Abstract
Mycoplasma genitalium is a leading pathogen of nongonoccocal chlamydia-negative urethritis, which has been implicated directly in numerous other genitourinary and extragenitourinary tract pathologies. The pathogenesis of infection is attributed in part to excessive immune responses. M. genitalium-derived lipid-associated membrane proteins (LAMPs) are a mixture of bacterial lipoproteins, exposed at the surface of mycoplasma, that are potent inducers of the host innate immune system. However, the interaction of M. genitalium-derived LAMPs as pathogenic agents with Toll-like receptors (TLRs) and the signaling pathways responsible for active inflammation and NF-κB activation have not been fully elucidated. In this study, LAMPs induced the production of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) in a dose-dependent manner. Blocking assays showed that TLR2- and CD14-neutralizing antibodies reduced the expression of TNF-α and IL-6 in THP-1 cells. Furthermore, LAMP-induced NF-κB activation was increased in 293T cells transfected with TLR2 plasmid. The activity of NF-κB was synergically augmented by cotransfected TLR1, TLR6, and CD14. Additionally, LAMPs were shown to inhibit NF-κB expression by cotransfection with dominant-negative MyD88 and TLR2 plasmids. These results suggest that M. genitalium-derived LAMPs activate NF-κB via TLR1, TLR2, TLR6, and CD14 in a MyD88-dependent pathway.
Mycoplasmas are the smallest of the known self-replicating parasitic microorganisms, and they lack a cell wall in cell-free medium. They are pervasive in nature, humans, and animals, but most mycoplasmas are nonpathogenic to the host (36). Lipid-associated membrane proteins (LAMPs) are a mixture of bacterial lipoproteins that are expressed on the surface, and they are the main structures of interaction with the host cells (37). They have been demonstrated to be biologically active and are the most potent initiator of inflammatory reactions in mycoplasma infection (9, 11, 34). Since Mycoplasma genitalium was first isolated from humans in 1981 (51), it has been considered an important pathogen that could emerge in sexually transmitted diseases, including acute endometritis, salpingitis, mucopurulent cervicitis, tubal factor infertility, and pelvic inflammatory disease, as well as a range of other pathologies, such as arthritis, AIDS progression, chronic fatigue, and autoimmune disorders (4, 10, 19). Recent evidence has demonstrated that M. pneumoniae-derived LAMPs can induce NF-κB activation in human acute monocytic leukemia cell lines (THP-1 cells) (42, 43).
Toll-like receptors (TLRs) play an essential role in initiating the inflammatory reactions, such as NF-κB activation (2, 22, 26). To date, 13 types of human TLRs have been identified and shown to be critical for signaling transduction in response to a number of pathogen-associated molecular patterns (PAMPs) (8, 44). PAMPs are recognized by different TLRs to form heteromeric complexes, among which the family of TLRs prominently features, for the cell to distinguish successfully among different pathogens. Among TLRs, TLR2, in tandem with TLR1 or TLR6, has been identified as a receptor that is important to the innate immune response against several gram-positive bacteria and for cellular signaling by components of gram-positive bacteria, such as peptidoglycan, lipoteichoic acid, and lipoproteins. The receptors transmit signaling pathways via interleukin-1R (IL-1R)-associated signal molecules, including myeloid differentiation factor 88 (MyD88), IL-1R-activated kinase, tumor necrosis factor (TNF) receptor-associated factor 6, and mitogen-activated protein kinases, leading to the activation of NF-κB and activating protein 1 (AP-1), which in turn triggers the expression of many proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8 (1, 20, 29, 47). MyD88 serves as the essential adaptor for other IL-1/TLR family members, including IL-1R and IL-18R. The activation of the intracellular pathway through MyD88 results in the activation of NF-κB. CD14 is a glycosylphosphatidylinositol surface-anchored molecule expressed in many kinds of cells as another accessory receptor of TLR2. It lacks an intracellular segment and functions as a coreceptor for numerous bacterial products, including peptidoglycan, lipopolysaccharide (LPS), and bacterial lipoproteins, thereby facilitating signaling through other receptors (23). Several studies have suggested that TLR2 functions as a signaling receptor for LPS in the presence of CD14 (50). Recently published data support the possibility that the multifunctional B class scavenger receptor CD36 also is involved in TLR2 transmembrane signal transducing (16). However, data available also have underscored the current lack of a complete understanding of the molecular mechanisms that link M. genitalium infection to the activation of the innate immune system, which is essential to the induction of the inflammation.
The LAMPs from Mycoplasma fermentans and Mycoplasma penetrans activate human immunodeficiency virus long terminal repeats through TLRs (41). To further understand how LAMPs activate the immune system of the cell, including THP-1 cells and human embryonic kidney cells (HEK293T cells), the present study was designed to investigate the interaction of M. genitalium-derived LAMPs with TLRs and CD14 and to clarify the role of MyD88 in activating the NF-κB signaling pathway.
MATERIALS AND METHODS
Cells.
The human monocytic cell line THP-1 (China Center for Type Culture Collection, Wuhan University) was cultured in RPMI 1640 (HyClone) containing 10% heat-inactivated fetal bovine serum (FBS) (HyClone), 2 mM l-glutamine, 100 U ml−1 penicillin G, and 100 μg ml−1 streptomycin at 37°C in a 5% CO2 humid atmosphere. The human kidney cell line HEK293T (China Center for Type Culture Collection, Wuhan University) was cultured in Dulbecco's modified Eagle's medium (DMEM) (HyClone) containing 10% heat-inactivated FBS (HyClone), 2 mM l-glutamine, 100 U ml−1 penicillin G, and 100 μg ml−1 streptomycin at 37°C in a 5% CO2 humid atmosphere. To exclude the possibility of mycoplasma infections in the cell cultures, the cell suspensions were inoculated onto pleuropneumonialike organism agar medium once a month. To check the contamination of mycoplasma, cells also were stained with 4,6-diamidino-2-phenylindole (DAPI) once a week.
Antibodies and reagents.
Neutralizing anti-human CD14 monoclonal antibody (MAb) (ab6083), neutralizing anti-human TLR2 MAb (ab45054), and isotype control MAb (immunoglobulin G [IgG]) as a blocking Ab were purchased from Abcam (51AB; China). Goat anti-mouse IgG-fluorescein isothiocyanate (FITC) was purchased from Sigma-Aldrich. Penicillin, ampicillin, polymyxin B, and LPS were purchased from Sigma-Aldrich. All other chemicals were obtained from commercial sources and were of analytical or reagent grade.
Mycoplasma culture and LAMP preparation.
M. genitalium (ATCC strain G37) was cultivated in modified SP-4 medium containing 20% newborn bovine serum, 10% yeast extract, and 1,000 U ml−1 penicillin. The culture was kept at 37°C and 5% CO2 until significant acid color change was noted in the culture medium and then was quantified as color changing units (CCU) per milliliter as described previously (14).
The preparation of LAMPs was performed as described previously (27). Briefly, M. genitalium was cultivated in modified SP-4 medium until the beginning of stationary phase, and then the sample was pelleted by centrifugation for 10 min at 12,000 × g. The pellets were washed with endotoxin-free phosphate-buffered saline (PBS) and resuspended in 5 ml of Tris-buffered saline (TBS; 50 mM Tris-Cl, pH 8.0, 0.15 M NaCl) containing 1 mM EDTA (TBSE), solubilized by adding Triton X-114 to a final concentration of 2%, and incubated at 4°C for 1 h. The lysate was incubated at 37°C for 10 min for phase separation. After centrifugation at 10,000 × g for 20 min, the upper aqueous phase was discarded and replaced by the same volume of TBSE. The solution then was vortexed and incubated at 4°C for 10 min, and the procedure of phase separation was repeated twice. The final Triton X-114 phase was resuspended in TBSE to the original volume, 2.5 volumes of ethanol were added to precipitate membrane components, and the solution was incubated at 20°C overnight. After centrifugation, the pellet was suspended in endotoxin-free PBS, followed by sonication for 30 s. The protein concentration of the suspension was measured with the Coomassie protein assay reagent (Pierce). For heat inactivation, M. genitalium (106 CCU ml−1) was isolated by centrifugation at 15,000 × g for 30 min and then washed and resuspended in Hayflick medium, followed by being heated at 60°C for 30 min. No growth was observed by the inoculation of heat inactivation during a 2-week period in SP-4 medium. The endotoxin concentration of LAMPs and heat-inactivated mycoplasmas was <0.04 endotoxin units ml−1, as determined by the Limulus amebocyte lysate assay (Associates of Cape Cod, Inc., MA). Both were stored at −70°C until needed.
Expression vectors.
pFLAG-TLR1, pFLAG-TLR2, and pFLAG-TLR6 expression vectors were kindly provided by M. Matsumoto (Hokkaido University, Japan). pcDNA3-CD14, pcDNA3-TLR1-YFP, pcDNA3-TLR2-CFP, and pcDNA3-TLR6-YFP were kindly provided by D. Golenbock (University of Massachusetts). pcDNA3-DN-TLR2 was kindly provided by S. Yokota (Sapporo Medical University, Japan), and dominant-negative MyD88 (DN-MyD88) was kindly provided by O. Equils (University of Califormia, Los Angeles). The NF-κB cis-reporting system containing pNF-κB-luc, a plasmid in which the luciferase reporter gene was fused to the NF-κB enhancer, was purchased from Stratagene. pRL-TK internal control plasmid was purchased from Promega. The plasmids used in transient transfections were prepared with an endotoxin-free plasmid mini-kit (Omega).
Blocking and cytokine assays.
THP-1 cells were cultivated in 24-well tissue culture plates and pretreated with 10 μg/ml mouse IgG1, anti-TLR2 MAb, or anti-CD14 MAb for 30 min at 37°C and then stimulated with PBS, LAMPs, or LPS (100 ng ml−1). After 8 h of stimulation, cells were lysed by two consecutive cycles of freezing/thawing; thus, the samples represented the total amount of cytokines produced (both intracellular and that released into the supernatant). The cytokine concentrations were measured by using human TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA) kits (Jingmei Biotech, China). The assays were performed according to the manufacturer's instructions, with all standards and samples run in duplicate.
Transfection and reporter gene assay.
HEK293T cells were transiently transfected using FuGENE HD (Roche) according to the manufacturer's instructions. HEK293T cells were plated at 2 × 105 cells per well in a 24-well plate. After incubation until they reached about 80% confluence, HEK293T cells were cotransiently transfected with different amounts of the indicated plasmids, together with pNF-kB-luc (0.05 μg ml−1) and pRL-TK (0.005 μg ml−1) for normalization. The total amount of transfected DNA was kept constant by adding empty vector. After 24 h, transfected cells were stimulated with LAMPs or inactive M. genitalium or PBS. After a further 8 h of incubation, cells were lysed and assayed for luciferase activity using a Dual-Luciferase Reporter assay system (Promega) according to the manufacturer's instructions. Both firefly and Renilla luciferase activities were monitored with a Lumat LB9507 luminometer (Berthold). Normalized reporter activity is expressed as the firefly luciferase value divided by the Renilla luciferase value for representative experiments from the means of three independent experiments.
Confocal microscopy.
The HEK293T cells were transiently transfected with the fluorescent protein TLR constructs using FuGENE HD. Confocal microscopy was performed with living cells that were seeded on 35-mm glass-bottomed tissue culture dishes (MatTek Corp). After stimulation, images were captured with a confocal microscope (TCS SP2 AOBS; Leica) equipped with an acousto-optical beamsplitter using version 2 of the Leica confocal software. The cells were kept at 37°C during imaging using a warm-stage apparatus. Cyan fluorescent protein (CFP)-tagged proteins were visualized using the 458-nm argon laser line, and the colors were transformed into red; for yellow fluorescent protein (YFP), the 514-nm line of a 100-mW argon laser was used and the colors were transformed into green. Cells expressing CFP and YFP were sequentially scanned with only one laser line active per scan.
Flow cytometry.
TLR2 expression on the surface of HEK293T cells was determined. HEK293T cells were pelleted by centrifugation at 4°C for 5 min at 1,000 × g and were washed twice in fluorescence-activated cell sorter buffer (Dulbecco's PBS containing 1% bovine serum albumin and 0.1% sodium azide). After 4% paraformaldehyde was added for 30 min at room temperature, cells were washed and treated with mouse anti-human TLR2 (ab45054; Abcam) MAb for 40 min at 4°C. Cells then were washed twice and incubated with FITC-labeled secondary Ab (0296G; Bios) for 40 min at 4°C. After the cells were washed and finally resuspended in 1% paraformaldehyde, they were stored protected from light at 4°C. Cells were analyzed on a FACSCalibur (BD Biosciences). For each assay condition, at least 100,000 cells were analyzed.
Statistical analysis.
All of the results are expressed as means value with standard errors of the means. The data were examined by a one-way analysis of variance test using SPSS 13.0 statistical software. Differences were considered significant at P < 0.05.
RESULTS
Anti-TLR2 and -CD14 MAbs inhibited LAMP-mediated production of cytokines.
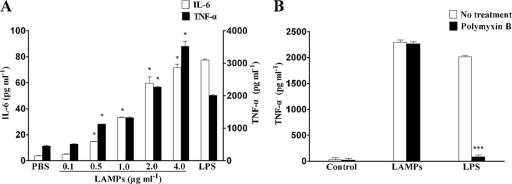
The pathology of M. genitalium infection seems to be related to chronic inflammation, and the activation of NF-κB is essential to cytokine production. As THP-1 cells resemble tissue monocytes and are commonly used as models for human monocytes/macrophages (45), we initially tested the activity of M. genitalium-derived LAMPs to stimulate the production of various proinflammatory cytokines in THP-1 cells. M. genitalium-derived LAMPs showed the release of TNF-α and IL-6 through activating THP-1 cells in a dose-dependent manner (Fig. 1A). LPS, used as positive controls, highly induced the release of proinflammatory cytokines. The level of cytokine production reached peak values at 4.0 μg ml−1 LAMPs, which was much higher than that of the unstimulated control. Based on these results and for practical reasons, the effect of LAMPs was further examined at 2.0 μg ml−1. Despite the fact that no endotoxin was detected by the Limulus amebocyte lysate assay in the purified preparation of LAMPs, the possibility that its proinflammatory activity was attributed to LPS contamination was investigated further by testing LAMP sensitivity to polymyxin B. As shown in Fig. 1B, LAMP pretreated with 100 μg ml−1 polymyxin B had no effect on the production of cytokines while also blocking most of the LPS-mediated stimulation activity.
FIG. 1.
Production of TNF-α and IL-6 by LAMP-induced THP-1 cells. (A) THP-1 cells were cultured in serum medium for 24 h in 24-well tissue culture plates, and the indicated concentrations of LAMPs were added to the medium (PBS or 100 ng ml−1 of LPS was used as the negative or positive control, respectively). After being treated for 8 h, THP-1 cells were lysed, and the culture supernatants were assayed for the proinflammatory cytokines by ELISA (IL-6, left y axis; TNF-α, right y axis). Values represent the means ± standard deviations from three independent experiments assayed in duplicate. P < 0.05 (*) was considered significant. (B) LAMPs (2.0 μg ml−1) or 100 ng ml−1 LPS was added to the medium. After 8 h of culture, supernatant was collected and the concentration of TNF-α in the medium was determined by ELISA as described in Materials and Methods. Values represent the means ± standard deviations from three independent experiments assayed in duplicate.
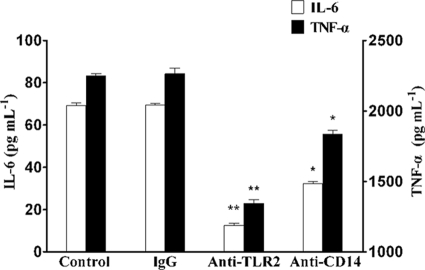
M. genitalium-derived LAMPs are able to induce proinflammatory cytokines in human monocytes/macrophages, and TLR2 and CD14 usually are involved in bacterial lipoprotein recognition. THP-1 cells were pretreated with anti-TLR2 or anti-CD14 MAb (10 μg ml−1) for 30 min and then stimulated with 2.0 μg ml−1 LAMPs for 8 h. Figure 2 shows that the expression of LAMP-induced proinflammatory cytokines was significantly decreased by pretreatment with anti-TLR2 Ab but only partly inhibited by anti-CD14 Ab. These results indicated that the production of cytokine by LAMPs was mediated by TLR2 and CD14.
FIG. 2.
Inhibitory effect of anti-TLR2 and anti-CD14 MAb on cytokine production. THP-1 cells were pretreated with 10 μg ml-1 mouse IgG1, anti-TLR2 MAb (IgG1), or anti-CD14 MAb (IgG1) for 30 min and then stimulated with 2.0 μg ml−1 LAMPs. After being treated for 8 h, THP-1 cells were lysed and the culture supernatant was assayed for the proinflammatory cytokines by ELISA (IL-6, left y axis; TNF-α, right y axis). Values represent the means ± standard deviations from three independent experiments assayed in duplicate.
Functional TLR2 is required for NF-κB activation by LAMPs.
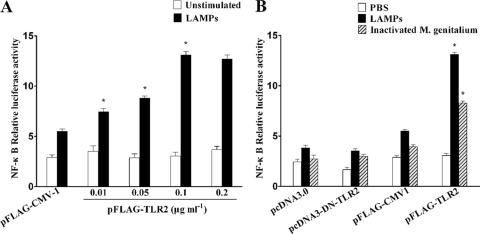
We then examined whether the activation of NF-κB by LAMPs was mediated by TLR2. The transfection of TLR2 plasmid restored the responsiveness of HEK293T cells to LAMPs, as measured by monitoring the expression of the NF-κB luciferase activity (NF-κB-luc). As shown in Fig. 3A, the cells activated by LAMPs were compared to unstimulated control cells, and the level of activity was increased for the activated cells, with an increase in the amount of a plasmid harboring the cDNA encoding TLR2. These results demonstrated that the expression of a functional TLR2 by the transfected cells is required for NF-κB activation that is responsive to LAMPs. This hypothesis was supported further by results showing that the transfection of HEK293T with a dominant-negative mutant of TLR2 (DN-TLR2) plasmid significantly attenuated NF-κB expression induced by LAMPs (Fig. 3B).
FIG. 3.
TLR2 is required for NF-κB activation by LAMPs. (A) HEKHKE293T cells were transiently cotransfected with the indicated concentrations of pFLAG-TLR2, 0.1 μg ml−1 pNF-κB-luc, and 0.01 μg ml−1 pRL-TK. After 24 h of incubation, the cells were stimulated for 8 h with 2.0 μg ml−1 LAMPs. All values represent the means and standard deviations from three assays. P < 0.05 (*) was considered significant. (B) HEK293T cells were transiently cotransfected with the indicated constructs, 0.01 μg ml−1 pNF-κB-luc, and 0.01 μg ml−1 pRL-TK. The total amount of cDNA transfected was kept constant with the level of control construct. After 24 h of incubation, the cells were stimulated for 8 h with 2.0 μg ml−1 LAMPs or inactivated M. genitalium. The cells then were lysed and assayed for luciferase reporter activity. Values represent the means ± standard deviations from three independent experiments assayed in duplicate.
When inactivated M. genitalium organisms were used as the stimulant, the activation response via TLR2 was similar to that observed in HEK293T cells (Fig. 3B).
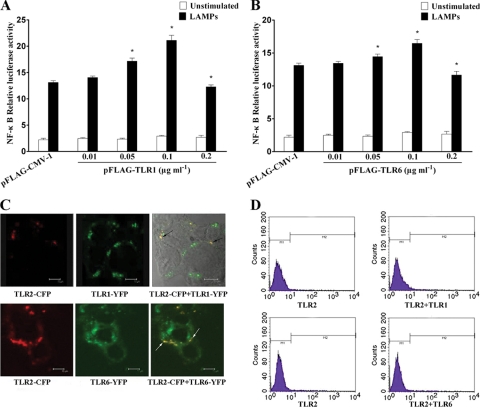
TLR1 and TLR6 enhanced TLR2-mediated NF-κB activation.
It has been reported that TLR1 and TLR2 were required for the recognition of triacylated lipopeptides such as Pam3CSK4, while TLR6 and TLR2 were required for the recognition of diacylated lipopeptides such as Pam2CSK4 (13, 52). To determine whether TLR1 or TLR6 could contribute to the TLR2-mediated recognition of LAMPs for NF-κB activation and to assess their respective contributions, we constructed two plasmids encoding TLR1 and TLR6 (pFLAG-TLR1 and pFLAG-TLR6), respectively. To better evaluate the role of TLR1 and TLR6, we adjusted our transfection conditions in which cells were fixed by dosage for TLR2 and cotransfected with various doses of TLR1 or TLR6. When the cotransfected cells were stimulated with LAMPs, the level of NF-κB activation was augmented (Fig. 4A and B). The level of NF-κB activation was almost at its highest when the concentration of TLR1 or TLR6 expression vector was at 0.1 μg ml−1. The effect of TLR1 or TLR6 on the expression of TLR2 was analyzed by flow cytometry (Fig. 4D). The level of TLR2 expression was almost constant irrespective of the expression of TLR1 or TLR6. In addition, to further determine whether TLR1 or TLR6 alone can mediate the activation of NF-κB by the LAMPs, HEK293T cells were transfected with TLR1 or TLR6 expression vector. The transfected cells were stimulated with LAMPs, and NF-κB was not activated in response to LAMPs by transfection with either TLR1 or TLR6 vector alone (Fig. 5B). These results indicate that TLR1 and TLR6 enhanced TLR2-mediated NF-κB activation by LAMPs.
FIG. 4.
TLR1 and TLR6 enhanced TLR2-mediated NF-κB activation. (A) HEK293T cells were transiently cotransfected with the indicated concentrations of pFLAG-TLR1, 0.1 μg ml−1 pFLAG-TLR2, 0.1 μg ml−1 pNF-κB-luc, and 0.01 μg ml−1 pRL-TK. After 24 h of incubation, the cells were stimulated for 8 h with 2.0 μg ml−1 LAMPs. The cells then were lysed and assayed for luciferase reporter activity. All values represent the means and standard deviations from three assays. P < 0.05 (*) was considered significant. (B) HEK293T cells were transiently cotransfected with the indicated concentrations of pFLAG-TLR6, 0.1 μg ml−1 pFLAG-TLR2, 0.1 μg ml−1 pNF-κB-luc, and 0.01 μg ml−1 pRL-TK. After 24 h of incubation, the cells were stimulated for 8 h with 2.0 μg ml−1 LAMPs. The cells then were lysed and assayed for luciferase reporter activity. All values represent the means and standard deviations from three assays. P < 0.05 (*) was considered significant. (C) HEK293T cells were transiently cotransfected with TLR2-CFP, and TLR1-YFP or TLR6-YFP was grown on glass-bottomed tissue culture dishes. After 24 h of incubation, the cells were stimulated for 8 h with 2.0 μg ml−1 LAMPs. The living cells then were analyzed by confocal microscopy as described in the text. To the left is the distribution of TLR2; in the center is the localization of TLR1 or TLR6; to the right is the colocalization of TLR2 with TLR1 or TLR6 (black arrows or white arrows). Representative confocal sections of cells are shown. (D) HEK293T cells were transiently cotransfected with the indicated constructs. After 24 h of incubation, the cells were stimulated for 8 h with 2.0 μg ml−1 LAMPs. The cells then were incubated with anti-TLR2 MAb and FITC-labeled secondary Ab. The cell surface expression of TLR2 was analyzed by flow cytometry. Representative confocal sections of cells are shown.
FIG. 5.
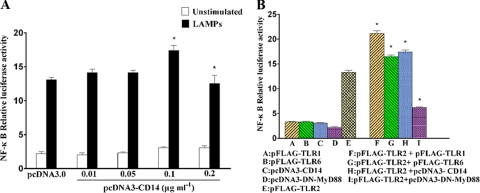
CD14 enhanced TLR2-mediated NF-κB activation. (A) HEK293T cells were transiently cotransfected with the indicated concentrations of pcDNA3-CD14, 0.1 μg ml−1 pFLAG-TLR2, 0.1 μg ml−1 pNF-κB-luc, and 0.01 μg ml−1 pRL-TK. After 24 h of incubation, the cells were stimulated for 8 h with 2.0 μg ml−1 LAMPs. The cells then were lysed and assayed for luciferase reporter activity. All values represent the means and standard deviations from three assays. P < 0.05 (*) was considered significant. (B) HEK293T cells were transiently cotransfected with the indicated plasmids (the concentration of each plasmid was 0.1 μg ml−1). After 24 h of incubation, the cells were stimulated for 8 h with 2.0 μg ml−1 LAMPs. The cells then were lysed and assayed for luciferase reporter activity. All values represent the means and standard deviations from three assays.
At the same time, we investigated the possible interaction between TLR1 and TLR2 or TLR6 and TLR2 by confocal microscopy. HEK293T cells were transiently cotransfected with vectors harboring cDNA encoding TLR proteins tagged with fluorescent probes (i.e., TLR1-YFP, TLR6-YFP, or TLR2-CFP). This method of epitope tagging appears to have no effect on TLR function (24, 38). Confocal microscopic images showed that TLR1, TLR2, and TLR6 were distributed discontinuously and were colocalized at the plasma membrane of HEK293T cells (Fig. 4C). There was no difference in the localization of TLR2, TLR1, or TLR6 between LAMP-stimulated and unstimulated cells (data not shown).
CD14 enhanced TLR2-mediated NF-κB activation.
CD14 has been shown to enhance signaling through TLR2 complexes in response to several ligands (23). The pretreatment of THP-1 cells with anti-CD14 MAb led to the inhibition of LAMP-mediated cytokine production. Obviously, CD14 may be involved in the LAMP-induced inflammatory response. We transiently cotransfected HEK293T cells with TLR2 and CD14, and LAMP-induced NF-κB activation was enhanced (Fig. 5A). In addition, the CD14-induced enhancement did not occur without the cotransfection of TLR2 (Fig. 5B). These results suggest that CD14 augments TLR2-mediated NF-κB activation.
Activation of NF-κB by LAMPs was TLR2 and MyD88 dependent.
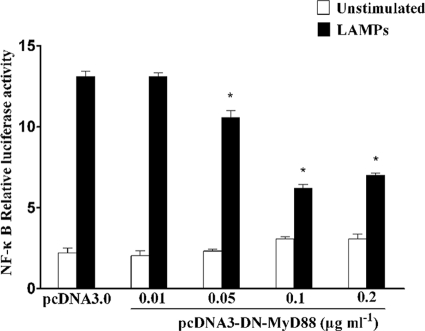
Taking it into consideration that MyD88 is the common adaptor protein shared by all TLR pathways, we sought to determine the role of MyD88 for NF-κB activation. To determine whether the induction of NF-κB occurred through MyD88, we transiently cotransfected HEK293T cells with DN-MyD88 constructs in a dose-dependent manner and with TLR2, and then we incubated them with 2.0 μg ml−1 LAMPs for 8 h and tested their response to LAMPs. As shown in Fig. 6, the overexpression of an intracellular deletion mutant of MyD88 with a COOH-terminal truncation led to the marked inhibition of TLR2-mediated NF-κB activation in HEK293T cells by LAMPs. When the concentration of DN-MyD88 was at 0.1 μg ml−1, the level of inhibition was decreased by 50%. Taken together, these results support the hypothesis that LAMP-induced NF-κB expression was mediated through the MyD88-dependent TLR2 signaling pathway in HEK293T cells.
FIG. 6.
Activation of NF-κB by LAMPs was TLR2 and MyD88 dependent. HEK93T cells were transiently cotransfected with the indicated concentrations of pcDNA3-DN-MyD88, 0.1 μg ml−1 pFLAG-TLR2, 0.1 μg ml−1 pNF-κB-luc, and 0.01 μg ml−1 pRL-TK. After 24 h of incubation, the cells were stimulated for 8 h with 2.0 μg ml−1 LAMPs. The cells then were lysed and assayed for luciferase reporter activity. All values represent the means and standard deviations from three assays. P < 0.05 (*) was considered significant.
DISCUSSION
Mycoplasmas have no cell walls; however, LAMPs, which are abundant on mycoplasma surfaces, may be the major proteins and may be responsible for the interaction with various components of the surrounding environment (36, 37). LAMPs can activate a variety of cells to produce a series of proinflammatory cytokines and induce apoptosis and necrosis because of the biological activity in mycoplasma (3, 5, 31). We chose THP-1 cells as the research model, because they demonstrate the high-level expression of TLRs and have typical signal transduction systems for characterizing the signaling pathways in response to extracellular stimuli (45). In this study, we found that M. genitalium-derived LAMPs could, in a dose-dependent manner, stimulate THP-1 cells to secrete profound amounts of TNF-α and IL-6. When the concentration was higher than 2 μg ml−1, the rate of increased cytokines was decreased (Fig. 1A); high concentrations of LAMPs may interfere with the biological function of the cells or may be toxic to them.
TLRs are transmembrane receptors in the IL-1 receptor superfamily and are regarded as the major molecules by which the hosts recognize invading microorganisms. Recent experiments have shown that the leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of TLR2 are involved in the recognition of LAMPs (12). In this experiment, we found that anti-TLR2 Ab significantly inhibited the emergence of cytokines by LAMPs in THP-1 cells. The results showed that TLR2 might participate in the induction of human inflammatory cytokines. Thereafter, we chose HEK293T cells as a research object, largely because of its lack of TLRs and CD14 and because it has a complete NF-κB signaling pathway that is conducive to the study of the TLR-induced signal transduction pathway by a variety of extracellular stimuli. The results indicated that LAMPs could induce the activation of NF-κB in a TLR2-dependent manner in HEK293T cells. Thus, cells that increase the expression of TLR2 may exhibit an enhanced response to LAMPs, although this study does not exclude a TLR2-independent process, because LAMPs also activated NF-κB in HEK293T cells but did not transfect TLRs. This finding was consistent with our previous findings, based on Ab inhibition data, which suggested that TLR2 is responsible for the inflammatory response induced by LAMPs. Upon the activation of TLR, MyD88 was recruited to TLR domains and links TLRs with the downstream intracellular signaling cascades (21, 46). This response was confirmed by our observation that the activation of NF-κB was significantly inhibited by transfection with DN-MyD88 plasmid, which lacks the COOH terminal.
Individual TLRs have a weak combination capacity for the recognition of PAMPs, and many receptors are involved in TLRs to enhance PAMP recognition in many cases (28, 33, 50). TLR1 and TLR6 exhibit 69.3% identity in overall amino acid sequence, but the TIR domains of both receptors are highly conserved, with more than 90% identity (49). The role of TLR1 and TLR6 usually was analyzed by introducing a dominant-negative form into the cell line. Interestingly, DN-TLR1 and DN-TLR6 could block the response to different extents in various cells expressing TLR2 (17). This suggests that the relative abundance of these TLRs within a cell plays a critical role in the response. It already has been reported that the relevance of TLR6 in the murine macrophage cell lines assists in the completion of functional TLR2 and enhancing TLR2 (15). In addition, experimental data also showed that fatty acids activated TLR2 and TLR6 or TLR1 heterodimers, but polyunsaturated fatty acids have an inhibitory effect (25, 35). Recent studies have demonstrated that TLR2 is associated with CD14 as a coreceptor to raise the sensitivity of pathogens to the outside world and facilitates ligand binding (28, 33). Our study has confirmed that TLR1, TLR6, and CD14 markedly enhanced TLR2-mediated NF-κB activation by LAMPs, and the level of activation was dose dependent according to their concentrations. The expression and colocation of TLR1 and TLR2 as well as TLR2 and TLR6 were observed by laser scanning, and flow analysis also confirmed that the expression of TLR1 or TLR6 had no effect on the expression of TLR2. The ability of LAMPs to activate HEK293T cells was CD14 dependent, which was consistent with CD14 Ab inhibition data demonstrated in our function-blocking assay. Interestingly, the excessive expression of cotransfection plasmids somewhat inhibited the induction of NF-κB by LAMPs. There are two possible reasons for this: first, receptor expression may be too saturated, and second, the high concentrations of TLR1, TLR6, and CD14 cause the competitive inhibition of TLR2 for LAMP recognition, which reduces the ability of TLR2 to be recognized. Furthermore, CD36 has been confirmed to be involved in TLR2-mediated responses in a manner analogous to that of CD14 (32), which will be clarified in subsequent studies.
During mycoplasma infection, LAMPs, as the mixtures of outer membrane proteins that interact with the host, are much more apparent than single lipoproteins (30, 40). Therefore, we used LAMPs as a whole to study the interaction between TLRs and lipoproteins. However, the recognition of TLRs is concerned with the types of lipoprotein or protein. It is widely known that triacylated lipoproteins are recognized by TLR1 and TLR2, whereas diacylated lipoproteins are recognized by TLR2 and TLR6. The N-acyltransferase gene has not been detected, and mycoplasma-derived LAMPs are not N-acylated by chemical identification, which supports the absence of triacylated lipoproteins (39, 40). However, the resistance to the Edman degradation of proteins from M. mycoides and the ratio of N-amide and O-ester bonds in M. gallisepticum and M. mycoides indicate the presence of triacylated lipoproteins (7, 18). Based on our results, LAMPs as a mixture of membrane proteins can activate NF-κB via TLR1, TLR2, and TLR6, but they are less likely to be activated in the presence of diacylated and triacylated lipoproteins in M. genitalium LAMPs. Recently, the peptide sequence as well as the whole molecular structure of lipoproteins were reported to be more responsible for TLR recognition than the number of acyl chains in LAMPs (6). Moreover, the differences in the amino acid sequences, as well as the levels of expression of TLRs in organs, might affect the NF-κB-inducing activity (48).
In summary, our results showed that M. genitalium-derived LAMPs in HEK293T cells could activate NF-κB through TLR1, TLR2, and TLR6 and CD14 in a MyD88-dependent pathway. If the interaction between TLRs and M. genitalium derivatives is taken into consideration, in a future study TLRs and LAMPs are expected to become therapeutic targets.
Acknowledgments
We thank Doug Golenbock, from the University of Massachusetts, for providing pcDNA3-CD14, pcDNA3-TLR1-YFP, pcDNA3-TLR2-CFP, and pcDNA3-TLR6-YFP. We also thank Misako Matsumoto, from Hokkaido University, for providing pFLAG-TLR1, pFLAG-TLR2, and pFLAG-TLR6; Shin-ichi Yokota, from Sapporo Medical University, for providing pcDNA3-DN-TLR2; and Ozlem Equils, from University of California at Los Angeles, for providing pcDNA3-DN-MyD88. We also thank Yuan Chunyang, from South China University, for modifying the manuscript.
We thank the National Natural Science Foundation of China for its financial support of this study (no. 30770115).
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2004. Toll-like receptor signaling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 3.Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 300:1524-1525. [DOI] [PubMed] [Google Scholar]
- 4.Baseman, J. B., M. Cagle, J. E. Korte, C. Herrera, W. G. Rasmussen, J. G. Baseman, R. Shain, and J. M. Piper. 2004. Diagnostic assessment of Mycoplasma genitalium in culture-positive women. J. Clin. Microbiol. 42:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler, B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430:257-263. [DOI] [PubMed] [Google Scholar]
- 6.Buwitt-Beckmann, U., H. Heine, K. H. Wiesmuller, G. Jung, R. Brock, S. Akira, and A. J. Ulmer. 2005. Toll-like receptor 6-independent signaling by diacylated lipopeptides. J. Eur. Immunol. 35:282-289. [DOI] [PubMed] [Google Scholar]
- 7.Chambaud, I., H. Wroblewski, and A. Blanchard. 1999. Interactions between Mycoplasma lipoproteins and the host immune system. Trends Microbiol. 7:493-499. [DOI] [PubMed] [Google Scholar]
- 8.Chu, H. W., S. Jeyaseelan, J. G. Rino, D. R. Voelker, R. B. Wexler, K. Campbell, R. J. Harbeck, and R. J. Martin. 2005. TLR2 signaling is critical for Mycoplasma pneumoniae-induced airway mucin expression. J. Immunol. 174:5713-5719. [DOI] [PubMed] [Google Scholar]
- 9.Dhandayuthapani, S., W. G. Rasmussen, and J. B. Baseman. 2002. Stability of cytadherence-related proteins P140/P110 in Mycoplasma genitalium requires MG218 and unidentified factors. Arch. Med. Res. 33:1-5. [DOI] [PubMed] [Google Scholar]
- 10.Falk, L., H. Fredlund, and S. Jensen. 2004. Symptomatic urethritis is more prevalent in men infected with Mycoplasma genitalium than with Chlamydia trachomatis. Sex. Transm. Infect. 80:289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleury, B., D. Bergonier, X. Berthelot, E. Peterhans, J. Frey, and E. M. Vilei. 2002. Characterization of P40, a cytadhesin of Mycoplasma agalactiae. Infect. Immun. 70:5612-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita, M., T. Into, M. Yasud, T. Okusawa, S. Hamahira, Y. Kuroki, A. Eto, T. Nisizawa, M. Morita, and K. I. Shibata. 2003. Involvement of leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of human Toll-like receptor 2 in the recognition of diacylated lipoproteins and lipopeptides and staphylococcus aureus peptidoglycans. J. Immunol. 171:3675-3683. [DOI] [PubMed] [Google Scholar]
- 13.Gay, N. J., and M. Gangloff. 2007. Structure and function of toll receptors and their ligands. Annu. Rev. Biochem. 76:141-165. [DOI] [PubMed] [Google Scholar]
- 14.Georges, R., R. Valerie, L. Brigitte, and R. Sergio. 1998. Activation of mitogen-activated protein kinase pathways by Mycoplasma fermentans membrane lipoproteins in murine macrophages: involvement in cytokine synthesis. J. Immunol. 160:1330-1339. [PubMed] [Google Scholar]
- 15.Hajjar, A. M., D. S. O'Mahony, A. Ozinsky, D. M. Underhill, A. Aderem, S. J. Klebanoff, and C. B. Wilson. 2001. Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166:15-19. [DOI] [PubMed] [Google Scholar]
- 16.Hoebe, K., P. Georgel, S. Rutschmann, X. Du, S. Mudd, K. Crozat, S. Sovath, L. Shamel, T. Hartung, U. Zähringer, and B. Beutler. 2005. CD36 is a sensor of diacylglycerides. Nature 433:523-527. [DOI] [PubMed] [Google Scholar]
- 17.Into, T., K. Kiura, M. Yasuda, H. Kataoka, N. Inoue, A. Hasebe, K. Takeda, S. Akira, and K. Shibat. 2004. Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-kB activation. Cell. Microbiol. 6:187-199. [DOI] [PubMed] [Google Scholar]
- 18.Jan, G., C. Fontenelle, F. Verrier, H. M. Le, and H. Wroblewski. 1996. Selective acylation of plasma membrane proteins of Mycoplasma mycoides subsp. mycoides SC, the contagious bovine pleuropneumonia agent. Curr. Microbiol. 32:38-42. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, J. S. 2004. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J. Eur. Acad. Dermatol. Venereol. 18:1-11. [DOI] [PubMed] [Google Scholar]
- 20.Jono, H., T. Shuto, H. Xu, H. Kai, D. J. Lim, J. J. Gum, Y. S. Kim, S. Yamaoka, X. H. Feng, and J. D. Li. 2002. Transforming growth factor-beta-Smad signaling pathway cooperates with NF-kappa B to mediate nontypeable Haemophilus influenzae-induced MUC2 mucin transcription. J. Biol. Chem. 277:45547-45557. [DOI] [PubMed] [Google Scholar]
- 21.Jordan, M. S., A. L. Singer, and G. A. Koretzky. 2003. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 4:110-116. [DOI] [PubMed] [Google Scholar]
- 22.Kufer, T. A., and P. J. Sansonetti. 2007. Sensing of bacteria: NOD a lonely job. Curr. Opin. Microbiol. 10:62-69. [DOI] [PubMed] [Google Scholar]
- 23.Landmann, R., B. Muller, and W. Zimmerli. 2000. CD14, new aspects of ligand and signal diversity. Microbes Infect. 2:295-304. [DOI] [PubMed] [Google Scholar]
- 24.Latz, E., A. Visintin, E. Lien, K. A. Fitzgerald, B. G. Monks, E. A. Kurt-Jones, D. T. Golenbock, and T. Espevik. 2002. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 277:47834-47843. [DOI] [PubMed] [Google Scholar]
- 25.Lee, J. Y., L. Zhao, H. S. Youn, A. R. Weatherill, R. Tapping, L. Feng, W. H. Lee, K. A. Fitzqerald, and D. H. Hwang. 2004. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J. Biol. Chem. 279:16971-16979. [DOI] [PubMed] [Google Scholar]
- 26.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 27.Lo, S. C., S. Tsai, J. R. Benish, J. W. Shih, D. J. Wear, and D. M. Wong. 1991. Enhancement of HIV-1 cytocidal effects in CD4+ lymphocytes by the AIDS-associated mycoplasma. Science 251:1074-1076. [DOI] [PubMed] [Google Scholar]
- 28.Manukyan, M., K. Triantafilou, M. Triantafilou, A. Mackie, N. Nilsen, T. Espevik, K. H. Wiesmuller, A. J. Ulmer, and H. Heime. 2005. Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. J. Eur. Immunol. 35:911-921. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. J. Janeway. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253-258. [DOI] [PubMed] [Google Scholar]
- 30.Mühlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muzio, M., N. Polentarutti, D. Bosisio, K. P. P. Manoj, and A. Mantovani. 2000. Toll-like receptor family and signalling pathway. Biochem. Soc. Trans. 28:563-566. [DOI] [PubMed] [Google Scholar]
- 32.Nadra, J. N., S. Deininger, U. Nonstad, F. Skjeldal, H. Husebye, D. Rodionov, S. V. Aulock, T. Hartung, E. Lien, O. Bakke, and T. Espevik. 2008. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling; role of CD14 and CD36. J. Leukoc. Biol. 84:280-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakata, T., M. Yasuda, M. Fujita, H. Kataoka, K. Kiura, H. Sano, and K. Shibata. 2006. CD14 directly binds to triacylated lipopeptides and facilitates recognition of the lipopeptides by the receptor complex of Toll-like receptors 2 and 1 without binding to the complex. Cell. Microbiol. 8:1899-1909. [DOI] [PubMed] [Google Scholar]
- 34.Narita, M., H. Tanaka, T. Togashi, and S. Abe. 2005. Cytokines involved in CNS manifestations caused by Mycoplasma pneumoniae. Pediatr. Neurol. 33:105-109. [DOI] [PubMed] [Google Scholar]
- 35.Omueti, K. O., J. M. Beyer, C. M. Johnson, E. A. Lyle, and R. I. Tapping. 2005. Domain exchange between human toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J. Biol. Chem. 280:36616-36625. [DOI] [PubMed] [Google Scholar]
- 36.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rottem, S. 2003. Interaction of Mycoplasmas with host cells. J. Physiol. Rev. 83:417-432. [DOI] [PubMed] [Google Scholar]
- 38.Sandor, F., E. Latz, L. Mandell, G. Repik, D. T. Golenbock, T. Espevik, E. A. Kurt-Jones, and R. W. Finberg. 2003. Importance of extra- and intracellular domains of TLR1 and TLR2 in NF-κB signaling. J. Cell Biol. 162:1099-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki, Y., J. Ishikawa, A. Yamashita, K. Oshima, T. Kenri, K. Furuya, C. Yoshino, A. Horino, T. Shiba, T. Sasaki, and M. Hattori. 2002. The complete genomic sequence of Mycoplasma penetrans, an intracellular bacterial pathogen in humans. Nucleic Acids Res. 30:5293-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata, K., A. Hasebe, T. Into, M. Yamada, and T. Watanabe. 2000. The N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal human gingival fibroblasts. J. Immunol. 165:6538-6544. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu, T., Y. Kida, and K. Kuwano. 2004. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through Toll-like receptors. Immunology 113:121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu, T., Y. Kida, and K. Kuwano. 2005. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-kappa B through TLR1, TLR2, and TLR6. J. Immunol. 175:4641-4646. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu, T., Y. Kida, and K. Kuwano. 2008. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect. Immun. 76:270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh, B. P., R. S. Chauhan, and L. K. Singhal. 2003. Toll-like receptors and their role in innate immunity. J. Curr. Sci. 85:1156-1164. [Google Scholar]
- 45.Sugawara, S., S. Yang, K. Iki, J. Hatakeyama, R. Tamai, O. Takeuchi, S. Akashi, T. Espevik, S. Akira, and H. Takada. 2001. Monocytic cell activation by nonendotoxic glycoprotein from Prevotella intermedia ATCC 25611 is mediated by Toll-like receptor 2. Infect. Immun. 69:4951-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki, N., S. Suzuki, and W. C. Yeh. 2002. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol. 23:503-506. [DOI] [PubMed] [Google Scholar]
- 47.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Muhlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554-557. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi, O., T. Kawai, H. Sanjo, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, K. Takeda, and S. Akira. 1999. TLR6: a novel member of an expanding Toll-like receptor family. Gene 231:59-65. [DOI] [PubMed] [Google Scholar]
- 50.Triantafilou, M., and K. Triantafilou. 2002. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends J. Immunol. 23:301-304. [DOI] [PubMed] [Google Scholar]
- 51.Tully, J. G., D. T. Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed] [Google Scholar]
- 52.West, A. P., K. A. A. Oblansky, and S. Ghosh. 2006. Recognition and signaling by toll-like receptors. Annu. Rev. Cell Dev. Biol. 22:409-437. [DOI] [PubMed] [Google Scholar]