Abstract
Cervid herpesvirus 2 (CvHV2) has been isolated from reindeer (Rangifer tarandus tarandus), and serological data indicate that in reindeer this virus is endemic in Fennoscandia, Alaska, Canada, and Greenland. CvHV2 has been described as a cause of subclinical genital infections in reindeer, but little information on primary infections exists. In this study, six seronegative and presumably pregnant reindeer were allocated to one of two groups. Two animals were inoculated with CvHV2 intratracheally, and two animals intravaginally, with one control animal in each group receiving sterile water. Mild hyperthermia and serous discharges from the vagina and nose were observed. No abortions were recorded, but one calf died shortly after birth. Inoculated animals seroconverted and had neutralizing antibodies after days 7 to 10 postinfection. CvHV2 was detected by PCR in nasal and vaginal swabs from animals in both groups but could be isolated only from nasal swabs in the respiratory group and from vaginal swabs in the genital group. CvHV2 was detected by PCR in various organs and tissues postmortem. In control animals, the virus could not be isolated in spite of PCR-positive nasal and vaginal swab samples and some degree of positive immunostaining. One of the animals that were inoculated intratracheally developed a hemorrhagic, necrotizing bronchopneumonia, which was CvHV2 positive by PCR and immunohistochemistry. We conclude that CvHV2 can cause systemic infection, that both genital and respiratory inoculations can lead to virus shedding, and that the virus can infect the fetus in utero.
Cervid herpesvirus 2 (CvHV2) belongs to the order Herpesvirales, family Herpesviridae, subfamily Alphaherpesvirinae, and genus Varicellovirus (6) and has been isolated from reindeer (Rangifer tarandus tarandus) after reactivation by dexamethasone treatment (11, 26). Serological evidence of CvHV2 or an antigenically related herpesvirus infection has been found in semidomesticated reindeer in Norway (31), in barren-ground caribou (Rangifer tarandus groenlandicus) in Greenland (1), and in Grant's caribou (Rangifer tarandus granti) in Alaska (9). Recent studies with semidomesticated reindeer in Norway revealed that CvHV2 is endemic, with a relatively high prevalence in the reindeer population (3, 4).
Ruminant varicelloviruses are antigenically and genetically closely related, as demonstrated by the gene encoding glycoprotein B (UL27), which shows 84% homology between bovine herpesvirus 1 (BoHV1) and CvHV2 (28). Serological cross-reactions between these viruses have likewise been shown (7, 19, 20, 25). BoHV1 causes infectious bovine rhinotracheitis and infectious pustular vulvovaginitis, as well as encephalitis and abortion, in cattle (12, 24), and such serological cross-reactions may hinder the efficiency of BoHV1 eradication or surveillance programs if similar viruses are circulating.
Experimentally, it has been demonstrated that CvHV2 can infect cattle and cause mild rhinotracheitis and induce a serological response, while BoHV1 infection in reindeer causes no clinical signs and only a weak serological response (22, 34). Reactivation was not detected in these cross-infection studies, supporting the notion that herpesvirus species are closely associated with their main respective hosts, as characterized by the ability to establish latency (5). This property is not demonstrated in every case of cross-infection, as recently evidenced by the establishment of latency of elk herpesvirus 1 in cattle (8). However, the genetic and antigenic similarities between these viruses imply that wild ruminants could be considered possible reservoirs for BoHV1 and bovines as reservoirs of alphaherpesviruses from other species (36).
Reactivation of BoHV1 usually causes a subclinical infection and the acquired immune response may prevent virus shedding during reactivation. Primary infection, on the other hand, may cause shedding and clinical signs, including abortion or perinatal mortality (12).
In experimental reactivation studies with reindeer, CvHV2 has caused asymptomatic genital infections, with virus being excreted in vaginal secretions (11, 35). No information has been published regarding primary infection. Recent field studies demonstrated that CvHV2 is present in the upper respiratory tract, with latency in the trigeminal ganglia, and that it can also be transmitted to the fetus via the placenta (3). The potential of CvHV2 to cause eventual abortion or calves that are born weak remains unknown.
In Norway, there were more than 275,000 reindeer in 2006 to 2007, of which almost 90% were semidomesticated and 10% were wild. Reindeer husbandry is of major economical and cultural importance, especially for the local indigenous Saami communities. The registered overall mortality of reindeer in Norway during the 2006 reindeer herding year (1 April 2005 to 31 March 2006) was 37% (2). Attacks by predators are considered the major cause of death, but approximately 11% of the losses are of unknown etiology. Semidomesticated reindeer usually give birth unattended while ranging free, which makes it difficult to assess the contribution of abortion or calves that are born weak to mortality (38).
The aim of this study was to carry out experimental infections with CvHV2 in pregnant seronegative reindeer to evaluate the pathogenesis of CvHV2. The clinical signs, different routes of infection (genital and respiratory), possible systemic infection with transmission to calves, and development of the humoral immune response were studied.
MATERIALS AND METHODS
Cells and virus.
Madin-Darby bovine kidney cells ([MDBK] ATCC CCL22) were maintained in Earle's minimum essential medium (EMEM) supplemented with 10% horse serum ([HS] LGC Standards, Teddington, UK).
The CvHV2 strain Salla 82 (11) was propagated in MDBK cells with EMEM supplemented with 2% HS and 2% penicillin-streptomycin ([PS] 10,000 U/ml penicillin and 10 mg/ml streptomycin; Sigma-Aldrich, Steinheim, Germany) at a multiplicity of infection of 0.1. At 48 h postinfection (p.i.), the culture medium was removed and centrifuged for 20 min at 1,500 × g. The supernatant was divided into aliquots and stored at −80°C. Virus titration was performed, and the 50% tissue culture infectious dose (TCID50) was calculated by the Spearman-Kärber method (37).
Animals.
In March 2008, 40 reindeer originating from one herd from Finnmark County, Norway, were screened for the presence of antibodies against CvHV2, using blocking enzyme-linked immunosorbent assay (ELISA) kits based on a viral glycoprotein (glycoprotein B [gB]) as the antigen, as previously described (4). Six seronegative and presumably pregnant reindeer (age range, 2 to 6 years) were chosen for the study. Animals were initially placed in a common fenced area for a 21-day adaptation period with both food (lichen, dry hay, and pelleted reindeer feed; Felleskjøpet, Norway) and water available ad libitum. During the adaptation period, animals were checked three times per day to monitor food intake, changes in behavior, and adaptation to the new environment.
Experimental design.
Animals were divided into two groups according to the inoculation method: one respiratory group (R1, R2, and R3) and one genital group (G1, G2, and G3). Prior to being inoculated with CvHV2, the animals where immobilized with an intramuscular administration of a combination of medetomidine hydrochloride (0.1 mg/kg Domitor; Orion Corporation, Turku, Finland) and ketamine hydrochloride (0.5 mg/kg Ketalar; Pfizer Inc., New York, NY) (29).
A dose of 2 × 106 TCID50 of CvHV2 in 1 ml of sterile water was administered intratracheally to animals R2 and R3 and intravaginally to animals G2 and G3. Control animals R1 (respiratory group) and G1 (genital group) were given 1 ml of sterile water only by the same methods as described for the other animals in their respective groups. After the inoculations, immobilization was reversed by intramuscular administration of atipamezole (Antisedan; Orion Corporation, Turku Finland) (5 mg per mg of previously administered medetomidine) (30).
Animals were checked by veterinarians three times a day during the entire experimental period. Samples of blood and nasal and vaginal swabs were taken at 21 and 10 days prior to inoculation, on days 0, 4, 7, 10, 16, 20, and 24 p.i., and on the day of euthanasia. Sera were stored at −20°C until tested. Nasal and vaginal swabs were shaken in a tube with 1.5 ml of EMEM containing 2% PS and kept at −80°C until further processing.
Food intake, behavior, and clinical signs such as discharges or bleeding, edema, lesions of the genital and nasal mucosas, and lesions in the gums, lips, or eyes were checked throughout the experiment. Animals were also checked for vulval enlargement as a sign of imminent birth. Calves were allowed to stay with their mothers throughout the study, and samples were taken from them only upon euthanasia. After day 24 p.i., samples were taken from the animals only on the day they were euthanized. All sampling was done after chemical immobilization of the animals.
Animals were euthanized at days 24 (R1 and R3), 28 (G3), 29 (G2), and 36 (R2 and G1) p.i. Calves (R2a and G1a) were euthanized on the same day as their mothers.
Experimental protocols were approved by the Norwegian Animal Research Authority in accordance with the Norwegian Animal Experimental and Scientific Purposes Act (1986).
Serology. (i) ELISA.
The presence of antibodies against CvHV2 in serum was monitored by using a blocking ELISA kit for BoHV1 (gB blocking LSI; Laboratoire Service International, France) previously validated for testing reindeer serum samples (4). All samples were tested in duplicate.
(ii) SNT.
CvHV2 neutralizing titers were measured by serum neutralization test (SNT) as previously described (4), using a 2-h initial serum-virus incubation period. Twofold dilutions (from 1:2 up to 1:2,048) of each sample were tested with six parallels, and the CvHV2-neutralizing titers were calculated as the initial dilution of serum that could neutralize the cytopathic effect (CPE) in 50% of the wells (50% effective dose) according to the Spearman-Kärber method (37).
CvHV2 detection in secretions.
The presence of CvHV2 in nasal and genital swabs and in swabs from mucosal erosions of two animals was evaluated by PCR, virus isolation in MDBK cells, and virus titration, using 50 μl of the swab sample. PCR was performed on the original swabs and on all samples after cell cultivation, irrespective of whether they caused CPE or not.
PCR.
DNA was extracted using DNeasy blood and tissue kits (Qiagen, Hilden, Germany). A nested panalphaherpesvirus PCR was carried out as previously described (27). The two primer sets amplified a region of 294 bp of the UL27 gene encoding gB, which is a highly conserved gene among ruminant alphaherpesviruses. Amplicons were purified and sequenced as previously described (3).
Isolation of CvHV2.
Each tube with a swab and EMEM was thawed and shaken before being centrifuged at 5,000 × g for 5 min at 4°C. A total of 500 μl of the supernatant was mixed with 1 ml of EMEM containing antibiotics (10 ml/liter of PS [10,000 units/ml penicillin and 10 mg/ml streptomycin], 1 ml/liter of gentamicin [50 mg/ml] and 10 ml/liter of amphotericin B [250 μg/ml]) and filtered through a 0.44-μM filter (Nunc, Roskilde, Denmark). The filtered supernatant (1.5 ml) was then added to MDBK cells grown on T25 cm2 flasks (Nunc, Roskilde, Denmark) and allowed to adsorb for 2 h at 37°C in a 5% CO2 atmosphere, followed by the addition of 7 ml EMEM with 2% HS and incubation at 37°C in a 5% CO2 atmosphere.
Monolayers were checked daily for the presence of CPE. If no CPE was observed after 6 days of incubation, a second passage in MDBK cells was done. After two blind passages without CPE, samples were considered to be negative. DNA was extracted from all samples inoculated on MDBK cells irrespective of the presence of CPE, and PCR was likewise carried out.
Necropsy and detection of CvHV2 in organs and tissues.
Necropsy was carried out according to standard procedures at the National Veterinary Institute, Norway. Samples of lung, heart, liver, spleen, kidney, brain, abomasum, rumen, uterus, and placenta were obtained, fixed in neutral buffered 10% formalin, sectioned, and stained with hematoxylin and eosin. Selected sections of lung were stained with periodic acid-Schiff, Van Gieson, or Gram-staining techniques.
Immunohistochemistry was performed using a mouse monoclonal antibody, clone 5G10, with specificity for the CvHV2 envelope gC (16). Paraffin-embedded lung sections of R3 and G1 and uterus sections of R3 were dewaxed in xylene and rehydrated through a graded ethanol series. Antigen was demasked by boiling the sections in 10 mM sodium citrate and 0.05% Tween 20 (pH 6.0) in a microwave oven four times, for 5 min each time. Endogenous peroxidase was quenched in 3% H2O2 and 1% sodium azide in phosphate-buffered saline (PBS) for 30 min, and unspecific binding was blocked by incubating the sections for 1 h at room temperature in 2% goat serum and 1% bovine serum albumin in PBS. The sections were then incubated with the primary antibody diluted 1:800 in blocking buffer overnight at 4°C. Washing steps were performed in PBS with 0.05% Tween 20. The specificity of the reaction was verified by labeling parallel sections with a mouse primary antibody isotype control (catalog number 08-6599; Invitrogen, Oslo, Norway). Reaction products were visualized using a Zymed SuperPicTure mouse DAB (3,3′-diaminobenzidine) kit (Invitrogen), and the sections were counterstained with hematoxylin.
Selected samples were examined for the presence and identification of bacteria at the National Veterinary Institute, Norway, according to standard routines. Fecal examinations for coccidia, helminth eggs, and larvae were performed for all adult animals at necropsy by modified McMaster (14) and Baermann techniques (32).
Samples of lung, liver, spleen, kidney, mesenteric lymph nodes, mediastinal lymph nodes, uterus mucosa, trigeminal ganglia, and, when available, milk, placenta, and mammary gland were taken for examination for viruses. Amniotic fluids and umbilical tissues were collected from the unborn fetus of R1 (R1a). DNA was extracted from the samples, and PCR was carried out as described above.
RESULTS
Clinical observations.
Abnormal behavior or signs of depression after inoculation were not observed in any animal. Rectal temperature was measured on the inoculation day and again on day 10 p.i. Mild hyperthermia, up to 40°C (normal temperature ranges from 38.6 to 39.0°C [10]), was observed in most animals, including the controls, on day 10 p.i., while reindeer G2 and G3 presented higher temperatures of 41.5°C and 42°C, respectively.
G3 had some serous vaginal discharges on day 4 p.i. and erosions on the vulva on day 10 p.i.
In the respiratory group, some slight serous nasal discharges were observed in animals R2 and R3 at days 4 to 8 p.i. A small erosion and erythema in the nasal cavity of R3 was observed, and a sample from it was taken on day 10 p.i.
On day 13 p.i., animal R3 gave birth to a small female calf (R3a) weighing 3.56 kg. The calf followed the mother immediately after birth and seemed to be in healthy condition but was found dead approximately 18 to 20 h later. R2 gave birth to a male calf (R2a) on day 14 p.i., while control animal G1 gave birth to a male calf on day 26 p.i. Both calves survived to the end of the study.
Serology.
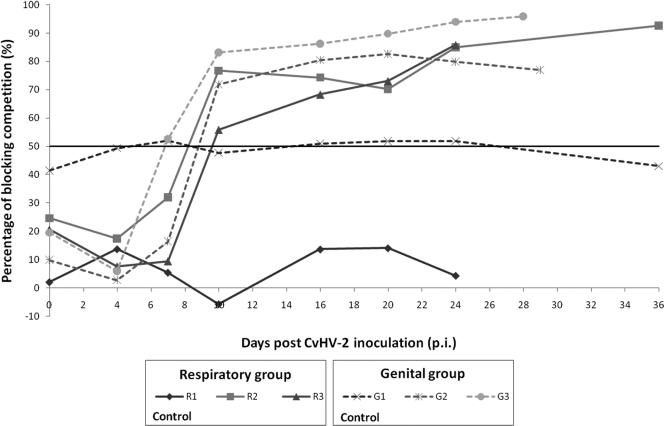
Animals inoculated with CvHV2 seroconverted between days 7 and 10 p.i. except for animal G3, which was already seropositive on day 7 p.i. (Fig. 1). On day 20 p.i., all virus-inoculated animals had percentages of competition above 70% in the ELISA and, on day 24 p.i., above 80%. Control animal R1 remained seronegative throughout the study, while control animal G1 had borderline results throughout the experiment.
FIG. 1.
Development of anti-gB antibody response in seronegative reindeer experimentally infected with CvHV2. The end point for each animal reflects the time of euthanasia. Measurements are presented as the percentage of competition in a BoHV1 gB-blocking ELISA. The horizontal black line marks the cutoff value (50%).
Only one of the three newborn calves (R2a) was classified as seropositive by ELISA (51% competition percentage; data not shown).
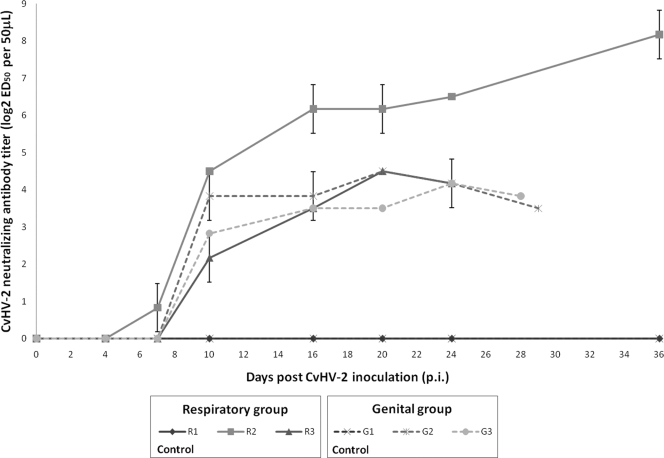
Neutralizing antibodies were observed in all animals inoculated with CvHV2 from day 10 p.i. onwards with the exception of animal R2, which already had a low neutralizing titer on day 7 p.i. (log2 = 0.83; 1:1.8 titer) (Fig. 2). However, day 10 p.i. was the first day on which an increase of two log2 steps was present in all virus-inoculated animals. For all virus-inoculated animals except R2, neutralizing titers increased until day 20 p.i. (maximum log2 = 4.5 for R3 and G3; 1:22) and decreased thereafter. In reindeer R2, neutralizing titers reached a log2 value of 6.17 (approximately 1:72) on day 16 p.i., and after a plateau of 4 days, increased gradually until reaching a log2 value of 8.17 (approximately 1:287) on day 36 p.i. Control animals R1 and G1 never showed any humoral response against CvHV2.
FIG. 2.
Kinetics of CvHV2-neutralizing antibody levels in experimentally infected seronegative reindeer. The end point for each animal reflects the time of euthanasia. A 48 h, SNT was performed, and titers are expressed as log2 50% effective dose per 50 μl of serum, calculated by the Spearman-Kärber method.
CvHV2 in nasal and vaginal swabs.
The results of PCR and the isolation of virus from nasal and vaginal swab samples are presented in Table 1.
TABLE 1.
Isolation and detection of CvHV2 in swab samples from experimentally infected seronegative reindeer
| Group | Animala | Clinical signs of infectionb | Day(s) p.i. on which virus was isolated from indicated sample type |
Day(s) p.i. on which viral DNA was detected by PCR in indicated sample typeb |
|||
|---|---|---|---|---|---|---|---|
| Nasal swab | Vaginal swab | Nasal swab | Vaginal swab | Lesions | |||
| Respiratory | R1 (C) | NO | 10 | 7 | NO | ||
| R2 | Serous nasal discharges, erosion in nasal mucosa | 4-7 | 4-24 | 4-10, 24 | NO | ||
| R3 | Serous nasal discharges and erythema in the nasal mucosa | 4-10 | 4-20 | 7-16 | 10 | ||
| Genital | G1 (C) | NO | 7 | NO | |||
| G2 | Hyperthermia | 7 | 4-10 | 4-7 | NO | ||
| G3 | Hyperthermia, serous vaginal discharges, vulval erosion | 4-7 | 4 | 4-7 | 4 | ||
(C), control animal.
NO, not observed.
In the respiratory group, CvHV2 was detected by PCR in nasal and vaginal swabs of virus-inoculated animals from day 4 p.i. up to day 24 p.i., but the virus could be isolated in cell culture only between days 4 and 10 p.i. and only from nasal swabs.
In the genital group, PCR positives were detected between days 4 and 10 p.i. from nasal and vaginal swabs from virus-inoculated animals, but the virus could be isolated in cell culture only on days 4 and 7 p.i. and only from genital swabs.
Control animals G1 (genital group) and R1 (respiratory group) had transient PCR-positive swabs, but CvHV2 was never isolated from these animals.
The results revealed a quick increase in the spread and replication of CvHV2 in swabs and lesions immediately after inoculation, as shown by early detection in cell culture. The presence of the virus subsided quickly after day 10 p.i., and there was no isolation of CvHV2 in cell culture after day 10 p.i.
Virus titration from the original swabs produced very low titers for all animals, with maximum titers of 102.2 TCID50/50 μl in the vaginal swab of animal G3 on day 4 p.i. and 101.9 TCID50/50 μl in the nasal swabs of animals R2 and R3 on days 4 and 10 p.i., respectively. Viral titers were not found for control animals or for animal G2.
Necropsy and macroscopic findings.
All animals were considered to be in good condition for the time of year. Warble fly larvae (Hypoderma tarandi) and throat bot larvae (Cephenemyia trompe) were detected in adult animals, as were nematode larvae of Dictyocaulus spp. and/or Elaphostrongylus rangiferi and eggs of Trichostrongylus spp. upon fecal examination. The described parasite infections are common in reindeer in northern Norway.
In contrast to the other animals, which showed no specific gross pathology except those related to parasite infections, R3 had several irregular, dark-red, scarcely exudative consolidations in the cranioventral and dorsocaudal parts of the lung (both sides). In the right cranioventral lobe, there was a well-circumscribed nodule about 2 cm in diameter within the consolidated tissue containing a yellow, purulent-like material. The mediastinal lymph nodes were slightly swollen.
Animal R1 was pregnant; the fetus was hairless and not fully developed, weighing 1 kg. Necropsy of the calf revealed no specific pathology. R2 gave birth to a normal calf 3 weeks before necropsy, and the uterus and udder appeared normal. Animal R3 gave birth 2 weeks before necropsy. The calf died at approximately 18 to 20 h, and necropsy showed emaciation and no milk in the stomachs. The R3 endometrium was mildly hyperemic, and a large fibrin clot was found in the lumen of the left uterus horn. Animals G2 and G3 showed no sign of recent pregnancy.
Histopathologic findings at necropsy.
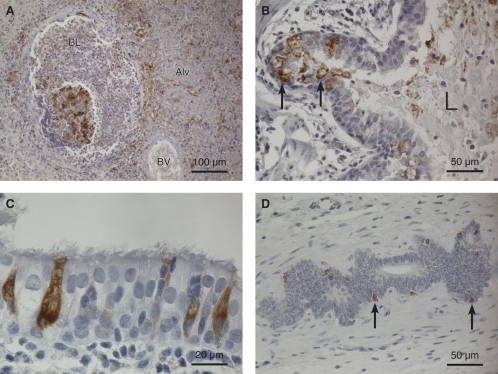
The adult animals had an interstitial, verminous pneumonia. In all animals except R3, the inflammation was chronic, with larvae and eggs of nematodes randomly distributed in the lung parenchyma, mild to moderate infiltration of predominantly mononuclear leukocytes, and some giant cells and eosinophilic granulocytes around the parasitic structures. Animal R3 showed a more severe and acute verminous pneumonia in the consolidated areas, with large amounts of eosinophilic granulocytes found both interstitially and around bronchioles. Besides the verminous pneumonia, an acute to subacute hemorrhagic, necrotizing bronchopneumonia was observed in the lesions in the cranioventral lobes of animal R3, with fibrin exudation, bleeding, and tissue destruction. Immune labeling of lung sections from R3 for the presence of CvHV2 gC revealed positive staining in areas with bronchopneumonia. The staining was most intense in connection with necrotized bronchioles (Fig. 3A). Positive staining was also observed in alveolar epithelial cells close to the necroses (not shown) and in bronchial epithelium throughout the lung (Fig. 3B and C), as well as in scattered mononuclear leukocytes in areas dominated by verminous pneumonia (data not shown). Interestingly, the CvHV2-positive cells in the respiratory epithelium were mostly goblet cells (Fig. 3C), which were often degenerated (Fig. 3B). Immune staining was also performed on lung sections from control G1 and revealed positive labeling for CvHV2 in goblet cells of several bronchi and desquamation of virus-stained cells to the bronchial lumen.
FIG. 3.
Immunohistochemistry of lung and uterus tissue from animal R3. The sections were labeled with a mouse monoclonal antibody to CvHV2 envelope gC, and positive labeling was visualized by a horseradish peroxidase-DAB method. Hematoxylin was used as a counterstain. (A) Cranioventral lung with a necrotic bronchiole in the area with bronchopneumonia. Positive immunolabeling for CvHV2 is seen as a brown stain in the tissues. BL, bronchiole lumen; BV, blood vessel; Alv, alveole filled with exudate. Magnification, ×200. (B) Bronchi in caudodorsal lung. Degenerated, CvHV2-positive cells (brown-stained cells, arrows) are seen in the respiratory epithelium, and positively stained cell debris (brown stain) is seen in the bronchial lumen (L). Magnification, ×400. (C) Highly magnified view of respiratory epithelium showing CvHV2-positive goblet cells (brown-stained cells). Magnification, ×1,000. (D) CvHV2-positive uterine cells (brown cells, arrows) in an endometrial gland. Magnification, ×400.
Another histopathological finding was that R3 had endometritis with neutrophil leukocyte infiltration in mucosa and fibrin exudation to the lumen of the previously pregnant horn. Positive immune staining was seen in scattered cells in the inflamed tissue and in endometrial glands in the adjacent tissue (Fig. 3D).
Bacteriologic findings at necropsy.
No specific pathogenic bacteria were detected after cultivation.
Virologic findings at necropsy.
The PCR results for CvHV2 from tissue samples collected during necropsies are shown in Table 2. CvHV2 DNA was amplified from all virus-infected animals, whereas samples from the control animals were PCR negative. Apart from the respiratory tract in the respiratory group and the uterus in the genital group, viral DNA was also amplified from other organs such as liver, spleen, lower digestive tract, mammary gland, and trigeminal ganglia. In R3, CvHV2 DNA was also amplified from lung lesions. In calf R3a, which died 18 to 20 h after birth, CvHV2 DNA was found in several organs, while in calf R2a, viral DNA was recovered only from a testicle sample.
TABLE 2.
Detection of CvHV2 DNA in tissues of experimentally infected reindeer by PCRa
| Tissue or fluid | Respiratory group |
Genital group |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1 (C) | R1a | R2 | R2a | R3 | R3a | G1 (C) | G1a | G2 | G3 | |
| Lung | − | − | + | − | + | + | − | − | − | − |
| Liver | − | − | − | − | − | + | − | − | − | − |
| Spleen | − | − | + | − | − | + | − | − | + | + |
| Kidney | − | − | − | − | − | − | − | − | − | − |
| Mesenteric lymph node | − | − | − | NS | − | − | − | − | − | − |
| Mediastinal lymph node | − | − | − | − | + | + | − | − | − | − |
| Uterus | − | NS | − | − | NS | − | + | + | ||
| Milk | − | − | − | − | ||||||
| Placenta | − | − | − | − | ||||||
| Trigeminal ganglia | − | NS | + | − | − | + | − | − | − | + |
| Mammary gland | − | + | − | NS | − | NS | NS | |||
| Other tissues | NS | NS | NS | +b | +c | +d | NS | NS | NS | +e |
Animals were divided into two groups (respiratory and genital) according to the place of inoculation. Animals R1 and G1 were the control animals of each group. R2a, R3a, and G1a were calves delivered from animals R2, R3, and G1, respectively. R1a was a fetus obtained from animal R1. All samples were collected during necropsy. +, virus detected; −, no virus detected; (C), control animal; NS, not sampled.
Testicles.
Lung lesion.
Nasal cavity.
Gastric lesion.
DISCUSSION
This is the first report of an experimental CvHV2 primary infection in reindeer. Virus isolation and PCR results strongly suggest that CvHV2 spread to different organs, also reaching the trigeminal ganglion. Therefore, CvHV-2 infection cannot be restricted to a localized genital infection as previously assumed (11, 35). Both the genital and respiratory infection routes induced systemic infection, with seroconversion and the detection of viral DNA in several organs. The clinical signs of CvHV2 infection were limited to transient hyperthermia, serous nasal and genital discharges, and focal erythematic erosion of the nasal and vaginal mucosa. Necropsy revealed a severe lung inflammation in one of the animals inoculated intratracheally with the virus. Transplacental infection of fetuses was shown by the presence of CvHV2 DNA in multiple organs of a calf that succumbed to death 18 to 20 h after birth.
The first positive PCRs from genital and respiratory swabs had already occurred by day 4 p.i. in both groups and lasted for a total of 6 days in the genital group and 12 days in the respiratory group. The shedding of CvHV2, as assessed by the presence of infectious virus on respiratory and genital mucosa and by clinical signs, was at its highest between days 4 and 10 p.i. Thereafter there was a sharp decline in the detection of CvHV2, as assessed by isolation in cell culture or PCR. A similar time course for virus isolation and PCR detection of herpesvirus infection has been found previously for BoHV1 in cattle, although in this case it lasted longer (17, 18, 40). At later stages, detection of CvHV2 was possible only by PCR, which was judged to reflect differences in sensitivity between cell culture and PCR.
The only clinical signs indicating virus infection were small erosions in the mucosa as well as serous nasal and vaginal discharges accompanied by moderate hyperthermia shortly after infection. The hyperthermia could also be linked to the stress caused by handling and sampling procedures. However, clinical findings, as well as positive immune staining for CvHV2 in inflamed lung tissue of one of the animals, indicate that CvHV2 infections are not necessarily asymptomatic. The lack of more-severe clinical signs could possibly have been the outcome of a low infective dose, but the virus dose inoculated was in the same range as infective doses used in similar studies with BoHV1 in cattle (18, 40). The determinants of CvHV2 virulence have never been studied, but ruminant alphaherpesviruses are reported to have different potentials to cause disease (35), and this could explain differences in clinical manifestations between ruminant species.
After an initial replication at the port of entry, ruminant alphaherpesviruses have been found to be restricted to the infection site or to spread systemically by viremia and neuroinvasion (12, 24). BoHV1 is mostly restricted to the port of entry, although with very high excretion titers, and then usually spreads by axonal transport to the local neuronal ganglia, where it becomes latent (12). Our results indicate that CvHV2 is not restricted to the port of entry, as it could be detected in samples from different organs. However, these differences between BoHV1 and CvHV2 could also be due to different sensitivities of the detection methods used, that is, PCR versus cell culture isolation.
CvHV2 titers in excretions did not surpass 102.2 TCID50/50 μl, which is lower than the titers generally reported for BoHV1 in similar excretions from cattle (15, 18). Nonetheless, maximum viral titers for animals R2, R3, and G3 coincided with the peak of “viral activity,” as shown in Table 1 in the virus isolation period. The lack of virus isolation from genital swabs of the respiratory group and vice versa indicates that despite systemic spread, replication and excretion occurred mostly at the original port of entry, findings similar to those for BoHV1 infections (21).
Despite the low titers in nasal and vaginal excretions, transmission of the virus to other animals occurred, as shown by positive CvHV2 PCR from nasal and vaginal swabs of the two control animals, as well as by positive immunostaining for CvHV2 in respiratory epithelium of the tested control, G1. However, the control animals did not seroconvert, and the virus was not isolated from these animals.
All experimentally infected animals seroconverted by day 10 p.i., and after day 20 p.i., all reached a plateau above 70% of competition percentage in the ELISA. Lemaire et al. (18) showed that after BoHV1 infection of cattle, ELISA results remained at a high competition percentage for at least 20 weeks, while SNT showed changes in antibody titers throughout the days of the study. In our study, an increase of neutralizing-antibody titers in all virus-inoculated animals was found by day 10 p.i. Neutralizing titers gradually increased until day 20 p.i. to a maximum of 1:22 for all experimentally infected animals except R2, whose neutralizing titer reached a plateau slightly above 1:64. These findings are similar to those from a study by Inman et al. (13), who reported a maximum neutralizing titer of 1:100 at 21 days p.i. in cattle infected with BoHV1 (13).
The neutralizing titer of animal R2 reached 1:64 on day 16 p.i. but increased further, to 1:256, by day 36 p.i., when the animal was euthanized. From the same animal, viral DNA amplification reoccurred from both the nasal and genital swabs on day 24 p.i. At the end of the study, no viral DNA could be amplified from any of this animal's swabs, but CvHV2 DNA was detected in the trigeminal ganglion. This may indicate that the transitory presence of CvHV2 in nasal and genital swabs at day 24 p.i. in R2 was caused by an early reactivation.
PCR amplification of viral DNA from different tissues from the virus-inoculated animals indicated spread of the virus beyond the port of entry.
The presence of CvHV2 in the trigeminal ganglia of animals R2 and G3 also showed that the viral infection reached the nervous system, where it most likely became latent.
The consistent presence of CvHV2 in the spleen might be of special interest for further studies. BoHV1 is reported to have the capability to infect CD4+ T lymphocytes, monocytes, and macrophages (23, 39). The capability of CvHV2 to infect similar cells could help to explain the presence of the virus in the spleen.
Animal R3, which was inoculated intratracheally, showed a more severe lung inflammation than the other animals. Histological examination of lung tissue from this animal showed both acute eosinophilic bronchointerstitial pneumonia and an acute to subacute fibrinohemorrhagic, necrotizing bronchopneumonia, the latter associated with intense positive CvHV2 immune staining, whereas scattered CvHV2 positive cells were seen in the inflamed tissue in areas dominated by verminous pneumonia. However, in either type of pneumonia, the immune staining revealed virus-infected cells in the respiratory epithelium of bronchi connected to the inflamed tissue. Considering that CvHV2 DNA was also amplified from areas dominated by verminous pneumonia, we speculate whether the severe eosinophilic reaction represents a herpesvirus infection-associated reactivation of a chronic verminous pneumonia.
Calf R3a, which died within 24 h of birth, had a systemic spread of CvHV2, including to the trigeminal ganglion, and it was seronegative, not having taken up any colostrum. Furthermore, CvHV2-immunopositive cells were seen in the endometrium of the mother, R3. These results indicate that the virus crossed the placenta and spread within the fetus, both systemically and by neuroinvasion. A postnatal infection would probably not have given the observed spread in such a short period of time. In bovines, calves that are born weak following BoHV1 infection may die shortly after birth. These calves often present necrotic foci in the liver, spleen, and kidney and sometimes even in the testicles (33). No such pathological lesions were found in calf R3a, and we cannot conclude that the CvHV2 infection contributed to the death of the calf. However, the observed endometritis in the previously pregnant horn of the mother and positive immune staining for virus in scattered cells in the inflamed tissue and in glands of adjacent tissue may indicate that an endometritis and/or placentitis caused by herpesvirus led to this calf being born weak. It is reasonable to assume that CvHV2 infections may have the potential to cause abortion, early birth, or disease in newborn calves, especially those deprived of colostrum uptake, like R3a.
In contrast to calf R3a, calf R2a was classified as seropositive, with a low ELISA score. However, it is uncertain whether this result reflects maternal antibodies in colostrum or the calf's own production after a postbirth infection.
The present study reports the first experimental primary infection of reindeer with CvHV2 and shows that this virus can cause not only local but also systemic infections that spread to multiple organs. Both the respiratory and genital inoculation routes led to excretion of the virus, as demonstrated by the isolation of the virus from experimentally infected animals, transmission to control animals, and development of a humoral immune response comparable to that found in bovines after BoHV1 infection.
Mother-to-offspring transmission was also confirmed, and the early death of an infected calf that did not receive maternal antibody protection should motivate further studies on the role of CvHV2 in abortion and early calf mortality, events likely to take place often in reindeer herding. Further studies of the virulence factors of CvHV2 are important to improve our knowledge about the pathogenesis of this virus.
Acknowledgments
We acknowledge Kristin Prestrud, Renate Sjølie, Kjetil Åsbakk, Terje Josefsen, and Andrea Balboni for their assistance during the experimental infection and the University of Tromsø and BioForsk for their technical and logistical support.
This project was partly funded by the Norwegian Reindeer Development Fund (RUF), the Norwegian School of Veterinary Science, and the Portuguese Science and Technology Foundation (FCT-Fundação para a Ciência e Tecnologia).
Footnotes
Published ahead of print on 21 October 2009.
REFERENCES
- 1.Anonymous. 1999, posting date. Report on the animal health situation in Greenland 1999. Ministry of Food, Agriculture and Fisheries- Greenland Homerule, Gothab, Greenland.
- 2.Anonymous. 2007. Ressursregnskap for reindriftsnæringen fra Reindriftsforvaltningen. Reindriftsforvaltningen/Norwegian Reindeer Husbandry Authority, Alta, Norway.
- 3.Das Neves, C. G., E. Rimstad, and M. Tryland. 2009. Cervid herpesvirus 2 causes respiratory and fetal infections in semidomesticated reindeer. J. Clin. Microbiol. 47:1309-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das Neves, C. G., M. Roger, N. G. Yoccoz, E. Rimstad, and M. Tryland. 2009. Evaluation of three commercial bovine ELISA kits for detection of antibodies against alphaherpesviruses in reindeer (Rangifer tarandus tarandus). Acta Vet. Scand. 51:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davison, A. J. 2002. Evolution of the herpesviruses. Vet. Microbiol. 86:69-88. [DOI] [PubMed] [Google Scholar]
- 6.Davison, A. J., R. Eberle, B. Ehlers, G. S. Hayward, D. J. McGeoch, A. C. Minson, P. E. Pellet, B. Roizman, M. J. Studdert, and E. Thiry. 2009. The order Herpesvirales. Arch. Virol. 154:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deregt, D., S. A. Gilbert, I. Campbell, K. M. Burton, H. W. Reid, S. V. D. Littel-van den Hurk, C. Penniket, and M. K. Baxi. 2005. Phylogeny and antigenic relationships of three cervid herpesviruses. Virus Res. 114:140-148. [DOI] [PubMed] [Google Scholar]
- 8.Deregt, D., S. V. Tessaro, and S. A. Gilbert. 2005. Serological evidence of latency in cattle experimentally infected with elk herpesvirus. Vet. Rec. 156:610-611. [DOI] [PubMed] [Google Scholar]
- 9.Dieterich, R. A. 1981. Respiratory viruses, p. 28-29. In Alaskan wildlife diseases. University of Alaska, Fairbanks, AK.
- 10.Dieterich, R. A. 1990. Reindeer health aide manual. University of Alaska Fairbanks and the U.S. Department of Agriculture, Fairbanks, AK.
- 11.Ek-Kommonen, C., S. Pelkonen, and P. F. Nettleton. 1986. Isolation of a herpesvirus serologically related to bovine herpesvirus 1 from a reindeer (Rangifer tarandus). Acta Vet. Scand. 27:299-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels, M., and M. Ackermann. 1996. Pathogenesis of ruminant herpesvirus infections. Vet. Microbiol. 53:3-15. [DOI] [PubMed] [Google Scholar]
- 13.Inman, M., L. Lovato, A. Doster, and C. Jones. 2002. A mutation in the latency-related gene of bovine herpesvirus 1 disrupts the latency reactivation cycle in calves. J. Virol. 76:6771-6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josefsen, T. D., K. K. Sørensen, T. Mørk, S. D. Mathiesen, and K. A. Ryeng. 2007. Pathological findings in completely emaciated carcasses. Acta Vet. Scand. 49:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaashoek, M. J., P. H. Straver, R. E. Van, J. Quak, and J. T. Van Oirschot. 1996. Virulence, immunogenicity and reactivation of seven bovine herpesvirus 1.1. strains: clinical and virological aspects. Vet. Rec. 139:416-421. [DOI] [PubMed] [Google Scholar]
- 16.Keuser, V., F. Schynts, B. Detry, A. Collard, B. Robert, A. Vanderplasschen, P. P. Pastoret, and E. Thiry. 2004. Improved antigenic methods for differential diagnosis of bovine, caprine, and cervine alphaherpesviruses related to bovine herpesvirus 1. J. Clin. Microbiol. 42:1228-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemaire, M., F. Schynts, G. Meyer, J. P. Georgin, E. Baranowski, A. Gabriel, C. Ros, S. Belak, and E. Thiry. 2001. Latency and reactivation of a glycoprotein E negative bovine herpesvirus type 1 vaccine: influence of virus load and effect of specific maternal antibodies. Vaccine 19:4795-4804. [DOI] [PubMed] [Google Scholar]
- 18.Lemaire, M., F. Schynts, G. Meyer, and E. Thiry. 1999. Antibody response to glycoprotein E after bovine herpesvirus type 1 infection in passively immunised, glycoprotein E-negative calves. Vet. Rec. 144:172-176. [DOI] [PubMed] [Google Scholar]
- 19.Lyaku, J. R. S., P. F. Nettleton, and H. Marsden. 1992. A comparison of serological relationships among 5 ruminant alphaherpesviruses by ELISA. Arch. Virol. 124:333-341. [DOI] [PubMed] [Google Scholar]
- 20.Martin, W. B., G. Castrucci, F. Frigeri, and M. Ferrari. 1990. A serological comparison of some animal herpesviruses. Comp. Immunol. Microbiol. Infect. Dis. 13:75-84. [DOI] [PubMed] [Google Scholar]
- 21.Muylkens, B., J. Thiry, P. Kirten, F. Schynts, and E. Thiry. 2007. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 38:181-209. [DOI] [PubMed] [Google Scholar]
- 22.Nettleton, P. F., C. Ek-Kommonen, R. Tanskanen, H. W. Reid, J. A. Sinclair, and J. A. Herring. 1988. Studies on the epidemiology and pathogenesis of alphaherpesvirus from red deer (Cervus elaphus) and reindeer (Rangifer tarandus), p. 143-148. In H. W. Reid (ed.), The management and health of farmed deer. Kluwer Academic Publishers, London, United Kingdom.
- 23.Nyaga, P. N., and D. G. McKercher. 1979. Pathogenesis of bovine herpesvirus-1 (BHV-1) infections: interactions of the virus with peripheral bovine blood cellular components. Comp. Immunol. Microbiol. Infect. Dis. 2:587-602. [DOI] [PubMed] [Google Scholar]
- 24.Pastoret, P. P., E. Thiry, B. Brochier, and G. Derboven. 1982. Bovid herpesvirus 1 infection of cattle: pathogenesis, latency, consequences of latency. Ann. Rech. Vet. 13:221-235. [PubMed] [Google Scholar]
- 25.Rimstad, E., R. Krona, and B. Hyllseth. 1992. Comparison of herpesviruses isolated from reindeer, goats, and cattle by restriction endonuclease analysis. Arch. Virol. 123:389-397. [DOI] [PubMed] [Google Scholar]
- 26.Rockborn, G., C. Rehbinder, B. Klingeborn, M. Lefler, K. Klintevall, T. Nikkilä, A. Landèn, and M. Nordkvist. 1990. The demonstration of a herpesvirus, related to bovine herpesvirus 1, in reindeer with ulcerative and necrotizing lesions of the upper alimentary tract and nose. Rangifer 3:373-384. [Google Scholar]
- 27.Ros, C., and S. Belak. 1999. Studies of genetic relationships between bovine, caprine, cervine, and rangiferine alphaherpesviruses and improved molecular methods for virus detection and identification. J. Clin. Microbiol. 37:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ros, C., and S. Belak. 2002. Characterization of the glycoprotein B gene from ruminant alphaherpesviruses. Virus Genes 24:99-105. [DOI] [PubMed] [Google Scholar]
- 29.Ryeng, K. A., J. M. Arnemo, and S. Larsen. 2001. Determination of optimal immobilizing doses of a medetomidine hydrochloride and ketamine hydrochloride combination in captive reindeer. Am. J. Vet. Res. 62:119-126. [DOI] [PubMed] [Google Scholar]
- 30.Ryeng, K. A., S. Larsen, B. Ranheim, G. Albertsen, and J. M. Arnemo. 2001. Clinical evaluation of established optimal immobilizing doses of medetomidine-ketamine in captive reindeer (Rangifer tarandus tarandus). Am. J. Vet. Res. 62:406-413. [DOI] [PubMed] [Google Scholar]
- 31.Stuen, S., J. Krogsrud, B. Hyllseth, and N. J. C. Tyler. 1993. Serosurvey of three virus infections in reindeer in northern Norway and Svalbard. Rangifer 13:215-219. [Google Scholar]
- 32.Thienpont, D., F. Rochette, and O. F. J. Vanparijs. 1979. Diagnosing helminthiasis through coprological examination 2nd (ed.), p. 36-38. Janssen Research Foundation, Beerse, Belgium.
- 33.Thiry, E. 2007. Infectious bovine rhinotracheitis (IBR), p. 19-31. In P. Vétérinaire (ed.), Clinical virology of ruminants. Wolters-Kluwer, Paris, France.
- 34.Thiry, E., M. Ackermann, M. Banks, K. Belak, S. Belak, I. Campbell, C. Ek-Kommonen, M. Engels, G. Meyer, H. Reid, C. Ros, and A. Six. 2001. Risk evaluation of cross-infection of cattle with ruminant alphaherpesviruses related to bovine herpesvirus type 1, p. 99-104. In R. Körber (ed.), Abstr. Third Int. Symp. zur BHV-1/BVD- Bekämpfung, Stendal, Germany.
- 35.Thiry, J., V. Keuser, B. Muylkens, F. Meurens, S. Gogev, A. Vanderplasschen, and E. Thiry. 2006. Ruminant alphaherpesviruses related to bovine herpesvirus 1. Vet. Res. 37:169-190. [DOI] [PubMed] [Google Scholar]
- 36.Thiry, J., B. Muylkens, and E. Thiry. 2008. Infectious bovine rhinotracheitis and the epidemiological role of the other ruminant species. Hung. Vet. J. 130:116-123. [Google Scholar]
- 37.Thrusfield, M. 1986. Serological epidemiology, p. 175-186. In M. Thrusfield (ed.), Veterinary epidemiology. Butterworths, London, United Kingdom.
- 38.Tveraa, T., P. Fauchald, C. Henaug, and N. G. Yoccoz. 2003. An examination of a compensatory relationship between food limitation and predation in semi-domesticated reindeer. Oecologia 137:370-376. [DOI] [PubMed] [Google Scholar]
- 39.Winkler, M. T. C., A. Doster, and C. Jones. 1999. Bovine herpesvirus 1 can infect CD4+ T lymphocytes and induce programmed cell death during acute infection of cattle. J. Virol. 73:8657-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler, M. T. C., A. Doster, J. H. Sur, and C. Jones. 2002. Analysis of bovine trigeminal ganglia following infection with bovine herpesvirus 1. Vet. Microbiol. 86:139-155. [DOI] [PubMed] [Google Scholar]