Abstract
We report a patient with microbiologically documented tuberculous meningitis showing that the therapeutic paradox, a therapy-induced switch to a neutrophil-predominant situation in the differential cell counts of cerebrospinal fluid specimens, had a correlation with an immunologic paradox, an increased Mycobacterium tuberculosis-specific gamma interferon-producing T-cell response.
CASE REPORT
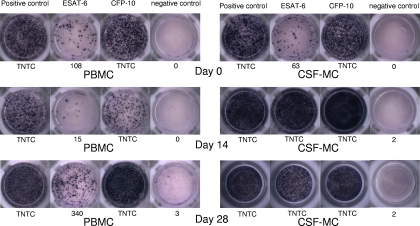
A 34-year-old female patient presented with a 1-week history of general malaise, headache, and fever of 38.5°C. Examination of the cerebrospinal fluid (CSF) on the first day revealed a lymphocytic pleocytosis (white blood cell count [WBC], 130/mm3; 75% lymphocytes and 7% polymorphonuclear cells), increased protein (134 mg/dl), decreased glucose (32 mg/dl; ratio of glucose concentration in CSF to that in serum, 0.3), and high adenosine deaminase levels (12 IU/liter). Microscopic examination of the CSF for acid-fast bacilli was negative. Serological testing for human immunodeficiency virus was negative. A brain magnetic resonance image showed suspicious tuberculous granulomas. A chest X ray was normal. A tuberculin skin test with 2 tuberculin units became negative (induration, 0 mm). From the time of admission, she was treated with isoniazid, rifampin, ethambutol, and pyrazinamide. Dexamethasone was given on the first day and tapered off over 4 weeks. After initiation of antituberculous therapy, her symptoms began gradually to improve. Follow-up examinations of the CSF on day 14 and day 28 revealed pleocytosis (WBC, 120/mm3; 49% lymphocytes and 42% polymorphonuclear cells; WBC, 42/mm3; 82% lymphocytes and 4% polymorphonuclear cells), normal protein levels (52 mg/dl and 43 mg/dl, respectively), and decreased glucose levels (30 mg/dl and 41 mg/dl, respectively). The CSF sample taken on day 0 grew Mycobacterium tuberculosis 4 weeks later, and an antituberculous susceptibility test revealed that the M. tuberculosis was susceptible to all drugs tested. On day 0, day 14, and day 28, we performed enzyme-linked immunospot (ELISPOT) assays to detect gamma interferon (IFN-γ)-secreting T cells in peripheral blood mononuclear cells (PBMC) and CSF mononuclear cells (CSF-MC), stimulated by two antigens, early secretory antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10). The ELISPOT assays (T-SPOT.TB; Oxford Immunotec, Abingdon, United Kingdom) were performed as described in a previous study (6). Briefly, PBMC were immediately (within 30 min) separated from 8-ml samples of peripheral venous blood. Concurrent with venous sampling, 5- to 10-ml samples of CSF were obtained, and CSF-MC were separated from the CSF within 30 min of sampling. The cells were suspended in AIM-V medium (GIBCO, Rockville, MD) at a concentration of 2.5 × 106 PBMC or CSF-MC/ml. The prepared PBMC and CSF-MC were plated (2.5 × 105 cells/well) on plates precoated with anti-human IFN-γ antibody and cultured for 18 h. Spots were then counted using an automated microscope (ELiSpot 04 HR; Autoimmune Diagnostika GmbH, Strasburg, Germany). The detailed results of the ELISPOT assays are shown in Fig. 1. The frequencies of IFN-γ-secreting T-cells in CSF-MC increased 2 weeks after antituberculous therapy despite clinical improvement and then slightly decreased 4 weeks after antituberculous therapy. However, the frequencies of IFN-γ-secreting T cells in PBMC slightly decreased 2 weeks after antituberculous therapy and then increased 4 weeks after antituberculous therapy.
FIG. 1.
Evolution of M. tuberculosis-specific T-cell responses in a patient with TBM. The ELISPOT assays were performed using 2.5 × 105 PBMC or 2.5 × 105 CSF-MC on day 0, day 14, and day 28 after antituberculous therapy. The diagnosis was confirmed by isolating M. tuberculosis from a culture of CSF. Data are presented as numbers of spot-forming cells/2.5 × 105 PBMC or CSF-MC. TNTC, too numerous to count accurately.
The diagnosis of tuberculous meningitis (TBM) is challenging. Therefore, if TBM is seriously suspected, many physicians usually begin empirical antituberculous therapy and reconsider the diagnosis a few weeks after treatment commences (3). In this problematic clinical situation, a phenomenon known as the “therapeutic paradox,” revealing a therapy-induced switch to a neutrophil-predominant situation in the differential cell count of CSF, has been regarded by some authors as pathognomonic of TBM (5, 12). It has been postulated that this phenomenon arises because of a hypersensitivity reaction related to the release of tubercular proteins during antituberculous therapy (2, 4). However, to our knowledge, there has been no report showing that this hypersensitive reaction has a correlation with evidence of in vitro cell-mediated immunity, such as a Mycobacterium tuberculosis-specific IFN-γ-producing T-cell response. In this report, we characterize an “immunologic paradox” in a patient with microbiologically documented TBM.
We used the term “immunologic paradox” as a phenomenon revealing a therapy-induced increase in the M. tuberculosis-specific T-cell response in the CSF or peripheral blood. Arias-Bouda et al. reported that an initial increase in antibody levels was observed in the early phase of treatment for 36% of all tuberculosis patients (1). Nicol et al. also showed an initial increased ELISPOT response to ESAT-6 during the first month of treatment, followed by a progressive decreased ELISPOT response to both ESAT-6 and CFP-10 (10). We assume that this is another representation of the immunologic paradox. These phenomena could be explained by an intense stimulation of the humoral and cell-mediated immune responses by antigens released from killed bacteria (1, 2).
Several reports on the therapeutic paradox in patients with TBM have been published. Sütlaçs et al. showed that the therapeutic paradox developed in one-third of patients with TBM, and clinical deterioration was found in half of such patients (12). Garcia-Monco et al. also reported a patient who developed the therapeutic paradox without clinical deterioration (5). However, they did not characterize any association between shifted responses of polymorphonuclear dominance and increased cell-mediated immune responses to M. tuberculosis antigens. In this case report, we clearly show that the therapeutic paradox was associated with the immunologic paradox of increased cell-mediated immune responses to M. tuberculosis-specific antigens. Interestingly, the immunologic paradox shown by CSF-MC preceded that exhibited by PBMC in our patient. This is plausible in view of our previous finding that M. tuberculosis-specific T cells are more compartmentalized to the CSF or peritoneal fluid than to the circulating blood in patients with TBM or tuberculous peritonitis (6, 7). However, further studies are needed to determine the proportion of patients with TBM who show immunologic paradoxes in CSF-MC or PBMC. It also remains to be determined whether these immunologic responses a few weeks after commencement of treatment could assist in differentiating TBM from other viral or bacterial meningitides.
Interleukin-8 (IL-8), a neutrophil-attracting chemokine, is known to be made by a variety of leukocyte populations following stimulation by M. tuberculosis (9). It is interesting that biomarkers such as IL-8 can predict patients with TB meningitis who will subsequently develop the therapeutic paradox after antituberculous therapy. Indeed, one study reported that IL-8 is elevated in tuberculous pleural effusions (11). NK cells provide a first-line defense against many infections by lysing infected cells as well as by secreting antiviral cytokines such as IFN-γ (8). So, IFN-γ-producing spots in the ELISPOT assay do not measure CD4+ or CD8+ T cells directly since other IFN-γ cells, such as NK or noncytotoxic cells, also contribute to the IFN-γ-producing spots. So, further studies are needed on these issues.
In conclusion, our study suggests that the appearance of the immunologic paradox in repeated spinal puncture or serial blood samples from a patient suspected of having TBM may give a promising clue to the presence of the most diagnostically difficult form of tuberculosis.
Acknowledgments
Neither author received financial support. There are no potential conflicts of interest.
Footnotes
Published ahead of print on 21 October 2009.
REFERENCES
- 1.Arias-Bouda, L. M., S. Kuijper, A. Van der Werf, L. N. Nguyen, H. M. Jansen, and A. H. Kolk. 2003. Changes in avidity and level of immunoglobulin G antibodies to Mycobacterium tuberculosis in sera of patients undergoing treatment for pulmonary tuberculosis. Clin. Diagn. Lab. Immunol. 10:702-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Be, N. A., K. S. Kim, W. R. Bishai, and S. K. Jain. 2009. Pathogenesis of central nerve system tuberculosis. Curr. Mol. Med. 9:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donald, P. R., and J. F. Schoeman. 2004. Tuberculous meningitis. N. Engl. J. Med. 351:1719-1720. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Monco, J. C. 1999. Central nervous system tuberculosis. Neurol. Clin. 17:737-759. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Monco, J. C., E. Ferreira, and M. Gomez-Beldarrain. 2005. The therapeutic paradox in the diagnosis of tuberculous meningitis. Neurology 65:1991-1992. [DOI] [PubMed] [Google Scholar]
- 6.Kim, S. H., K. Chu, S. J. Choi, K. H. Song, H. B. Kim, N. J. Kim, S. H. Park, B. W. Yoon, M. D. Oh, and K. W. Choe. 2008. Diagnosis of central nervous system tuberculosis by T-cell-based assays on peripheral blood and cerebrospinal fluid mononuclear cells. Clin. Vaccine Immunol. 15:1356-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim, S. H., O. H. Cho, S. J. Park, B. D. Ye, H. Sung, M. N. Kim, S. O. Lee, S. H. Choi, J. H. Woo, and Y. S. Kim. Diagnosis of abdominal tuberculosis by T-cell-based assays on peripheral blood and peritoneal fluid mononuclear cells. J. Infect., in press. doi: 10.1016/j.jinf.2009.09.006. [DOI] [PubMed]
- 8.Kirwan, S., D. Merriam, N. Barsby, A. McKinnon, and D. N. Burshtyn. 2006. Vaccinia virus modulation of natural killer cell function by direct infection. Virology 30:75-87. [DOI] [PubMed] [Google Scholar]
- 9.Lyon, M. J., T. Yoshimura, and D. N. McMurray. 2004. Interleukin (IL)-8 (CXCL8) induces cytokine expression and superoxide formation by guinea pig neutrophils infected with Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 84:283-292. [DOI] [PubMed] [Google Scholar]
- 10.Nicol, M. P., D. Pienaar, K. Wood, B. Eley, R. J. Wilkinson, H. Henderson, L. Smith, S. Samodien, and D. Beatty. 2005. Enzyme-linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin. Infect. Dis. 40:1301-1308. [DOI] [PubMed] [Google Scholar]
- 11.Supriya, P., P. Chandrasekaran, and S. D. Das. 2008. Diagnostic utility of interferon-γ-induced protein of 10 kDa (IP-10) in tuberculous pleurisy. Diagn. Microbiol. Infect. Dis. 62:186-192. [DOI] [PubMed] [Google Scholar]
- 12.Sütlaçs, P. N., A. Unal, H. Forta, S. Senol, and D. Kirbaçs. 2003. Tuberculous meningitis in adults: review of 61 cases. Infection 31:387-391. [DOI] [PubMed] [Google Scholar]