Abstract
Despite their potential role in the spread of foot-and-mouth disease (FMD), the immune response and viral persistence in FMD virus (FMDV)-infected Indian buffaloes (Bubalus bubalis) have been unexplored. We found similar kinetics of neutralizing antibody responses in the sera and secretory fluids of buffaloes following experimental FMDV Asia 1 infection, but the lymphocyte-proliferative response in infected buffaloes was of low magnitude. Despite inducing a significant systemic and secretory immune response, viral persistence seems to be a common outcome in buffaloes following FMDV Asia 1 infection, which is associated with a weak cellular immune response.
Foot-and-mouth disease (FMD) is an acute, highly contagious viral disease that affects a wide range of hosts, including Indian buffaloes (Bubalus bubalis) (1, 12, 16). Buffaloes contribute significantly to the livestock industry in India in terms of milk (52%) and meat production (30%) (6). FMD virus (FMDV) infection causes a drastic drop in productivity, resulting in severe financial losses. Hence, FMD in buffaloes has a huge impact on the economy of the country (12).
Developing countries where FMD is endemic have mixed-husbandry practices of rearing cattle, buffaloes, sheep, and goats together, which favors FMDV persistence and spread in nature. Furthermore, FMDV transmission between cattle and buffaloes has been observed in natural outbreaks (12, 30) and in experimental studies (15, 23). The susceptibility of buffaloes to FMD has been found to vary according to the country (buffalo subspecies) and serotype and strain of the virus (6, 30, 31). Of significant importance, the clinical disease pattern in buffaloes is distinct from that in cattle, where buffaloes exhibit a covert nature of disease with unique lesions at different sites in buffaloes (21, 23). In addition to causing acute clinical disease, FMDV causes persistent asymptomatic infection in ruminants, including buffaloes. Persistently infected animals (carriers) are considered to be the source of virus for later outbreaks (1). Thus, buffaloes serve as an unapparent reservoir of FMDV for the spread of disease and represent a major host with a potential role in the epidemiology of FMD.
The immune response to FMDV has been extensively studied following infection and also following vaccination in cattle, pigs, sheep, and goats. Infection induces antibody responses in serum and secretory fluids and a cellular immune response, which is distinct from that after vaccination (24). Moreover, the immune response to persistent FMDV infection in cattle seems to be distinct (22, 26, 27). In this context, information on the kinetics of the immune response in FMDV-infected buffaloes and viral persistence is lacking. Furthermore, FMDV Asia 1 is believed to be restricted mainly to the Asian continent. However, the recent spread of the disease to European countries and the emergence of new variants have pointed to the potential role of type Asia 1 in global FMD (19, 33). Despite its significance, the immune response to persistent FMDV Asia 1 infection has been unexplored. The present experimental study was therefore designed to investigate the systemic (serum) and secretory antibody response, the cellular immune response to FMDV Asia 1 infection, and viral persistence in Indian buffaloes following experimental inoculation.
Five male Indian buffaloes, aged 10 to 14 months, free from serum-neutralizing antibodies to FMDV, were infected by intradermolingual inoculation of 104 50% infective doses of cattle tongue-adapted FMDV type Asia 1 strain 8/79. All the experimental procedures of the study were reviewed by the institutional animal ethics committee for the control and supervision of animal experiments of the Indian Veterinary Research Institute, Bangalore, India. Buffaloes infected with FMDV Asia 1 developed vesicular-to-erosive lesions in their feet, although tongue lesions were observed in only three of them. Thus, development of clinical disease following experimental infection with cattle-adapted FMDV Asia 1 supports the idea that virus is transmitted between cattle and buffaloes (15, 23). To assess the immune response to FMDV Asia 1 in buffaloes, blood, esophageal-pharyngeal fluid (OPF) and oronasal fluid (ONF) were collected preinfection and at 7- to 15-day intervals up to 65 days postinfection (DPI).
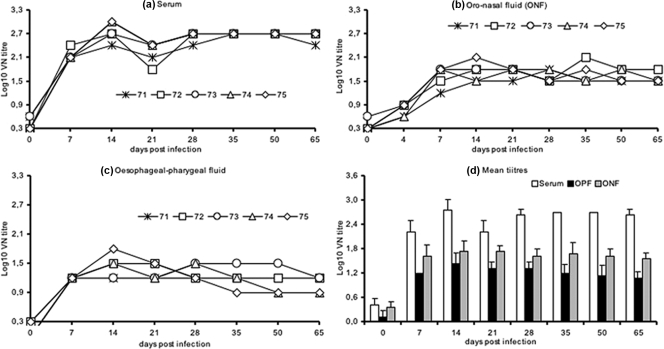
Virus-neutralizing antibody (VN) titers in serum were determined by a virus microneutralization test as described in the OIE manual (2) using BHK21 clone 13 cells and 100 50% tissue culture infective doses of FMDV Asia1 8/79. The kinetics of the neutralizing antibody response in sera of FMDV Asia 1-infected buffaloes is depicted in Fig. 1a and d. All infected buffaloes developed significant VN titers in serum, which reached a peak (log10 2.71) around 14 DPI. The appearance of serum-neutralizing antibodies coincided with the healing of lesions, and complete recovery from disease was evident by 14 DPI, supporting the role of neutralizing antibodies in the clearance of virus from the site of predilection (1). The titers continued to be high until the end of experiment at 65 DPI. There was no significant difference in the VN titers among the infected buffaloes (Fig. 1a), despite the differences in lesion development. Although, buffaloes exhibit distinct covert disease, the kinetics of the serum antibody response to FMDV Asia 1 infection in buffaloes is similar to that of cattle observed in our previous studies (21, 22). Further, Gomes and coworkers (15) have also reported similarity in the kinetics of VN titers in the sera of FMDV type O-infected Indian buffaloes and cattle. Taken together, our results indicate that the development of serum-neutralizing antibodies in FMDV-infected cattle is similar to that in buffaloes, irrespective of their clinical disease.
FIG. 1.
FMD VN titer in Indian buffaloes (Bubalus bubalis) at different DPI following FMDV type Asia 1 experimental inoculation in serum (a), ONF (b), and OPF (c). (d) Mean VN titers (± standard deviations) in serum, ONF, and OPF.
To evaluate the secretory antibody response, ONF and OPF were collected and processed as described previously (22). VN titers in secretory fluids (OPF and ONF) was determined by a virus microneutralization test (2) using BHK21 clone 13 cells and 10 50% tissue culture infective doses of FMDV Asia 1 8/79 (22). The kinetics of neutralizing antibody response in secretory fluids of FMDV Asia 1-infected buffaloes is depicted in Fig. 1b, c, and d. The secretory fluids of buffaloes were negative for neutralizing antibody prior to experimental infection. However, significant VN titers were observed in the OPF and ONF of all the buffaloes following FMDV Asia 1 infection. There was no significant difference in VN titers of secretory fluids among the infected buffaloes (Fig. 1b and c). The peak mean titer was reached around 14 DPI in both secretory fluids, which coincided with the peak serum VN titers (Fig. 1d). Further, there was a steady, but moderate, decline in the antibody titer in secretory fluids until the end of the experiment (Fig. 1d). The VN titers of ONF were higher than those of OPF throughout the observation period in the present experiment (Fig. 1d). The difference between the OPF and ONF titers in the present study is in agreement with findings for FMDV Asia 1-infected cattle (22) and might be related to stimulation of different immunosecretory compartments (7, 20).
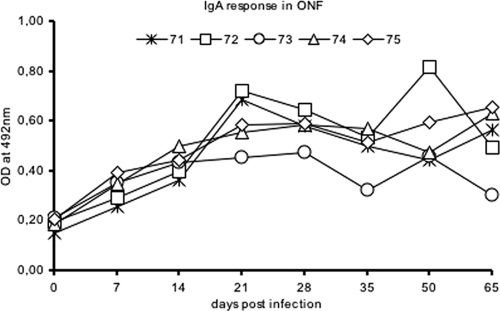
The neutralizing activity of secretory fluids is due mainly to secretory immunoglobulin A (IgA), although IgM and IgG antibodies also contribute (7). Similarly, FMDV-specific antibodies in secretory fluids are mainly IgA antibodies, and their levels are significantly high following infection in cattle and pigs (13, 29). Henceforth, to evaluate the secretory IgA response, an indirect enzyme-linked immunosorbent assay (ELISA) for the detection of IgA antibodies to structural proteins was performed as described previously (22). In the present study, IgA antibodies were not detected in the secretory fluids of buffaloes collected prior to infection. Following infection, significant IgA levels were observed in ONF, with a peak around 21 DPI, and the response was sustained until the end of the experiment. All buffaloes, except one (buffalo 73), had sustained high responses (Fig. 2). In contrast, Maroudam and coworkers (23) failed to detect any IgA antibodies in the saliva of FMDV type O-infected Indian cattle and buffaloes. Further, in the present study, the IgA response in OPF was very low and did not show a significant rise following infection. This difference is in agreement with observations of VN titers of secretory fluids. Commensurably, the secretory IgA response to FMDV Asia 1 in infected buffaloes is similar to that in infected cattle observed in our previous study (22). Thus, our results are suggestive of similar kinetics of the secretory antibody response in FMDV Asia 1-infected Indian buffaloes and cattle.
FIG. 2.
IgA antibody response in the ONF of Indian buffaloes (Bubalus bubalis) at different DPI following FMDV type Asia 1 experimental inoculation.
Although neutralizing antibodies clear virus from its site of predilection, the cellular immune response is involved in the clearance of intracellular viral infection. Therefore, a lymphocyte proliferation test was performed to assess the cellular immune response in FMDV Asia 1-infected Indian buffaloes. Heparinized blood (10 IU/ml) was collected for separation of peripheral blood mononuclear cells (PBMC) by centrifugation over Histopaque 1.077. PBMC obtained were suspended in RPMI 1640 medium with supplements. The lymphocyte proliferation test was performed using the 3-(4,5 dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) dye method (18), with some modifications. PBMC (2 × 105/well) were stimulated in duplicate with inactivated FMDV Asia 1 antigen (Ag) (10 μg/ml) and concanavalin A (ConA), a T-cell mitogen (10 μg/ml). For in vitro lymphocyte stimulation and proliferation, the plates were incubated at 37°C for 96 h in a humidified atmosphere with 5% CO2. MTT (10 μl) solution (10 mg/ml in sterile phosphate-buffered saline) was added to each well and incubated for 4 h at 37°C in 5% CO2. Dimethyl sulfoxide (100 μl) was added to each well, and the plates were further incubated for 15 min at 37°C to dissolve the formazan crystals formed by metabolization of MTT. The optical density (OD) was recorded at 540 nm with reference reduction of background color in an ELISA reader. The lymphocyte-proliferative (LP) response, depicted as a percentage of proliferation, is calculated according to the formula [(OD of stimulated cells − OD of unstimulated cells)/OD of unstimulated cells] × 100.
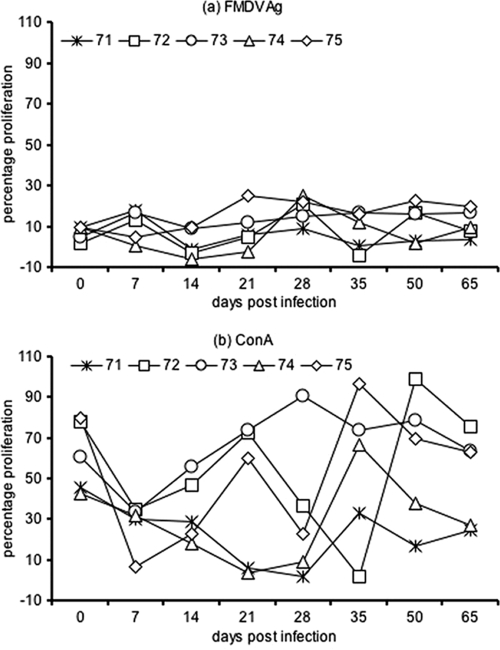
The LP responses of infected buffaloes are depicted in the Fig. 3. The response was variable at different DPI among the individual buffaloes. The LP response to FMDV Asia 1 Ag in the present study was of low magnitude, indicating a weak primary cellular immune response following infection (Fig. 3a). The peak LP response was observed on 28 DPI. Furthermore, the magnitudes of the cellular responses have been found to vary with the virus serotype, strain, and species and the breed of the host (due to the major histocompatibility complex haplotype) (3, 9, 14, 17, 25, 27). In our study, the kinetics of the LP response to FMDV Ag did not correlate with the VN titer in the sera (Pearson correlation coefficient [r] = 0.18, P = 0.65) or secretory fluids (r = 0.12, P = 0.75) of infected buffaloes. A similar lack of correlation between the cellular immune response and VN titer in serum has been reported in previous studies of FMDV (serotype O and A/SAT)-vaccinated and -infected cattle (14, 25). Thus, kinetics of the neutralizing antibody response and cellular immune response to FMDV may not correlate among the infected animals.
FIG. 3.
LP responses of in vitro-stimulated PBMC cultures from Indian buffaloes (Bubalus bubalis) at different DPI following experimental FMD virus type Asia 1 inoculation. (a) LP response to FMDV Asia 1 Ag (10 μg/ml) stimulation; (b) LP response to ConA (10 μg/ml) stimulation.
The LP response to ConA decreased significantly following infection and recovered by 35 DPI (Fig. 3b). These findings of a low-magnitude cellular immune response observed following FMDV Asia 1 infection in the present study is suggestive of a suppressed cellular immune response in buffaloes. The recent studies have also observed inhibited cellular immune responses among infected pigs (5, 10, 11), cattle (25, 27), and sheep (3). Interestingly, recent findings have implicated a role of interleukin 10 in the suppressed cellular responses of FMDV-infected pigs (5, 10, 11). In contrast, vaccination with FMDV serotypes (O, A, Asia 1) has been shown to induce a significant cellular immune response in buffaloes (8). Thus, our results are supportive of a suppressed cellular immune response due to extensive viral replication in FMDV-infected naïve animals.
Indian buffaloes are one of the major hosts of FMDV and are believed to carry virus asymptomatically (persistent infection) (1). To investigate the development of persistent infection, OPF samples were collected and analyzed for the presence of FMDV RNA by Ag capture reverse transcription-PCR (Ag RT-PCR) as described previously (22). Briefly, a mixture of OPF and phosphate-buffered saline-Tween-20 containing 3% bovine serum albumin was incubated in FMDV Asia 1-specific Ab-coated microcentrifuge tubes at 37°C for 3 h. Following virus capture, RT-PCR were done as described previously (32) to yield the 330-bp amplification product of VP1 in the virus-positive samples. Ag RT-PCR is found to be at least 125-fold-more sensitive than ELISA and has sensitivity comparable to that of other RT-PCR protocols for the detection of FMDV (28, 32).
All inoculated Indian buffaloes were found positive for FMDV Asia 1 in OPF on or beyond 28 DPI and hence termed carriers. This finding is in agreement with previous studies in which all infected buffaloes were found to be carriers (4, 15, 23). Taken together, our results suggest that development of viral persistence among the buffaloes is a common sequel to FMDV infection and that the frequency of carriage is much higher than among cattle, as observed in previous studies (4, 15, 23).
In the present study, although infected buffaloes developed high titers of virus neutralizing antibody in serum and secretory fluids, FMDV persistence was observed in all the animals. This is in agreement with the opinion that neutralizing antibodies are ineffective in clearing virus from the site of persistence, the pharyngeal region (1, 26). However, the sustained high IgA levels in persistently infected Indian buffaloes, as with carrier cattle, support the opinion of secretory IgA as an indicator of viral persistence (22, 26, 29). Furthermore, IgA detection can serve as alternate test to identify carriers. Intriguingly, persistent infection was associated with a suppressed cellular immune response, which plays a major role in the clearance of persistent viral infection. Similarly, recent studies have observed suppressed cellular immune responses among persistently infected cattle and sheep (3, 25, 27). Thus, the findings of the present study are in agreement with the hypothesis that suppression of the cellular immune response by FMDV favors viral persistence.
To conclude, despite a significant systemic and secretory antibody response following infection, FMDV Asia 1 persistence associated with a weak cellular immune response seems to be a common outcome in buffaloes. These findings suggest the need for further in-depth studies to investigate viral persistence in relation to the immune response in buffaloes. A better understanding of this issue might improve vaccination and surveillance strategies, leading to better control of global FMD.
Acknowledgments
We are grateful to the animal attendees at the Isolation Unit, Yelahanka, Indian Veterinary Research Institute, for their assistance in sample collection. We thank Jagadeesh Bayry and Srini V Kaveri (INSERM, Paris) for critical reading of the manuscript.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Alexandersen, S., Z. Zhang, A. I. Donaldson, and A. J. Garland. 2003. The pathogenesis and diagnosis of foot-and-mouth disease. J. Comp. Pathol. 129:1-36. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2005. Foot-and-mouth disease. Manual of standards for diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees), 6th ed., vol. 1. Office International des Epizooties, Paris, France.
- 3.Barnett, P. V., P. Keel, S. Reid, R. M. Armstrong, R. J. Statham, C. Voyce, N. Aggarwal, and S. J. Cox. 2004. Evidence that high potency foot-and-mouth disease vaccine inhibits local virus replication and prevents the “carrier” state in sheep. Vaccine 22:1221-1232. [DOI] [PubMed] [Google Scholar]
- 4.Barros, J. J., V. Malirat, M. A. Rebello, E. V. Costa, and I. E. Bergmann. 2007. Genetic variation of foot-and-mouth disease virus isolates recovered from persistently infected water buffalo (Bubalus bubalis). Vet. Microbiol. 120:50-62. [DOI] [PubMed] [Google Scholar]
- 5.Bautista, E. M., G. S. Ferman, and W. T. Golde. 2003. Induction of lymphopenia and inhibition of T cell function during acute infection of swine with foot and mouth disease virus (FMDV). Vet. Immunol. Immunopathol. 92:61-73. [DOI] [PubMed] [Google Scholar]
- 6.Borghese, A. (ed.). 2005. Buffalo production and research. Food and Agriculture Organization of the United Nations, Rome, Italy.
- 7.Brandtzaeg, P. 2007. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25:5467-5484. [DOI] [PubMed] [Google Scholar]
- 8.Chhabra, R., R. Sharma, and N. K. Kakker. 2004. Comparative immunogenicity of foot and mouth disease virus antigens in FMD-haemorrhagic septicaemia combined vaccine and FMD vaccine alone in buffalo calves. Indian J. Exp. Biol. 42:259-264. [PubMed] [Google Scholar]
- 9.Childerstone, A. J., L. Cedillo-Baron, M. Foster-Cuevas, and R. M. Parkhouse. 1999. Demonstration of bovine CD8+ T-cell responses to foot-and-mouth disease virus. J. Gen. Virol. 80:663-669. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-San Segundo, F., T. Rodriguez-Calvo, A. de Avila, and N. Sevilla. 2009. Immunosuppression during acute infection with foot-and-mouth disease virus in swine is mediated by IL-10. PLoS One 4:e5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-San Segundo, F., F. J. Salguero, A. de Avila, M. M. de Marco, M. A. Sanchez-Martin, and N. Sevilla. 2006. Selective lymphocyte depletion during the early stage of the immune response to foot-and-mouth disease virus infection in swine. J. Virol. 80:2369-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta, P. K., G. Sarma, and S. K. Das. 1983. Foot-and-mouth disease in Indian buffaloes. Vet. Rec. 113:134. [DOI] [PubMed] [Google Scholar]
- 13.Eble, P. L., A. Bouma, K. Weerdmeester, J. A. Stegeman, and A. Dekker. 2007. Serological and mucosal immune responses after vaccination and infection with FMDV in pigs. Vaccine 25:1043-1054. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Valcarcel, M., T. Doel, T. Collen, M. Ryan, and R. M. Parkhouse. 1996. Recognition of foot-and-mouth disease virus and its capsid protein VP1 by bovine peripheral T lymphocytes. J. Gen. Virol. 77:727-735. [DOI] [PubMed] [Google Scholar]
- 15.Gomes, I., A. K. Ramalho, and P. A. de Mello. 1997. Infectivity assays of foot-and-mouth disease virus: contact transmission between cattle and buffalo (Bubalus bubalis) in the early stages of infection. Vet. Rec. 140:43-47. [DOI] [PubMed] [Google Scholar]
- 16.Grubman, M. J., and B. Baxt. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17:465-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzman, E., G. Taylor, B. Charleston, M. A. Skinner, and S. A. Ellis. 2008. An MHC-restricted CD8+ T-cell response is induced in cattle by foot-and-mouth disease virus (FMDV) infection and also following vaccination with inactivated FMDV. J. Gen. Virol. 89:667-675. [DOI] [PubMed] [Google Scholar]
- 18.Heaney, J., S. L. Cosby, and T. Barrett. 2005. Inhibition of host peripheral blood mononuclear cell proliferation ex vivo by Rinderpest virus. J. Gen. Virol. 86:3349-3355. [DOI] [PubMed] [Google Scholar]
- 19.Lee, K. N., J. K. Oem, J. H. Park, S. M. Kim, S. Y. Lee, S. Tserendorj, R. Sodnomdarjaa, Y. S. Joo, and H. Kim. 2009. Evidence of recombination in a new isolate of foot-and-mouth disease virus serotype Asia 1. Virus Res. 139:117-121. [DOI] [PubMed] [Google Scholar]
- 20.Liebler-Tenorio, E. M., and R. Pabst. 2006. MALT structure and function in farm animals. Vet. Res. 37:257-280. [DOI] [PubMed] [Google Scholar]
- 21.Maddur, M. S., M. R. Gajendragad, S. Gopalakrishna, and N. Singh. 2008. Comparative study of experimental foot-and-mouth disease in cattle (Bos indicus) and buffaloes (Bubalis bubalus) (sic). Vet. Res. Commun. 32:481-489. [DOI] [PubMed] [Google Scholar]
- 22.Maddur, M. S., M. R. Gajendragad, S. Kishore, A. K. Chockalingam, V. V. Suryanarayana, S. Gopalakrishna, and N. Singh. 2008. Enhanced mucosal immune response in cattle persistently infected with foot-and-mouth disease virus. Vet. Immunol. Immunopathol. 125:337-343. [DOI] [PubMed] [Google Scholar]
- 23.Maroudam, V., S. B. Nagendrakumar, M. Madhanmohan, P. Santhakumar, D. Thiagarajan, and V. A. Srinivasan. 2008. Experimental transmission of foot-and-mouth disease among Indian buffalo (Bubalus bubalis) and from buffalo to cattle. J. Comp. Pathol. 139:81-85. [DOI] [PubMed] [Google Scholar]
- 24.McCullough, K. C., F. De Simone, E. Brocchi, L. Capucci, J. R. Crowther, and U. Kihm. 1992. Protective immune response against foot-and-mouth disease. J. Virol. 66:1835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh, Y., B. Charleston, D. J. Paton, P. Jong-Hyun, P. V. Barnett, J. Yi-Seok, and S. Parida. 2006. Importance of cell mediated immunity for protection against foot and mouth disease, p. 230-239. In Report of session of the research group of the standing technical committee of the European Commission for the control of foot-and-mouth disease, Appendix 34, 17 to 20 October 2006, Paphos, Cyprus. Food and Agriculture Organization, Rome, Italy.
- 26.Parida, S., J. Anderson, S. J. Cox, P. V. Barnett, and D. J. Paton. 2006. Secretory IgA as an indicator of oro-pharyngeal foot-and-mouth disease virus replication and as a tool for post vaccination surveillance. Vaccine 24:1107-1116. [DOI] [PubMed] [Google Scholar]
- 27.Parida, S., Y. Oh, S. M. Reid, S. J. Cox, R. J. Statham, M. Mahapatra, J. Anderson, P. V. Barnett, B. Charleston, and D. J. Paton. 2006. Interferon-gamma production in vitro from whole blood of foot-and-mouth disease virus (FMDV) vaccinated and infected cattle after incubation with inactivated FMDV. Vaccine 24:964-969. [DOI] [PubMed] [Google Scholar]
- 28.Reid, S. M., G. H. Hutchings, and N. P. Ferris. 2000. Evaluation of RT-PCR procedures for diagnosis of clinical samples of foot-and-mouth disease virus (serotypes O, A, C and Asia 1) under the European Union Concerted Action Group Project Pl 98-4032, p. 181-187. In Report of session of the research group of the standing technical committee of the European Commission for the control of foot-and-mouth disease, Appendix 21, 5 to 8 September 2000, Borovets, Bulgaria. Food and Agriculture Organization, Rome, Italy.
- 29.Salt, J. S., G. Mulcahy, and R. P. Kitching. 1996. Isotype-specific antibody responses to foot-and-mouth disease virus in sera and secretions of “carrier” and “non-carrier” cattle. Epidemiol. Infect. 117:349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samara, S. I., and A. A. Pinto. 1983. Detection of foot-and-mouth disease carriers among water buffalo (Bubalus bubalis) after an outbreak of the disease in cattle. Vet. Rec. 113:472-473. [DOI] [PubMed] [Google Scholar]
- 31.Sharma, S. K. 1983. Foot-and-mouth disease in Indian buffaloes. Vet. Rec. 113:478. [DOI] [PubMed] [Google Scholar]
- 32.Suryanarayana, V., B. Madanamohan, P. Bist, C. Natarajan, and J. D. Tratschin. 1999. Serotyping of foot-and-mouth disease virus by antigen capture reverse transcriptase/polymerase chain reaction. J. Virol. Methods 80:45-52. [DOI] [PubMed] [Google Scholar]
- 33.Valarcher, J. F., N. J. Knowles, N. P. Ferris, D. J. Paton, V. Zakharov, A. Sherbakov, Y. J. Shang, Z. X. Liu, X. T. Liu, A. Sanyal, D. Hemadri, C. Tosh, and T. J. Rasool. 2005. Recent spread of FMD virus serotype Asia 1. Vet. Rec. 157:30. [DOI] [PubMed] [Google Scholar]