Abstract
Veillonella parvula is an anaerobic gram-negative coccus that is part of the normal flora of the animal and human mouth and gastrointestinal and genitourinary tracts. Oral V. parvula is involved in the development of early periodontal disease as well as different types of serious infections. Present data on molecular mechanisms responsible for innate immune response against Veillonella are very scanty. The aim of this study was to investigate the Toll-like receptor (TLR) pathways responsible for V. parvula lipopolysaccharide (LPS) and to identify the intracellular pathways induced by this recognition. V. parvula LPS stimulated tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) release in human peripheral blood mononuclear cells (PBMC) in a dose-dependent manner. Pretreatment of cells with a TLR4 antagonist significantly reduced TNF-α and IL-6 production in PBMC stimulated with either Veillonella or Escherichia coli LPS. However, V. parvula LPS was 10- to 100-fold less active than E. coli LPS for cytokine induction. TNF-α, IL-1β, IL-6, and IL-10 were released in wild-type and TLR2−/−, but not TLR4−/−, mouse macrophage cultures. V. parvula LPS was able to activate the human PBMC p38 mitogen-activated protein kinase (MAPK). A specific p38 MAPK inhibitor strongly inhibited V. parvula LPS-induced TNF-α, IL-1β, IL-6, and IL-10. In conclusion, V. parvula LPS is able to induce cytokine production in both human and murine in vitro models, although it is less effective than Enterobacteriaceae LPS. V. parvula LPS-stimulated cytokine induction, as well as p38 MAPK activation, are TLR4-dependent features.
Veillonella organisms are small, nonfermentative, strictly anaerobic, gram-negative cocci which form part of the normal flora of the oral, genitourinary, respiratory, and intestinal tracts of humans and animals (10). The genus Veillonella was first isolated by Veillon and Zuber in 1898 and currently consists of eight species (28).
Veillonella species have been reported as causes of serious infections, including meningitis (6), osteomyelitis and discitis (7, 28), prosthetic joint infection (26), and acute and chronic pleuropulmonary infection (33).
Risk factors for Veillonella infection include periodontal disease, immunodeficiency, intravenous drug use, and premature birth (28). V. parvula is an important pathogen implicated in periodontitis and other dental infections (3, 18), and it is one of the most common anaerobic pathogens in chronic maxillary sinusitis and deep neck infections (9, 37). V. parvula has also been reported as a pathogen for osteomyelitis (34) and abscessed orchiepididymitis with sepsis (4). Endovascular infections reportedly may range from bacteremia to severe endocarditis and fatal cases of sepsis (8, 14, 25).
Lipopolysaccharides (LPS) are major pathogenic factors of gram-negative bacteria. LPS from aerobic and facultative bacteria have been extensively studied (5). On the contrary, very little is known regarding the biological activity of LPS from anaerobic microorganisms such as Veillonella (10, 24, 29, 32). In addition, little is known about cellular and molecular mechanisms responsible for innate immune response against V. parvula, as well as for inflammatory reactions leading to severe periodontitis or sinusitis. Toll-like receptors (TLRs) recognize microbial compounds (36) and trigger the inflammatory and immune responses against pathogens. Immunohistochemical localization of TLR2 and TLR4 in gingival tissue of periodontitis patients has been reported (30). In mammals, engagement of TLRs by LPS results in the recruitment of cytoplasmic signaling molecules (12, 36), which eventually activates mitogen-activated protein kinases (MAPKs), including p38, JNK, and extracellular signal-regulated kinase (ERK). MAPK activation leads to cytokine release (2).
However, the interaction between oral V. parvula LPS and TLRs has not been directly studied yet. The aim of this study was to investigate the potential role of TLR2 and TLR4 for the recognition of Veillonella parvula LPS in both human peripheral blood mononuclear cells (PBMC) and in TLR2 and TLR4 knockout (KO) mouse macrophages, as well as the intracellular kinase signaling pathways induced after challenge of monocytes with Veillonella parvula LPS.
MATERIALS AND METHODS
Veillonella parvula culture and LPS purification.
V. parvula ATCC 10790 (American Type Culture Collection, Rockville, MD) was grown anaerobically in modified lactate broth at 37°C. After V. parvula reached the early stationary phase (about 40 h of incubation), bacterial cells were harvested by centrifugation and washed twice in 50 mM potassium phosphate buffer (pH 7.0) containing 20 mM 2-mercaptoethanol (PPB). Cells were extracted twice with phenol-water (38). Briefly the aqueous layers were combined and dialyzed against 20 liters of distilled water at 4°C. The crude LPS was lyophilized and then dissolved in distilled water and centrifuged once at 80,000 × g for 7 h at 4°C. The pellet was suspended in distilled water and recentrifuged twice at 105,000 × g for 3 h at 4°C. The LPS was dissolved in PPB with RNase (Sigma, Chemical Co., St. Louis, MO) and DNase (Sigma) at 20 μg/ml each. After 2 h of incubation at 37°C, the solution was centrifuged at 105,000 × g for 3 h at 4°C. The pellet was washed in distilled water and centrifuged twice at 105,000 × g at 4°C. The final pellet was dissolved in distilled water, lyophilized, and stored at −20°C as once-purified LPS. Double-step-purified LPS (repurified LPS) was obtained following the method described by Hirschfeld et al. (17).
Isolation of peripheral blood mononuclear cells and stimulation of cytokine production.
Isolation of PBMC was performed as described elsewhere (1), with minor modifications. All volunteers signed an inform consent form, according to the institutional guidelines and procedures. A total of 5 × 105 PBMC in a 100-μl volume were added to round-bottom 96-well plates (Greiner) and incubated with either 100 μl of culture medium or the various stimuli: highly purified Veillonella LPS (1 to 100 ng/ml), highly purified E. coli LPS (1 ng/ml; Sigma), or Pam3Cys (10 μg/ml; EMC Microcollections). In some experimental sets, cells were pretreated with or without double-extracted Bartonella quintana LPS (1 μg/ml), a TLR4 antagonist (31), 30 min before treatment with V. parvula LPS (100 ng/ml). Specific signal transduction inhibitors of p38 MAPK (SB202190; 25 μm), ERK1/2 (U0126; 25 μm), JNK1/2/3 kinase (SP600125; 25 μm) (all inhibitors purchased from Superarray Bioscience Corporation, Bethesda, MD) were added to the cultures for 45 min prior to addition of Veillonella LPS. All unstimulated and control samples received an equal amount of inhibitor solvent, dimethyl sulfoxide (0.1% [vol/vol]). After 24 h of culture, the supernatants were collected, centrifuged at 1,200 rpm, and stored at −80°C until assayed.
Isolation of peritoneal macrophages and stimulation of cytokine production.
For isolation of mouse peritoneal macrophages and spleen cells, groups of five TLR2−/− and TLR4−/− mice (kindly provided by S. Akira, Osaka, Japan) and their control littermates were sacrificed, and resident peritoneal macrophages were harvested by injecting 4 ml of sterile phosphate-buffered saline containing 0.38% sodium citrate. After washing, the cells were resuspended in RPMI 1640 medium containing 1 mM pyruvate, 2 mM l-glutamine, 100 μg/ml gentamicin, and 2% fresh mouse plasma (culture medium). Cells were cultured in 96-well microtiter plates (Greiner, Alphen, The Netherlands) at 105 cells per well, in a final volume of 200 μl. The cells were stimulated with either control medium or highly purified Veillonella LPS (1 μg/ml), highly purified E. coli LPS (1 ng/ml), or Pam3Cys (10 ng/ml). After 24 h of incubation at 37°C, the plates were centrifuged (500 × g, 10 min), and the supernatant and cell lysate (three freeze-thaw cycles) were collected and stored at −80°C until cytokine assays were performed.
Cytokine determinations.
Human tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-10 were measured by using either specific or commercial enzyme-linked immunosorbent assay (ELISA) kits (from R&D Systems, Abingdon, United Kingdom, and the Pelikine Compact from Sanquin, Amsterdam, The Netherlands). Sensitivities of both ELISAs were below 5 pg/ml (13). Murine IL-1α and TNF-α were measured with a radioimmunoassay, and murine IL-6 and IL-10 were determined using ELISA kits (R&D Systems, Abingdon, United Kingdom).
Western blot analysis of Veillonella LPS signal transduction.
After the isolation of human PBMC, aliquots of the cells were placed in 3-cm sterile tissue cultures plates in 1 ml of RPMI 1640 medium complemented with RPMI 1640 containing 10% fetal bovine serum, 10 mM l-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin (all obtained from Invitrogen, Carlsbad, CA). The cells were immediately pretreated with or without the TLR4 antagonist B. quintana LPS (1 μg/ml) (31) added 30 min before treatment with V. parvula LPS (100 ng/ml).
At different time points the cells were collected and the proteins were extracted using a lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 10 mM EDTA (pH 8), 10 mM Na4P2O7, 0.5% NP-40, 10% glycerol, 0.5 mM dithiothreitol, 10 mM NaVO3, 100 mM NaF, 100 μg/ml leupeptin, 0.5 mM phenylmethylsulfonyl fluoride. After incubation for 30 min on ice, lysates were centrifuged at 10,000 × g for 10 min at 4°C. A 30-μg portion of cell extract was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a nitrocellulose membrane. Blots were incubated with anti-p38, anti-phospho-p38, anti-ERK, anti-phospho-ERK, anti-JNK, or anti-phospho-JNK (1:1,000; Cell Signaling), or anti-actin (1:200; Santa Cruz) antibodies and then with anti-rabbit or anti-mouse horseradish peroxidase-coupled secondary antibody (Amersham, Buckinghamshire, United Kingdom). Immune complexes were identified by using an enhanced chemiluminescence system (Amersham, Buckinghamshire, United Kingdom).
Statistical analysis.
The data are expressed as mean ± standard errors of the means unless indicated otherwise. Differences between experimental groups were tested using the Mann-Whitney U-test, performed using GraphPad Prism 4.0 software. P values of 0.05 or less were considered significant.
RESULTS
V. parvula LPS is a weak stimulator of cytokine production in human PBMC.
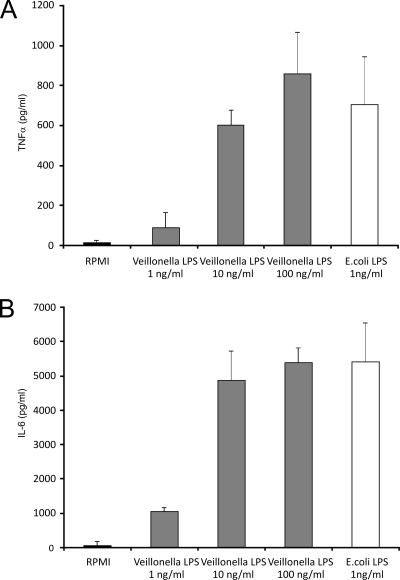
Following stimulation with increasing amounts of V. parvula LPS (1 to 100 ng/ml), human PBMC released TNF-α and IL-6 in a dose-related fashion. The highest concentration of V. parvula LPS used (100 ng/ml) released an amount of the above cytokines that was comparable to that produced by 1 ng/ml of the reference E. coli LPS (Fig. 1A and B).
FIG. 1.
(A) TNF-α production after exposure to Veillonella LPS. Human PBMC were stimulated with a dose of purified Veillonella LPS for 24 h. E. coli LPS served as a positive control. Data represent the means ± standard deviations for cytokine production by at least four healthy volunteers. (B) IL-6 production. Cytokine levels were determined by an ELISA, and the detection limit was 20 pg/ml for both cytokines.
A TLR4 antagonist blocks V. parvula LPS-induced cytokine production.
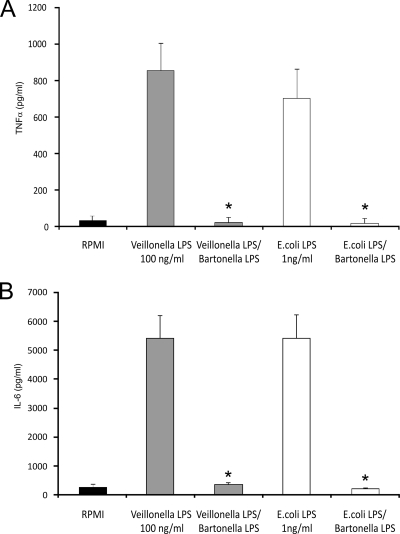
When human PBMC were pretreated with a TLR4 antagonist (B. quintana LPS at 1 μg/ml), the release of TNF-α and IL-6 following stimulation with V. parvula LPS (1 to 100 ng/ml), as well as with 1 ng/ml of the reference E. coli LPS, was strongly inhibited (Fig. 2A and B).
FIG. 2.
Inhibition of Veillonella LPS-induced TNF-α (A) and IL-6 (B) production by a TLR4 antagonist. PBMC were pretreated with Bartonella quintana LPS for 1 h and then either Veillonella LPS or E. coli LPS was added. Data represent the means ± standard deviations for cytokine production of at least four healthy volunteers. Note the complete suppression of cytokine production after TLR4 blockade. *, P < 0.01 (Wilcoxon rank test).
TLR4 is the major receptor for V. parvula LPS-induced cytokine production in mice.
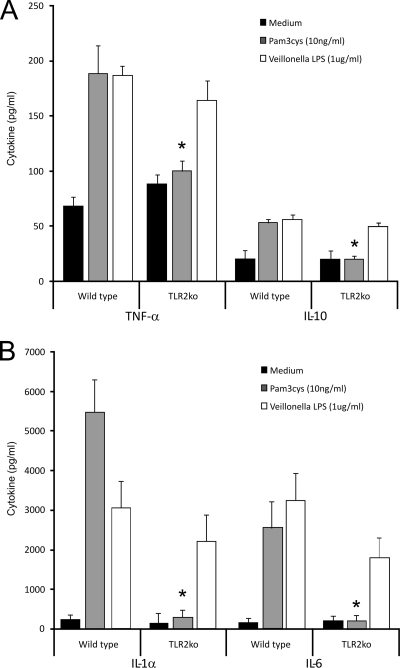
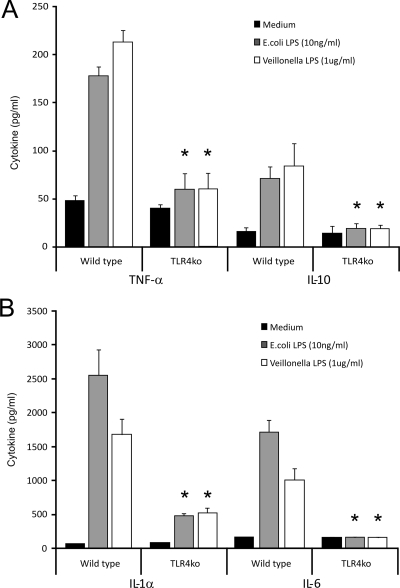
Resident peritoneal macrophages were harvested from TLR2−/− and TLR4−/− mice and their control littermates and stimulated in vitro with either control medium or highly purified Veillonella LPS (1 μg/ml), highly purified E. coli LPS (1 ng/ml), or Pam3Cys (10 ng/ml). The three stimuli used were able to produce a cytokine release substantially higher than medium alone in control macrophage cultures. Although Veillonella LPS caused a significant release of cytokines in the macrophages from TLR2−/− mice, it was not able to induce a relevant release of cytokines among the macrophages from TLR4−/− mice (Fig. 3A and B and 4A and B).
FIG. 3.
TLR2 is not activated by Veillonella LPS. Peritoneal macrophages from wild-type and TLR2 gene-deficient mice were stimulated with Veillonella LPS for 24 h. As a control, cells were stimulated with Pam3Cys (a potent TLR2 ligand). (A) Murine TNF-α and IL-10 production induced by Veillonella LPS. (B) Murine IL-1α and IL-6 levels. Note that Pam3Cys-induced cytokine production was abrogated in TLR2 knockout (TLR2ko) cells. Data represent the means ± standard deviations for cytokine production by at least five mice. *, P < 0.01 (Wilcoxon rank test).
FIG. 4.
Veillonella LPS-induced cytokine production is TLR4 dependent. Peritoneal macrophages from wild-type and TLR4 gene-deficient mice were stimulated with Veillonella LPS for 24 h. As a control, cells were stimulated with E. coli LPS (a potent TLR4 ligand). (A) Murine TNF-α and IL-10 production induced by Veillonella LPS. (B) Murine IL-1α and IL-6. Note that both Veillonella LPS and E. coli LPS induction of cytokine production was abrogated in TLR4 knockout (ko) cells. Data represent the means ± standard deviations for cytokine production by at least five mice. *, P < 0.01 (Wilcoxon rank test).
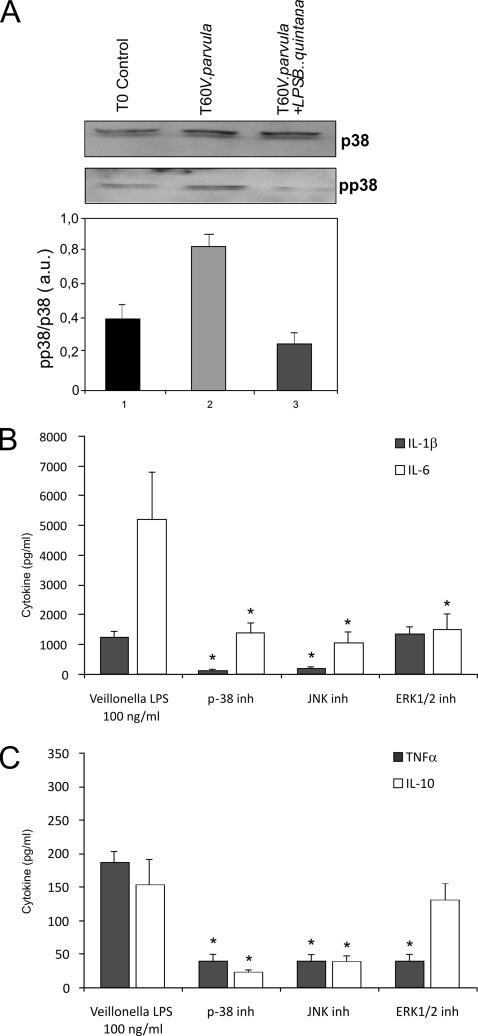
Veillonella-induced activation of p38 MAPK, which mediates cytokine production.
V. parvula LPS was able to activate the human PBMC p38 MAPK after 60 minutes of incubation. When B. quintana double-purified LPS was used to inhibit TLR4 on PBMC, activation of p38 MAPK was hardly inhibited after 60 minutes (Fig. 5A).
FIG. 5.
Veillonella LPS activates p38 MAPK. (A) Effects of TLR4 inhibition by B. quintana LPS on Veillonella LPS-stimulated p38 activation in human PBMC. Protein extracts (30 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blotting using specific anti-p38 and anti-phopsho-p38 antibodies. The intensity of phospho-p38-specific bands was measured by densitometry and is reported as arbitrary units (a.u.) of phosho-p38 expression after normalization with p38 levels. The means ± standard errors of the means of three independent experiments are shown. (B and C) Effects of pharmacological inhibition of p38 MAPK-, JNK-, and ERK-mediated pathways during Veillonella LPS stimulation of PBMC; the graphs show IL-1β and IL-6 production after inhibition of p38 MAPK, JNK, and ERK (B) or TNF-α and IL-19 production (C). Data represent the means ± standard deviations for cytokine production of at least four healthy volunteers. Note the strong suppression of cytokine production after inhibition of p38 MAPK and JNK. *, P < 0.01 (Wilcoxon rank test).
The activation of JNK and ERK1/2 after the V. parvula LPS stimulus was very inconsistently observed regardless of the time point considered (15, 30, and 60 min) (data not shown). In line with these data, a p38 MAPK inhibitor strongly reduced cytokine production induced by V. parvula LPS (Fig. 5B and C). Similar results were observed with an inhibitor of JNK kinase, while inhibition of ERK had a more modest effect (Fig. 5B and C).
DISCUSSION
In the present paper we demonstrate that V. parvula LPS is recognized by TLR4 in both humans and mice and that the activation of MAP kinases plays an important role in V. parvula LPS-stimulated human PBMC signaling.
Previous studies on the in vitro and in vivo activities of the LPS from Veillonella parvula demonstrated that such an endotoxin has biological effects comparable to enterobacterial LPS (10, 29). Earlier studies reported on fibroblast collagenase-inducing PBMC cytokines stimulated by Veillonella spp. LPS; however, such cytokines were not directly evaluated (16). Formalin-killed V. parvula has been demonstrated to induce the release of IL-1β in human blood monocytes at levels comparable to those released by Porphryomonas gingivalis, Bacteroides forsythus, and Prevotella intermedia (27). More recent investigations showed that the concentrations of TNF-α, IL-6, and IL-10 released from umbilical cord and adult mononuclear cells following in vitro challenge with UV-killed V. parvula were comparable or even higher than cytokine concentrations evaluated in Pseudomonas aeruginosa- and E. coli-stimulated cord and adult cells (19).
The lower cytokine production of PBMC induced by Veillonella LPS (approximately 20 to 30% of that induced by E. coli LPS) is mirrored in the type of pathology induced by these microorganisms. On the one hand, gram-negative sepsis with enterobacteriaceae induces a superacute inflammatory reaction, followed sometimes by organ failure and even death. On the other hand, Veillonella is present in the mouth flora and is an important pathogen of periodontal disease, a chronic inflammatory condition. Thus, the low cytokine production by Veillonella is certainly important in inducing the low-grade inflammation in periodontitis.
To the best of our knowledge, very few papers have reported on the role of TLRs for the pathogenesis of Veillonella infection. In one study treatment of dendritic cells with an anti-TLR4 antibody decreased cytokine production induced by UV-treated V. parvula (20), but the nature of the Veillonella ligand recognized by TLR4 was not identified.
Similarly, TLR2- and TLR4-transfected human embryonic kidney cells responded to sonicated Veillonella bacteria stimulation (21). In both these studies, Veillonella seemed to stimulate both TLR4 and TLR2. However, the bacterial product responsible for the TLR engagement was not addressed, and the models used were only from murine species. In the present study, we demonstrate that V. parvula LPS is recognized by TLR4 in both human and murine cells, and we show the MAPK role in Veillonella LPS-stimulated human PBMC signaling.
V. parvula has been implicated in both dental/periodontal diseases (3, 18) and joint disorders (26). Periodontal disease and rheumatoid arthritis have remarkably similar inflammatory mediator profiles (22). Within periodontal lesions, activated monocytes, macrophages, and fibroblasts produce cytokines, such as TNF-α, IL-1β, and IL-6, which have been found to be significantly elevated in diseased periodontal sites compared with healthy or inactive sites (11). These cytokines orchestrate the cascade of destructive events that occur in the periodontal tissues and trigger the production of an array of inflammatory enzymes and mediators, resulting in irreversible hard and soft tissue damage (15). By exploiting an in vitro model of periodontal diseases, it has been found that upregulation of IL-6 by lipopolysaccharide treatment is TLR4 dependent. This pattern of gene expression indicates that pathogens may trigger TLR4 signaling and cause periodontitis (35). Therefore, our present findings may have sound clinical impact. Moreover, due to the similarity of pathogenesis between periodontitis and rheumatoid arthritis, antagonists of TLR4, as well as p38 inhibitors, have the potential to ameliorate progression of periodontal disease (23), rheumatic pathologies, and other chronic inflammatory/degenerative disorders. The TLR4 antagonist B. quintana LPS (31) has been demonstrated to dramatically improve the evolution of experimental arthritis in the mouse (1). Further studies are warranted in order to exploit the modulation of TLR4 and p38 MAPK in the therapy of chronic inflammatory/degenerative diseases.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Abdollahi-Roodsaz, S., L. A. Joosten, M. F. Roelofs, T. R. Radstake, G. Matera, C. Popa, J. W. van der Meer, M. G. Netea, and W. B. van den Berg. 2007. Inhibition of TLR4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 56:2957-2967. [DOI] [PubMed] [Google Scholar]
- 2.Arancibia, S. A., C. J. Beltrán, I. M. Aguirre, P. Silva, A. L. Peralta, F. Malinarich, and M. A. Hermoso. 2007. Toll-like receptors are key participants in innate immune responses. Biol. Res. 40:97-112. [DOI] [PubMed] [Google Scholar]
- 3.Arif, N., E. C. Sheehy, T. Do, and D. Beighton. 2008. Diversity of Veillonella spp. from sound and carious sites in children. J. Dent. Res. 87:278-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrosagaray, P. M., C. Salas, M. Morales, M. Correas, J. M. Barros, and M. L. Cordon. 1987. Bilateral abscessed orchiepididymitis associated with sepsis caused by Veillonella parvula and Clostridium perfringens: case report and review of the literature. J. Clin. Microbiol. 25:1579-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler, B., and E. T. Rietschel. 2003. Innate immune sensing and its roots: the story of endotoxins. Nat. Rev. Immunol. 3:169-176. [DOI] [PubMed] [Google Scholar]
- 6.Bhatti, M. A., and M. O. Frank. 2000. Veillonella parvula meningitis: case report and review of Veillonella infections. Clin. Infect. Dis. 31:839-840. [DOI] [PubMed] [Google Scholar]
- 7.Bongaerts, G. P., B. W. Schreurs, F. V. Lunel, J. A. Lemmens, M. Pruszczynski, and M. A. Merkx. 2004. Was isolation of Veillonella from spinal osteomyelitis possible due to poor tissue perfusion? Med. Hypoth. 63:659-661. [DOI] [PubMed] [Google Scholar]
- 8.Boo, T. W., B. Cryan, A. O'Donnel, and G. Fahy. 2005. Prosthetic valve endocarditis caused by Veillonella parvula. J. Infect. 50:81-83. [DOI] [PubMed] [Google Scholar]
- 9.Brook, I. 1996. Veillonella infections in children. J. Clin. Microbiol. 34:1283-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delwiche, E. A., J. J. Pestka, and M. L. Tortorello. 1985. The Veillonellae: gram negative cocci with a unique physiology. Annu. Rev. Microbiol. 39:175-193. [DOI] [PubMed] [Google Scholar]
- 11.Ejeil, A. L., F. Gaultier, S. Igondjo-Tchen, K. Senni, B. Pellat, G. Godeau, and B. Gogly. 2003. Are cytokines linked to collagen breakdown during periodontal disease progression? J. Periodontol. 74:196-201. [DOI] [PubMed] [Google Scholar]
- 12.Elson, G., I. Dunn-Siegrist, B. Daubeuf, and J. Pugin. 2007. Contribution of Toll-like receptors to the innate immune response to gram- negative and gram-positive bacteria. Blood 109:1574-1583. [DOI] [PubMed] [Google Scholar]
- 13.Endres, S., R. Ghorbani, G. Lonnemann, J. W. van der Meer, and C. A. Dinarello. 1988. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin. Immunol. Immunopathol. 49:424-438. [DOI] [PubMed] [Google Scholar]
- 14.Fisher, R. G., and M. R. Denison. 1996. Veillonella parvula bacteremia without an underlying source. J. Clin. Microbiol. 34:3235-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graves, D. T. 1999. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin. Infect. Dis. 28:482-490. [DOI] [PubMed] [Google Scholar]
- 16.Heath, J. K., S. J. Atkinson, R. M. Hembry, J. J. Reynolds, and M. C. Meikle. 1987. Bacterial antigens induce collagenase and prostaglandin E2 synthesis in human gingival fibroblasts through a primary effect on circulating mononuclear cells. Infect. Immun. 55:2148-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 18.Hughes, C. V., P. E. Kolenbrander, R. N. Andersen, and L. V. Moore. 1988. Coaggregation properties of human oral Viellonella spp.: relationship to colonization site and oral ecology. Appl. Environ. Microbiol. 54:1957-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson, H., C. Hessle, and A. Rudin. 2002. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 70:6688-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson, H., P. Larsson, A. E. Wold, and A. Rudin. 2004. Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect. Immun. 72:2671-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikkert, R., M. L. Laine, L. A. Aarden, and A. J. van Winkelhoff. 2007. Activation of Toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiol. Immunol. 22:145-151. [DOI] [PubMed] [Google Scholar]
- 22.Kirkwood, K. L., J. A. Cirelli, J. E. Rogers, and W. V. Giannobile. 2007. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol. 2000 43:294-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkwood, K. L., F. Li, J. E. Rogers, J. Otremba, D. D. Coatney, J. M. Kreider, N. J. D'Silva, S. Chakravarty, S. Dugar, L. S. Higgins, A. A. Protter, and S. Medicherla. 2007. A p38α selective mitogen-activated protein kinase inhibitor prevents periodontal bone loss. J. Pharmacol. Exp. Ther. 320:56-63. [DOI] [PubMed] [Google Scholar]
- 24.Liu, H., R. W. Redline, and Y. W. Han. 2007. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J. Immunol. 179:2501-2508. [DOI] [PubMed] [Google Scholar]
- 25.Liu, J. W., J. J. Wu, L. R. Wang, L. J. Teng, and T. C. Huang. 1998. Two fatal cases of Veillonella bacteraemia. Eur. J. Clin. Microbiol. Infect. Dis. 17:62-64. [DOI] [PubMed] [Google Scholar]
- 26.Marchandin, H., H. Jean-Pierre, C. Carrière, F. Canovas, H. Daras, and E. Jumas-Bilak. 2001. Prosthetic joint infection due to Veillonella dispar. Eur. J. Clin. Microbiol. Infect. Dis. 20:340-342. [DOI] [PubMed] [Google Scholar]
- 27.Mark, L. L., A. D. Haffajee, S. S. Socransky, R. L. Kent, Jr., D. Guerrero, K. Kornman, M. Newman, and P. Stashenko. 2000. Effect of interleukin-1 genotype on monocyte IL-1β expression in subjects with adult periodontitis. J. Periodontal Res. 35:172-177. [DOI] [PubMed] [Google Scholar]
- 28.Marriott, D., D. Stark, and J. Harkness. 2007. Veillonella parvula discitis and secondary bacteremia: a rare infection complicating endoscopy and colonoscopy? J. Clin. Microbiol. 45:672-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matera, G., M. C. Liberto, M. C. Berlinghieri, and A. Focà. 1991. Biological effects of Veillonella parvula and Bacteroides intermedius lipopolysaccharides. Microbiologica 14:315-323. [PubMed] [Google Scholar]
- 30.Mori, Y., A. Yoshima, T. Ukai, E. Lien, T. Espevik, and Y. Hara. 2003. Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol. Immunol. 18:54-58. [DOI] [PubMed] [Google Scholar]
- 31.Popa, C., S. Abdollahi-Roodsaz, L. A. Joosten, N. Takahashi, T. Sprong, G. Matera, M. C. Liberto, A. Focà, M. van Deuren, B. J. Kullberg, W. B. van den Berg, J. W. van der Meer, and M. G. Netea. 2007. Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect. Immun. 75:4831-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawada, N., T. Ogawa, Y. Asai, Y. Makimura, and A. Sugiyama. 2007. Toll-like receptor 4-dependent recognition of structurally different forms of chemically synthesized lipid As of Porphyromonas gingivalis. Clin. Exp. Immunol. 148:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah, A., C. Panjabi, V. Nair, R. Chaudhry, and S. S. Thukral. 2008. Veillonella as a cause of chronic anaerobic pneumonitis. Int. J. Infect. Dis. 12:e115-e117. [DOI] [PubMed] [Google Scholar]
- 34.Singh, N., and V. L. Yu. 1992. Osteomyelitis due to Veillonella parvula: case report and review. Clin. Infect. Dis. 14:361-363. [DOI] [PubMed] [Google Scholar]
- 35.Sun, Y., R. Shu, M. Z. Zhang, and A. P. Wu. 2008. Tool-like receptor 4 signaling plays a role in triggering periodontal infection. FEMS Immunol. Med. Microbiol. 52:362-369. [DOI] [PubMed] [Google Scholar]
- 36.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 37.Tan, P. T., L. Y. Chang, Y. C. Huang, C. H. Chiu, C. R. Wang, and T. Y. Lin. 2001. Deep neck infections in children. J. Microbiol. Immunol. Infect. 34:287-392. [PubMed] [Google Scholar]
- 38.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides. Extraction with phenol water and further applications of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]