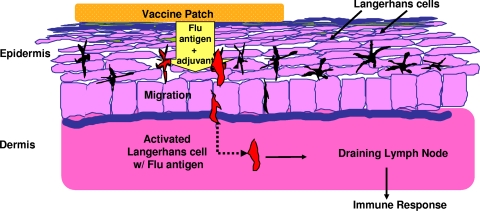
The new route that has become practical is transcutaneous administration. The skin contains numerous Langerhan cells, which carry antigens to neighboring lymph nodes, as depicted in Fig. 9 (40). Although most of these cells are in the dermis, they can migrate into the epidermis (85). Transcutaneous immunization relies on delivery of antigen by patches containing an antigen with or without an adjuvant that promotes passage through the epidermis (76), on microneedles that bear an antigen and penetrate only into the epidermis, or on antigen delivered simultaneously with a small electric current. The microneedle system has been applied to administration of influenza vaccine, for which equivalence or even superiority has been demonstrated in comparison with intramuscular injection (6, 52, 66, 117). Fig. 10 illustrates one device used for microneedle administration. The needle is 30 gauge and is 1.5 mm long.
FIG. 9.
Principle of transcutaneous immunization, in this case antigen and adjuvant delivery via a patch: a patch on the skin contains an influenza virus antigen and E. coli labile toxin as an adjuvant. The antigen migrates through the epidermis to reach the Langerhans cells, which carry it to the lymph nodes. (Reprinted from reference 40 with permission of the publisher.)
FIG. 10.
BD Soluvia microneedle device for transcutaneous immunization. (Courtesy of Becton Dickinson, reproduced with permission).
VACCINE MANUFACTURE
Techniques for vaccine manufacture have been constrained by the regulatory acceptability of newer substrates. Until recently, the only acceptable substrates have been cells cultured from the organs of animals, human diploid cell strains, and embryonated avian eggs, although Vero cells, a cell line derived from the African green monkey kidney, have achieved respectability for the delivery of inactivated polio and rabies vaccines. However, the practical and economic limitations of use of those substrates have encouraged investigation of other means for vaccine production. Pressure to make larger numbers of doses of influenza vaccines has been instrumental in driving the new technologies (116).
Foremost among the new resources are cell lines, including spontaneously or artificially transformed continuous cell lines. Thus, cell lines from canine, chicken, insect, and human cells are all candidates for acceptance (60, 90). Transformation through transfection with the adenovirus 5 E1 gene appears to be safe and allows the cultivation of agents that are ordinarily difficult to replicate in vitro. Among the cell lines that are receiving the most attention are Madin-Darby canine kidney cells (30), HEK293 and PerC6 human retinal cells (65, 113), and Spodoptera frugiperda insect cells (24).
An intriguing potential technique is the possible production of proteins in plant cells by the use of an Agrobacterium vector. In this technology, tobacco plants are grown in the laboratory, and then the leaves are infiltrated under pressure with a suspension of Agrobacterium bacteria containing the gene for an influenza virus HA. The suspension infiltrates the entire leaf, and after 4 days, the plant cells produce influenza virus HA. The leaves can then be ground up and the HA concentrated and purified by the usual techniques to yield vaccine antigen (18, 105).
CONCLUDING THOUGHTS
Influenza vaccine is an example of how modern vaccinology can potentially improve an old biological tool, as shown in Table 5. First, new antigens can be added to the HA. These include defined amounts of neuraminidase; M2e, a protein from the virus that elicits broad neutralization (82); conserved epitopes, including the newly described conserved fusion peptide in the stem of H1 and H5 HA (9, 32, 49, 78, 111); bivalent peptide conjugate that also may give broad protection (4); nucleoprotein that generates cellular immune responses; and multiple T-helper epitopes to potentiate those responses (14, 49).
TABLE 5.
New vaccine approaches for treatment of pandemic and seasonal influenza
| Category and example of new approach |
|---|
| New influenza antigen(s) |
| Conserved HA stem epitopes |
| Bivalent peptide conjugate |
| Multiple Th epitopes |
| New formulations |
| HA-DNA plasmids |
| VLPs |
| Nanoparticles with liposomes |
| Antigens linked to CpG TLR-9 ligands |
| Live vaccine: |
| Attenuated |
| NS-1 deleted |
New formulations of influenza vaccine that could improve immunity are those employing DNA plasmids coding for HA, VLPs, nanoparticles with liposomes, and HA to which new adjuvants have been added using either oil in water or TLR ligands (125).
Live influenza virus vaccine produced on the basis of reassortment is already in use, and NS1-deleted strains that do not elicit interferon responses are under investigation (109).
Finally, combinations of the techniques described above can be used in a prime-boost strategy.
Although this review has not focused on safety, it is clear that the progress of vaccinology depends on vaccines that are not only effective but also free of serious reactions. Although a zero reaction rate is an impossible goal, the new strategies described above could potentially improve vaccine safety through attenuation of live agents and avoidance of allergic and autoimmune reactions through the employ of purer and better-characterized antigens.
In the 21st century, a number of problems stand out as the biggest in vaccinology. Among them I would cite five: immaturity of responses in the newborn owing to poor antigen processing; postmaturity of responses in the elderly owing to deficiencies of naïve T cells; maintenance of both effector and central memory cells after the antigen is no longer present; adjuvants capable of selectively stimulating distinct cell types such as B, Th1, Th2, Th17, Treg, CD4+, CD8+, or dendritic cells; and mucosal immunization with nonreplicating antigens. Although these are large problems, I am not pessimistic about their solution. As Maurice Hilleman stated, “Vaccinology is a field in which dreams may be turned into realities. It is an activity which is heavily overshadowed by uncertainties, but can be conquered by persistent rational pursuits and by selective choices needed to surmount the hills and mountains in the quest” (50).
Biography
 Stanley A. Plotkin is an Emeritus Professor of the University of Pennsylvania and Executive Advisor to Sanofi Pasteur. Until 1991, he was Professor of Pediatrics and Microbiology at the University of Pennsylvania and Professor of Virology at the Wistar Institute and at the same time Director of Infectious Diseases and Senior Physician at the Children's Hospital of Philadelphia. In 1991, Stanley Plotkin left the University to join the vaccine manufacturer Pasteur-Mérieux-Connaught, where for 7 years he was Medical and Scientific Director and was based at Marnes-la-Coquette, outside Paris, France. The company is now named Sanofi Pasteur; he is now Executive Advisor to the Chief Executive Officer.
Stanley A. Plotkin is an Emeritus Professor of the University of Pennsylvania and Executive Advisor to Sanofi Pasteur. Until 1991, he was Professor of Pediatrics and Microbiology at the University of Pennsylvania and Professor of Virology at the Wistar Institute and at the same time Director of Infectious Diseases and Senior Physician at the Children's Hospital of Philadelphia. In 1991, Stanley Plotkin left the University to join the vaccine manufacturer Pasteur-Mérieux-Connaught, where for 7 years he was Medical and Scientific Director and was based at Marnes-la-Coquette, outside Paris, France. The company is now named Sanofi Pasteur; he is now Executive Advisor to the Chief Executive Officer.
Stanley Plotkin's career included internship at Cleveland Metropolitan General Hospital, residency in pediatrics at the Children's Hospital of Philadelphia and the Hospital for Sick Children in London, and 3 years in the Epidemic Intelligence Service of the Centers for Disease Control of the U.S. Public Health Service.
He has been chairman of the Infectious Diseases Committee and the AIDS Task Force of the American Academy of Pediatrics, a liaison member of the Advisory Committee on Immunization Practices, and Chairman of the Microbiology and Infectious Diseases Research Committee of the National Institutes of Health. Stanley Plotkin received the Bruce Medal in Preventive Medicine of the American College of Physicians, the Distinguished Physician Award of the Pediatric Infectious Diseases Society, the Clinical Virology Award of the Pan-American Society for Clinical Virology, the Richard Day Master Teacher in Pediatrics Award of the Alumni Association of New York Downstate Medical College, and the Marshall Award of the European Society for Pediatric Infectious Diseases. In June 1998, he received the French Legion of Honor Medal; in June 2001, the Distinguished Alumnus Award of the Children's Hospital of Philadelphia, in September 2006, the gold medal from the same hospital; the Sabin Gold Medal in May 2002; in September 2004, the Fleming (Bristol) Award of the Infectious Diseases Society of America; in May 2007, the medal of the Fondation Mérieux; in March 2009, the Finland Award of the National Foundation for Infectious Diseases; and in May 2009, the Hilleman Award of the American Society for Microbiology. He was elected to the Institute of Medicine of the National Academy of Sciences in 2005 and to the French Academy of Medicine in 2007. Stanley Plotkin holds an honorary degree from the University of Rouen (France) and from the Complutense University of Madrid. Named lectures in his honor have been established at the Pediatric Academic Societies annual meeting and at the International Advanced Vaccinology Course in Annecy, France. His bibliography includes over 675 articles, and he has edited several books, including the standard textbook on vaccines. He developed the rubella vaccine now in standard use throughout the world, was codeveloper of the newly licensed pentavalent rotavirus vaccine, and has worked extensively on the development and application of other vaccines, including polio, rabies, varicella, and cytomegalovirus vaccines.
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Atkins, G. J., M. N. Fleeton, and B. J. Sheahan. 2008. Therapeutic and prophylactic applications of alphavirus vectors. Expert. Rev. Mol. Med. 10:e33. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, D., M. Kunitomi, M. Vignuzzi, K. Saksela, and R. Andino. 2008. Harnessing endogenous miRNAs to control virus tissue tropism as a strategy for developing attenuated virus vaccines. Cell Host Microbe 4:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, M., and C. Cooper. 2007. Review of hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine safety and efficacy. Expert. Opin. Biol. Ther. 7:1731-1737. [DOI] [PubMed] [Google Scholar]
- 4.Beernink, P. T., D. A. Caugant, J. A. Welsch, O. Koeberling, and D. M. Granoff. 2009. Meningococcal factor H-binding protein variants expressed by epidemic capsular group A, W-135, and X strains from Africa. J. Infect. Dis. 199:1360-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belshe, R. B., K. M. Edwards, T. Vesikari, S. V. Black, R. E. Walker, M. Hultquist, G. Kemble, and E. M. Connor. 2007. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 356:685-696. [DOI] [PubMed] [Google Scholar]
- 6.Belshe, R. B., F. K. Newman, K. Wilkins, I. L. Graham, E. Babusis, M. Ewell, and S. E. Frey. 2007. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine 25:6755-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Yehuda, A., A. Joseph, Y. Barenholz, E. Zeira, S. Even-Chen, I. Louria-Hayon, I. Babai, Z. Zakay-Rones, E. Greenbaum, I. Galprin, R. Gluck, R. Zurbriggen, and E. Kedar. 2003. Immunogenicity and safety of a novel IL-2-supplemented liposomal influenza vaccine (INFLUSOME-VAC) in nursing-home residents. Vaccine 21:3169-3178. [DOI] [PubMed] [Google Scholar]
- 8.Berencsi, K., Z. Gyulai, E. Gonczol, S. Pincus, W. I. Cox, S. Michelson, L. Kari, C. Meric, M. Cadoz, J. Zahradnik, S. Starr, and S. Plotkin. 2001. A canarypox vector-expressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting cytotoxic T cell responses in human CMV-seronegative subjects. J. Infect. Dis. 183:1171-1179. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi, E., P. Ingallinella, M. Finotto, J. Joyce, X. Liang, M. D. Miller, G. G. Kinney, G. Ciliberto, J. W. Shiver, and A. Pessi. 2009. Synthetic peptide vaccines: the quest to develop peptide vaccines for influenza, HIV, and Alzheimer's disease. Adv. Exp. Med. Biol. 611:121-123. [DOI] [PubMed] [Google Scholar]
- 10.Brockstedt, D. G., K. S. Bahjat, M. A. Giedlin, W. Liu, M. Leong, W. Luckett, Y. Gao, P. Schnupf, D. Kapadia, G. Castro, J. Y. Lim, A. Sampson-Johannes, A. A. Herskovits, A. Stassinopoulos, H. G. Bouwer, J. E. Hearst, D. A. Portnoy, D. N. Cook, and T. W. Dubensky, Jr. 2005. Killed but metabolically active microbes: a new vaccine paradigm for eliciting effector T-cell responses and protective immunity. Nat. Med. 11:853-860. [DOI] [PubMed] [Google Scholar]
- 11.Broers, A. E., R. van Der Holt, J. W. van Esser, J. W. Gratama, S. Henzen-Logmans, V. Kuenen-Boumeester, B. Lowenberg, and J. J. Cornelissen. 2000. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 95:2240-2245. [PubMed] [Google Scholar]
- 12.Bukreyev, A., A. Marzi, F. Feldmann, L. Zhang, L. Yang, J. M. Ward, D. W. Dorward, R. J. Pickles, B. R. Murphy, H. Feldmann, and P. L. Collins. 2009. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology 383:348-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns, C. C., J. Shaw, R. Campagnoli, J. Jorba, A. Vincent, J. Quay, and O. Kew. 2006. Modulation of poliovirus replicative fitness in HeLa cells by deoptimization of synonymous codon usage in the capsid region. J. Virol. 80:3259-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton, D. R. 2002. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2:706-713. [DOI] [PubMed] [Google Scholar]
- 15.Cannon, M. J., and P. E. Pellett. 2005. Risk of congenital cytomegalovirus infection. Clin. Infect. Dis. 40:1701-1702. [DOI] [PubMed] [Google Scholar]
- 16.Chambers, T. J., A. Nestorowicz, P. W. Mason, and C. M. Rice. 1999. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J. Virol. 73:3095-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Che, X., M. Reichelt, M. H. Sommer, J. Rajamani, L. Zerboni, and A. M. Arvin. 2008. Functions of the ORF9-to-ORF12 gene cluster in varicella-zoster virus replication and in the pathogenesis of skin infection. J. Virol. 82:5825-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chichester, J. A., L. R. Haaheim, and V. Yusibov. 2009. Using plant cells as influenza vaccine substrates. Expert Rev. Vaccines 8:493-498. [DOI] [PubMed] [Google Scholar]
- 19.Clark, H. F., P. A. Offit, S. A. Plotkin, and P. M. Heaton. 2006. The new pentavalent rotavirus vaccine composed of bovine (strain WC3)-human rotavirus reassortants. Pediatr. Infect. Dis. J. 25:577-583. [DOI] [PubMed] [Google Scholar]
- 20.Coffin, J. M. 2008. Attenuation by a thousand cuts. N. Engl. J. Med. 359:2283-2285. [DOI] [PubMed] [Google Scholar]
- 21.Coleman, J. R., D. Papamichail, S. Skiena, B. Futcher, E. Wimmer, and S. Mueller. 2008. Virus attenuation by genome-scale changes in codon pair bias. Science 320:1784-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colugnati, F. A., S. A. Staras, S. C. Dollard, and M. J. Cannon. 2007. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC. Infect. Dis. 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comeron, J. M., and M. Aguade. 1998. An evaluation of measures of synonymous codon usage bias. J. Mol. Evol. 47:268-274. [DOI] [PubMed] [Google Scholar]
- 24.Cox, M. M., and J. R. Hollister. 2009. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals 37:182-189. [DOI] [PubMed] [Google Scholar]
- 25.Crough, T., and R. Khanna. 2009. Immunobiology of human cytomegalovirus: from bench to bedside. Clin. Microbiol. Rev. 22:76-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dale, J. B. 2008. Current status of group A streptococcal vaccine development. Adv. Exp. Med. Biol. 609:53-63. [DOI] [PubMed] [Google Scholar]
- 27.De Gregorio, E., U. D'Oro, and A. Wack. 2009. Immunology of TLR-independent vaccine adjuvants. Curr. Opin. Immunol. 21:339-345. [DOI] [PubMed] [Google Scholar]
- 28.Deauvieau, F., V. Sanchez, C. Balas, A. Kennel, A. De Montfort, J. Lang, and B. Guy. 2007. Innate immune responses in human dendritic cells upon infection by chimeric yellow-fever dengue vaccine serotypes 1-4. Am. J. Trop. Med. Hyg. 76:144-154. [PubMed] [Google Scholar]
- 29.Donnelly, J. J., B. Wahren, and M. A. Liu. 2005. DNA vaccines: progress and challenges. J. Immunol. 175:633-639. [DOI] [PubMed] [Google Scholar]
- 30.Doroshenko, A., and S. A. Halperin. 2009. Trivalent MDCK cell culture-derived influenza vaccine Optaflu (Novartis vaccines). Expert Rev. Vaccines 8:679-688. [DOI] [PubMed] [Google Scholar]
- 31.Ehrenfeld, E., J. Modlin, and K. Chumakov. 2009. Future of polio vaccines. Expert Rev. Vaccines 8:899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekiert, D. C., G. Bhabha, M. A. Elsliger, R. H. Friesen, M. Jongeneelen, M. Throsby, J. Goudsmit, and I. A. Wilson. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Excler, J. L., and S. Plotkin. 1997. The prime-boost concept applied to HIV preventive vaccines. AIDS 11(Suppl. A):S127-S137. [PubMed] [Google Scholar]
- 34.Fraillery, D., D. Baud, S. Y. Pang, J. Schiller, M. Bobst, N. Zosso, F. Ponci, and D. Nardelli-Haefliger. 2007. Salmonella enterica serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin. Vaccine Immunol. 14:1285-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-Piñeres, A. J., A. Hildesheim, L. Dodd, T. J. Kemp, J. Yang, B. Fullmer, C. Harro, D. R. Lowy, R. A. Lempicki, and L. A. Pinto. 2009. Gene expression patterns induced by HPV-16 L1 virus-like particles in leukocytes from vaccine recipients. J. Immunol. 182:1706-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garland, S. M., M. Hernandez-Avila, C. M. Wheeler, G. Perez, D. M. Harper, S. Leodolter, G. W. Tang, D. G. Ferris, M. Steben, J. Bryan, F. J. Taddeo, R. Railkar, M. T. Esser, H. L. Sings, M. Nelson, J. Boslego, C. Sattler, E. Barr, and L. A. Koutsky. 2007. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 356:1928-1943. [DOI] [PubMed] [Google Scholar]
- 37.Girard, M. P., and W. C. Koff. 2008. Human immunodeficiency virus vaccines, p. 1213-1252. In S. Plotkin, W. A. Orenstein, and P. A. Offit (ed.), Vaccines. Elsevier-Saunders, Philadelphia, PA.
- 38.Girard, M. P., A. Osterhaus, Y. Pervikov, L. Palkonyay, and M. P. Kieny. 2008. Report of the third meeting on influenza vaccines that induce broad spectrum and long-lasting immune responses, World Health Organization, Geneva, Switzerland, 3-4 December 2007. Vaccine 26:2443-2450. [DOI] [PubMed] [Google Scholar]
- 39.Giuliani, M. M., J. du-Bobie, M. Comanducci, B. Arico, S. Savino, L. Santini, B. Brunelli, S. Bambini, A. Biolchi, B. Capecchi, E. Cartocci, L. Ciucchi, F. Di Marcello, F. Ferlicca, B. Galli, E. Luzzi, V. Masignani, D. Serruto, D. Veggi, M. Contorni, M. Morandi, A. Bartalesi, V. Cinotti, D. Mannucci, F. Titta, E. Ovidi, J. A. Welsch, D. Granoff, R. Rappuoli, and M. Pizza. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 103:10834-10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glenn, G. M., S. A. Frech, K. Kennedy, and L. Ellingsworth. 2005. Transcutaneous immunization: antigen delivery to the skin, p. 797. In R. L. Bronaugh and H. I. Maibach (ed.), Percutaneous absorption: drugs, cosmetics, mechanisms, methods, 4th ed. Informa Healthcare, New York, NY.
- 41.Glenn, J. 1981. Cytomegalovirus infections following renal transplantation. Rev. Infect. Dis. 3:1151-1178. [DOI] [PubMed] [Google Scholar]
- 42.Gonczol, E., and S. Plotkin. 2001. Development of a cytomegalovirus vaccine: lessons from recent clinical trials. Expert Opin. Biol. Ther. 1:401-412. [DOI] [PubMed] [Google Scholar]
- 43.Guirakhoo, F., J. Arroyo, K. V. Pugachev, C. Miller, Z. X. Zhang, R. Weltzin, K. Georgakopoulos, J. Catalan, S. Ocran, K. Soike, M. Ratterree, and T. P. Monath. 2001. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J. Virol. 75:7290-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guirakhoo, F., S. Kitchener, D. Morrison, R. Forrat, K. McCarthy, R. Nichols, S. Yoksan, X. Duan, T. H. Ermak, N. Kanesa-Thasan, P. Bedford, J. Lang, M. J. Quentin-Millet, and T. P. Monath. 2006. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum. Vaccines 2:60-67. [DOI] [PubMed] [Google Scholar]
- 45.Guy, B. 2007. The perfect mix: recent progress in adjuvant research. Nat. Rev. Microbiol. 5:505-517. [DOI] [PubMed] [Google Scholar]
- 46.Harari, A., P. A. Bart, W. Stohr, G. Tapia, M. Garcia, E. Medjitna-Rais, S. Burnet, C. Cellerai, O. Erlwein, T. Barber, C. Moog, P. Liljestrom, R. Wagner, H. Wolf, J. P. Kraehenbuhl, M. Esteban, J. Heeney, M. J. Frachette, J. Tartaglia, S. McCormack, A. Babiker, J. Weber, and G. Pantaleo. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J. Exp. Med. 205:63-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassett, D. E., J. Zhang, and J. L. Whitton. 1997. Neonatal DNA immunization with a plasmid encoding an internal viral protein is effective in the presence of maternal antibodies and protects against subsequent viral challenge. J. Virol. 71:7881-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haynes, J. R., L. Dokken, J. A. Wiley, A. G. Cawthon, J. Bigger, A. G. Harmsen, and C. Richardson. 2009. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine 27:530-541. [DOI] [PubMed] [Google Scholar]
- 49.Heiny, A. T., O. Miotto, K. N. Srinivasan, A. M. Khan, G. L. Zhang, V. Brusic, T. W. Tan, and J. T. August. 2007. Evolutionarily conserved protein sequences of influenza A viruses, avian and human, as vaccine targets. PLoS One 2:e1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hilleman, M. R. 2003. Personal reflections on twentieth century vaccinology. Southeast Asian J. Trop. Med. Public Health 34:244-248. [PubMed] [Google Scholar]
- 51.Hirao, L. A., L. Wu, A. S. Khan, A. Satishchandran, R. Draghia-Akli, and D. B. Weiner. 2008. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 26:440-448. [DOI] [PubMed] [Google Scholar]
- 52.Holland, D., R. Booy, F. De Looze, P. Eizenberg, J. McDonald, J. Karrasch, M. McKeirnan, H. Salem, G. Mills, J. Reid, F. Weber, and M. Saville. 2008. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J. Infect. Dis. 198:650-658. [DOI] [PubMed] [Google Scholar]
- 53.Hovav, A. H., M. W. Panas, C. E. Osuna, M. J. Cayabyab, P. Autissier, and N. L. Letvin. 2007. The impact of a boosting immunogen on the differentiation of secondary memory CD8+ T cells. J. Virol. 81:12793-12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huleatt, J. W., V. Nakaar, P. Desai, Y. Huang, D. Hewitt, A. Jacobs, J. Tang, W. McDonald, L. Song, R. K. Evans, S. Umlauf, L. Tussey, and T. J. Powell. 2008. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 26:201-214. [DOI] [PubMed] [Google Scholar]
- 55.Ishikawa, T., D. G. Widman, N. Bourne, E. Konishi, and P. W. Mason. 2008. Construction and evaluation of a chimeric pseudoinfectious virus vaccine to prevent Japanese encephalitis. Vaccine 26:2772-2781. [DOI] [PubMed] [Google Scholar]
- 56.Jagusztyn-Krynicka, E. K., M. Dadlez, A. Grabowska, and P. Roszczenko. 2009. Proteomic technology in the design of new effective antibacterial vaccines. Expert. Rev. Proteomics 6:315-330. [DOI] [PubMed] [Google Scholar]
- 57.Jennings, G. T., and M. F. Bachmann. 2008. The coming of age of virus-like particle vaccines. Biol. Chem. 389:521-536. [DOI] [PubMed] [Google Scholar]
- 58.Johnson, P. R., B. C. Schnepp, J. Zhang, M. J. Connell, S. M. Greene, E. Yuste, R. C. Desrosiers, and C. K. Reed. 2009. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med. 15:901-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones, B., X. Zhan, V. Mishin, K. S. Slobod, S. Surman, C. J. Russell, A. Portner, and J. L. Hurwitz. 2009. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine 27:1848-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jordan, I., A. Vos, S. Beilfuss, A. Neubert, S. Breul, and V. Sandig. 2009. An avian cell line designed for production of highly attenuated viruses. Vaccine 27:748-756. [DOI] [PubMed] [Google Scholar]
- 61.Kelly, E. J., E. M. Hadac, S. Greiner, and S. J. Russell. 2008. Engineering microRNA responsiveness to decrease virus pathogenicity. Nat. Med. 14:1278-1283. [DOI] [PubMed] [Google Scholar]
- 62.Kenneson, A., and M. J. Cannon. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17:253-276. [DOI] [PubMed] [Google Scholar]
- 63.Köping-Höggård, M., A. Sanchez, and M. J. Alonso. 2005. Nanoparticles as carriers for nasal vaccine delivery. Expert Rev. Vaccines 4:185-196. [DOI] [PubMed] [Google Scholar]
- 64.Laddy, D. J., J. Yan, A. S. Khan, H. Andersen, A. Cohn, J. Greenhouse, M. Lewis, J. Manischewitz, L. R. King, H. Golding, R. Draghia-Akli, and D. B. Weiner. 2009. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J. Virol. 83:4624-4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ledwith, B. J., C. L. Lanning, L. A. Gumprecht, C. A. Anderson, J. B. Coleman, N. T. Gatto, G. Balasubramanian, G. M. Farris, R. K. Kemp, L. B. Harper, A. B. Barnum, S. J. Pacchione, K. L. Mauer, P. F. Troilo, E. R. Brown, J. J. Wolf, J. A. Lebronl, J. A. Lewis, and W. W. Nichols. 2006. Tumorigenicity assessments of Per.C6 cells and of an Ad5-vectored HIV-1 vaccine produced on this continuous cell line. Dev. Biol. (Basel) 123:251-263. [PubMed] [Google Scholar]
- 66.Leroux-Roels, I., E. Vets, R. Freese, M. Seiberling, F. Weber, C. Salamand, and G. Leroux-Roels. 2008. Seasonal influenza vaccine delivered by intradermal microinjection: a randomised controlled safety and immunogenicity trial in adults. Vaccine 26:6614-6619. [DOI] [PubMed] [Google Scholar]
- 67.Liu, H. C., J. Hicks, and D. Yoo. 2008. Proteomic dissection of viral pathogenesis. Dev. Biol. (Basel) 132:43-53. [DOI] [PubMed] [Google Scholar]
- 68.Ljungberg, K., A. C. Whitmore, M. E. Fluet, T. P. Moran, R. S. Shabman, M. L. Collier, A. A. Kraus, J. M. Thompson, D. C. Montefiori, C. Beard, and R. E. Johnston. 2007. Increased immunogenicity of a DNA-launched Venezuelan equine encephalitis virus-based replicon DNA vaccine. J. Virol. 81:13412-13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Locher, C. P., M. Paidhungat, R. G. Whalen, and J. Punnonen. 2005. DNA shuffling and screening strategies for improving vaccine efficacy. DNA Cell Biol. 24:256-263. [DOI] [PubMed] [Google Scholar]
- 70.Low, N., S. Kraemer, M. Schneider, and A. M. Restrepo. 2008. Immunogenicity and safety of aerosolized measles vaccine: systematic review and meta-analysis. Vaccine 26:383-398. [DOI] [PubMed] [Google Scholar]
- 71.Luckay, A., M. K. Sidhu, R. Kjeken, S. Megati, S. Y. Chong, V. Roopchand, D. Garcia-Hand, R. Abdullah, R. Braun, D. C. Montefiori, M. Rosati, B. K. Felber, G. N. Pavlakis, I. Mathiesen, Z. R. Israel, J. H. Eldridge, and M. A. Egan. 2007. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 81:5257-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macadam, A. J., G. Ferguson, D. M. Stone, J. Meredith, S. Knowlson, G. Auda, J. W. Almond, and P. D. Minor. 2006. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication. J. Virol. 80:8653-8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maione, D., I. Margarit, C. D. Rinaudo, V. Masignani, M. Mora, M. Scarselli, H. Tettelin, C. Brettoni, E. T. Iacobini, R. Rosini, N. D'Agostino, L. Miorin, S. Buccato, M. Mariani, G. Galli, R. Nogarotto, D. Nardi, F. Vegni, C. Fraser, G. Mancuso, G. Teti, L. C. Madoff, L. C. Paoletti, R. Rappuoli, D. L. Kasper, J. L. Telford, and G. Grandi. 2005. Identification of a universal group B streptococcus vaccine by multiple genome screen. Science 309:148-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malyala, P., and M. Singh. 2008. Formulations and delivery systems for mucosal vaccines, p. 499-511. In M. Vajdy (ed.), Immunity against mucosal pathogens. Springer Science+Business Media B.V., New York, NY.
- 75.Mann, J. F., R. Acevedo, J. D. Campo, O. Perez, and V. A. Ferro. 2009. Delivery systems: a vaccine strategy for overcoming mucosal tolerance? Expert Rev. Vaccines 8:103-112. [DOI] [PubMed] [Google Scholar]
- 76.McKenzie, R., A. L. Bourgeois, S. A. Frech, D. C. Flyer, A. Bloom, K. Kazempour, and G. M. Glenn. 2007. Transcutaneous immunization with the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine 25:3684-3691. [DOI] [PubMed] [Google Scholar]
- 77.McLachlan, J. B., C. P. Shelburne, J. P. Hart, S. V. Pizzo, R. Goyal, R. Brooking-Dixon, H. F. Staats, and S. N. Abraham. 2008. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat. Med. 14:536-541. [DOI] [PubMed] [Google Scholar]
- 78.McMurry, J. A., B. E. Johansson, and A. S. De Groot. 2008. A call to cellular & humoral arms: enlisting cognate T cell help to develop broad-spectrum vaccines against influenza A. Hum. Vaccines 4:148-157. [DOI] [PubMed] [Google Scholar]
- 79.Men, R., M. Bray, D. Clark, R. M. Chanock, and C. J. Lai. 1996. Dengue type 4 virus mutants containing deletions in the 3′ noncoding region of the RNA genome: analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in rhesus monkeys. J. Virol. 70:3930-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Monath, T. P., F. Guirakhoo, R. Nichols, S. Yoksan, R. Schrader, C. Murphy, P. Blum, S. Woodward, K. McCarthy, D. Mathis, C. Johnson, and P. Bedford. 2003. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J. Infect. Dis. 188:1213-1230. [DOI] [PubMed] [Google Scholar]
- 81.Moriel, D., M. Scarselli, et al. 2008. Genome-based vaccine development. Hum. Vaccines 4:184-188. [DOI] [PubMed] [Google Scholar]
- 82.Mozdzanowska, K., D. Zharikova, M. Cudic, L. Otvos, and W. Gerhard. 2007. Roles of adjuvant and route of vaccination in antibody response and protection engendered by a synthetic matrix protein 2-based influenza A virus vaccine in the mouse. Virol. J. 4:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mueller, S., D. Papamichail, J. R. Coleman, S. Skiena, and E. Wimmer. 2006. Reduction of the rate of poliovirus protein synthesis through large-scale codon deoptimization causes attenuation of viral virulence by lowering specific infectivity. J. Virol. 80:9687-9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neumann, G., M. A. Whitt, and Y. Kawaoka. 2002. A decade after the generation of a negative-sense RNA virus from cloned cDNA—what have we learned? J. Gen. Virol. 83:2635-2662. [DOI] [PubMed] [Google Scholar]
- 85.Nicolas, J. F., and B. Guy. 2008. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev. Vaccines 7:1201-1214. [DOI] [PubMed] [Google Scholar]
- 86.Oliveira, M. L., A. P. Areas, and P. L. Ho. 2007. Intranasal vaccines for protection against respiratory and systemic bacterial infections. Expert Rev. Vaccines 6:419-429. [DOI] [PubMed] [Google Scholar]
- 87.Paavonen, J., D. Jenkins, F. X. Bosch, P. Naud, J. Salmeron, C. M. Wheeler, S. N. Chow, D. L. Apter, H. C. Kitchener, X. Castellsague, N. S. de Carvalho, S. R. Skinner, D. M. Harper, J. A. Hedrick, U. Jaisamrarn, G. A. Limson, M. Dionne, W. Quint, B. Spiessens, P. Peeters, F. Struyf, S. L. Wieting, M. O. Lehtinen, and G. Dubin. 2007. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 369:2161-2170. [DOI] [PubMed] [Google Scholar]
- 88.Pan, C. H., G. S. Jimenez, N. Nair, Q. Wei, R. J. Adams, F. P. Polack, A. Rolland, A. Vilalta, and D. E. Griffin. 2008. Use of Vaxfectin adjuvant with DNA vaccine encoding the measles virus hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques against measles virus. Clin. Vaccine Immunol. 15:1214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peek, L. J., C. R. Middaugh, and C. Berkland. 2008. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 60:915-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petricciani, J., and R. Sheets. 2008. An overview of animal cell substrates for biological products. Biologicals 36:359-362. [DOI] [PubMed] [Google Scholar]
- 91.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 92.Plotkin, S. A. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47:401-409. [DOI] [PubMed] [Google Scholar]
- 93.Plotkin, S. L., and S. Plotkin. 2008. A short history of vaccination, p. 1-16. In S. Plotkin, W. A. Orenstein, and P. A. Offit (ed.), Vaccines. Elsevier-Saunders, Phildadelphia, PA.
- 94.Querec, T. D., R. S. Akondy, E. K. Lee, W. Cao, H. I. Nakaya, D. Teuwen, A. Pirani, K. Gernert, J. Deng, B. Marzolf, K. Kennedy, H. Wu, S. Bennouna, H. Oluoch, J. Miller, R. Z. Vencio, M. Mulligan, A. Aderem, R. Ahmed, and B. Pulendran. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10:116-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Radosević, K., A. Rodriguez, A. Lemckert, and J. Goudsmit. 2009. Heterologous prime-boost vaccinations for poverty-related diseases: advantages and future prospects. Expert Rev. Vaccines 8:577-592. [DOI] [PubMed] [Google Scholar]
- 96.Rappuoli, R. 2000. Reverse vaccinology. Curr. Opin. Microbiol. 3:445-450. [DOI] [PubMed] [Google Scholar]
- 97.Reddy, S. T., and H. C. Ertl. 1999. The potential use of DNA vaccines for neonatal immunization. Curr. Opin. Mol. Ther. 1:22-29. [PubMed] [Google Scholar]
- 98.Reed, S. G., S. Bertholet, R. N. Coler, and M. Friede. 2009. New horizons in adjuvants for vaccine development. Trends Immunol. 30:23-32. [DOI] [PubMed] [Google Scholar]
- 99.Robinson, H. L. 2008. Viral attenuation by design. Nat. Biotechnol. 26:1000-1001. [DOI] [PubMed] [Google Scholar]
- 100.Roux, X., C. Dubuquoy, G. Durand, T. L. Tran-Tolla, N. Castagne, J. Bernard, A. Petit-Camurdan, J. F. Eleouet, and S. Riffault. 2008. Sub-nucleocapsid nanoparticles: a nasal vaccine against respiratory syncytial virus. PLoS One 3:e1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schell, J., N. F. Rose, N. Fazo, P. A. Marx, M. Hunter, E. Ramsburg, D. Montefiori, P. Earl, B. Moss, and J. K. Rose. 2009. Long-term vaccine protection from AIDS and clearance of viral DNA following SHIV89.6P challenge. Vaccine 27:979-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schiller, J. T., I. H. Frazer, and D. R. Lowy. 2008. Human papillomavirus vaccines, p. 243-258. In S. Plotkin, W. A. Orenstein, and P. A. Offit (ed.), Vaccines, 5th ed. Elsevier-Saunders, Philadelphia, PA.
- 103.Schmidt, A. C., D. R. Wenzke, J. M. McAuliffe, M. St. Claire, W. R. Elkins, B. R. Murphy, and P. L. Collins. 2002. Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone. J. Virol. 76:1089-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Serruto, D., L. Serino, V. Masignani, and M. Pizza. 2009. Genome-based approaches to develop vaccines against bacterial pathogens. Vaccine 27:3245-3250. [DOI] [PubMed] [Google Scholar]
- 105.Shoji, Y., H. Bi, K. Musiychuk, A. Rhee, A. Horsey, G. Roy, B. Green, M. Shamloul, C. E. Farrance, B. Taggart, N. Mytle, N. Ugulava, S. Rabindran, V. Mett, J. A. Chichester, and V. Yusibov. 2009. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine 27:1087-1092. [DOI] [PubMed] [Google Scholar]
- 106.Sizemore, D. R., A. A. Branstrom, and J. C. Sadoff. 1997. Attenuated bacteria as a DNA delivery vehicle for DNA-mediated immunization. Vaccine 15:804-807. [DOI] [PubMed] [Google Scholar]
- 107.Skoberne, M., A. Yewdall, K. S. Bahjat, E. Godefroy, P. Lauer, E. Lemmens, W. Liu, W. Luckett, M. Leong, T. W. Dubensky, D. G. Brockstedt, and N. Bhardwaj. 2008. KBMA Listeria monocytogenes is an effective vector for DC-mediated induction of antitumor immunity. J. Clin. Investig. 118:3990-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stahl-Hennig, C., M. Eisenblatter, E. Jasny, T. Rzehak, K. Tenner-Racz, C. Trumpfheller, A. M. Salazar, K. Uberla, K. Nieto, J. Kleinschmidt, R. Schulte, L. Gissmann, M. Muller, A. Sacher, P. Racz, R. M. Steinman, M. Uguccioni, and R. Ignatius. 2009. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 5:e1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steel, J., A. C. Lowen, L. Pena, M. Angel, A. Solorzano, R. Albrecht, D. R. Perez, A. Garcia-Sastre, and P. Palese. 2009. Live attenuated influenza viruses containing NS1 truncations as vaccine candidates against H5N1 highly pathogenic avian influenza. J. Virol. 83:1742-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suhrbier, A. 2002. Polytope vaccines for the codelivery of multiple CD8 T-cell epitopes. Expert Rev. Vaccines 1:207-213. [DOI] [PubMed] [Google Scholar]
- 111.Sui, J., W. C. Hwang, S. Perez, G. Wei, D. Aird, L. M. Chen, E. Santelli, B. Stec, G. Cadwell, M. Ali, H. Wan, A. Murakami, A. Yammanuru, T. Han, N. J. Cox, L. A. Bankston, R. O. Donis, R. C. Liddington, and W. A. Marasco. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang, R. S., M. MacPhail, J. H. Schickli, J. Kaur, C. L. Robinson, H. A. Lawlor, J. M. Guzzetta, R. R. Spaete, and A. A. Haller. 2004. Parainfluenza virus type 3 expressing the native or soluble fusion (F) Protein of Respiratory Syncytial Virus (RSV) confers protection from RSV infection in African green monkeys. J. Virol. 78:11198-11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thomas, P., and T. G. Smart. 2005. HEK293 cell line: a vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 51:187-200. [DOI] [PubMed] [Google Scholar]
- 114.Tobin, G. J., J. D. Trujillo, R. V. Bushnell, G. Lin, A. R. Chaudhuri, J. Long, J. Barrera, L. Pena, M. J. Grubman, and P. L. Nara. 2008. Deceptive imprinting and immune refocusing in vaccine design. Vaccine 26:6189-6199. [DOI] [PubMed] [Google Scholar]
- 115.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, and A. Friedman. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1749. [DOI] [PubMed] [Google Scholar]
- 116.Ulmer, J. B., U. Valley, and R. Rappuoli. 2006. Vaccine manufacturing: challenges and solutions. Nat. Biotechnol. 24:1377-1383. [DOI] [PubMed] [Google Scholar]
- 117.Van Damme, P., F. Oosterhuis-Kafeja, M. Van der Wielin, Y. Almagor, O. Sharon, and Y. Levin. 2009. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 27:454-459. [DOI] [PubMed] [Google Scholar]
- 118.Verstraeten, T., D. Descamps, M. P. David, T. Zahaf, K. Hardt, P. Izurieta, G. Dubin, and T. Breuer. 2008. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 26:6630-6638. [DOI] [PubMed] [Google Scholar]
- 119.Vesikari, T., H. F. Clark, P. A. Offit, M. J. Dallas, D. J. DiStefano, M. G. Goveia, R. L. Ward, F. Schodel, A. Karvonen, J. E. Drummond, M. J. DiNubile, and P. M. Heaton. 2006. Effects of the potency and composition of the multivalent human-bovine (WC3) reassortant rotavirus vaccine on efficacy, safety and immunogenicity in healthy infants. Vaccine 24:4821-4829. [DOI] [PubMed] [Google Scholar]
- 120.Vignuzzi, M., E. Wendt, and R. Andino. 2008. Engineering attenuated virus vaccines by controlling replication fidelity. Nat. Med. 14:154-161. [DOI] [PubMed] [Google Scholar]
- 121.Wang, L., and R. L. Coppel. 2008. Oral vaccine delivery: can it protect against non-mucosal pathogens? Expert Rev. Vaccines 7:729-738. [DOI] [PubMed] [Google Scholar]
- 122.Widman, D., I. Frolov, and P. Mason. 2009. Third-generation flavivirus vaccines based on single-cycle, encapsidation-defective viruses, p. 77-126. In K. Mamamorosch, A. Shatkin, and F. Murphy (ed.), Advances in virus research, vol. 72. Elsevier, Philadelphia, PA. [DOI] [PubMed] [Google Scholar]
- 123.Winston, D. J., W. G. Ho, and R. E. Champlin. 1990. Cytomegalovirus infections after allogeneic bone marrow transplantation. Rev. Infect. Dis. 12(Suppl. 7):S776-S792. [DOI] [PubMed] [Google Scholar]
- 124.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468. [DOI] [PubMed] [Google Scholar]
- 125.Zhao, L., Z. Liu, and D. Fan. 2008. Overview of mimotopes and related strategies in tumor vaccine development. Expert Rev. Vaccines 7:1547-1555. [DOI] [PubMed] [Google Scholar]