Abstract
Natural killer (NK) cells provide one of the initial barriers of cellular host defense against pathogens, in particular intracellular pathogens. The role of these cells in foot-and-mouth disease virus (FMDV) infection is unknown. Previously, we characterized the phenotype and function of NK cells from swine (F. N. Toka et al., J. Interferon Cytokine Res. 29:179-192, 2009). In the present study, we report the analysis of NK cells isolated from animals infected with FMDV and tested ex vivo and show that NK-dependent cytotoxic activity against tumor cells as targets was impaired. More relevantly to this infection, the killing of target cells infected with FMDV also was inhibited. Further, the proportion of NK cells capable of producing gamma interferon and storing perforin was reduced. Peripheral blood mononuclear cells isolated from infected animals are not productively infected, but virus exposure in vivo resulted in the significant induction of NKp30 and Toll-like receptor 3 expression and the moderate activation of SOCS3 and interleukin-15 receptor mRNA. However, there was little alteration of mRNA expression from a number of other receptor genes in these cells, including SH2D1B and NKG2A (inhibitory) as well as NKp80, NKp46, and NKG2D (activating). These data indicate that this virus infection influences the ability of NK cells to recognize and eliminate FMDV-infected cells. In addition, a reduction in NK cell cytotoxicity coincided with the increase in virus titers, indicating the virus blocking of NK cell-associated innate responses, albeit temporarily. These effects likely culminate in brief but effective viral immune evasion, allowing the virus to replicate and disseminate within the host.
Innate immunity is a vital part of the overall host immune response to invasion by pathogens, particularly during virus infections. Natural killer (NK) cells occupy a critical position in the initial host responses against infection. Originally, NK cells were discovered on the basis of their capability to kill certain tumors without prior activation. Now the role of NK cells has been defined in virus infections such as human cytomegalovirus (8, 11, 55), murine cytomegalovirus virus (2, 32), influenza virus (28, 35), herpes simplex virus (44, 52), ectromelia virus (16, 41), and human immunodeficiency virus (HIV) (14, 50, 56). In two of these infections, a lack or deficiency in NK cell function leads to increased susceptibility to infection (6, 10).
The initiation of NK cell responses is thought to originate from signals delivered by the professional pathogen-sensing system, which is comprised mainly of dendritic cells (DC) (21, 47, 57). Although the evidence is not yet definitive, the direct activation of NK cells also may occur through pathogen recognition receptors expressed by NK cells (49, 51). The cross-talk between NK cells and DC leads to the activation of NK cells, after which they operate in a manner that is dependent on the sensing expression of specific molecules induced on virus-infected cells through receptors present on the NK cell surface. In part, the recognition of an infected cell by NK cells relies on the detection of the missing self, i.e., the lack of major histocompatibility complex class I expression on the infected cell surface. Ultimately, the balance between signals from both inhibiting and activation receptors (34) on NK cells control NK cell function in response to infection. Subsequently, NK cells engage in cytokine secretion and, upon the encounter of a virus-infected cell, release cytotoxic granule contents or induce apoptosis. These mechanisms lead to the elimination of virus-infected cells. Whereas the discovery of activating or inhibitory receptors on NK cells has progressed tremendously, the identification of respective ligands on infected or transformed cells has been difficult (reviewed in reference 12).
Although much is known about the function of NK cells in humans and mice, NK cell activity in swine or cattle remains preliminary, and their role in animal viral diseases still is obscure. The recent progress in these animal species has been reviewed by Boysen and Storset (9) and Gerner et al. (20). In pigs, NK cells may account for a total of 5 to 10% of circulating lymphocytes and currently are identified as belonging to a subset of cells that coexpress CD2 and CD8 molecules (17). Although mRNAs of many activating and inhibitory receptors have been detected, no studies have been conducted to define their role in the generic function of porcine NK cells. But it is known that porcine NK cells can secret gamma interferon (IFN-γ), store perforin, and kill in vitro targets (54). Their function can be modulated by direct stimulation with cytokines such as interleukin-2 (IL-2), IL-12, IL-15, IL-18 (42, 54), or IFN-α, or Toll-like receptor (TLR) agonists such as polyinosinic:poly(C) (pI:C), CpG, imiquimod, and resiquimod in humans (23).
In this study, we examine NK cell responses during infection with foot-and-mouth disease virus (FMDV). FMDV is a contagious disease of cloven-hoofed animals caused by a picornavirus (25). Infection with FMDV presents as an acute disease characterized by fever, short-lived viremia, and the occurrence of lesions on feet and tongue (reviewed in reference 25). The control of FMDV in certain regions of the world depends on the use of inactivated vaccines. The use of these as emergency vaccines, however, is compromised by the time from vaccination to protection (7 days in cattle) (22) and the difficulty in distinguishing infected animals from vaccinated animals. In FMDV-free countries vaccination is not practiced, leading to outbreak responses relying mostly on the elimination of all susceptible animals within the areas of outbreaks (15).
The immune response to FMDV infection in pigs has not been fully dissected and remains an area of speculation. Whereas B-cell responses are activated early and neutralizing antibody titers correlate with protection (36), there is no clear picture regarding the induction of functional T-cell immunity in infected animals. Bautista et al. (4) found the inhibition of T-cell responses to mitogens during the infection of pigs with FMDV. Some groups have reported the induction of antigen-specific T cells reactive with nonstructural proteins of the virus (7). More recently, antigen-specific, major histocompatibility complex-restricted CD8+ T-cell responses were detected following infection with FMDV (26).
The importance of innate immunity in FMDV infection is perhaps the most overlooked systemic response to FMDV in susceptible species and remains largely undefined. To date, there is no comprehensive study addressing the role of NK cells during infection with FMDV in either swine or cattle. A more sophisticated understanding of the innate response mechanisms involved in infection with FMDV is pertinent to the design of potent emergency vaccines that can protect against infection.
In these studies, we characterized the response of NK cells derived from swine peripheral blood following infection with the O1 Campos strain of FMDV. Results demonstrate that these cells are compromised in the killing of either tumor cells or FMDV-infected target cells ex vivo. Moreover, this dysfunction includes a reduction in the proportion of IFN-γ-producing and perforin-storing cells and an alteration in the expression pattern of activating and inhibitory receptor genes. These data suggest a profound effect of FMDV infection on porcine NK cell function. Taken together, this abnormal functional status likely contributes to the acute nature of FMDV infection in this species and toward making it one of the most contagious viral infections of swine.
MATERIALS AND METHODS
Animals and viruses.
Yorkshire pigs were purchased from Animal Biotech Inc. (Danboro, PA) at the age of 3 to 4 months and acclimated before use in the experiments described here. All procedures performed on these animals were approved by the Plum Island Animal Disease Center Institutional Animal Care and Use Committee. Pigs were infected in the heel bulb intradermally with 104 PFU/ml of FMDV strain 01 Campos A12 or 105 PFU/ml LL-A12 (an attenuated derivative of A12 achieved by removing the leader protease [13]). Body temperature was taken daily.
Preparation of PBMC and serum.
Blood samples from animals before infection and after infection were drawn into heparin-containing vacuum tubes, diluted with phosphate-buffered saline (PBS) 1:1, and layered onto Lymphoprep (Axis-Shield; PoC AS, Oslo, Norway). Cells were centrifuged for 20 min at 20°C. Peripheral blood mononuclear cells (PBMC) were collected and washed in PBS twice followed by one was with RPMI 1640, and finally they were suspended in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), HEPES, l-glutamine, and antibiotic/antimycotic solutions. Whole-blood analysis was performed on fresh blood samples in an AcT hematology analyzer (Beckman Coulter, Hialeah, FL) according to the manufacturer's procedures.
Blood for serum was collected into separate tubes containing clot-activating factor and later centrifuged for 20 min at room temperature. Serum was separated and immediately stored at −70°C until use. All cell-based assays described in subsequent experiments were performed with freshly isolated cells.
Enrichment of porcine NK cells.
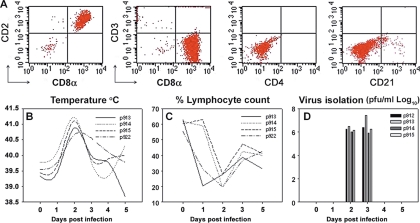
NK cells were enriched based on the characterization by Denyer et al. (17), Pintaric et al. (42), and Gerner et al. (20), in which porcine NK cells are defined as CD3−/CD2+/CD8a+/CD8b−. Negative selection was performed by removing adherent macrophages (MΦ) and CD172+, CD3+, CD4+, and CD21+ cells. Magnetic-activated cell sorting (Miltenyi Biotech, Germany) was used to separate the cells according to the manufacturer's instructions. Mouse primary antibodies against porcine CD3 (clone PPT3), CD4 (clone 74-12-4), CD21 (clone BB6-11C9.6), and CD172 (clone 74-22-15) were purchased from Southern Biotech (Burmingham, AL). Briefly, PBMC were labeled with primary antibodies for 10 min at 4°C. Subsequently, cells were bound by microbeads coated with goat anti-mouse immunoglobulin G and further incubated for 15 min at 4°C. Cells were washed and passed through a magnetic field for separation. This procedure was repeated twice to ensure the complete removal of labeled cells. After separation, cells were washed twice and resuspended in RPMI 1640 supplemented with 10% FBS. Purity was checked by staining with CD2 (clone MSA4; Accurate Chemicals) and CD8 (clone 76-2-11; Southern Biotech). Enrichment usually reached 85 to 95% of CD2+/CD8+/CD3− cells containing the majority of porcine NK cells (Fig. 1A).
FIG. 1.
Enrichment of porcine NK cells and clinical parameters during FMDV infection in swine. (A) PBMC were isolated and depleted of CD3, CD4, CD172, and CD21 by a magnetic-activated cell sorting system. A different set of clones of anti-CD3, anti-CD4, and anti-CD21 (BB238E6; Southern Biotech; and PT90A VMRD and B-ly4; BD Biosciences, respectively) were used to check the accuracy of depletion, and representative plots are shown. (B) Body temperature was taken daily after infection until 7 days. (C) Blood was drawn daily, and lymphocyte counts were performed in a Coulter AcT hematology analyzer. Data are presented as percentages of lymphocytes. (D) Serum samples were collected and virus isolation done as described in Materials and Methods. Virus titers are given as log10 values. The data shown represent a single experiment from a total of three performed. Day 0 depicts data from noninfected animals. Numbers in the graph legend denote individual animal identification tags.
Virus isolation and quantification.
The viral load in serum was determined by a standard assay for isolating virus (40). Tenfold serial dilutions of serum samples were prepared and added to monolayers of BHK21 cells in 12-well plates, and adsorption was carried out for 1 h at 37°C. An overlay of 2 ml tragacanth was added later, and plates were incubated further for 24 h. Finally, the monolayers in the plates were stained with crystal violet in a fixative for 5 min and then washed and dried at room temperature. Plaques were counted, and results are expressed as PFU/milliliter.
NK cell lytic activity assay.
A flow cytometry-based assay was employed to analyze the NK cell cytotoxicity. K562 tumors cells (human erythroleukemia cell line), stably transfected with the green fluorescent protein (GFP) (referred to here as K562-GFP), were kindly provided by Michael Olin (University of Minnesota, School of Veterinary Medicine, St. Paul, MN). Bulk assays or assays with sorted CD2+/CD8+/CD3− cells were performed by incubating cells from healthy or FMDV-infected animals with target cells (K562-GFP) at effector:target (E:T) ratios of 50:1 to 12:1, followed by the addition of 7AAD (the dead/live discriminating dye 7 aminoactinomycin D; BD Biosciences, San Diego CA). Cells then were mixed, spun briefly at 233 × g, and placed in 5% CO2 for 3 to 4 h at 37°C. Data were acquired using a FACSCalibur flow cytometer and later analyzed with CellQuest Pro software (BD Biosciences, San Jose, CA) by establishing a gate on K562-GFP to display cells reflecting the killed population (cells stained by both GFP and 7AAD). The lysis level was determined by the formula R1/(R1+R2) × 100 = % lysis, where R1 is GFP-positive cells plus 7AAD-positive cells and R2 is GFP positive cells.
Stimulation of NK cells within PBMC.
NK cells within PBMC preparations were stimulated with TLR agonists. pI:C was purchased from Amersham Biosciences, Piscataway, NJ, and used at 2 μg/ml. CpG2216 was purchased from InvivoGen (San Diego, CA) and used at 10 μg/ml. The TLR7/8 agonist 3M-011 was a gift from Richard Miller of 3M Pharmaceuticals, Inc. (Minneapolis, MN), and was used at 1 μM. PBMC were stimulated with these agonists for 18 h and later used to assess the NK cell cytotoxicity.
NK cell cytotoxicity against FMDV-infected targets.
A genetically attenuated strain of FMDV was used in this assay. LL-KGE virus is O1 Campos structural proteins inserted into the A12 strain backbone with a positive-charge mutation and another mutation in the receptor binding (RGD) sequence to KGE to allow for the binding of the heparin sulfate receptor so as to increase the cell host range. Additionally, the leader protease, which determines the in vitro attenuation, has been removed. To measure the cytotoxicity against an in vitro-infected target, SK6 cells (a porcine kidney fibroblast cell line) were infected with LL-KGE virus at a multiplicity of infection of 10 for 4 h. Later, the cells were labeled with 5 μM of carboxy fluorescein succinimidyl ester (CFSE) and used as target cells in the 4-h killing assay at an E:T ratio of 50:1. CFSE here was used to identify the target cells by flow cytometry.
Expression of SLA-I on SK6 and VP1 detection.
SK6 cells were grown in minimum essential medium (MEM) to confluence in culture flasks and then detached with cell dissociation medium for 5 min at 37°C. The cells were further pipetted repeatedly to allow for thorough dissociation into single-cell suspension. Cells (1 × 107) were infected with LL-KGE at a multiplicity of infection of 10 in MEM with 0.5% FBS and incubated for 4 h with shaking every 15 min. At the end of the incubation period, cells were washed four times in fluorescence-activated cell sorting (FACS) buffer and surface stained with SLA-I-fluorescein isothiocyanate for 30 min at 4°C, followed by three washes in FACS buffer. Cells then were permeabilized with BD Cytoperm/Cytofix (BD Biosciences) for 30 min at 4°C, washed in BD Perm/Wash three times, and finally stained intracellularly with 12FB2.2.2 monoclonal antibody against VP1 of FMDV for 45 min at 4°C. After three washes in BD Perm/Wash, the cells were stained with a secondary antibody against mouse immunoglobulin G2a conjugated to phycoerythrin (PE) and incubated for 45 min at 4°C. Cells then were washed three times with BD Perm/Wash and three times with FACS buffer, and the fluorescence intensity was determined in a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA).
CD107a flow cytometry.
To determine if the PBMC contained cells that recently had been engaged in cytolysis, we stained for CD107a on the cell surface. Cells that have released their cytotoxic molecules (perforin, granulysin, or granzymes, which previously were stored in endosomes) express CD107a. We analyzed cells from infected and noninfected animals stained with mouse anti-porcine CD107a-FITC (AbD; Serotec, Oxford, United Kingdom) in the presence or absence of K562 cells for 4 h. Data were acquired using a FACSCalibur and analyzed in CellQuest software (BD Biosciences, San Jose, CA).
Intracellular staining for IFN-γ and perforin.
PBMC from infected or noninfected animals were placed in 96-well plates at 1 × 106 per well and incubated for 5 h with 3 μg/ml brefeldin A (eBioscience, San Diego, CA). Cells for positive controls additionally were treated with a mixture of phorbol myristate acetate and ionomycin (Sigma, St. Louis, MO) at 100 and 10 ng/ml, respectively. Later, cells first were stained with CD2, CD3, and CD8a and then fixed and permeabilized, followed by staining with anti-porcine IFN-γ-PE (clone P2G10, BD Bioscience, San Diego CA) at 1 μg/ml or anti-perforin-PE at the concentration suggested by the manufacturer (clone δG9; BD Biosciences, San Diego CA). Data were acquired and analyzed on a FACSCalibur using CellQuest Pro software (BD Biosciences, San Diego CA).
Serum IL-12, IL-15, IL-18, and IFN-α.
We determined the serum levels of the cytokines by antigen capture enzyme-linked immunosorbent assay (ELISA). Capture and detecting antibodies against porcine or human cytokines were purchased from Biosource (human IL-15; Camarillo, CA), R&D Systems Inc. (porcine IL-12; Minneapolis, MN), Endogen/Pierce (porcine IFN-α; Rockford, IL), and Bender MedSystems (porcine IL-18; Vienna, Austria). ELISAs were performed as described by the manufacturers of the coating and detecting antibodies. Optical densities were read on an ELISA reader (Bio-Tek, Winooski, VT), and data were corrected with a linear regression equation in Microsoft Excel.
qrtRT-PCR.
RNA were isolated from sorted CD2+/CD8+/CD3− cells originating from noninfected or FMDV-infected pigs, treated with DNase, and then transcribed into cDNA using the following reaction mixture: 5× reaction buffer, 10 mM deoxynucleoside triphosphates, 50 ng/μl random primers, 0.1 M dithiothreitol, 40 U RNase inhibitor, and 200 U Moloney murine leukemia virus RT. The cDNA templates were used later in the quantitative real-time RT-PCRs (qrtRT-PCRs) in duplicate. The TaqMan RT-PCR system was used for detection. Primers and probes were designed at the Beltsville Human Nutrition Research Center (http://www.ars.usda.gov/Services/docs.htm?docid=6065; ARS, USDA), and sequences are detailed elsewhere (53, 54). PCRs were run in the 7700 ABI Prism Sequence Detector (Applied Biosystems, Foster City, CA) with the following cycling parameters: hold at 50°C for 2 min, hold at 95°C for 10 min, and then 40 cycles at 95°C for 15 s (denature) and 60°C for 1 min (anneal and extension). Ubiquitin or cyclophilin was used as the reference gene to normalize gene expression. Relative expression was calculated by 2−ΔΔCT, and data are expressed as ratios relative to the calibrator (CD2+/CD8+/CD3− cells from noninfected pigs).
Statistics.
Statistical evaluation was applied using a two-tailed t test. Values of P < 0.05 were considered significant.
RESULTS
Clinical disease following FMDV O1 Campos infection.
To put into perspective the conditions under which porcine NK cells are called to function during infection with FMDV, we monitored multiple clinical parameters from days 0 to 7. Figure 1B, C, and D summarize fever, peripheral blood lymphocyte count profiles (lymphopenia) and virus titers on a sample set of animals infected with FMDV O1 Campos. In all experiments, peak viremia and fever were observed between days 2 and 3, after which vesicular lesions formed on the feet and snout (data not shown). These symptoms were concurrent with significant lymphopenia as previously reported (4). All parameters analyzed appeared only transiently and had resolved by day 4 or 5. As reviewed elsewhere (25), during infection with FMDV, lesions appear rapidly on the feet and snout, and the virus titers rise and fall quickly. Such conditions have been reported to be limiting for the function of NK cells (27, 46).
FMDV infection of swine inhibits NK cell lytic activity.
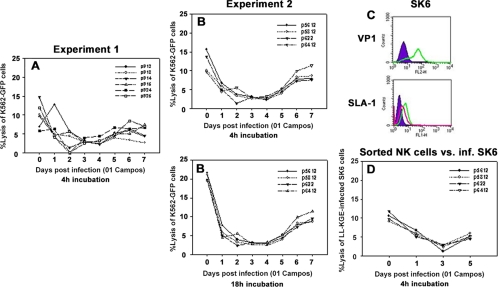
To assess the influence of FMDV infection on NK cell function in swine, we examined the capability of NK cells harvested from Yorkshire pigs infected with the O1 Campos strain of FMDV to lyse target cells ex vivo. The inability of porcine NK cells to lyse a tumor target in vitro is shown in Fig. 2A and B. This was evident beginning 24 h postinfection, when lytic levels dropped at least 1.5-fold compared to baseline levels (day 0). The reduction in cytotoxic activity against K562-GFP on day 2 or 3 was consistent in all animals examined but usually rebounded by day 5. By day 14 (data not shown), most animals had recovered the cytolytic activity of NK cells. Occasionally, NK cells from a donor pig reacted by increasing the NK cell activity 24 h after infection but abruptly declined by day 2.
FIG. 2.
Cytotoxic activity of porcine peripheral blood NK cells. PBMC were isolated from noninfected pigs or pigs infected with O1 Campos strain of FMDV, and NK cell lytic assays against K562-GFP cells in a 4-h assay were performed daily until day 7. (A and B) Two different sets of animal groups were analyzed in two different experiments out a total of three separate experiments. (C) SK6 cells infected (inf.) with LL-KGE, VP1, and SLA-I were assessed by flow cytometry. The shaded area of the upper panel is VP1 staining in noninfected cells, and the green line is VP1 staining in infected cells; the shaded area of the lower panel is the isotype monoclonal antibody control, the green line is the SLA-I staining of noninfected cells, and the red line is the SLA-I staining of virus-infected cells. (D) CD2+/CD8+/CD3− cells were purified from PBMC on days 0, 1, 3, and 5, and the killing assay was performed on SK6 cells previously infected with LL-KGE virus. Data represent a single experiment from a total of two separate determinations. Data represent a single experiment from a total of two. Percent lysis at an E:T ratio of 50:1 is shown. Numbers in the graph legend denote individual animal identification tags.
Because earlier studies (24) have reported increased levels of NK cell cytotoxicity when incubations were prolonged beyond the 4 h of the standard assays, in the second experiment (Fig. 2B) we also compared killing at 4 to that at 18 h. Although the level of killing was higher at 18 h, relative killing by cells at different days following FMDV infection were very similar regardless of how long we incubated effector cells with tumor cell targets.
We were curious to know if this inability to kill a tumor target also was applicable to in vitro FMDV-infected targets. To test this, first SK6 cells were examined for the level of SLA-I expression after infection with the attenuated FMDV strain, LL-KGE. Figure 2C shows the reduction of SLA-I following a 4-h infection. Later, SK6 cells were incubated with sorted CD2+/CD8+/CD3− cells (a population that constitutes porcine NK cells) from infected animals, and the NK cell lytic activity was analyzed after 4 h. A similar trend of NK cell dysfunction was observed but was more prominent on the third day postinfection (Fig. 2D). Therefore, the inability of NK cells from infected animals to engage in lytic activity was consistent when using either FMDV-infected porcine fibroblasts or human leukemia target cells.
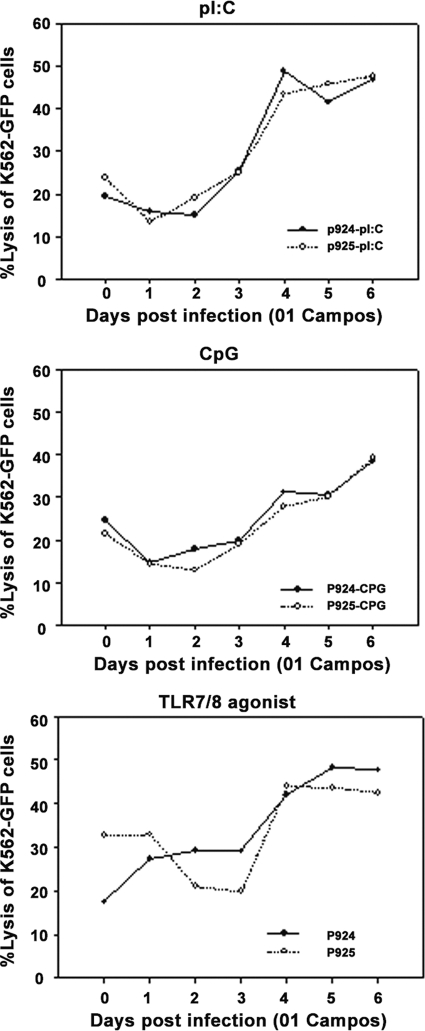
Furthermore, the inhibition of cytolytic activity could not be restored with additional in vitro stimulation. We tested the addition of TLR agonists (pI:C/TLR3, CpG2216/TLR9, or 3M-011/TLR7 and 3M-011/TLR8 agonists) to PBMC from FMDV-infected or noninfected animals and cultured them for 18 h before the NK assay. It was not possible to completely restore the NK cell activity with pI:C or CpG2216, particularly on days 1 and 2 of infection (Fig. 3A and B). NK cell activation with both agonists (pI:C and CpG2216) could be demonstrated from day 3 onwards. A slightly different pattern of activation was observed for the TLR7/8 agonists, with increased activity on day 1 followed by stagnation on days 2 and 3 and a rapid increase thereafter (Fig. 3C). These data indicate that the infection with FMDV induces a profound defect in the function of NK cells during the acute phase of the disease.
FIG. 3.
Restimulation of PBMC does not fully restore the NK cell cytotoxic activity against K562-GFP. PBMC were isolated from infected or noninfected cells and restimulated with either pI:C (A), CpG (B), or TLR7/8 agonists (C) for 18 h and then incubated with K562-GFP cells for 4 h to assay the lytic capability of NK cells. Data are from a single experiment, and lytic levels are presented at an E:T ratio of 50:1. Numbers in the graph legend denote individual animal identification tags.
Effect of FMDV infection on NK cell degranulation.
The fact that we did not detect the cytolytic activity of NK cells from FMDV-infected animals could indicate an already exhausted population of peripheral blood NK cells that no longer was reactive to targets in vitro. When NK cells engage in cytotoxic activity, they degranulate to release their cytotoxic arsenal, which leaves a trace on the cell surface in the form of a lysosome-associated membrane protein (LAMP-1), or CD107a. This protein is expressed mainly in endosome-lysosome membranes before degranulation (1). Initially, we isolated PBMC from healthy pigs, stimulated them with IL-15, and assessed CD107a expression after incubation with or without K562 cells. To determine the level of degranulation in vivo, PBMC from pigs infected with FMDV were isolated and directly stained for CD107a or restimulated with K562 and then examined for CD107a expression.
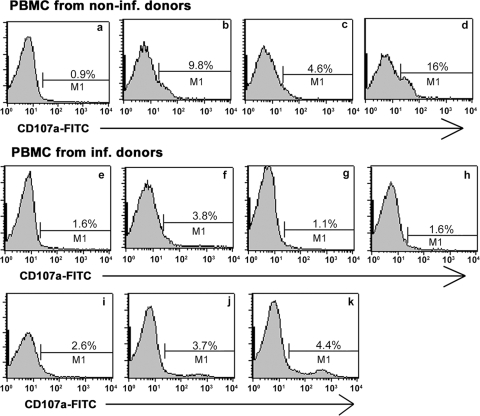
Results presented in Fig. 4 show virtually no constitutive expression of CD107a detected on cells from healthy animals (Fig. 4a) and low levels of expression following stimulation with a porcine IL-15 (Fig. 4c). However, incubation with K562 cells increased the surface expression of CD107a both in cytokine-stimulated cells and in resting cells (Fig. 4b and d). In contrast, the direct staining of cells isolated from infected animals showed little difference in the expression of CD107a through the 7 days of assessment compared to that of cells isolated from noninfected donors (Fig. 4e to k). Furthermore, in vitro restimulation with K562 cells did not induce the degranulation of porcine NK cells from infected animals (data not shown). However, beginning on day 1 postinfection, the expression of CD107a could be detected on another population of lymphocytes, which was neither CD2+ nor CD8+ (data not shown). This pattern of CD107a expression currently is under investigation to identify the CD107a-expressing cells during FMDV infection. Overall, we could not detect a significant presence of CD2+/CD8+-expressing CD107a in the peripheral blood of FMDV-infected pigs. The result strongly suggests the likelihood that NK cells did not engage in lytic activity during the acute phase of infection.
FIG. 4.
Degranulation of NK cells. To show evidence of NK cell release of cytotoxic granule contents, cells were stained for CD107a following incubation with K562 cells or were left unstained not. (a) PBMC from noninfected (non-inf.) animals stained for CD107a; (b) PBMC from noninfected animals incubated with K562 cells and stained for CD107a; (c) PBMC from noninfected animals stimulated with IL-15 for 18 h and stained for CD107a; and (d) IL-15-stimulated PBMC from noninfected animals incubated with K562 and stained with CD107a. (e to k) PBMC from O1 Campos FMDV-infected pigs days 1 to 7 after infection, respectively. Histograms are from a single determination from two separate experiments.
IFN-γ production and perforin storage are influenced by FMDV infection.
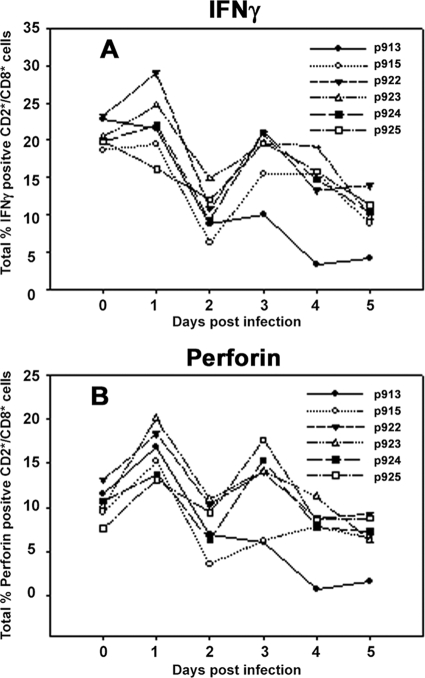
The increased secretion of IFN-γ in NK cells is said to coincide with the enhanced activity of these cells. We examined this in CD2+/CD8+/CD3− cells from O1 Campos-infected pigs. Interestingly, three phases of the response were seen in cells from infected animals. The first phase was the initial transient increase of the total percentage of NK cells producing IFN-γ after 24 h of infection, followed by a rapid decline to below the levels detected before infection by day 2 (Fig. 5A). The reduction on day 2 after infection was concurrent with the reduction in cytolytic activity (Fig. 2A and B), the occurrence of lymphopenia, and an increase in virus titer (Fig. 1). These cells recovered IFN-γ expression on day 3 in most animals but began to decline in expression by day 4 or 5. The same CD2+/CD8+ cells were examined for the storage of perforin, an important effector molecule in the function of NK cells. A similar pattern was observed in the total percentages of perforin-positive cells (Fig. 5B).
FIG. 5.
IFN-γ and perforin profile in infected animals. PBMC were isolated on 5 consecutive days from infected animals and stained intracellularly for IFN-γ and perforin as described in Materials and Methods. CD3+ cells were gated out, and another gate was created on either IFN-γ- or perforin-positive cells and then applied to CD3−/CD2+/CD8+ cells to give the percentage of CD2+/CD8+ positive cells for either IFN-γ (A) or perforin (B). Data represent a single example from five separate experiments. Day 0 represents data derived from noninfected animals and serves as a point of reference. Numbers in the graph legend denote individual animal identification tags.
After examining the IFN-γ production and perforin storage, it appeared that following infection with FMDV, the total percentage of porcine NK cells positive for IFN-γ and perforin was reduced on day 2, a time at which most of the responses in these cells were low. The increase and decrease in cytokine production after day 2 did not correlate with the cytolytic activity of the NK cells. Similar measurements performed on cells restimulated with TLR agonists also did not change the pattern of response (data not shown). These results suggest that FMDV influences the function of porcine NK cells by affecting the number of cells in the circulation and their capability to secrete cytokines or store cytolytic molecules such as perforin.
Serum cytokine profile in FMDV-infected pigs.
IL-2, IL-12, IL-15, IL-18, and IFN-α play an important role in activating porcine NK cells. After infection, serum samples were collected throughout the 7-day period of assessment and were analyzed for the levels of cytokines that are critical to the function of NK cells. ELISA was performed to detect IL-2, IL-12, IL-15, IL-18, and IFN-α. Indeed, the levels of IFN-α were appreciably higher on days 2 and 3 after infection with O1 Campos (Fig. 6). This result confirms our other studies showing the induction of IFN-α that was detectable in serum of swine infected with FMDV regardless of which serotype (A, O, or C) was analyzed (39a). The reduced NK cell function could result from a lack of NK cell-activating cytokines or from initial high levels of IFN-α early in the FMDV infection of swine, as has been reported by others (18). Levels of IL-12, IL-I5, and IL-18 in serum of O1 Campos-infected pigs were below detection (data not shown). Also, we could not detect any IL-2 in the serum of any of the experimentally infected animals. Taken together, this result indicates that FMDV affects the cells responsible for innate cytokine production during infection, which leads to inadequate NK cell activation, therefore rendering them dysfunctional.
FIG. 6.
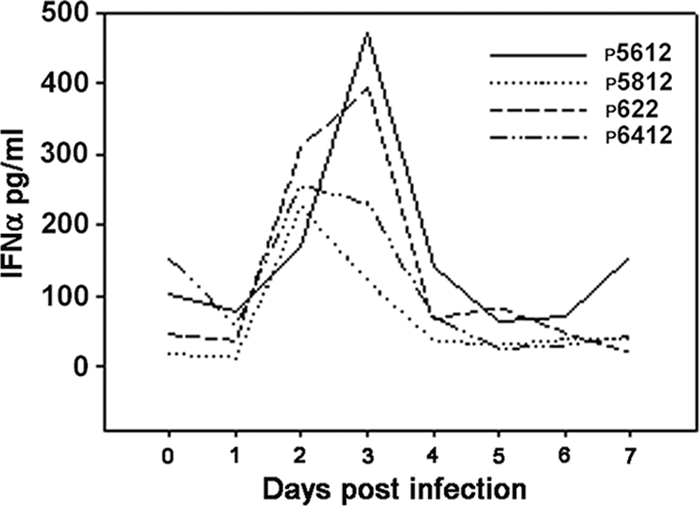
Serum level of IFN-α. Serum was prepared from blood samples of infected animals and tested for the presence of IFN-α using ELISA. Data represent a single determination from three separate experiments. Numbers in the graph legend denote individual animal identification tags.
Differential expression of NK cell-associated genes in CD2+/CD8+/CD3− cells from infected pigs.
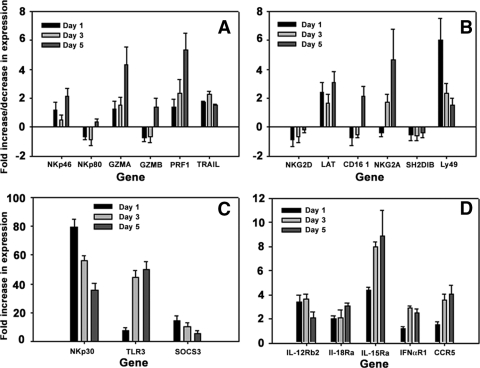
NK cells express multiple activating and inhibitory receptors, as well as effector molecules, that direct the activity of NK cells in a given environment. Therefore, it would be assumed that in a situation where the function of NK cells is hampered, the expression of NK cell receptors is skewed in favor of an inhibitory phenotype. Ideally, the measurement of the surface expression of the receptors would be more informative, but reagents are not available for the porcine homologues of these molecules on NK cells, and furthermore, not all molecules are known in the porcine model. To learn if FMDV infection alters the expression of NK cell receptor genes, we used qrtRT-PCR to measure the mRNA expression of a selected set of inhibitory and activating receptor genes and effector molecules, such as granzymes and perforin. RNA was isolated from sorted CD2+/CD8+/CD3− cells of FMDV-infected or noninfected pigs. Of the six activating receptors examined, only NKp30 was considerably upregulated 24 h after infection with FMDV, and Ly49 was moderately upregulated (Fig. 7A, B, and C). NKp30 and Ly49 gradually reduced expression at all three time points analyzed, although expression still remained at an appreciable level. NKG2D, NKp80, and granzyme B were little changed at all time points examined, whereas the expression of two other important effector molecules, granzyme A and perforin, gradually increased. One inhibitory receptor, NKG2A, was moderately increased by day 3 in all animals tested. Results show the varied expression of NK cell receptor mRNA during infection, which does not correlate with the dysfunctional status of the NK cells.
FIG. 7.
mRNA expression by qrtRT-PCR. CD2+/CD8+/CD3− cells were purified from PBMC of infected animals on days 1, 3, and 5. RNA was isolated, and qrtRT-PCR was performed on the transcribed cDNA. (A to C) NK cell-associated genes; (D) cytokine receptor genes. Data are presented as the mean expression levels relative to those of CD2+/CD8+/CD3− cells from noninfected animals. The experiment was repeated three times.
The inability of NK cells to function also could result from inadequate cytokine receptor expression. Therefore, we investigated the mRNA expression of IL-12Rβ2, IL-15Rα, IL-18Rα, and IFN-αR1 on the CD2+/CD8+/CD3− cell subset during infection with FMDV. In cells isolated from infected animals, all five cytokine receptors were moderately upregulated at all time points examined compared to results for cells from noninfected animals. IL-15Rα mRNA showed the highest increase in expression, increasing each day to nearly 10-fold (Fig. 7D). There also was significant upregulation of TLR3 mRNA and more moderate activation of CCR5 (Fig. 7C, D). Of interest was the nearly 20-fold induction of SOCS3 mRNA by 24 h, which rapidly waned thereafter. These results minimally demonstrate that porcine NK cells do transcribe the genes for the receptors for these cytokines. The cytokine receptor mRNA expression pattern shown here appears to be appropriate for the activated environment such that of as a viral infection, therefore cytokine receptors may not participate in the aberrant function of porcine NK cells during infection with FMDV.
Influence of viral proteins on function of porcine NK cells.
We investigated whether the observed dysfunction of NK cells could be attributed to virulence factors encoded by the virus. Using a genetically attenuated strain of FMDV, LL-A12 virus, and the parent strain, wild-type A12, we compared the activity of NK cells isolated from pigs infected with these viruses. The attenuation is due to a lack of the leader protease encoded by the virus, thus the designation leaderless (LL) for this attenuation. The leader protease blocks cap-dependent mRNA translation in infected cells by cleaving elongation factor 4G in the ribosomal assembly (19).
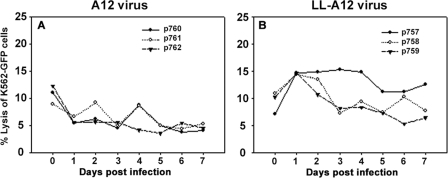
NK cell activity from animals infected with the wild-type virus showed a trend between days 1 and 3 (Fig. 8A) that is dysfunctional similarly to the previous experiments using FMDV strain O1 Campos. In contrast, NK cells derived from LL-A12-infected pigs increased the cytolytic activity within 24 h of infection (Fig. 8B). The supposition that some viral proteins are involved in impairing the functions of porcine NK cells also was evident in the serum cytokine profile of IL-15 and IL-18 of the same animals. IL-15 was detected at a higher level in animals infected with LL-A12 than in A12-infected pigs from day 3 onwards (Fig. 9A and B). However, the difference between the two groups was not statistically significant (P = 0.065) due to high variability among individual animals. Also, there was no significant difference observed for IL-18 (Fig. 9C and D) between wild-type and attenuated virus-infected animals (P = 0.071). Surprisingly, we could not detect IL-12 and IFN-α in either group of animals. Also, the difference in the induction of IL-15 and IL-18 or a lack thereof during infection with 01 Campos and A12 viruses is not understood and may be virus strain dependent. These results indicated that the leader protease is involved in downregulating the NK cell activity following infection with FMDV, possibly by blocking cytokine-inducing signals from infected cells.
FIG. 8.
Comparison of NK cell activity of two strains of FMDV. Two groups of three pigs each were infected with either A12 or LL-A12 (a laboratory genetically attenuated strain). PBMC were isolated on 7 consecutive days and incubated with K562-GFP cells to assess the lytic activity. (A) A12; (B) LL-A12. Data are representative of a single experiment. Day 0 denotes samples from the same animals before infection. Numbers in the graph legend denote individual animal identification tags.
FIG. 9.
Serum levels of IL-15 and IL-18. Serum was prepared from blood samples collected from either infected or noninfected pigs, and ELISA was performed for IL-15 and IL-18. Shown are levels of IL-15 in A12 (A)- and LL-A12 (B)-infected pigs as well as levels of IL-18 in A12 (C)- and LL-A12 (D)-infected pigs. Representative data are from a single experiment. Day 0 denotes samples from the same animals before infection. Numbers in the graph legend denote individual animal identification tags.
DISCUSSION
These studies have characterized the activity of NK cells from animals infected with FMDV. We show that NK-dependent cytotoxicity against FMDV-infected cells as the killing target or a standard tumor cell target is impaired. Also, the proportion of NK cells capable of producing IFN-γ and storing perforin is reduced. In vivo, the virus replicating in susceptible tissue appears to create an activated environment for porcine NK cells. The loss of function therefore is not anticipated, as there is no detectable infection of the circulating NK cells, although that does not rule out a low level of abortive infection. Few NK cell receptors are modulated in expression, but initially, there is a significant induction of one receptor, NKp30, which then decreases through day 5. This is known to be an activating receptor in other species.
Reduction in NK cell cytotoxicity coincides with the elevation in virus titer in peripheral blood. For instance, cells were not reactive to an in vitro-infected target, a target cell type that most reflected an in vivo encounter. Further, prolonging the incubation of these NK cells with targets did not improve the cytolytic activity, and additional stimulation with exogenous cytokines could not rescue the killing capability of NK cells. An exception was observed only when TLR7/8 agonist was used to restimulate the cells, illuminating the potential of these agonists as biotherapeutics.
In analyzing the dysfunction of porcine NK cells during FMDV, it is worth considering at least three factors that converge on the role of these cells in infection, i.e., viremia, lymphopenia, and high fever. All of the functions assessed on the NK cells were at their lowest at the time of high virus load in circulation, a lower number of lymphocytes in the blood, and high body temperature. The effects of these three factors could give way to defects in generic NK cell functions, such as cytotoxicity, cytokine and chemokine secretion, and NK cell proliferation. Viremia is associated with poor NK cell performance in HIV infection in humans as well (37). In fact, the capability of NK or CD8+ T cells to suppress endogenous HIV replication inversely correlated with virus load such that high viral titers coincide with less NK cell suppression of viral replication. Also, the viral load negatively influenced the secretory function of NK and CD8+ T cells from viremic individuals as opposed to that of aviremic patients (30).
An increase in temperature led to a reduction in the activity of human NK cells incubated in vitro at 42°C for 1 h, just as intentional hyperthermia for cancer therapy at 42°C produced a similar reduction of NK cell cytotoxicity. In both instances NK cell function was restored after the temperatures were returned to normal (27). In those studies, the authors attributed the perturbation in NK cell function to the downregulation of perforin and granzyme B (29) by CD56dim cells, a population of NK cells in humans that is characterized by high cytotoxicity. A similar example of multifactorial influence on early immune responses is described for Ebola hemorrhagic fever virus infection in cynomologous macaques, where the numbers as well as function of NK cells were low, and there was no indication of a robust immune response to infection (46).
Previously, we reported the induction of lymphopenia during the acute phase of FMDV infection (4), but this report is the first to connect the impact of lymphopenia in FMDV to the dysfunction of NK cells in the infected pigs. A recent report by Mohan et al. (38) indicates the occurrence of a similar phenomenon in cattle and buffaloes and indicates the correlation between an increase in body temperature and leukocyte reduction. Regarding lymphocyte kinetics after infection, FMDV differs from other viral infections in that NK cell numbers appear to be reduced already in the acute phase. In HIV, initially there is a selective increase of CD3−/CD56dim/CD16+ cells and a depletion of CD3−/CD56bright/CD16− cells, but as the infection progresses, CD3−/CD56dim/CD16+ cells are depleted while CD3−/CD56bright/CD16− cells become anergic (29). Therefore, these three factors, viremia, lymphopenia, and fever, induced concurrently during acute FMDV infection, appear to exert a strong inhibitory effect on the cytolytic and secretory function of porcine NK cells.
The activation of NK cells is said to require cytokines such as IFN-α (56). Indeed, earlier in infection, FMDV induces the production of IFN-α, which is detectable in serum on days 2 and 3 postinfection in swine. However, it is surprising that even though this cytokine is present in the peripheral blood, the NK cells from FMDV-infected pigs fail to function. This observation is not attributable simply to the loss of NK cells from peripheral blood, as NK cells (CD2+/CD8+/CD3−) isolated on these days following infection are inactive. Apparently, other mechanisms are required to fully restore and maintain the functional status of these cells. On the other hand, the higher levels of IFN-α may be incapacitating for NK cells due to strong stimulation. The repeated administration of pI:C in piglets, which was intended to stimulate NK cells, only rendered them hyporeactive due to higher levels of IFN-α in the serum (18). However, the mechanism leading to hyporeactivity remains undefined.
In the case of FMDV, a negative feedback mechanism could be implicated in this event, such as the upregulation of the SOCS genes. Increased levels of IFN-α could signal through IFN-αR1, inducing the upregulation of SOCS3 and leading to a blockade in IFN-α-induced gene expression programs (33). Alternatively, since FMDV is an RNA virus, an increase in SOCS3 mRNA could be mediated through RIG-I or TLR3 sensing, as suggested by Pothlichet et al. (43). In our results, both TLR3, and more transiently, SOCS3 mRNAs were upregulated, indicating the possibility of such a mechanism for the inhibition of NK cell response.
Regarding other cytokines of importance to NK cell activation, we could not detect IL-12, IL-15, and IL-18 in the serum of pigs infected with FMDV strain O1 Campos, but the cytokines could be detected in the serum of animals infected with the A12 strain and the attenuated derivative, LL-A12. Presumably, the efficiency with which each strain effects host cell protein synthesis was different. The lack of these cytokines could further contribute to the lack of the cytotoxic potential of NK cells after infection with FMDV. Barnett et al. (3) reported no elevation of IL-12 in pigs challenged with FMDV strain O1 Lausanne. The lack of NK cell-activating cytokines in serum may indicate an expected lack of protein synthesis due to the manner in which FMDV alters the cell protein synthesis machinery.
The leader protease cleaves elongation factor 4G (elF-4G), therefore shutting down the cap-dependent translation of cellular mRNA (19). Consequently, the infected cells may fail to translate new proteins (48). However, this mechanism is likely only in the case of an infected cell. There is no clear evidence that innate cytokine-producing cells are infected with FMDV, yet the inhibition of cytokine secretion has been reported (5, 39). The infection of pigs with the attenuated so-called leaderless virus is marked by the activation of NK cell activity, while the parent wild-type virus inhibits NK cell function in vivo. Kottilil et al. (31) found that HIV envelope proteins derived from R5 or X4 subtype isolates altered the expression of genes associated with NK cell function. They upregulated genes involved in apoptosis and downregulated genes involved in cell proliferation. Such NK cells were dysfunctional regarding NK cell cytotoxicity and cytokine secretion.
One alternative explanation for a lack of NK cell cytotoxic activity when testing cells isolated from infected pigs is that NK cells encountered target cells in vivo, preceding isolation and analysis ex vivo. NK cells from HIV-infected individuals display a substantial expression of CD107a ex vivo without in vitro stimulation with target cells (45). We did a similar assessment of CD107a to discriminate several populations of NK cells (1) beyond the killing assays and cytokine secretion. We were unable to detect CD107a expressed at significant levels on cells from infected animals, meaning that the cells likely were not previously engaged in cytolysis. These results indicate that rather than exhausting NK cell function by interaction with infected cells, a strong inhibition of NK cell activation occurs in vivo during FMDV infection.
An array of surface receptors modulates the general function of NK cells through a balance of the inhibitory and activating receptors that recognize ligands on infected cells. The measurement of mRNA expression for some of these molecules shows little alteration in gene expression in cells from infected animals, including NKG2D, NKp80, and granzyme B. On the other hand, the expression of another receptor, NKp30, was significantly induced, an expected result for cells originating from an activated environment. However, these cells were dysfunctional.
In animal species where these molecules have been studied in depth, the downregulation of activating receptors was associated with defective NK cell cytotoxicity (37). The upregulation of CCR5 has been reported in HIV infection that is associated with assisting virus entry into CD4+ T cells. Its expression on NK cells is not clearly understood, but possibly it interacts with viral envelope protein to cause defects in their function (31). Unfortunately, no information is available yet about the precise function of the swine homologues of these genes expressed by NK cells. Therefore, it is difficult to speculate how this pattern of expression relates to the observed dysfunctional nature of porcine NK cells during infection with FMDV. Moreover, the narrow selection of receptors analyzed does not allow the drawing of definitive conclusions on a possible tipping of the balance toward a cell phenotype that is dysfunctional. Although cytokine receptor mRNAs were appreciably expressed, we could not detect appropriate cytokines in the serum. This observation could argue in favor of NK cell dysfunction, since no cytokine signals would be transduced, even though cytokine receptor expression was intact. However, due to the lack of reagents, we could not examine the NK cell surface cytokine receptor expression.
Although the mechanisms of NK cell dysfunction during FMDV infection remain unresolved, it is certain that FMDV infection is associated with functional perturbations in NK cells, which include reduced cytotoxicity, the altered expression of NK cell receptors, and a reduced ability to secrete cytokines. This dysfunction may result from the direct effect of FMDV on NK cells, although these cells are not productively infected. The nonproductive infection of these cells, below the detection levels of our assays, is a situation that cannot be ruled out. Alternatively, the virus may induce the deregulation of signals from infected cells, resulting in the induction of an anergic state in the NK cells. Potential mechanisms of such a situation presently are under investigation. In conclusion, these results illustrate another aspect of the evasion of host antiviral responses by FMDV infection.
Acknowledgments
This work was supported by CRIS 1940-32000-052-00D (W.T.G.) and 1235-51000-051-00 (H.D.) from the Agricultural Research Service, USDA, and interagency agreement number 60-1940-8-037 between the Department of Homeland Security, Science, and Technology Directorate and the USDA (W.T.G.).
C. Nfon was the recipient of a Plum Island Animal Disease Center Research Participation Program fellowship, administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and the USDA.
We thank Mary Kenney for her technical assistance, Kathy Apicelli for assistance with illustrations, and the animal care staff at the Plum Island Animal Disease Center for their professional support and assistance. We thank Richard Miller of 3M Pharmaceuticals, Inc. (Minneapolis, MN) for the generous gift of the TLR7/8 agonist compounds. Finally, we thank D. Mark Estes, University of Texas Medical Branch at Galveston, for his consultation and discussion of this work.
No competing financial interests exist.
Footnotes
Published ahead of print on 14 October 2009.
REFERENCES
- 1.Alter, G., J. M. Malenfant, and M. Altfeld. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15-22. [DOI] [PubMed] [Google Scholar]
- 2.Arase, H., E. S. Mocarski, A. E. Campbell, A. B. Hill, and L. L. Lanier. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296:1323-1326. [DOI] [PubMed] [Google Scholar]
- 3.Barnett, P. V., S. J. Cox, N. Aggarwal, H. Gerber, and K. C. McCullough. 2002. Further studies on the early protective responses of pigs following immunisation with high potency foot and mouth disease vaccine. Vaccine 20:3197-3208. [DOI] [PubMed] [Google Scholar]
- 4.Bautista, E. M., G. S. Ferman, and W. T. Golde. 2003. Induction of lymphopenia and inhibition of T cell function during acute infection of swine with foot and mouth disease virus (FMDV). Vet. Immunol. Immunopathol. 92:61-73. [DOI] [PubMed] [Google Scholar]
- 5.Bautista, E. M., G. S. Ferman, D. Gregg, M. C. Brum, M. J. Grubman, and W. T. Golde. 2005. Constitutive expression of alpha interferon by skin dendritic cells confers resistance to infection by foot-and-mouth disease virus. J. Virol. 79:4838-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittleman, D. B., K. K. Maves, J. A. Bertolatus, S. M. Bonsib, P. Densen, Z. K. Ballas, and J. M. Weiler. 1994. Recurrent infections, pericarditis and renal disease in a patient with total C2 deficiency and decreased NK cell function consistent with acute rheumatic fever and systemic lupus erythematosus. Ann. Rheum. Dis. 53:280-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco, E., M. Garcia-Briones, A. Sanz-Parra, P. Gomes, E. De Oliveira, M. L. Valero, D. Andreu, V. Ley, and F. Sobrino. 2001. Identification of T-cell epitopes in nonstructural proteins of foot-and-mouth disease virus. J. Virol. 75:3164-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borysiewicz, L. K., B. Rodgers, S. Morris, S. Graham, and J. G. Sissons. 1985. Lysis of human cytomegalovirus infected fibroblasts by natural killer cells: demonstration of an interferon-independent component requiring expression of early viral proteins and characterization of effector cells. J. Immunol. 134:2695-2701. [PubMed] [Google Scholar]
- 9.Boysen, P., and A. K. Storset. 2009. Bovine natural killer cells. Vet. Immunol. Immunopathol. 130:163-177. [DOI] [PubMed] [Google Scholar]
- 10.Carr, D. J. J., T. Wuest, and J. Ash. 2008. An increase in herpes simplex virus type 1 in the anterior segment of the eye is linked to a deficiency in NK cell infiltration in mice deficient in CXCR3. J. Interferon Cytokine Res. 28:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr, W. H., A.-M. Little, E. Mocarski, and P. Parham. 2002. NK cell-mediated lysis of autologous HCMV-infected skin fibroblasts is highly variable among NK cell clones and polyclonal NK cell lines. Clin. Immunol. 105:126-140. [DOI] [PubMed] [Google Scholar]
- 12.Cerwenka, A., and L. L. Lanier. 2001. Ligands for natural killer cell receptors: redundancy or specificity. Immunol. Rev. 181:158-169. [DOI] [PubMed] [Google Scholar]
- 13.Chinsangaram, J., M. Koster, and M. J. Grubman. 2001. Inhibition of L-deleted foot-and-mouth disease virus replication by alpha/beta interferon involves double-stranded RNA-dependent protein kinase. J. Virol. 75:5498-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 15.Cottam, E. M., D. T. Haydon, D. J. Paton, J. Gloster, J. W. Wilesmith, N. P. Ferris, G. H. Hutchings, and D. P. King. 2006. Molecular epidemiology of the foot-and-mouth disease virus outbreak in the United Kingdom in 2001. J. Virol. 80:11274-11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delano, M. L., and D. G. Brownstein. 1995. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus replication by cells with an NK phenotype. J. Virol. 69:5875-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denyer, M. S., T. E. Wileman, C. M. Stirling, B. Zuber, and H. H. Takamatsu. 2006. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet. Immunol. Immunopathol. 110:279-292. [DOI] [PubMed] [Google Scholar]
- 18.Derbyshire, J. B., and C. E. Lesnick. 1990. Hyporeactivity to interferon induction in newborn piglets. J. Interferon Res. 10:47-53. [DOI] [PubMed] [Google Scholar]
- 19.Devaney, M. A., V. N. Vakharia, R. E. Lloyd, E. Ehrenfeld, and M. J. Grubman. 1988. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J. Virol. 62:4407-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerner, W., T. Käser, and A. Saalmüller. 2009. Porcine T lymphocytes and NK cells—an update. Dev. Comp. Immunol. 33:310-320. [DOI] [PubMed] [Google Scholar]
- 21.Gerosa, F., B. Baldani-Guerra, C. Nisii, V. Marchesini, G. Carra, and G. Trinchieri. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golde, W. T., J. M. Pacheco, H. Duque, T. Doel, B. Penfold, G. S. Ferman, D. R. Gregg, and L. L. Rodriguez. 2005. Vaccination against foot-and-mouth disease virus confers complete clinical protection in 7 days and partial protection in 4 days: use in emergency outbreak response. Vaccine 23:5775-5782. [DOI] [PubMed] [Google Scholar]
- 23.Gorski, K. S., E. L. Waller, J. Bjornton-Severson, J. A. Hanten, C. L. Riter, W. C. Kieper, K. B. Gorden, J. S. Miller, J. P. Vasilakos, M. A. Tomai, and S. S. Alkan. 2006. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int. Immunol. 18:1115-1126. [DOI] [PubMed] [Google Scholar]
- 24.Gray, J. D., C. G. Brooks, and R. W. Baldwin. 1981. Detection of either rapidly cytolytic macrophages or NK cells in “activated” peritoneal exudates depends on the method of analysis and the target cell type. Immunology 42:561-568. [PMC free article] [PubMed] [Google Scholar]
- 25.Grubman, M. J., and B. Baxt. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17:465-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzman, E., G. Taylor, B. Charleston, M. A. Skinner, and S. A. Ellis. 2008. An MHC-restricted CD8+ T-cell response is induced in cattle by foot-and-mouth disease virus (FMDV) infection and also following vaccination with inactivated FMDV. J. Gen. Virol. 89:667-675. [DOI] [PubMed] [Google Scholar]
- 27.Harada, H., T. Murakami, S. S. Tea, A. Takeuchi, T. Koga, S. Okada, M. A. Suico, T. Shuto, and H. Kai. 2007. Heat shock suppresses human NK cell cytotoxicity via regulation of perforin. Int. J. Hyperthermia 23:657-665. [DOI] [PubMed] [Google Scholar]
- 28.He, X. S., M. Draghi, K. Mahmood, T. H. Holmes, G. W. Kemble, C. L. Dekker, A. M. Arvin, P. Parham, and H. B. Greenberg. 2004. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J. Clin. Investig. 114:1812-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koga, T., H. Harada, T. S. Shi, S. Okada, M. A. Suico, T. Shuto, and H. Kai. 2005. Hyperthermia suppresses the cytotoxicity of NK cells via down-regulation of perforin/granzyme B expression. Biochem. Biophys. Res. Commun. 337:1319-1323. [DOI] [PubMed] [Google Scholar]
- 30.Kottilil, S., T. W. Chun, S. Moir, S. Liu, M. McLaughlin, C. W. Hallahan, F. Maldarelli, L. Corey, and A. S. Fauci. 2003. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 187:1038-1045. [DOI] [PubMed] [Google Scholar]
- 31.Kottilil, S., K. Shin, J. O. Jackson, K. N. Reitano, M. A. O'Shea, J. Yang, C. W. Hallahan, R. Lempicki, J. Arthos, and A. S. Fauci. 2006. Innate immune dysfunction in HIV infection: effect of HIV envelope-NK cell interactions. J. Immunol. 176:1107-1114. [DOI] [PubMed] [Google Scholar]
- 32.Krmpotiæ, A., D. H. Busch, I. Bubic, F. Gebhardt, H. Hengel, M. Hasan, A. A. Scalzo, U. H. Koszinowski, and S. Jonjic. 2002. MCMV glycoprotein gp40 confers virus resistance to CD8+ T cells and NK cells in vivo. Nat. Immunol. 3:529-535. [DOI] [PubMed] [Google Scholar]
- 33.Lang, R., A.-L. Pauleau, E. Parganas, Y. Takahashi, J. Mages, J. N. Ihle, R. Rutschman, and P. J. Murray. 2003. SOCS3 regulates the plasticity of gp130 signaling. Nat. Immunol. 4:546-550. [DOI] [PubMed] [Google Scholar]
- 34.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225-274. [DOI] [PubMed] [Google Scholar]
- 35.Long, B. R., J. Michaelsson, C. P. Loo, W. M. Ballan, B.-A. N. Vu, F. M. Hecht, L. L. Lanier, J. M. Chapman, and D. F. Nixon. 2008. Elevated frequency of gamma interferon-producing NK cells in healthy adults vaccinated against influenza virus. Clin. Vaccine Immunol. 15:120-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mateu, M. G. 1995. Antibody recognition of picornaviruses and escape from neutralization: a structural view. Virus Res. 38:1-24. [DOI] [PubMed] [Google Scholar]
- 37.Mavilio, D., J. Benjamin, M. Daucher, G. Lombardo, S. Kottilil, M. A. Planta, E. Marcenaro, C. Bottino, L. Moretta, A. Moretta, and A. S. Fauci. 2003. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl. Acad. Sci. USA 100:15011-15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohan, M., M. Gajendragad, S. Gopalakrishna, and N. Singh. 2008. Comparative study of experimental foot-and-mouth disease in cattle (Bos indicus) and buffaloes (Bubalis bubalus). Vet. Res. Comm. 32:481-489. [DOI] [PubMed] [Google Scholar]
- 39.Nfon, C. K., G. S. Ferman, F. N. Toka, D. A. Gregg, and W. T. Golde. 2008. Interferon-alpha production by swine dendritic cells is inhibited during acute infection with foot-and-mouth disease virus. Viral Immunol. 21:68-77. [DOI] [PubMed] [Google Scholar]
- 39a.Nfon, C. K., F. N. Toka, M. Kenney, J. M. Pacheco, and W. T. Golde. Loss of plasmacytoid dendritic cell function coincides with lymphopenia and viremia during foot-and-mouth disease virus (FMDV) infection. Viral Immunol., in press. [DOI] [PubMed]
- 40.Pacheco, J. M., T. M. Henry, V. K. O'Donnell, J. B. Gregory, and P. W. Mason. 2003. Role of nonstructural proteins 3A and 3B in host range and pathogenicity of foot-and-mouth disease virus. J. Virol. 77:13017-13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker, A. K., S. Parker, W. M. Yokoyama, J. A. Corbett, and R. M. L. Buller. 2007. Induction of natural killer cell responses by ectromelia virus controls infection. J. Virol. 81:4070-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pintaric, M., W. Gerner, and A. Saalmüller. 2008. Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-gamma production of porcine natural killer cells. Vet. Immunol. Immunopathol. 121:68-82. [DOI] [PubMed] [Google Scholar]
- 43.Pothlichet, J., M. Chignard, and M. Si-Tahar. 2008. Cutting edge: innate immune response triggered by influenza a virus is negatively regulated by SOCS1 and SOCS3 through a RIG-I/IFNAR1-dependent pathway. J. Immunol. 180:2034-2038. [DOI] [PubMed] [Google Scholar]
- 44.Rager-Zisman, B., P. C. Quan, M. Rosner, J. R. Moller, and B. R. Bloom. 1987. Role of NK cells in protection of mice against herpes simplex virus-1 infection. J. Immunol. 138:884-888. [PubMed] [Google Scholar]
- 45.Ravet, S., D. Scott-Algara, E. Bonnet, H. K. Tran, T. Tran, N. Nguyen, L. X. Truong, I. Theodorou, F. Barre-Sinoussi, G. Pancino, and P. Paul. 2007. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood 109:4296-4305. [DOI] [PubMed] [Google Scholar]
- 46.Reed, D. S., L. E. Hensley, J. B. Geisbert, P. B. Jahrling, and T. W. Geisbertl. 2004. Depletion of peripheral blood T lymphocytes and NK cells during the course of Ebola hemorrhagic fever in cynomolgus macaques. Viral Immunol. 17:390-400. [DOI] [PubMed] [Google Scholar]
- 47.Reschner, A., P. Hubert, P. Delvenne, J. Boniver, and N. Jacobs. 2008. Innate lymphocyte and dendritic cell cross-talk: a key factor in the regulation of the immune response. Clin. Exp. Immunol. 152:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanz-Parra, A., F. Sobrino, and V. Ley. 1998. Infection with foot-and-mouth disease virus results in a rapid reduction of MHC class I surface expression. J. Gen. Virol. 79:433-436. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt, K. N., B. Leung, M. Kwong, K. A. Zarember, S. Satyal, T. A. Navas, F. Wang, and P. J. Godowski. 2004. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J. Immunol. 172:138-143. [DOI] [PubMed] [Google Scholar]
- 50.Scott-Algara, D., F. Vuillier, A. Cayota, and G. Dighiero. 1992. Natural killer (NK) cell activity during HIV infection: a decrease in NK activity is observed at the clonal level and is not restored after in vitro long-term culture of NK cells. Clin. Exp. Immunol. 90:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sivori, S., M. Falco, M. D. Chiesa, S. Carlomagno, M. Vitale, L. Moretta, and A. Moretta. 2004. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc. Natl. Acad. Sci. USA 101:10116-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thapa, M., R. S. Welner, R. Pelayo, and D. J. J. Carr. 2008. cxcl9 and cxcl10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J. Immunol. 180:1098-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toka, F. N., C. K. Nfon, H. Dawson, and W. T. Golde. 2009. Accessory-cell-mediated activation of porcine NK cells by Toll-like receptor 7 (TLR7) and TLR8 agonists. Clin. Vaccine Immunol. 16:866-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toka, F. N., C. K. Nfon, H. Dawson, D. Mark Estes, and W. T. Golde. 2009. Activation of Porcine Natural Killer (NK) Cells and Lysis of Foot-and-Mouth Disease Virus (FMDV) Infected Cells. J. Interferon Cytokine Res. 29:179-192. [DOI] [PubMed] [Google Scholar]
- 55.Tomasec, P., V. M. Braud, C. Rickards, M. B. Powell, B. P. McSharry, S. Gadola, V. Cerundolo, L. K. Borysiewicz, A. J. McMichael, and G. W. Wilkinson. 2000. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science 287:1031-1033. [DOI] [PubMed] [Google Scholar]
- 56.Tomescu, C., J. Chehimi, V. C. Maino, and L. J. Montaner. 2007. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: requirement for IFN-mediated NK activation by plasmacytoid dendritic Cells. J. Immunol. 179:2097-2104. [DOI] [PubMed] [Google Scholar]
- 57.Zitvogel, L., M. Terme, C. Borg, and G. Trinchieri. 2006. Dendritic cell-NK cell cross-talk: regulation and physiopathology. Curr. Top. Microbiol. Immunol. 298:157-174. [DOI] [PubMed] [Google Scholar]