Abstract
Adequately sensitive and specific methods to diagnose pertussis in adolescents and adults are not widely available. Currently, no Food and Drug Administration-approved diagnostic assays are available for the serodiagnosis of Bordetella pertussis. Since concentrations of B. pertussis-specific antibodies tend to be high during the later phases of disease, a simple, rapid, easily transferable serodiagnostic test was developed. This article describes test development, initial evaluation of a prototype kit enzyme-linked immunosorbent assay (ELISA) in an interlaboratory collaborative study, and analytical validation. The data presented here demonstrate that the kit met all prespecified criteria for precision, linearity, and accuracy for samples with anti-pertussis toxin (PT) immunoglobulin G (IgG) antibody concentrations in the range of 50 to 150 ELISA units (EU)/ml, the range believed to be most relevant for serodiagnosis. The assay met the precision and linearity criteria for a wider range, namely, from 50 to 200 EU/ml; however, the accuracy criterion was not met at 200 EU/ml. When the newly adopted World Health Organization International Standard for pertussis antiserum (human) reference reagent was used to evaluate accuracy, the accuracy criteria were met from 50 to 200 international units/ml. In conclusion, the IgG anti-PT ELISA met all assay validation parameters within the range considered most relevant for serodiagnosis. This ELISA was developed and analytically validated as a user-friendly kit that can be used in both qualitative and quantitative formats. The technology for producing the kit is transferable to public health laboratories.
Despite effective immunization with pertussis vaccines, pertussis remains endemic. Recently reported disease has increased in adolescents and adults, among whom the diagnosis of pertussis is especially problematic (7, 8, 10, 16, 30). Routine surveillance, outbreak investigations, and implementation of control measures have been limited by a lack of validated and standardized tests to identify suspected cases of pertussis (4, 29, 32, 36, 38). By the time health care is sought and pertussis is suspected, isolation of Bordetella pertussis by culture or confirmation by PCR is uncommon (24, 29, 31, 36). Although detecting the physical presence of the bacterium is difficult, antibody titers directed to B. pertussis tend to be elevated during the later phase of illness and persist after the infection is resolved, so the development and validation of a simple and rapid serodiagnostic test has practical utility for diagnosing pertussis infections. The ability to confirm outbreaks quickly using a serologic test has the potential to conserve resources that have been used for outbreaks mistakenly attributed to pertussis (4). Additionally, the routine availability of a serodiagnostic test for pertussis would provide significant public health benefit by furnishing public health officials with a more accurate assessment of the burden of disease in the United States and with a better understanding of the role of adolescents and adults in the transmission of pertussis. Acellular pertussis vaccines for adults and adolescents have recently been licensed (5, 6); however, the absence of readily available, validated and standardized tests to confirm suspected cases in these older age groups has hampered the collection of the epidemiological data required to guide developers and public health officials in effective utilization of these vaccines (11, 12, 32). A serodiagnostic test could supply these data and allow the design and evaluation of control strategies.
A large body of evidence is now available to demonstrate that measurement of specific antibodies could assist in the laboratory confirmation of pertussis (8, 13-15, 17, 20); however, the criteria defining the infection threshold are not well agreed upon by international and national health organizations. One proposal for threshold values was based on the measurement of antibodies against pertussis toxin (PT), filamentous hemagglutinin, and fimbria types 2 and 3 in a population of more than 6,000 U.S. residents of ages 6 to 49 years who participated in the Third National Health and Nutrition Examination Survey (2). Based on the mixture modeling of these data to identify hypothesized exposure groups, an anti-PT immunoglobulin G (IgG) level of >94 ELISA units (EU)/ml was proposed as the diagnostic cutoff point for recent infection, with a lower value of >49 EU/ml as an intermediate cutoff that suggested possible infection (3). Alternate diagnostic thresholds have been developed and applied. Specifically, the Massachusetts State laboratory has utilized a cutoff value of 200 EU/ml for almost 20 years (23), and De Melker et al. (9) adopted a value of 125 EU/ml for routine use in The Netherlands. Thus, the above studies established a variety of threshold cutoffs for anti-PT titers that range from 49 to 200 EU/ml. Final assessment of these proposed diagnostic cutoff points requires a prospective clinical study including patients with confirmed B. pertussis infection. By establishing accurate cutoff values for anti-PT titers for patients currently or recently ill, serological detection may provide a qualitative assessment of whether a test sample has anti-PT titers that are higher or lower than appropriately defined positive and negative control values.
Despite these potential benefits, no Food and Drug Administration (FDA)-approved diagnostic assays are currently available for the serodiagnosis of B. pertussis infection, and none of the published methods (1, 9, 17, 19, 23, 25-27, 33-35, 37) have been demonstrated to be readily transferable to public health laboratories. Thus, the overall goal of this project is to develop a simple and readily transferable enzyme-linked immunosorbent assay (ELISA) for the measurement of anti-PT IgG in human serum samples that subsequently could be subjected to an appropriate clinical assessment. A single-serum dilution-based ELISA procedure with ready-to-use reagents was designed and optimized to quantify the anti-PT range thought relevant for diagnosing late-stage pertussis infections. We describe the initial assay development, initial evaluation of the prototype kit by an interlaboratory collaborative study, and assay validation study.
MATERIALS AND METHODS
Human sera.
Human sera that were either positive or negative for IgG antibodies to PT were obtained by recalcification of plasma. The Centers for Disease Control and Prevention (CDC) provided the plasma, which was obtained from screened donors. Positive plasmas were collected from adult donors with documented pertussis identified through surveillance activities. The donors were culture positive for B. pertussis or epidemiologically linked to a culture-confirmed pertussis case. Specimens were collected 4 to 6 weeks after onset of cough. Negative specimens were collected from adults without pertussis enrolled through a blood bank. Negative serum had anti-PT concentrations below the limit of detection of 2 EU/ml in a conventional ELISA (25). Positive and negative specimens were used as samples for the collaborative study and the analytical validation. Human specimens were collected in compliance with ethical and regulatory requirements. The Center for Biologics Evaluation and Research (CBER) provided the U.S. Reference Pertussis Antiserum (human), lot 3 (CBER3), which has an assigned unitage of 200 EU/ml of IgG anti-PT (25, 39). Kathryn Edwards of Vanderbilt University provided the Vanderbilt pertussis reference serum, a secondary reference that was calibrated in EU/ml using CBER3 (2). The WHO pertussis antiserum (human) reference reagent, which became available in January 2009, was obtained from the National Institute for Biological Standards and Control (NIBSC, Potters Bar, United Kingdom). The reagent, NIBSC code 06/140, has been assigned a value of 335 IU of IgG anti-PT per ampoule (39). Based on an international collaborative study (39), 1 EU of IgG anti-PT is approximately equal to 1 IU of IgG anti-PT.
Lyophilized standards and controls.
Various quantities of 14 positive sera were combined to prepare a pertussis master serum pool (PMP-1). PMP-1 was calibrated against the Vanderbilt pertussis reference serum (2) and assigned a unitage of 619 EU/ml. Six kit standards, with concentrations of 15, 30, 60, 120, 240, and 480 EU/ml, and three kit controls were prepared: negative control I (CI) with nondetectable concentrations of anti-PT IgG, control II (CII) with 49 EU/ml, and control III (CIII) with 94 EU/ml. Standards and controls were prepared by combining PMP-1 with negative sera and then diluting the sera 1:100 using preparation buffer (phosphate-buffered saline [1× PBS], pH 7.4, with 4% bovine serum albumin [Sigma-Aldrich, St. Louis, MO]). PBS (1×) was prepared by dilution of 10-times-concentrated PBS (0.1 M sodium phosphate, 1.45 M sodium chloride, pH 7.4, when diluted for use). One-ml aliquots of the diluted sera were lyophilized on either a 1- or 2-day cycle. Standard and control batch 04, used in the interlaboratory collaborative study, was lyophilized on a 2-day cycle (CBER, Rockville, MD), and standard and control batch 08, used in the analytical validation, was lyophilized on a 1-day cycle (CDC, Atlanta, GA). The concentrations of anti-PT IgG in standards and controls following lyophilization were verified by comparison to a reference serum. The Vanderbilt reference serum, which had been calibrated using CBER3, was used for verification of standard and control batch 04. Because the supply of the Vanderbilt reference had been depleted, CBER3 was used for verification of standard and control batch 08. Concentrations of standards and controls were defined in EU/ml because they were calibrated using CBER3.
Detection antibody.
These studies used a horseradish peroxidase (HRP)-conjugated anti-human IgG(1,2,3,4) Fc-specific mouse isotype IgG1 monoclonal antibody (clone HP6043; Hybridoma Reagent Laboratory, Baltimore, MD). For the prototype kit used in the interlaboratory collaborative study, conjugate was diluted 1:4,200 in HRP stabilizer (KPL, Gaithersburg, MD), lyophilized in 1-ml aliquots using a 2-day lyophilization schedule, and stored at 2 to 8°C. Vials were reconstituted with 8.0 ml of assay buffer (1× PBS containing 4% bovine serum albumin and 0.05% Tween 20 [Spectrum, Gardena, CA]) on the day of use for a final dilution of 1:33,600. The kit used in the analytical validation contained conjugate diluted 1:5,000 in preparation buffer containing 50% glycerol and stored at −20°C. On the day of use, the conjugate was diluted to 1:70,000 using assay buffer. The conjugate stored in 50% glycerol was used in lieu of lyophilized conjugate because it was more stable and easier to prepare.
ELISA.
The ELISA was adapted from methods reported previously (2, 25) with minor modifications. All plated volumes were 100 μl per well. Purified PT antigens (80 μg/ml; lot PT/Ag/03/97 for the interlaboratory collaborative study and lot APT0BAA241 at 95.5 μg/ml for the analytical validation; GlaxoSmithKline, Rixensart, Belgium) were stored at −20°C in 50 mM phosphate buffer (pH 7.6) and 500 mM NaCl that contained 50% glycerol. The PT was diluted to approximately 2 μg/ml (range of 2.0 to 2.3 μg/ml) in coating buffer (0.05 M carbonate-bicarbonate buffer, pH 9.6; Sigma-Aldrich, St. Louis, MO) and added to each well of a microtiter strip (Immunolon 2HB; Thermo Scientific, Waltham, MA) prior to incubation for 14 to 24 h at 2 to 8°C. On the day of an assay, test serum samples were diluted 1:100 in assay buffer. Lyophilized standards and controls were reconstituted with standard and kit control diluent (deionized water containing 0.05% Tween 20). After the coated microtiter strips were washed three times with wash solution (1× PBS containing 0.05% Tween 20), test sera, standards, and controls were added and plates were incubated for 2 h ± 15 min at room temperature, approximately 22°C. Following a second wash, anti-human IgG conjugate was added and plates were incubated again at room temperature for 2 h ± 15 min. After a final wash, TMB substrate (SureBlue Reserve [3,3′,5,5′ tetramethylbenzidine]; KPL, Inc., Gaithersburg, MD) was added, followed by incubation at room temperature for 9 to 11 min. The reaction was terminated by adding stop solution (1 N hydrochloric acid; VWR Scientific, West Chester, PA). Microtiter strips were read at a wavelength of 450 nm. ELISA unitage for each sample was calculated using a four-parameter logistic reference curve. For the interlaboratory collaborative study, each site used the commercially available four-parameter logistic software available in their laboratory. Gen5 software (BioTek, Winooski, VT) was used to calculate unitage during the analytical validation. An assay in the collaborative section was considered valid if the following criteria were met: the standard curve had a smooth sigmoidal shape and had a correlation coefficient of >0.95, the percent coefficient of variation (%CV) of the optical density (OD) of the replicate wells for all standards and controls was <15%, the OD of the assay buffer wells was <0.200 and the OD of CI was <0.400, the OD of 480 EU/ml standard was between 1.200 and 3.000, and the calculated concentrations of CII and CIII fell within prespecified limits (for CII, 26 to 68 EU/ml; for CIII, 56 to 134 EU/ml). The criteria for the analytical validation were the same as those for the collaborative study except the criterion for the %CV of the OD for the standard and control replicate wells was changed to be less than or equal to 20%.
Interlaboratory collaborative study design.
The collaborative study included eight analysts from four laboratories (one analyst from Vanderbilt University, two each from Colorado State Laboratory and the CDC, and three from CBER), who each performed five independent assays using identical kits and aliquots of the same test samples provided by the coordinating laboratory. The analysts and laboratories had relevant experience performing ELISAs; however, there was heterogeneity in the extent of recent experience and in the equipment available at each laboratory. Each assay was performed on a different day. In each assay, one negative sample and seven serum samples of various concentrations were tested. The seven positive test samples were targeted at the following concentrations: 30, 55, 85, 105, 105, 170, and 275 EU/ml. The two 105-EU/ml samples were prepared from the same serum. Four of the samples were tested once per assay, and four were independently diluted and tested three times per assay. The analyst was blinded to identification of samples prior to study initiation, and sample placement on each assay plate was randomly assigned to generate a different layout for each of the five assays. Prior to initiating the study, each analyst was required to successfully complete one qualifying assay. Data were analyzed qualitatively and quantitatively. A total of 741 results were reported from assay plates that met the prespecified validity criteria. A test result was included in the analysis if the reported value fell within the assay range of 15 to 480 EU/ml and the %CV of the concentration measured in replicate wells did not exceed 25%. With these exclusions, 585 values were available for analysis, 63 were excluded because the %CV was greater than 25%, and 93 were excluded because they fell outside the working range of the assay. Of those excluded, 78 values that came from antibody-negative samples were <15 EU/ml.
Analytical validation.
The analytical validation study evaluated assay precision (intra- and interassay and reproducibility), accuracy, dilutional linearity, and recovery. Since the ELISA was not designed to evaluate the lowest analyte potency, the limit of quantitation and limit of detection were not assessed. Acceptance criteria were specified prior to the start of the study and are defined in Results. Precision was evaluated at CBER and CDC laboratories using a 3 × 3 × 3 × 3 design; specifically, each sample was diluted and tested three times per assay with three assays performed per day over a total of 3 days by three analysts per site. In each assay, seven samples were analyzed; these samples had target concentrations of 49, 58, 83, 100, 135, 204, and 217 EU/ml. Assays evaluating linearity, accuracy, and recovery were performed at the CDC laboratory. Linearity was assessed by diluting three high-concentration serum samples twofold, fourfold, and eightfold in antibody-negative serum; each dilution was then treated as a test sample. The geometric mean of the observed values was plotted versus the expected values and fit to a straight line. The 95% confidence limit for the slope and the coefficient of determination (r2) were calculated. Accuracy was evaluated by diluting CBER3 with antibody-negative serum to final concentrations of 50, 100, 150, and 200 EU/ml. Subsequent to the primary validation study, an additional accuracy evaluation was performed using NIBSC 06/140 at final concentrations of 50, 100, 150, and 200 IU/ml. The measured concentration for three independent preparations was compared to the expected concentration. Recovery was assessed by spiking three clinical serum samples with different reconstituted standards to prepare six spiked serum samples. These spiked serum samples were independently spiked three times to yield a total of 18 samples with targeted concentrations ranging from 59 to 248 EU/ml. Recoveries were calculated as the observed concentration divided by the expected concentration. The expected concentration was determined as the mean standard concentration value for each undiluted clinical sample analyzed as two unknown samples in the assay. A test result was excluded from the analysis if the %CV of the concentration measured in replicate wells exceeded 25%. After the data exclusions were carried out, 1,095 reported values were available for analysis.
Statistical analysis.
For the interlaboratory collaborative study (IC), the %CV (IC %CV) was calculated for each study sample and the two positive control samples by dividing the standard deviation of the values for the study sample by the mean value for the sample. Mandel's k statistic was used to evaluate within-analyst variability (21, 22).
Analysis of the analytical validation evaluated repeatability (intra-assay precision), intermediate precision (interassay), and reproducibility (interlaboratory) using the variance components approach to estimate the variability from various sources. This analysis was performed on the log-transformed values. Repeatability was analyzed for each analyst and each sample using a statistical model that included the terms of day and plate within-day. The intra-assay %CV was calculated as [Exp (within-plate variance) − 1]1/2 × 100, where Exp is exponent. Intermediate precision was evaluated for each sample at each laboratory using a statistical model that included terms analyst, day within analyst, and plate within analyst by day. The intermediate %CV was calculated as [Exp (sum of all variance components) − 1]1/2 × 100. Reproducibility was evaluated for each sample using a statistical model that included site, analyst within site, day within site by analyst, and plate within site by analyst by day terms. The reproducibility %CV was calculated as [Exp (sum of all variance components) − 1]1/2 × 100. In addition, overall precision estimates across all samples or samples within the 50 to 200 EU/ml concentration range were obtained.
A qualitative approach was used to evaluate the assays performed in the collaborative study and the assays used for evaluating precision in the analytical validation. ODs instead of the calculated sample concentrations were used to define a concentration range for each test sample. Within each assay plate, the OD value for a sample was compared to the OD values for the controls CII and CIII (target values of 49 and 94 EU/ml, respectively). If the mean sample OD was less than the mean OD of CII, then the sample was interpreted as negative. If the mean sample OD was greater than or equal to the mean OD of CII but less than the mean OD of CIII, then the sample was interpreted as intermediate. Finally, if the mean sample OD was greater than or equal to the mean OD of CIII, then the sample was interpreted as positive.
RESULTS
Interlaboratory collaborative study.
An IC was carried out to evaluate the performance and transferability of the kit design. The assay met acceptance criteria between 32 EU/ml and 100 EU/ml; specifically, when the results of the eight analysts were combined, the two controls and four control serum samples had an overall IC %CV of ≤26%. More variability was observed for three control sera samples having concentrations greater than 100 EU/ml. These samples had an IC %CV range between 29 and 36% (Table 1).
TABLE 1.
IC data: descriptive statistics for quantitative resultsa
| Sample | No. of values reported | No. of values used in analysesb | Expected sample value (EU/ml)b | Mean sample value (EU/ml)c | IC %CV |
|---|---|---|---|---|---|
| S1 | 39 | 0 | <15 | <15 | |
| S2 | 39 | 35 | 30 | 32 | 23 |
| S3 | 117 | 108 | 55 | 53 | 26 |
| S4 | 117 | 107 | 85 | 74 | 25 |
| S5 | 39 | 36 | 105 | 100 | 21 |
| S6 | 39 | 37 | 105 | 109 | 36 |
| S7 | 117 | 102 | 170 | 148 | 31 |
| S8 | 117 | 85 | 275 | 230 | 29 |
| CI | 39 | 0 | <15 | <15 | |
| CII | 39 | 37 | 49 | 49 | 18 |
| CIII | 39 | 38 | 94 | 85 | 17 |
Controls CI, CII, and CIII and samples S1, S2, S5, and S6 were tested one time per assay plate; whereas, samples S3, S4, S7, and S8 were tested three times per assay plate.
A result was included in the analysis if the reported value fell within the assay range of 15 to 480 EU/ml and the %CV did not exceed 25%. Results were excluded as follows: 78 values were <15 EU/ml, 15 values were >480 EU/ml, and 63 values had a %CV of >25%.
ELISA units/ml.
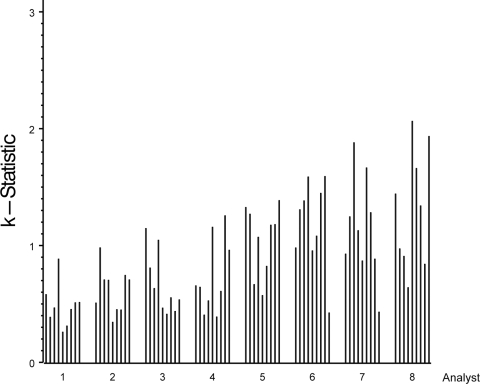
Since the IC %CV for samples of >100 EU/ml was higher than our targeted value of 25%, we assessed a variety of potential factors that might affect assay variability. Mandel k statistics indicated that heterogeneity among the analysts in assay precision was the most likely factor that contributed to the high IC %CV (Fig. 1) (21, 22). Evaluation by k statistics generates a mathematical assessment of the consistency of each analyst's results compared to the average consistency across all analysts. Because the number of analysts varied among the laboratories, results were reported by analyst rather than by laboratory to maintain blinding. In Fig. 1, the height of a bar represents the precision achieved for each sample by each analyst. When the k statistic for a given sample was greater than 1, the variability for that analyst was higher than the average variability across all analysts for that sample. Conversely, a k statistic less than 1 indicates that the variability for that analyst was less than the average variability across all analysts for that sample. For each analyst, the height of the bar appeared similar for the majority of samples (Fig. 1). Interestingly, the four analysts (analysts 5 through 8) with the highest variability were distributed among three laboratories, suggesting that the analytical performance of the analyst was more important than variability emanating from the laboratory. The assay met the acceptance criteria, especially in the range anticipated to be most important for diagnosis (i.e., between 50 and 100 EU/ml). However, differences among the analysts' experience levels suggests improving training and technical proficiency would improve the robustness of this assay.
FIG. 1.
Plot of Mandel's k statistic by sample within analyst (1 to 8). Analyst order was determined by visual observation of least-variable analyst to most-variable analyst; sample order is the same as shown in Table 1 (samples with values of <15 EU/ml were excluded from this analysis). The height of the bar is proportional to the analyst's variability per sample.
The interlaboratory data were also evaluated using a qualitative approach in which the test samples were classified as negative, intermediate, or positive based on direct comparison of the ODs of test and control samples as described in Materials and Methods above (Table 2). Overall, the results were consistent with the quantitative results: the low-concentration samples were most frequently classified as negative, high-concentration samples as positive, and remaining samples as intermediate. For example, the antibody-negative sample was classified as negative 100% of the time, and a low-concentration sample (mean concentration of 32 EU/ml) was negative 91% of the time. For the two samples (5 and 6) with concentrations slightly above the CIII value of 94 EU/ml, the test samples were positive 74% and 76% of the time, respectively, while the two samples (7 and 8) with the highest anti-PT concentrations were positive 100 and 99% of the time, respectively. These data indicate that a qualitative approach is feasible for this assay once appropriate anti-PT threshold values are established using positive and negative serum samples collected and evaluated in clinical studies.
TABLE 2.
Qualitative evaluation results of the IgG anti-PT ELISA test during an interlaboratory collaborative studya
| Sample identifier | Mean sample concentrations (EU/ml)b | No. of resultsc | % of determinations scored as: |
||
|---|---|---|---|---|---|
| Negative (ODS < ODCII) | Intermediate (ODS ≥ ODCII and < ODCIII) | Positive (ODS ≥ ODCIII) | |||
| S1 | < 15 | 39 | 100 | 0 | 0 |
| S2 | 32 | 32 | 91 | 9 | 0 |
| S3 | 53 | 104 | 42 | 54 | 4 |
| S4 | 74 | 99 | 3 | 71 | 26 |
| S5 | 100 | 34 | 0 | 26 | 74 |
| S6 | 109 | 34 | 3 | 21 | 76 |
| S7 | 148 | 97 | 0 | 0 | 100 |
| S8 | 230 | 93 | 1 | 0 | 99 |
The OD of samples (ODS) were compared with the OD of controls CII and CIII (ODCII and ODCIII, respectively). Boldface (samples S3 and S4) indicates those samples with an IgG anti-PT concentration between those of CII (49 EU/ml) and CIII (94 EU/ml).
ELISA units/ml.
All results from valid plates were used provided that the %CV criteria were met for CII, CIII, and sample. Qualitative analyses were based solely on ODs; no results were excluded based on calculated values of <15 EU/ml or >480 EU/ml.
Technical proficiency requirements were refined. Procedures to qualify each analyst were updated to allow for additional training and a more rigorous demonstration of technical proficiency prior to test implementation. Reagents were optimized to ensure that final OD readings were approximately 2.0, which is within the working range for most spectrophotometers. Additionally, longer-term stability studies suggested that the lyophilized HRP-conjugated secondary antibody had lost activity following a year of storage. To address the stability issues and to facilitate production, the conjugate storage buffer was changed to 50% glycerol. The conjugate stored in 50% glycerol was used in the subsequent analytical validation study. Based on these refinements, a new set of prototype kits was produced for the analytical validation study.
Analytical validation.
Our prespecified acceptance criterion for intra-assay precision required that the intra-assay %CV would not exceed 25% for each sample with mean concentrations between 50 and 200 EU/ml. The intra-assay variability ranged from 8% to 25% when samples ranged from 50 to 200 EU/ml and from 8% to 27% when samples ranged from 49 to 217 EU/ml (Table 3). Intermediate precision was used to assess interassay variability and analyst-to-analyst variability, while reproducibility was used to evaluate the amount of variability between laboratories. The acceptance criteria for intermediate precision and reproducibility stated that for each sample with study mean concentrations between 50 and 200 EU/ml, neither the analyst-to-analyst %CV nor the site-to-site %CV, respectively, would exceed 30%, as estimated through the statistical model. For 50- to 200-EU/ml samples, the intermediate precision ranged from 22% to 29%CV and reproducibility %CV ranged from 23% to 28%. Thus, all prespecified precision criteria were met (Table 3).
TABLE 3.
Precision results of IgG anti-PT ELISAa
| Parameter | %CV for sample (geometric mean concn [EU/ml]) |
||||||
|---|---|---|---|---|---|---|---|
| S1 (49) | S2 (58) | S3 (83) | S4 (100) | S5 (135) | S6 (204) | S7 (217) | |
| Intra-assay precision | |||||||
| Analyst 1 | 22 | 25 | 18 | 15 | 24 | 15 | 21 |
| Analyst 2 | 16 | 12 | 8 | 10 | 13 | 15 | 13 |
| Analyst 3 | 12 | 10 | 15 | 10 | 14 | 14 | 13 |
| Analyst 4 | 12 | 12 | 11 | 12 | 14 | 12 | 14 |
| Analyst 5 | 16 | 16 | 18 | 21 | 17 | 19 | 18 |
| Analyst 6 | 17 | 14 | 13 | 13 | 16 | 12 | 27 |
| Interassay precision | |||||||
| Site A | 22 | 23 | 22 | 24 | 23 | 23 | 26 |
| Site B | 21 | 29 | 23 | 21 | 25 | 21 | 26 |
| Site Bb | 16 | 17 | 15 | 16 | 18 | 19 | 22 |
| Reproducibility | |||||||
| Site-to-site | 23 | 28 | 23 | 23 | 27 | 25 | 29 |
| Site-to-sitec | 20 | 22 | 20 | 21 | 23 | 23 | 27 |
Samples are ordered from low to high concentrations. Boldface (samples 2 to 5) indicates those samples with an IgG anti-PT concentration in the range analyzed under the prespecified acceptance criteria (50 to 200 EU/ml).
Interassay precision without analyst 1 from site B.
Reproducibility without analyst 1 from site B.
Studies conducted during the assay trial revealed that the electronic pipette used by analyst 1 at site B had been appropriately calibrated in the single dispense mode but not in the multiple dispense mode. The multiple dispense mode was used during the analytical validation. Although these data were included in the primary analysis, when data from analyst 1 at site B were removed during secondary analysis, the intermediate precision %CV for samples in the range of 50 to 200 EU/ml decreased from 24% to 15% and the reproducibility %CV decreased from 23% to 20% (Table 3). Other analysis indicated that major sources of variability were the intra-assay, plate-to-plate, and analyst-to-analyst variations. The day-to-day and site-to-site variations made little contribution to the total variability.
Accuracy was evaluated using U.S. reference serum CBER3 (25, 39). According to the preestablished acceptance criteria, the geometric mean concentrations from three independent assays for samples containing 50 and 100 EU/ml had to be within 80% and 125% of the true value. Three assay determinations of samples containing 150 and 200 EU/ml had to be within 74% and 135% of the true value. These accuracy criteria were met for samples containing 50 to 150 EU/ml, which are concentrations deemed most critical. However, the measured value for the 200-EU/ml sample had a percent accuracy of 72 and did not meet the acceptance criteria (Table 4). When the newly adopted WHO pertussis reference reagent, NIBSC 06/140, became available, a secondary evaluation was performed using this reagent. Specifically, when the concentrations of diluted NIBSC 06/140 samples were measured in the ELISA, the concentration measured in EU closely matched the assigned value in IU (Table 5). Under the assumption that 1 EU is approximately equal to 1 IU (39), the percent accuracy for the NIBSC 06/140 samples was 100% to 112% for concentrations between 50 and 200 EU/ml (Table 5).
TABLE 4.
IgG anti-PT ELISA accuracy results obtained from analytical validation
| Anti-PT IgG concn (EU/ml) for CBER3 (reference) | Anti-PT IgG concn (EU/ml) |
% Accuracy | ||||
|---|---|---|---|---|---|---|
| Replicate 1 | Replicate 2 | Replicate 3 | Geometric mean | Expected value | ||
| 50 | 52 | 50 | 41 | 47 | 50 | 95 |
| 100 | 85 | 92 | 81 | 86 | 100 | 86 |
| 150 | 120 | 124 | 113 | 119 | 150 | 79 |
| 200 | 142 | 139 | 151 | 144 | 200 | 72 |
TABLE 5.
IgG anti-PT ELISA accuracy results using WHO pertussis antiserum (human) reference reagent
| Anti-PT IgG concn (IU/ml) for NIBSC 06/140 (reference)a | Anti-PT IgG concn (EU/ml) |
% Accuracyb | ||||
|---|---|---|---|---|---|---|
| Replicate 1 | Replicate 2 | Replicate 3 | Geometric mean | Expected valueb | ||
| 50 | 56 | 54 | 57 | 56 | 50 | 112 |
| 100 | 109 | 101 | 104 | 105 | 100 | 105 |
| 150 | 152 | 147 | 151 | 150 | 150 | 100 |
| 200 | 197 | 247 | 196 | 212 | 200 | 106 |
WHO Pertussis antiserum (human) reference reagent, NIBSC 06/140.
Expected value and accuracy calculations were based on the assumption that 1 IU = 1 EU (39).
Recovery was analyzed by spiking 6 positive serum samples independently three times with reconstituted standards, yielding 18 samples with final concentrations ranging from 42 to 214 EU/ml. The acceptance criteria stated that 15 out of these 18 samples must have an estimated recovery within 75% to 133%. All 18 samples met this criterion, with percent recoveries ranging from 90% to 117%; thus, the recovery criterion was met.
Linearity was assessed by measuring the concentration of samples prepared by diluting high-concentration serum samples with antibody-negative serum and then plotting the observed verses expected values. The acceptance criteria stated that the 95% confidence limits for the slope of this plot should be contained in the interval between 0.67 and 1.50 and the r2 value should not be less than 0.95. For all three samples, the measured slope was 1.0 with all 95% confidence intervals within the range from 0.9 to 1.2; the r2 value was 1.0 (Table 6). Thus, the linearity criterion was met, and the data suggest excellent linearity for the range from 40 to 338 EU/ml.
TABLE 6.
IgG anti-PT ELISA linearity results obtained from analytical validation
| Sample | Dilution | Observed value (EU/ml) | Expected value (EU/ml) | r2 valuea | Slope (95% CIb) |
|---|---|---|---|---|---|
| S1 | Undiluted | 352 | 352 | 1.0 | 1.0 (1.0-1.1) |
| 1:2 | 163 | 176 | |||
| 1:4 | 82 | 88 | |||
| 1:8 | 40 | 44 | |||
| S2 | Undiluted | 323 | 323 | 1.0 | 1.0 (0.9-1.2) |
| 1:2 | 128 | 161 | |||
| 1:4 | 61 | 81 | |||
| 1:8 | 31 | 40 | |||
| S3 | Undiluted | 338 | 338 | 1.0 | 1.0 (1.0-1.0) |
| 1:2 | 174 | 169 | |||
| 1:4 | 87 | 84 | |||
| 1:8 | 44 | 42 |
Coefficient of determination.
CI, confidence interval.
The data from the analytical validation study were also evaluated using the qualitative approach described for the interlaboratory collaborative study. The data are similar to those presented in the collaborative study, providing further support for the conclusion that the assay could easily be adapted to a qualitative format once clinically validated cutoff values for infection are obtained.
DISCUSSION
The overall goal of this project was to develop an ELISA kit that could accurately and precisely measure the concentrations of IgG anti-PT in the range of interest for diagnostic assays. Critical reagents were prequalified so that the assay could be performed by any laboratory with immunoassay equipment and experience. Because a variety of clinical laboratories have adopted proposed threshold cutoffs of 49 to 200 EU/ml, the assay and validation protocol were designed to measure samples within this range. We sought optimal performance around 100 EU/ml. Based on these considerations, an assay kit was assembled containing six lyophilized serum standards that tested the limits of the assay from 15 to 480 EU/ml. The kit also included a commercially available, well-characterized monoclonal antibody and lyophilized positive, negative, and intermediate controls. The controls were devised to test whether the assay could be used in either a quantitative or qualitative manner to aid disease diagnosis. Because the most appropriate cutoffs have yet to be confirmed, the current kit employs assay controls targeting the concentrations of 0, 49, and 94 EU/ml as proposed by Baughman et al. (2). When studies to confirm the cutoffs are completed, the assay controls will be revised.
Several benefits were obtained by performing an interlaboratory collaborative study prior to the analytical validation. Most importantly, we learned that the assay could be transferred and that analysts with no experience with this specific assay could perform the test successfully in both the quantitative and qualitative formats. Additionally, the results and feedback from the participants provided valuable insight for improvements in the method. Specifically, the written procedures were refined and clarified, the number of successful assays required for qualification of an analyst was increased, and reagents were adjusted to bring the OD readings into a narrower range.
The data presented here demonstrate that the kit met all prespecified criteria for precision, linearity, and accuracy for samples with IgG anti-PT antibody concentrations in the range of 50 to 150 EU/ml, the range believed to be most relevant for serodiagnosis. The assay met the precision and linearity criteria for a wider range, 50 to 200 EU/ml; however, the primary accuracy criterion was not met for the sample at 200 EU/ml. Specifically, the concentration of the 200-EU/ml sample of CBER3 was underestimated by approximately 28%. The reason for the underestimation of CBER3 at 200 EU/ml remains unknown; however, a follow-up study using NIBSC 06/140 demonstrated that the assay met the predefined criterion at all concentrations tested, including 200 IU/ml. Additionally, the linearity assessment with three positive sera (Table 6) indicated excellent linearity over the range from 42 to 352 EU/ml. These data suggest that the underestimation is limited to the CBER3 samples, and an investigation has begun to determine whether the results for CBER3 were influenced by the age or storage conditions of the preparations tested. Testing to understand the discrepancy between high-titer samples of NIBSC 06/140 and CBER3 are ongoing. The failure of CBER3 to meet acceptance criteria should not compromise the applicability of this assay provided future diagnostic thresholds are limited to values lower than 200 EU/ml.
Together, the interlaboratory collaborative and analytical validation studies indicate that the kit is relatively easy to transfer and perform. Both studies suggest the importance of establishing training and technical proficiency requirements prior to test implementation. For future studies, we recommend that one or more known positive serum samples be added to each assay in addition to the lyophilized controls. The serum control would be diluted along with the unknown samples and tracked over time to assess the stability of the kit and continued qualification of the analyst. In addition, a proficiency panel of serum should be established and provided to all testing laboratories to evaluate the continued performance of each testing laboratory. Results of the proficiency panel and the positive-control serum can be used to qualify new personnel and evaluate the ongoing competency of personnel performing the assay.
Tetanus-diphtheria-acellular pertussis vaccines, which were introduced into the adult population in 2005 (5, 6) may induce elevated anti-PT IgG titers for a short time after vaccination, requiring reevaluation of the diagnostic threshold (18, 28). Finally, while the assay was designed and evaluated to quantify anti-PT titers, the preliminary results presented here demonstrate that the assay can serve in a qualitative format once optimal threshold cutoffs are defined, thus eliminating the need for a full set of standards. Such a format would reduce the number of procedural manipulations needed to physically perform the assay, and it would simplify the interpretation of the results by eliminating the need for a statistical analysis program. This would facilitate adoption and use by regional or state health departments to assess pertussis outbreaks and measure the burden of disease in adolescents and adults. In fact, the possibility of making this kit widely available has been proposed for future evaluation.
In conclusion, the anti-PT IgG ELISA met all assay validation parameters within a range of 50 to 150 EU/ml. This ELISA was developed and analytically validated to be a user-friendly kit that can be used for both qualitative and quantitative purposes. With the analytical validation of this test, the first part of a two-phase project has been completed. In the second phase, now under way at CDC, the test is being evaluated in a prospective multisite clinical trial that will evaluate the sensitivity, specificity, and predictive values of this kit and two other diagnostic tools (PCR and culture). Once subjected to appropriate clinical assessment and once diagnostic thresholds are established, this assay should be considered for wide-scale use in public health, which would add an important tool for pertussis diagnosis.
Acknowledgments
This project was funded in part by an interagency agreement between the CDC and the FDA/CBER.
We acknowledge GlaxoSmithKline for providing the purified pertussis toxin antigens used in this study. We also acknowledge Trudy Murphy, CDC, Atlanta, GA, for assistance in obtaining funding for this project; Kristine Bisgard, CDC, Atlanta, GA, for procurement of sera and other reagents; Pamela Cassiday, CDC, Atlanta, GA, for collection and handling of reagents; Teresa Kenney, CDC, Atlanta, GA, for input on the analytical validation protocol and for serving as an analyst in the analytical validation; Freyja Lynn, FDA/CBER, Rockville, MD, for discussions on assay design; Lokesh Bhattacharyya, FDA/CBER, Rockville, MD, for input on the development of the analytical validation protocol; Christine Anderson FDA/CBER, Rockville, MD, for reagent lyophilization and shipping of supplies and kits to study collaborators; Bruce Lowe, FDA/CBER, Rockville, MD, for reagent preparation and lyophilization; Lindsay Swanson, Colorado Department of Public Health, Denver, CO, for serving as an analyst in the collaborative study; and Kris Ehrismann, Cynthia Kenyan, and Claudia Miller, Minnesota Department of Health, St. Paul, MN, and Stephanie Schauer, Susan Lett, and Linda Han, Massachusetts Department of Health, Boston, MA, for collection and handling of positive plasma.
The Pertussis Assay Working Group included Gary Sanden, CDC, Atlanta, GA (discussions on design and methodology and procurement and storage of sera and other reagents); Barbara A. Slade, CDC, Atlanta, GA (coordination of the assay development working group); Patricia P. Wilkins, CDC, Atlanta, GA (discussions on design and methodology; also analyst in the collaborative study); Jody Peters, Vanderbilt University, Nashville, TN, Gregory L. Waidmann, Colorado Department of Public Health, Denver, CO, and Leslie Wagner, CBER, FDA, Bethesda, MD (analysts in the collaborative study); and Nicolette deVore and Anita Verma, CBER, FDA, Bethesda, MD (analysts in the analytical validation).
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration or the Centers for Disease Control and Prevention and should not be construed as representing any agency determination or policy.
Footnotes
Published ahead of print on 28 October 2009.
REFERENCES
- 1.Andre, P., V. Caro, E. Njamkepo, A. M. Wendelboe, A. Van Rie, and N. Guiso. 2008. Comparison of serological and real-time PCR assays to diagnose Bordetella pertussis infection in 2007. J. Clin. Microbiol. 46:1672-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baughman, A. L., K. M. Bisgard, K. M. Edwards, D. Guris, M. D. Decker, K. Holland, B. D. Meade, and F. Lynn. 2004. Establishment of diagnostic cutoff points for levels of serum antibodies to pertussis toxin, filamentous hemagglutinin, and fimbriae in adolescents and adults in the United States. Clin. Diagn. Lab. Immunol. 11:1045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baughman, A. L., K. M. Bisgard, F. Lynn, and B. D. Meade. 2006. Mixture model analysis for establishing a diagnostic cut-off point for pertussis antibody levels. Stat. Med. 25:2994-3010. [DOI] [PubMed] [Google Scholar]
- 4.CDC. 2007. Outbreaks of respiratory illness mistakenly attributed to pertussis—-New Hampshire, Massachusetts, and Tennessee, 2004-2006. MMWR 56:837-842. [PubMed] [Google Scholar]
- 5.CDC. 2006. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 55:1-34. [PubMed] [Google Scholar]
- 6.CDC. 2006. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm. Rep. 55:1-37. [PubMed] [Google Scholar]
- 7.Cherry, J. D. 2006. Epidemiology of pertussis. Pediatr. Infect. Dis. J. 25:361-362. [DOI] [PubMed] [Google Scholar]
- 8.Cherry, J. D. 2005. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics 115:1422-1427. [DOI] [PubMed] [Google Scholar]
- 9.de Melker, H. E., F. G. Versteegh, M. A. Conyn-Van Spaendonck, L. H. Elvers, G. A. Berbers, A. van Der Zee, and J. F. Schellekens. 2000. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J. Clin. Microbiol. 38:800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards, K. M. 2005. Overview of pertussis: focus on epidemiology, sources of infection, and long term protection after infant vaccination. Pediatr. Infect. Dis. J. 24:S104-S108. [DOI] [PubMed] [Google Scholar]
- 11.Forsyth, K., M. Nagai, A. Lepetic, and E. Trindade. 2005. Pertussis immunization in the global pertussis initiative international region: recommended strategies and implementation considerations. Pediatr. Infect. Dis. J. 24:S93-S97. [DOI] [PubMed] [Google Scholar]
- 12.Forsyth, K. D., C. H. Wirsing von Konig, T. Tan, J. Caro, and S. Plotkin. 2007. Prevention of pertussis: recommendations derived from the second Global Pertussis Initiative roundtable meeting. Vaccine 25:2634-2642. [DOI] [PubMed] [Google Scholar]
- 13.Fry, N. K., O. Tzivra, Y. T. Li, A. McNiff, N. Doshi, P. A. Maple, N. S. Crowcroft, E. Miller, R. C. George, and T. G. Harrison. 2004. Laboratory diagnosis of pertussis infections: the role of PCR and serology. J. Med. Microbiol. 53:519-525. [DOI] [PubMed] [Google Scholar]
- 14.Giammanco, A., A. Chiarini, P. A. Maple, N. Andrews, R. Pebody, N. Gay, R. M. Olander, F. Fivet-Groyne, S. Baron, A. Tischer, S. Swidsinski, J. Schellekens, and E. Reizenstein. 2003. European Sero-Epidemiology Network: standardisation of the assay results for pertussis. Vaccine 22:112-120. [DOI] [PubMed] [Google Scholar]
- 15.Giammanco, A., A. Nardone, R. Pebody, G. Kafatos, N. Andrews, A. Chiarini, S. Taormina, F. de Ory, K. Prosenc, B. Krize, H. Hallander, M. Ljungman, E. Marva, A. Tsakris, D. O'Flanagan, F. Schneider, A. Griskevicius, R. Vranckx, and I. Karacs. 2008. European Sero-Epidemiology Network 2: standardisation of immunoassay results for pertussis requires homogeneity in the antigenic preparations. Vaccine 26:4486-4493. [DOI] [PubMed] [Google Scholar]
- 16.Guris, D., P. M. Strebel, B. Bardenheier, M. Brennan, R. Tachdjian, E. Finch, M. Wharton, and J. R. Livengood. 1999. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin. Infect. Dis. 28:1230-1237. [DOI] [PubMed] [Google Scholar]
- 17.Kosters, K., M. Riffelmann, B. Dohrn, and C. H. von Konig. 2000. Comparison of five commercial enzyme-linked immunosorbent assays for detection of antibodies to Bordetella pertussis. Clin. Diagn. Lab. Immunol. 7:422-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le, T., J. D. Cherry, S. J. Chang, M. D. Knoll, M. L. Lee, S. Barenkamp, D. Bernstein, R. Edelman, K. M. Edwards, D. Greenberg, W. Keitel, J. Treanor, and J. I. Ward. 2004. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT Study. J. Infect. Dis. 190:535-544. [DOI] [PubMed] [Google Scholar]
- 19.Litt, D. J., D. Samuel, J. Duncan, A. Harnden, R. C. George, and T. G. Harrison. 2006. Detection of anti-pertussis toxin IgG in oral fluids for use in diagnosis and surveillance of Bordetella pertussis infection in children and young adults. J. Med. Microbiol. 55:1223-1228. [DOI] [PubMed] [Google Scholar]
- 20.Lynn, F., G. F. Reed, and B. D. Meade. 1996. Collaborative study for the evaluation of enzyme-linked immunosorbent assays used to measure human antibodies to Bordetella pertussis antigens. Clin. Diagn. Lab. Immunol. 3:689-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandel, J. 1991. Evaluation and control of measurements. Marcel Dekker, Inc., New York, NY.
- 22.Mandel, J. 1991. The validation of measurement through interlaboratory studies. Chemom. Intell. Lab. Syst. 11:109-119. [Google Scholar]
- 23.Marchant, C. D., A. M. Loughlin, S. M. Lett., C. W. Todd, L. H. Wetterlow, R. Bicchieri, S. Higham, P. Etkind, E. Silva, and G. R. Siber. 1994. Pertussis in Massachusetts, 1981-1991: incidence, serologic diagnosis, and vaccine effectiveness. J. Infect. Dis. 169:1297-1305. [DOI] [PubMed] [Google Scholar]
- 24.Mattoo, S., and J. D. Cherry. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meade, B. D., A. Deforest, K. M. Edwards, T. A. Romani, F. Lynn, C. H. O'Brien, C. B. Swartz, G. F. Reed, and M. A. Deloria. 1995. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics 96:570-575. [PubMed] [Google Scholar]
- 26.Prince, H. E., M. Lape-Nixon, and J. Matud. 2006. Evaluation of a tetraplex microsphere assay for Bordetella pertussis antibodies. Clin. Vaccine Immunol. 13:266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reder, S., M. Riffelmann, C. Becker, and C. H. Wirsing von Konig. 2008. Measuring immunoglobulin G antibodies to tetanus toxin, diphtheria toxin, and pertussis toxin with single-antigen enzyme-linked immunosorbent assays and a bead-based multiplex assay. Clin. Vaccine Immunol. 15:744-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riffelmann, M., M. Littmann, C. Hulsse, and C. H. von Konig. 2009. Antibody decay after immunisation of health-care workers with an acellular pertussis vaccine. Eur. J. Clin. Microbiol. Infect. Dis. 28:275-279. [DOI] [PubMed] [Google Scholar]
- 29.Riffelmann, M., C. H. Wirsing von Konig, V. Caro, and N. Guiso. 2005. Nucleic acid amplification tests for diagnosis of Bordetella infections. J. Clin. Microbiol. 43:4925-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothstein, E., and K. Edwards. 2005. Health burden of pertussis in adolescents and adults. Pediatr. Infect. Dis. J. 24:S44-S47. [DOI] [PubMed] [Google Scholar]
- 31.Tatti, K. M., K. H. Wu, M. L. Tondella, P. K. Cassiday, M. M. Cortese, P. P. Wilkins, and G. N. Sanden. 2008. Development and evaluation of dual-target real-time polymerase chain reaction assays to detect Bordetella spp. Diagn. Microbiol. Infect. Dis. 61:264-272. [DOI] [PubMed] [Google Scholar]
- 32.Tondella, M. L., G. M. Carlone, N. Messonnier, C. P. Quinn, B. D. Meade, D. L. Burns, J. D. Cherry, N. Guiso, E. L. Hewlett, K. M. Edwards, D. Xing, A. Giammanco, C. H. Wirsing von Konig, L. Han, L. Hueston, J. B. Robbins, M. Powell, C. M. Mink, J. T. Poolman, S. W. Hildreth, F. Lynn, and A. Morris. 2009. International Bordetella pertussis assay standardization and harmonization meeting report. Centers for Disease Control and Prevention, Atlanta, Georgia, United States, 19-20 July 2007. Vaccine 27:803-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trollfors, B., T. Lagergard, E. Gunnarsson, and J. Taranger. 2003. Determination of pertactin IgG antibodies for the diagnosis of pertussis. Clin. Microbiol. Infect. 9:585-589. [DOI] [PubMed] [Google Scholar]
- 34.van Gageldonk, P. G., F. G. van Schaijk, F. R. van der Klis, and G. A. Berbers. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 335:79-89. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe, M., B. Connelly, and A. A. Weiss. 2006. Characterization of serological responses to pertussis. Clin. Vaccine Immunol. 13:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wendelboe, A. M., and A. Van Rie. 2006. Diagnosis of pertussis: a historical review and recent developments. Expert Rev. Mol. Diagn. 6:857-864. [DOI] [PubMed] [Google Scholar]
- 37.Wirsing von Konig, C. H., D. Gounis, S. Laukamp, H. Bogaerts, and H. J. Schmitt. 1999. Evaluation of a single-sample serological technique for diagnosing pertussis in unvaccinated children. Eur. J. Clin. Microbiol. Infect. Dis. 18:341-345. [DOI] [PubMed] [Google Scholar]
- 38.Wirsing von Konig, C. H., S. Halperin, M. Riffelmann, and N. Guiso. 2002. Pertussis of adults and infants. Lancet Infect. Dis. 2:744-750. [DOI] [PubMed] [Google Scholar]
- 39.Xing, D., C. H. Wirsing von Konig, P. Newland, M. Riffelmann, B. D. Meade, M. Corbel, and R. Gaines-Das. 2009. Characterization of reference materials for human antiserum to pertussis antigens by an international collaborative study. Clin. Vaccine Immunol. 16:303-311. [DOI] [PMC free article] [PubMed] [Google Scholar]