Abstract
Forty-nine typical and atypical enteropathogenic Escherichia coli (EPEC) strains belonging to different serotypes and isolated from humans, pets (cats and dogs), farm animals (bovines, sheep, and rabbits), and wild animals (monkeys) were investigated for virulence markers and clonal similarity by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST). The virulence markers analyzed revealed that atypical EPEC strains isolated from animals have the potential to cause diarrhea in humans. A close clonal relationship between human and animal isolates was found by MLST and PFGE. These results indicate that these animals act as atypical EPEC reservoirs and may represent sources of infection for humans. Since humans also act as a reservoir of atypical EPEC strains, the cycle of mutual infection of atypical EPEC between animals and humans, mainly pets and their owners, cannot be ruled out since the transmission dynamics between the reservoirs are not yet clearly understood.
Enteropathogenic Escherichia coli (EPEC) strains are among the major causes of infantile diarrhea in developing countries (71) and can be classified as typical and atypical, depending on the presence or absence of the E. coli adherence factor plasmid (pEAF), respectively (39).
The pathogenesis of EPEC resides in the ability to cause the attaching and effacing (A/E) lesion in the gut mucosa of human or animal hosts, leading to diarrheal illness (40). The genes responsible for the A/E lesion formation are located in a chromosomal pathogenicity island of ∼35 kb, known as the locus of enterocyte effacement (LEE) (23, 47). LEE encodes an adhesin called intimin (38), its translocated receptor (Tir) (42), components of a type III secretion system (36), and effector molecules, named E. coli-secreted proteins (Esp proteins) (41). These virulence factors have a crucial role in A/E lesion formation, and their detection in EPEC strains is an indicator of their potential to produce these lesions (19, 56).
Atypical EPEC strains have been associated with diarrhea outbreaks in developed countries (31, 73, 77) and with sporadic cases of diarrhea in developing and developed countries (1, 12, 26, 52, 55). At present, the prevalence of atypical EPEC is higher than that of typical EPEC in several countries (1, 12, 26, 52, 55, 65).
Different from the situation in developed countries, where atypical EPEC outbreaks and sporadic infections are associated with children and adults, atypical EPEC infection in Brazil is mainly associated with children's illnesses (32, 71).
Typical EPEC strains are rarely isolated from animals, and humans are the major natural reservoir for these pathogens (14, 32, 53, 71). In contrast, atypical EPEC strains are present in both healthy and diseased animals (dog, monkey, cats, and bovines) and humans (4, 6, 18, 28, 71). Some studies have associated pets and farm and wild animals as reservoirs and infection sources of atypical EPEC strains for humans (32). However, these studies did not compare atypical EPEC strains isolated from humans and animals by gold-standard molecular methods like multilocus sequence typing (MLST) or pulsed-field gel electrophoresis (PFGE) (15, 35, 43, 53). For this reason, there are some doubts about whether atypical EPEC strains isolated from animals represent risks for human health and whether animals really play the role of reservoirs of atypical EPEC.
The aim of this study was to compare atypical EPEC strains isolated from humans and different animals, including pets (cats and dogs), farm animals (bovines, ovines, and rabbits), and wild animals (monkeys), by molecular phylogenetic techniques to verify the role of animals as reservoirs of and sources of infection with atypical EPEC in humans.
MATERIALS AND METHODS
Bacterial strains.
This study was based on the analysis of 42 atypical EPEC strains isolated from humans (n = 20) and from pets and farm and wild animals (cats, dogs, rabbits, bovines, ovines, and monkeys; n = 22) described in previous studies (3, 13, 14, 19, 21, 29, 44, 46, 51, 53, 59, 60, 66, 72). Seven typical EPEC strains isolated from humans and animals, belonging to serotypes O127:H40 and O142:H6 (14, 46), were also included to be compared with atypical EPEC strains. All selected isolates belong to classical EPEC serotypes that commonly cause disease in both humans and animals and were isolated in unrelated epidemiological studies from diseased or healthy humans and animals between 1954 and 2003 in Brazil and other countries (Table 1).
TABLE 1.
Origin, clinical data, and genotypic and phenotypic characteristics of typical and atypical EPEC strains used in this study
| Strain | Source | Origin, year of isolation, and clinical dataa | PCR detection of the indicated virulence geneb,c |
MLST profile | PFGE pattern | Serotype | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eae subtype | tir subtype | bfpA | espA | espB | espD | espFd | EHEC hlyA | ||||||
| HSP20 | Human | BRA, 1985, D | ζtw | α | − | − | +e | +e | ‡ | − | ST1 | PP4 | O111:H[9] |
| CDC2416 | Human | USA, 1970, D | ζtw | α | − | − | +e | +e | ‡ | − | ST2 | PP4 | O111:H[9] |
| HE8 | Dog | BRA, 1999, D | θ/γ2 | α | − | + | +s | +e | ‡ | − | ST3 | PP7 | O111:H25 |
| HE9 | Dog | BRA, 1999, D | θ/γ2 | α | − | + | +e | +s | + | − | ST4 | PP3 | O111:H25 |
| UIPA17(8) | Cat | BRA, 2004, ND | θ/γ2 | α | − | − | +e | +s | + | − | ST4 | PP7 | O111:H25 |
| CDC2858 | Human | USA, 1971, D | θ/γ2 | − | − | + | +e | +s | + | − | ST4 | PP3 | O111:H25 |
| HSP105 | Human | BRA, 1985,D | θ/γ2 | − | − | + | +e | +s | ‡ | − | ST5 | PP7 | O111:H25 |
| B1 col1 | Dog | BRA, 1998, D | θ/γ2 | α | − | + | +s | +s | + | − | ST6 | PP7 | O111:H25 |
| SPA14 | Dog | BRA, 1998, D | β1 | β | − | + | +s | +e | − | − | ST7 | PP1 | O119:H2 |
| BIO12 | Dog | BRA, 1999, D | β1 | β | − | + | +s | +e | − | − | ST7 | PP1 | O119:H2 |
| 34 | Human | BRA, 1993, D | β1 | β | − | + | +s | +e | − | − | ST7 | PP1 | O119:H2 |
| 30 | Human | BRA, 1993, D | β1 | β | − | + | +s | +e | − | − | ST7 | PP1 | O119:H2 |
| DIF11 | Human | BRA, unknown, D | β1 | β | − | + | +s | +e | − | − | ST8 | PP10 | O119:H2 |
| OG1999 | Human | BRA, unknown, D | β1 | β | − | + | +s | +e | − | − | ST7 | PP10 | O119:H2 |
| 147 | Rabbit | BRA, 2000, D | β1 | β | − | + | +O26 | +e | − | − | ST9 | PP10 | O119:H2 |
| CB3114 | Human | AUS, 1993, D | α2 | γ | − | + | +e | +e | + | − | ST10 | PP8 | O125:H[6] |
| CB10.101 | Cat | BRA, 2004, D | α2 | α | − | + | +e | +s | + | − | ST10 | PP8 | O125:H6 |
| CB1924 | Human | GER, 1992, D | α2 | − | − | + | +e | +e | + | − | ST10 | PP8 | O125:H6 |
| EC292/92 | Human | BRA, 1993, D | α2 | β | − | + | +e | +e | + | − | ST10 | PP8 | O125:H6 |
| 3 | Monkey | BRA, 2003, D | θ/γ2 | α | − | + | +e | +e | ‡ | − | ST11 | PP2 | O127:H40 |
| 12A | Monkey | BRA, 2003, D | θ/γ2 | α | − | + | +e | +e | ‡ | − | ST12 | PP2 | O127:H40 |
| 15A | Monkey | BRA, 2003, unknown | θ/γ2 | − | + | + | +e | +e | ‡ | − | ST13 | PP2 | O127:H40 |
| 174 | Human | BRA, 1988, D | θ/γ2 | α | + | + | +e | +e | − | − | ST14 | PP2 | O127:H40 |
| 154 | Rabbit | BRA, 2000, D | β1 | β | − | + | +s | +s | − | − | ST9 | PP6 | O128:H[2] |
| 155 | Rabbit | BRA, 2000, D | β1 | β | − | + | +s | +s | − | − | ST9 | PP6 | O128:H[2] |
| 157 | Rabbit | BRA, 2000, D | β1 | β | − | − | +O26 | +s | − | − | ST15 | PP6 | O128:H[2] |
| 1 | Monkey | BRA, 2002, ND | β1 | β | − | + | +s | +s | − | − | ST16 | PP6 | O128:H2 |
| 163-3 | Sheep | BRA, 2002, unknown | β1 | β | − | + | +s | +s | − | − | ST17 | PP6 | O128:H2 |
| 143 | Rabbit | BRA, 2000, D | β1 | β | − | + | +s | +s | − | − | ST18 | PP6 | O128:H2 |
| 144 | Rabbit | BRA, 2000, ND | β1 | β | − | + | +O26 | +s | − | − | ST9 | PP6 | O128:H2 |
| 2254 | Human | BRA, 1975, D | β1 | β | − | + | +s | +s | − | − | ST18 | PP6 | O128:H2 |
| 3733 | Human | BRA, 1971, D | β1 | β | − | + | +s | +s | − | − | ST19 | P11 | O128:H2 |
| EC276 | Human | BRA, 1988, D | β1 | β | − | + | +s | +s | − | − | ST20 | P11 | O128:H2 |
| 15C | Monkey | BRA, 2002, unknown | β1 | β | − | + | +O26 | +s | − | − | ST9 | P11 | O128:H2 |
| 16A | Monkey | BRA, 2003, unknown | α1 | α | + | + | +e | +e | ‡ | − | ST21 | PP5 | O142:H6 |
| 16B | Monkey | BRA, 2003, unknown | α1 | α | + | + | +e | +e | ‡ | − | ST21 | PP5 | O142:H6 |
| 277 | Human | BRA, unknown, D | α1 | α | + | + | +e | +e | − | − | ST21 | PP9 | O142:H6 |
| 115 | Human | BRA, unknown, D | α1 | α | + | + | +e | +e | + | − | ST21 | PP9 | O142:H6 |
| 56 | Human | BRA, unknown, D | α1 | α | + | + | +e | +e | ‡ | − | ST22 | PP9 | O142:H6 |
| 3451-3 | Human | BRA, 1986, D | β1 | β | − | − | +s | +e | − | + | ST23 | PP13 | O26:H[11] |
| O26TREMP | Human | BRA, 2003, D | β1 | β | − | + | +O26 | +e | − | − | ST24 | PP12 | O26:H[11] |
| 30FG(5) | Bovine | BRA, 2003, unknown | β1 | α | − | + | +s | +e | − | + | ST23 | PP12 | O26:H11 |
| 20F(10) | Bovine | BRA, 2003, D | β1 | α | − | + | +O26 | +e | − | + | ST23 | PP15 | O26:H11 |
| 20F(5) | Bovine | BRA, 2003, D | β1 | β | − | + | +O26 | +e | − | + | ST25 | PP15 | O26:H11 |
| 20F(8) | Bovine | BRA, 2003, D | β1 | α | − | + | +s | +e | − | + | ST26 | PP15 | O26:H11 |
| 105B3 | Human | BRA, 1984, D | β1 | β | − | − | +s | +e | − | − | ST27 | PP13 | O26:H11 |
| 9100 | Human | PER, 1983, D | γ1 | γ | − | − | +s | +s | + | − | ST28 | PP14 | O55:H[7] |
| C997 | Human | IR, 1963, D | γ1 | γ | − | − | +s | +s | + | − | ST28 | PP14 | O55:H7 |
| 1381PSHC | Human | BRA, 1981, D | γ1 | γ | − | − | +s | +s | ‡ | − | ST29 | PP14 | O55:H7 |
BRA, Brazil; USA, United States; AUS, Australia; GER, Germany; PER, Peru; IR, Iran; D, diarrheic; ND, nondiarrheic.
Virulence genes were investigated by PCR as described in the text. All 49 strains were positive for sepL and negative for stx1, stx2, stx2f, and astA.
−, PCR negative; +, PCR positive; +e, PCR positive with primers based on EPEC E2348/69 sequence (GenBank accession no. AF022236); +s, PCR positive with primers based on EHEC EDL933 sequence (GenBank accession no. Y13068); +O26, PCR-positive with primers based on STEC O26:H sequence (GenBank accession no. Y13859).
‡, PCR generated a fragment of different size when compared with amplified fragment of the prototype strain E2348/69.
Serotyping.
The somatic (O) and flagellar (H) antigen typing was performed as described by Ørskov and Ørskov (57). Strains classified as nonmotile were analyzed by using a fliC PCR-restriction fragment length polymorphism (RFLP) method as previously described (24).
Detection of virulence factors.
PCR tests were carried out as previously described (45). DNA to be amplified was obtained from whole cells by boiling. The PCR mixture consisted of a 25-pmol sample of each primer and the following reagents (Invitrogen, Carlsbad, CA): Taq DNA polymerase (1.5 U); 10× PCR buffer (5.0 μl); dATP, dCTP, dGTP, and dTTP (0.1 mM each); MgCl2 (2.0 mM); and 1 μl of cell lysate containing template DNA. Sequences of primers, annealing temperatures, and amplicon sizes were described previously, as follows: eae (58); astA (76); bfpA (30); enterohemorrhagic E. coli (EHEC) hlyA (69); espA, espB, espD, espF, and sepL (46); pEAF (25); stx1 and stx2 (10); stx2f (70); and tir-α, tir-β and tir-γ (17) (Table 2). The PCR products were separated by electrophoresis in 1.5% agarose gels, stained with 0.5 μg/ml ethidium bromide, and visualized under UV light.
TABLE 2.
Genes encoding virulence markers investigated by PCR
| Gene coding for virulence marker (strain or serotype)a | Primer |
Annealing temp (°C) | Size of amplified fragment (bp) | Reference | |
|---|---|---|---|---|---|
| Name | Sequence 5′-3′ | ||||
| eae | SK1 | CCCGAATTCGGCACAAGCATAAGC | 52 | 863 | 58 |
| SK2 | CCCGGATCCGTCTCGCCAGTATTCG | ||||
| astA | astA1 | CCATCAACACAGTATATCCGA | 55 | 111 | 76 |
| astA2 | GGTCGCGAGTGACGGCTTTGT | ||||
| bfpA | bfpA A | AATGGTGCTTGCGCTTGCTGC | 65 | 326 | 30 |
| bfpA B | GCCGCTTTATCCAACCTGGTA | ||||
| EHEC hlyA | A1 | CACACGGAGCTTATAATATTATGTCA | 57 | 321 | 70 |
| A4 | AATGTTATCCCATTGACATCATTTGACT | ||||
| espA | espAF | TTGTTATTCCCGGTTATTTACCAAGGGAT | 50 | 570 | 46 |
| espAR | TAGTTATCTCCGGTTATTTACCAAGGGAT | ||||
| espB (EPEC E2348/69) | espB/F | CGGGATCCCGTGAGATGGTCAC | 55 | 600 | 46 |
| espB/R | CGCTCGAGGGTTGGACTTGACA | ||||
| espB (EHEC EDL933) | espBO157/F | GATAATACTCAAGTAACGATGGTT | 59 | 920 | 46 |
| espBO157/R | CCCAGCTAAGCGACCCGATT | ||||
| espB (O26:HNM) | espBO26/F | AATCAAGTAATGACGGTTAAT | 50 | 900 | 46 |
| espBO26/R | AGCTAAGCGAACCGATTG | ||||
| espD | espD/F | GTGACCATCTCACGCAAC | 50 | 1,070 | 46 |
| espD/R | CCTTGGTAAATAACCGGA | ||||
| espD (EHEC EDL933) | espDO157/F | ATGCTTAACGTAAATAACGATACC | 59 | 120 | 46 |
| espDO157/R | AATTCGGCCACTAACAATACGACT | ||||
| espF | espF/F | CCCCTCAGCCTGTTCGTCGTCTTACT | 54 | 520 | 46 |
| espF/R | TACCCTTTCTTCGTTGCTCATAG | ||||
| pEAF | eafA | CAGGGTAAAAGAAAGATGATAA | 52 | 397 | 25 |
| eafB | TATGGGGACCATGTATTATCA | ||||
| stx1 | stx1A | CAGTTAATGTGGTGGCGAAG | 55 | 894 | 10 |
| stx1B | CTGCTAATAGTTCTGCGCATC | ||||
| stx2 | stx2A | CTTCGGTATCCTATTCCCGG | 55 | 478 | 10 |
| stx2B | GGATGCATCCTGGTCATTG | ||||
| stx2f | 128-1 | AGATTGGGCGTAATTCACTGGTTG | 57 | 428 | 70 |
| 128-2 | TACTTTAATGGCCGCCCTGTCTCC | ||||
| tir-α | tirA/F | CRCCKCCAYTACCTTCACA | 54 | 342 | 17 |
| tirA/R | CGCTAACCTCCAAACCATT | ||||
| tir-β | tirB/F | CRCCKCCAYTACCTTCACA | 54 | 560 | 17 |
| tirB/R | GATTTTTCCCTCGCCACTA | ||||
| tir-γ | tirG/F | CRCCKCCAYTACCTTCACA | 54 | 781 | 17 |
| tirG/R | GTCGGCAGTTTCAGTTTCAC | ||||
| sepL | sepL/F | ACCCCGCATCTGTTTTTA | 55 | 980 | 46 |
| sepL/R | TGTATTACTCCTCTGCTCGTTATC | ||||
Where indicated, primers were based on the espB and espD sequences of a particular strain or serotype.
eae gene subtyping.
Intimin subtyping was performed based on the eae RFLP protocol described by Jenkins et al. (37). The intimin genes classified as α, β, and γ were also classified as the α1, α2, β1, β2, γ1, and γ2 types, respectively, by the PCR-RFLP protocol, as described by Oswald et al. (58).
EHEC hemolysin production.
EHEC hemolysin production was detected by a method previously described by Beutin et al. (5), using sheep blood agar containing 10% CaCl2. These assays were performed only with the EHEC hlyA-positive strains.
MLST.
MLST was performed on seven conserved housekeeping genes (arcA, cyaA, fadD, icdA, lysP, mtlD, and rpoS). These genes were selected on the basis of the most variable loci found in previous studies (7, 45). A detailed protocol of the MLST procedure, including primers and PCR and DNA sequencing conditions, is published at the EcMLST website (http://www.shigatox.net/mlst). Single colonies of E. coli strains grown on tryptic soy agar plates were used as target DNA sources. PCR products were purified with a QIAquick PCR Purification Kit (Qiagen, Germany). Sequencing reactions were carried out using a DYEnamic ET Dye Terminator Kit with Thermo Sequenase II DNA Polymerase (GE Healthcare, Milwaukee, MK), and the obtained molecules were separated on an automatic DNA sequencer (DNA MegaBACE 1000; GE Healthcare, Milwaukee, WI).
DNA sequence analysis.
The sequences were reviewed and edited by visual inspection using the SeqMan module of Lasergene (version 7.1.0) software (DNAStar, Inc. Madison, WI). After being edited, the sequences were exported to BioEdit, version 7.0.9 (http://www.mbio.ncsu.edu/BioEdit/BioEdit.html), and aligned with the ClustalW module. Differences of only 1 nucleotide allowed classification of the sequences in different alleles. The different alleles of each housekeeping gene were randomly numbered, and the allelic profiles or sequence types (STs) were determined based on the seven studied loci. Strains belonging to the same ST were considered the same clone, and just one member of each ST was used in the phylogenetic analysis.
Phylogenetic analysis.
Phylogeny was based on a supergene constructed by concatenating the individual gene sequences in the following order: arcA, cyaA, fadD, icdA, lysP, mtlD, and rpoS. The phylogenetic trees were rooted with homologous sequences from E. coli strains EDL933 and E2348/69 and Salmonella enterica strain Ty2 as an outgroup, extracted from the GenBank database. Phylogenetic trees were inferred by distance, maximum-likelihood (ML), and Bayesian methods.
The phylogenetic tree inferred by distance was constructed using the neighbor-joining (NJ) algorithm, the Kimura 2-parameter algorithm of nucleotide substitution, and bootstrapping of 1,000 replications, using MEGA4 software (http://www.megasoftware.net).
ML analysis was performed with bootstrapping of 1,000 replications using PAUP*, version 4.0b10, software (75). The best tree search was realized by heuristic methods, using tree bisection and reconnection as a branch-swapping method. Bayesian analysis was performed with MrBayes software, version 3.1 (68), for 10 million generations, sampling every 1,000 generations, with the final tree being constructed from generations 2.5 to 10 million. The Bayesian tree was inferred to validate the bootstrapping values found on NJ and ML analyses. The best-fit model of DNA substitution and the parameter estimates used for tree reconstruction on ML and Bayesian analysis were chosen by performing hierarchical likelihood ratio tests, which were implemented in PAUP* (75) with Modeltest, version 1.05 (61), and MrMODELST, version 2.3 (J. A. A. Nylander, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden), respectively.
Phylogenetic network analysis was performed with SplitsTree4 software (33) using the neighbor-net algorithm and untransformed distances (p distances). To distinguish between recurrent mutation and recombination in the genotypic diversity a pairwise homoplasy index (Φw) test was performed.
PFGE.
Preparation of genomic DNA for PFGE was performed according to the protocol of the Centers for Disease Control (Atlanta, GA), with slight modification (64). The samples were digested with 20 U of XbaI restriction enzyme (Invitrogen), and DNA fragments were resolved in 1% agarose gels in a CHEF-DR-II system (Bio-Rad Laboratories, Germany). Lambda concatemers (New England BioLabs, Ipswich, MA) with a molecular size range of 50 to 1,000 kb were used as DNA weight markers. Electrophoresis was performed for 27 h at 14°C, with a constant voltage of 6 V/cm2, using a linear pulse ramp of 3 s to 1 min 23 s in running buffer (0.5× Tris-borate-EDTA and 50 mM thiourea). Evaluation of PFGE profiles for similarity was performed with Gel Works 1D Advanced (version 3.01) software (Ultra Violet Products, Cambridge, United Kingdom). An unweighted-pair group method using average linkages tree was constructed using Dice similarity indices, complete linkage, and optimization of 1%, and position tolerance of 1.3%, as previously described (7).
Nucleotide accession numbers.
The nucleotide sequences obtained for each housekeeping allele have been submitted to the GenBank database under the accession numbers FJ561750 to FJ562092.
RESULTS
Serotypes and fliC RFLP typing.
Most O and H types of atypical EPEC strains studied in this study were determined by conventional serological methods. Nine strains classified as nonmotile had their flagellar antigen determined by fliC RFLP typing. The strains belonged to nine different serotypes, previously characterized as atypical EPEC (Table 1).
Detection of LEE, bfpA, and toxin-encoding genes.
All strains presented positive PCRs for the eae gene, and none was positive for genes encoding Shiga-toxins (stx1, stx2, and stx2f), the enteroaggregative heat-stable toxin (astA), or for the pEAF plasmid. The presence of bfpA was detected only in the seven strains previously classified as typical EPEC. The remaining 42 strains were classified as atypical EPEC strains, i.e., having the genetic profile eae positive, pEAF positive, and bfpA negative (Table 1).
Intimin typing resulted in six eae genotypes including α1 (5 strains), α2 (4 strains), β1 (25 strains), γ1 (3 strains), γ2/θ (10 strains), and ζtw (2 strains). The tir subtyping identified tir-β as the most common intimin receptor subtype. Specifically, tir-β was detected in 23 of 49 (46.9%) strains, followed by tir-α in 18 (36.7%) and tir-γ in 4 (8.2%) strains. Four (8.2%) strains showed negative PCRs for all tir subtypes studied (Table 1).
Genes encoded by LEE4 were detected with different frequencies. The presence of espB, espD, and sepL genes was detected in all strains, whereas espA was detected in 40 (81.6%) strains. espF was detected in only 22 (42.8%) strains. Amplified espF fragments of 11 strains were distinct in size compared to espF fragments of strain E2348/69 (520 bp). These fragments ranged from 400 to 600 bp.
Detection and expression of EHEC hlyA.
The presence of the EHEC hlyA gene was detected in five strains of serotype O26:H11 (Table 1). The expression of this gene was detected in these strains by the formation of a hemolytic zone around the spots of inoculation in blood agar plates after 24 h of incubation at 37°C.
MLST.
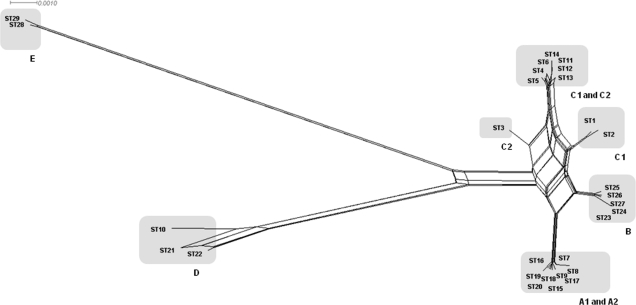
In all strains, internal fragments of the seven housekeeping genes could be sequenced with acceptable raw traces. The analysis of multiple-sequence alignment of nucleotide sequences and of inferred amino acid sequences showed a total of 184 variable sites among 4,080 nucleotides of the supergene, 25 of which involved in amino acid replacements. SplitsTree analysis revealed several parallel paths, indicating phylogenetic incompatibility in the divergence of atypical EPEC clones, caused by recombination (Fig. 1). Using the Φw test, statistical significance of recombination was demonstrated for concatenated sequences (P = 1.98 × 10−5). The analysis of the seven isolated genes revealed evidence of recombination for only icdA (P = 0.0125).
FIG. 1.
The phylogenetic (splits) network was based on the neighbor-net algorithm using a p distance matrix. The clusters of strains found on Bayesian analysis are indicated by gray boxes.
The allelic frequency of housekeeping genes ranged from 6 to 13 alleles per locus. The most variable loci were icdA and rpoS, with 10 and 13 alleles, respectively. The different alleles classified the strains in 29 STs (Tables 1 and 3). All STs found, with the exception of ST9, were represented by only one serotype. ST9 was represented by five strains of both O128:H[2] (where brackets indicate antigens identified by fliC RFLP) and O119:H2 serotypes (Table 1 and Fig. 2).
TABLE 3.
Allelic profile and relative frequency of ST obtained by MLST analysis
| ST | No. of alleles of the indicated housekeeping gene |
Relative frequency (no. of strains) | ||||||
|---|---|---|---|---|---|---|---|---|
| arcA | cyaA | fadD | icdA | lysP | mtlD | rpoS | ||
| ST1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ST2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 |
| ST3 | 2 | 2 | 3 | 2 | 2 | 3 | 1 | 1 |
| ST4 | 2 | 2 | 3 | 3 | 3 | 3 | 1 | 3 |
| ST5 | 2 | 2 | 3 | 4 | 3 | 3 | 1 | 1 |
| ST6 | 2 | 2 | 3 | 5 | 3 | 3 | 1 | 1 |
| ST7 | 1 | 1 | 4 | 6 | 2 | 4 | 1 | 5 |
| ST8 | 1 | 1 | 4 | 6 | 2 | 4 | 2 | 1 |
| ST9 | 1 | 1 | 4 | 2 | 2 | 4 | 1 | 5 |
| ST10 | 3 | 3 | 5 | 7 | 4 | 5 | 3 | 4 |
| ST11 | 2 | 2 | 3 | 5 | 2 | 6 | 4 | 1 |
| ST12 | 2 | 2 | 3 | 5 | 2 | 6 | 1 | 1 |
| ST13 | 1 | 2 | 3 | 3 | 2 | 6 | 1 | 1 |
| ST14 | 2 | 2 | 3 | 5 | 5 | 6 | 4 | 1 |
| ST15 | 1 | 1 | 4 | 2 | 2 | 4 | 5 | 1 |
| ST16 | 1 | 1 | 4 | 2 | 2 | 4 | 6 | 1 |
| ST17 | 1 | 1 | 4 | 2 | 2 | 4 | 7 | 1 |
| ST18 | 1 | 1 | 4 | 2 | 2 | 4 | 8 | 2 |
| ST19 | 1 | 1 | 4 | 2 | 2 | 4 | 9 | 1 |
| ST20 | 1 | 1 | 4 | 2 | 2 | 4 | 10 | 1 |
| ST21 | 4 | 4 | 6 | 8 | 4 | 7 | 10 | 4 |
| ST22 | 4 | 4 | 6 | 8 | 6 | 7 | 10 | 1 |
| ST23 | 2 | 1 | 4 | 9 | 2 | 8 | 1 | 3 |
| ST24 | 2 | 5 | 4 | 9 | 2 | 8 | 11 | 1 |
| ST25 | 2 | 6 | 4 | 9 | 2 | 8 | 1 | 1 |
| ST26 | 2 | 1 | 4 | 9 | 2 | 8 | 12 | 1 |
| ST27 | 2 | 1 | 4 | 9 | 2 | 8 | 1 | 1 |
| ST28 | 5 | 7 | 7 | 10 | 7 | 9 | 13 | 2 |
| ST29 | 6 | 7 | 7 | 10 | 7 | 9 | 13 | 1 |
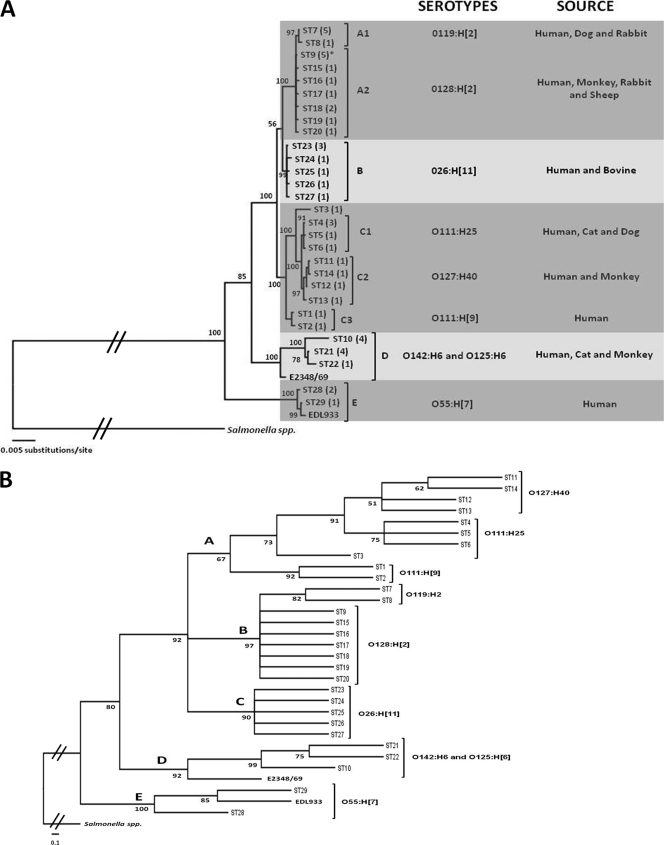
FIG. 2.
Inferred phylogeny of 49 typical and atypical EPEC strains isolated from humans and different animal species. The strains were rooted with E. coli strains EDL933 and E2348/69; S. enterica strain Ty2 was included as an outgroup standard. (A) Phylogenetic tree inferred by Bayesian analysis. The serotypes and sources of the strains are listed. The nucleotide substitution model best fit was constructed using GTR+I+G (where GTR is general time reversible, I is the proportion of invariable sites, and G is gamma) where G = 0.6931 and I = 0.5112. In the majority of cases, the tree is supported by Bayes credibility values higher than 90%. ST9 was the only ST representing two serotypes. (B) ML-inferred tree with similar topology and groups found on Bayesian analysis. The serotypes of each group found are listed. The ML tree was constructed with the model TrN+I+G, where TrN is Tamura-Nei, G = 0.6876, and I = 0.5139. In the majority, the tree is supported by bootstrapping values higher than 80%.
The rooted trees inferred by NJ, ML, and Bayesian methods were constructed with the sequences of the seven housekeeping genes. All phylogenetic trees grouped the strains in well-defined clusters based on their serotypes, indicating that recombination events did not affect our analysis or that they occurred early in the divergence of the EPEC strains.
All three methods of phylogenetic inference yielded similar trees, with strains clustering into similar groups supported by high bootstrapping or Bayes credibility values. These high values obtained in different types of analyses validate the phylogenetic relationship of the strains analyzed (Fig. 2).
The ML and Bayesian trees divided the strains into five clusters (A to E). The Bayesian tree cluster A included strains of serotypes O119:H2 and O128:H[2] isolated from humans, dogs, rabbits, sheep, and monkeys. Cluster B comprised exclusively O26:H[11] strains isolated from humans and bovines. Cluster C comprised strains of O111:H[9], O111:H25, and O127:H40 isolated from humans, cats, dogs, and monkeys. Although these strains shared the same cluster, they formed three distinct minor clusters (C1 to C3) separating these serotypes (Fig. 2).
Some strains showed close proximity to strains of different pathotypes. All O125:H[6] strains belonged to the same cluster of typical EPEC strains, and O55:H[7] strains were identified as belonging to the cluster of the EHEC EDL933 strain. In the ML tree shown in Fig. 2B, the strains are grouped as in the tree obtained in Bayesian analysis. The NJ tree also obtained similar results (data not shown).
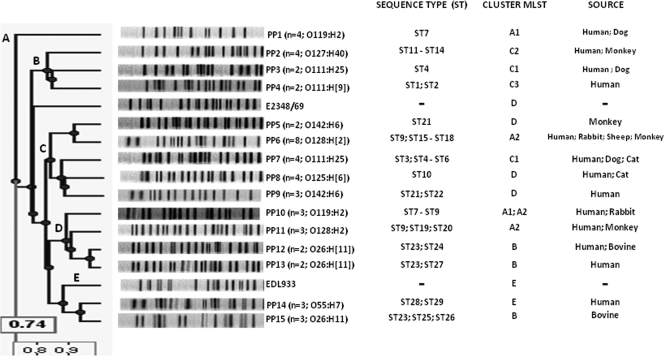
PFGE.
PFGE typing of 49 EPEC strains resulted in 15 pulsed-field patterns (PPs). The comparison of patterns revealed five clusters (A to E) with general similarity of 74% in the unweighted-pair group method using average linkages tree. Each cluster comprised strains belonging to different serotypes isolated from animals and humans (Fig. 3). One exception was cluster A, which consisted of four strains of serotype O119:H2. Among the 15 patterns found, PP14 and PP15 formed an isolated group with the EHEC strain EDL933. PP1 was the most divergent pattern, constituting a separated group from other strains analyzed. PP4, PP9, PP13, and PP14 were formed exclusively by strains isolated from humans, and PP5 and PP15 were recovered from monkey and bovine isolates, respectively. All other patterns were represented by human and animal isolates.
FIG. 3.
PFGE profiles and clusters of human and animal typical and atypical EPEC strains. The corresponding MLST sequence types, as well as the serotypes and sources of the strains for each PP, are listed.
DISCUSSION
This study describes the role of some animals that act as reservoirs of and possible sources of infection with atypical EPEC in humans. In several countries, the frequency of atypical EPEC is increasing compared to typical EPEC, and it is currently considered an important emerging diarrheagenic pathogen for children (1, 12, 26, 32, 55, 65).
The LEE-encoded proteins are responsible for the A/E lesion formation. In some strains of our study, espA, espF, and tir were not detected by PCR analysis although this does not prevent these strains from causing A/E lesions, as indicated by their ability to cause A/E lesion in vitro (data not shown). For instance, the UIPA17 strain isolated from a cat was negative for espA but was positive in a fluorescent actin staining test, an indication of the ability to produce the A/E lesions in vitro (51). The unsuccessful detection of LEE genes in some strains may indicate the presence of gene subtypes not detected by the primers employed (46). In addition to PCR, DNA hybridization and DNA sequencing would be necessary either to confirm or to exclude the presence of the respective LEE genes in the PCR-negative isolates. Studies utilizing these techniques will be performed in forthcoming experiments.
Some reports have identified polymorphisms and different subtypes of LEE genes (2, 17, 54). As a matter of fact, some reports (20, 27) have detected molecular differences between espA genes according to the origin of the sequences used to design the primers for the PCRs. Neves et al. (54) showed also that an antiserum against EspA of EPEC E2348/69 did not react with EspA filaments of EHEC O157:H7. Similarly, an antiserum against EspA of EHEC 85-170 did not react with EspA filaments derived from typical and some atypical EPEC strains, with the exception of O55:H7 strains known to be the ancestors of the O157:H7 EHEC serotype. Interestingly, several strains belonging to different serotypes of typical and atypical EPEC strains, though able to show a positive fluorescent actin staining reaction and therefore harboring the espA gene and the respective filament, did not react with either antibody. Based upon these reports, we can assume that our negative PCR results for espA were due to the polymorphisms within these gene sequences.
The espB and espD genes were detected in all strains. To reach this frequency, we used a different set of primers based on different E. coli pathotypes and serotypes (Tables 1 and 2). These data indicate that atypical EPEC can acquire the entire LEE region or just single genes of this region from different origins, as previously suggested (2, 16). This hypothesis is feasible on the basis of the present study since strains harboring distinct subtypes of eae and tir were found (Table 1).
In contrast to some previous reports, the astA gene was not detected in the atypical EPEC strains studied (22, 77). The presence of the stx2f gene has been detected in diarrheagenic E. coli strains, previously classified as atypical EPEC strains by some authors (63). To exclude the presence of Shiga toxin-producing E. coli (STEC) strains in our analysis, PCR with specific primers for stx2f was conducted, and the presence of this gene was not detected.
The virulence factor profiles of strains isolated from humans and animals were similar, indicating the potential of animal strains to cause disease in humans.
MLST and PFGE were performed on all 49 atypical EPEC strains. In both techniques it was possible to identify strains isolated from humans and animals that shared similar clonal origins. MLST was more discriminative than PFGE since each PP was represented by more than one ST. The better discriminative power of MLST was restricted to detecting minor clones among strains of the same serotype. This indicates, as previously suggested, that each serotype has more than one clonal origin (13, 29, 59, 66, 74).
Some strains revealed a close relationship to typical EPEC or EHEC strains. In PFGE analysis, two groups of O26 strains (PP12 and PP13) were closely related to typical EPEC strains, and one O26 group (PP15) was close to the O55:H7 strains and to EDL933 (O157:H7). Based on these data, these strains and pathotypes may have evolved either by acquisition or loss of the stx or bfp genes (11). Some studies have demonstrated the loss of stx genes by EHEC strains associated with hemolytic-uremic syndrome during human infection, indicating that some atypical EPEC serotypes, like O26:H11, O119:H2, and O128:H2, may be EHEC strains that lost stx genes (8, 9, 48, 49, 50). Despite these reports, some atypical EPEC serotypes studied by us were phylogenetically unrelated to typical EPEC or EHEC strains, thus indicating that they are true atypical EPEC strains.
Strains of serogroups O111, O119, O125, and O128, unlike O26 strains, shared clonal relationships only with typical EPEC strains. Interestingly, O119:H2 strains were divided into two groups by PFGE. These results may indicate the acquisition of new virulence factors by these strains not detected in the MLST analysis.
The differences between MLST and PFGE may be the result of the type of analysis. While PFGE detects differences in the genome, MLST analyzes just small fragments of conserved metabolic genes. Therefore, events like recent acquisition of virulence factors cannot be detected by MLST.
In general, both in MLST or PFGE analysis, the animal and human strains showed close proximity. Therefore, animals can play a role as reservoirs and sources of infection of atypical EPEC for humans, as previously suggested by other authors (3, 15, 45, 51, 53, 72). This indicates that in contrast to typical EPEC strains, diarrhea caused by atypical EPEC can be considered zoonosis.
On the other hand, the possibility cannot be excluded that some animals, mainly pets, had contracted their atypical EPEC strains through contact with human feces. Atypical EPEC strains are known to play a role as pathogens in cats and other animals (14, 42, 62). Investigations of cycles of mutual transmission of pathogenic E. coli between humans and different animals have been performed mainly with dogs. EPEC has been isolated from a diarrheic child and a diarrheic dog living in the same house (67). Hence, fecal E. coli strains as potential agents of disease may be transmitted directly or indirectly between humans and animals (53, 67). Farm animals carrying atypical EPEC strains may represent indirect risk since E. coli pathotypes have been found in fresh animal-derived food (34).
Few studies are available on animals as possible sources of infection of diarrheagenic E. coli in humans. However, the presence of potentially human pathogenic EPEC types among EPEC strains from pets, farm animals, and wild animals indicates that transmission of pathogens between animals and humans can occur and have an impact on public health. The role of these animals as carriers of atypical EPEC should be considered in investigations of outbreaks.
Acknowledgments
Financial support was received from the Fundação de Apoio à Pesquisa do Estado de São Paulo (grant 2004/12136-5 and fellowship to R.A.M.) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant 303826-2006 to A.F.P.D.C.).
We thank Helge Karch (Institute for Hygiene and the National Consulting Laboratory on Hemolytic Uremic Syndrome, University of Münster, Germany) for kindly providing the positive control for stx2f PCRs. We also thank Silvia Y. Bando for helping with the bioinformatics analysis and A. Leyva for English editing of the manuscript.
Footnotes
Published ahead of print on 2 October 2009.
REFERENCES
- 1.Afset, J. E., L. Bevanger, P. Romundstad, and K. Bergh. 2004. Association of atypical enteropathogenic Escherichia coli (EPEC) with prolonged diarrhea. J. Med. Microbiol. 53:1137-1144. [DOI] [PubMed] [Google Scholar]
- 2.Afset, J. E., E. Anderssen, G. Bruant, J. Harel, L. Wieler, and K. Bergh. 2008. Phylogenetic backgrounds and virulence profiles of atypical enteropathogenic Escherichia coli strains from a case-control study using multilocus sequence typing and DNA microarray analysis. J. Clin. Microbiol. 46:2280-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aidar-Ugrinovich, L., J. Blanco, M. Blanco, J. E. Blanco, L. Leomil, G. Dahbi, A. Mora, D. L. Onuma, W. D. Silveira, and A. F. Pestana de Castro. 2007. Serotypes, virulence genes, and intimin types of Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) isolated from calves in São Paulo, Brazil. Int. J. Food Microbiol. 115:297-306. [DOI] [PubMed] [Google Scholar]
- 4.Aktan, I., K. A. Sprigings, R. M. La Ragione, L. M. Faulkner, G. A. Paiba, and M. J. Woodward. 2004. Characterization of attaching-effacing Escherichia coli isolated from animals at slaughter in England and Wales. Vet. Microbiol. 103:43-53. [DOI] [PubMed] [Google Scholar]
- 5.Beutin, L., M. A. Montenegro, I. Orskov, F. Orskov, J. Prada, S. Zimmermann, and R. Stephan. 1989. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J. Clin. Microbiol. 27:2559-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutin, L., O. Marches, K. A. Bettelheim, K. Gleir, S. Zimmermann, H. Schmidt, and E. Oswald. 2003. HEp-2 cell adherence, actin aggregation, and intimin types of attaching and effacing Escherichia coli strains isolated from healthy infants in Germany and Australia. Infect. Immun. 71:3995-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beutin, L., S. Kaulfuss, S. Herold, E. Oswald, and H. Schmidt. 2005. Genetic analysis of enteropathogenic and enterohemorrhagic Escherichia coli serogroup O103 strains by molecular typing of virulence and housekeeping genes and pulsed-field gel electrophoresis. J. Clin. Microbiol. 43:1552-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielaszewska, M., R. Prager, R. Kock, A. Mellmann, W. Zhang, H. Tschape, P. I. Tarr, and H. Karch. 2007. Shiga toxin gene loss and transfer in vitro and in vivo during enterohemorrhagic Escherichia coli O26 infection in humans. Appl. Environ. Microbiol. 73:3144-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielaszewska, M., B. Middendorf, R. Kock, A. W. Friedrich, A. Fruth, H. Karch, M. A. Schmidt, and A. Mellmann. 2008. Shiga toxin-negative attaching and effacing Escherichia coli: distinct clinical associations with bacterial phylogeny and virulence traits and inferred in-host pathogen evolution. Clin. Infect. Dis. 47:208-217. [DOI] [PubMed] [Google Scholar]
- 10.Blanco, M., J. E. Blanco, A. Mora, J. Rey, J. M. Alonso, M. Hermoso, J. Hermoso, M. P. Alonso, G. Dahbi, E. A. Gonzalez, M. I. Bernardez, and J. Blanco. 2003. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from healthy sheep in Spain. J. Clin. Microbiol. 41:1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bortolini, M. R., L. R. Trabulsi, R. Keller, G. Frankel, and V. Sperandio. 1999. Lack of expression of bundle-forming pili in some clinical isolates of enteropathogenic Escherichia coli (EPEC) is due to a conserved large deletion in the bfp operon. FEMS Microbiol. Lett. 179:169-174. [DOI] [PubMed] [Google Scholar]
- 12.Bueris, V., M. P. Sircili, C. R. Taddei, M. F. dos Santos, M. R. Franzolin, M. B. Martinez, S. R. Ferrer, M. L. Barreto, and L. R. Trabulsi. 2007. Detection of diarrheagenic Escherichia coli from children with and without diarrhea in Salvador, Bahia, Brazil. Mem. Inst. Oswaldo Cruz 102:839-844. [DOI] [PubMed] [Google Scholar]
- 13.Campos, L. C., T. S. Whittam, T. A. Gomes, J. R. Andrade, and L. R. Trabulsi. 1994. Escherichia coli serogroup O111 includes several clones of diarrheagenic strains with different virulence properties. Infect. Immun. 62:3282-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho, V. M., C. L. Gyles, K. Ziebell, M. A. Ribeiro, J. L. Catão-Dias, I. L. Sinhorini, J. Otman, R. Keller, L. R. Trabulsi, and A. F. Pestana de Castro. 2003. Characterization of monkey enteropathogenic Escherichia coli (EPEC) and human typical and atypical EPEC serotype isolates from neotropical nonhuman primates. J. Clin. Microbiol. 41:1225-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho, V. M., K. Irino, D. Onuma, and A. F. Pestana de Castro. 2007. Random amplification of polymorphic DNA reveals clonal relationships among enteropathogenic Escherichia coli isolated from non-human primates and humans. Braz. J. Med. Biol. Res. 40:237-241. [DOI] [PubMed] [Google Scholar]
- 16.Castillo, A., L. E. Eguiarte, and V. Souza. 2005. A genomic population genetics analysis of the pathogenic enterocyte effacement island in Escherichia coli: the search for the unit of selection. Proc. Natl. Acad. Sci. USA 102:1542-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.China, B., F. Goffaux, V. Pirson, and J. Mainil. 1999. Comparison of eae, tir, espA and espB genes of bovine and human attaching and effacing Escherichia coli by multiplex polymerase chain reaction. FEMS Microbiol. Lett. 178:177-182. [DOI] [PubMed] [Google Scholar]
- 18.Cid, D., J. A. Ruiz-Santa-Quiteria, I. Marin, R. Sanz, J. A. Orden, R. Amils, and R. De La Fuente. 2001. Association between intimin (eae) and espB gene subtypes in attaching and effacing Escherichia coli strains isolated from diarrheic lambs and goat kids. Microbiology 147:2341-2353. [DOI] [PubMed] [Google Scholar]
- 19.Cleary, J., L. C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527-538. [DOI] [PubMed] [Google Scholar]
- 20.Crepin, V. F., R. Shaw, S. Knutton, and G. Frankel. 2005. Molecular basis of antigenic polymorphism of EspA filaments: development of a peptide display technology. J. Mol. Biol. 350:42-52. [DOI] [PubMed] [Google Scholar]
- 21.do Valle, G. R., T. A. Gomes, K. Irino, and L. R. Trabulsi. 1997. The traditional enteropathogenic Escherichia coli (EPEC) serogroup O125 comprises serotypes which are mainly associated with the category of enteroaggregative E. coli. FEMS Microbiol. Lett. 152:95-100. [DOI] [PubMed] [Google Scholar]
- 22.Dulguer, M. V., S. H. Fabbricotti, S. Y. Bando, C. A. Moreira-Filho, U. Fagundes-Neto, and I. C. A. Scaletsky. 2003. Atypical enteropathogenic E. coli strains: phenotypic and genetic profiling reveals a strong association between enteroaggregative E. coli heat-stable enterotoxin and diarrhea. J. Infect. Dis. 188:1685-1694. [DOI] [PubMed] [Google Scholar]
- 23.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 24.Fields, P. I., K. Blom, H. J. Hughes, L. O. Helsel, P. Feng, and B. Swaminathan. 1997. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J. Clin. Microbiol. 35:1066-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke, J., S. Franke, H. Schmidt, A. Schwarzkopf, L. H. Wieler, G. Baljer, L. Beutin, and H. Karch. 1994. Nucleotide sequence analysis of enteropathogenic Escherichia coli (EPEC) adherence factor probe and development of PCR for rapid detection of EPEC harboring virulence plasmids. J. Clin. Microbiol. 32:2460-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franzolin, M. R., R. C. Alves, R. Keller, T. A. Gomes, L. Beutin, M. L. Barreto, C. Milroy, A. Strina, H. Ribeiro, and L. R. Trabulsi. 2005. Prevalence of diarrheagenic Escherichia coli in children with diarrhea in Salvador, Bahia, Brazil. Mem. Inst. Oswaldo Cruz 100:359-363. [DOI] [PubMed] [Google Scholar]
- 27.Garrido, P., M. Blanco, M. Moreno-Paz, C. Briones, G. Dahbi, J. Blanco, and V. Parro. 2006. STEC-EPEC oligonucleotide microarray: a new tool for typing genetic variants of the LEE pathogenicity island of human and animal Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) strains. Clin. Chem. 52:192-201. [DOI] [PubMed] [Google Scholar]
- 28.Goffaux, F., B. China, L. Janssen, and J. Mainil. 2000. Genotypic characterization of enteropathogenic Escherichia coli (EPEC) isolated in Belgium from dogs and cats. Res. Microbiol. 151:865-871. [DOI] [PubMed] [Google Scholar]
- 29.Gonçalves, A. G., L. C. Campos, T. A. Gomes, J. Rodrigues, V. Sperandio, T. S. Whittam, and L. R. Trabulsi. 1997. Virulence properties and clonal structures of strains of Escherichia coli O119 serotypes. Infect. Immun. 65:2034-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunzburg, S. T., N. G. Tornieporth, and L. W. Riley. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedberg, C. W., S. J. Savarino, J. M. Besser, C. J. Paulus, V. M. Thelen, L. J. Myers, D. N. Cameron, T. J. Barrett, J. B. Kaper, and M. T. Osterholm. 1997. An outbreak of foodborne illness caused by Escherichia coli O39:NM, an agent not fitting into the existing scheme for classifying diarrheogenic E. coli. J. Infect. Dis. 176:1625-1628. [DOI] [PubMed] [Google Scholar]
- 32.Hernandes, R. T., W. P. Elias, M. A. M. Vieira, and T. A. T. Gomes. 2009. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 297:137-149. [DOI] [PubMed] [Google Scholar]
- 33.Huson, D. H., and D. Bryant. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23:254-267. [DOI] [PubMed] [Google Scholar]
- 34.Hussein, H. S., and L. M. Bollinger. 2005. Prevalence of Shiga toxin-producing Escherichia coli in beef. Meat Sci. 71:676-689. [DOI] [PubMed] [Google Scholar]
- 35.Ishii, S., K. P. Meyer, and M. J. Sadowsky. 2007. Relationship between phylogenetic groups, genotypic clusters, and virulence gene profiles of Escherichia coli strains from diverse human and animal sources. Appl. Environ. Microbiol. 73:5703-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarvis, K. G., J. A. Girón, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins, C., A. J. Lawson, T. Cheasty, G. A. Willshaw, P. Wright, G. Dougan, G. Frankel, and H. R. Smith. 2003. Subtyping intimin genes from enteropathogenic Escherichia coli associated with outbreaks and sporadic cases in the United Kingdom and Eire. Mol. Cell Probes 4:149-156. [DOI] [PubMed] [Google Scholar]
- 38.Jerse, A., and J. B. Kaper. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect. Immun. 59:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaper, J. B. 1996. Defining EPEC. Proceedings of the 2nd International Symposium on Enteropathogenic Escherichia coli (EPEC), 1995. Rev. Microbiol. 27:130-133. [Google Scholar]
- 40.Kaper, J. B., J. B. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 41.Kenny, B., and B. B. Finlay. 1995. Protein secretion by enteropathogenic E. coli is essential for transducing signals to epithelial cells. Proc. Natl. Acad. Sci. USA 92:7991-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenny, B., R. Devinney, M. Stein, D. J. Reinscheld, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli (EPEC) transfer its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 43.Krause, G., S. Zimmermann, and L. Beutin. 2005. Investigation of domestic animals and pets as a reservoir for intimin- (eae) gene positive Escherichia coli types. Vet. Microbiol. 106:87-95. [DOI] [PubMed] [Google Scholar]
- 44.Leomil, L., L. Aidar-Ugrinovich, B. E. Guth, K. Irino, M. P. Vettorato, D. L. Onuma, and A. F. Pestana de Castro. 2003. Frequency of Shiga toxin-producing Escherichia coli (STEC) isolates among diarrheic and non-diarrheic calves in Brazil. Vet. Microbiol. 97:103-109. [DOI] [PubMed] [Google Scholar]
- 45.Leomil, L., A. F. Pestana de Castro, G. Krause, H. Schmidt, and L. Beutin. 2005. Characterization of two major groups of diarrheagenic Escherichia coli O26 strains which are globally spread in human patients and domestic animals of different species. FEMS Microbiol. Lett. 249:335-342. [DOI] [PubMed] [Google Scholar]
- 46.Mairena, E. C., B. C. Neves, L. R. Trabulsi, and W. P. Elias. 2004. Detection of LEE 4 region-encoded genes from different enteropathogenic and enterohemorrhagic Escherichia coli serotypes. Curr. Microbiol. 48:412-418. [DOI] [PubMed] [Google Scholar]
- 47.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellmann, A., M. Bielaszewska, L. B. Zimmerhackl, R. Prager, D. Harmsen, H. Tschape, and H. Karch. 2005. Enterohemorrhagic Escherichia coli in human infection: in vivo evolution of a bacterial pathogen. Clin. Infect. Dis. 41:785-792. [DOI] [PubMed] [Google Scholar]
- 49.Mellmann, A., M. Bielaszewska, R. Köck, A. W. Friedrich, A. Fruth, B. Middendorf, D. Harmsen, M. A. Schmidt, and H. Karch. 2008. Analysis of collection of hemolytic uremic syndrome-associated enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 14:1287-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mellmann, A., M. Bielaszewska, and H. Karch. 2009. Intrahost genome alterations in enterohemorrhagic Escherichia coli. Gastroenterology 136:1925-1938. [DOI] [PubMed] [Google Scholar]
- 51.Morato E. C. P., L. Leomil, G. Krause, L. Beutin, R. A. Moura, and A. F. Pestana de Castro. 2009. Domestic cats constitute a natural reservoir of human enteropathogenic Escherichia coli types. Zoonoses Public Health 56:229-237. [DOI] [PubMed] [Google Scholar]
- 52.Moreno, A. C., A. F. Filho, T. D. Gomes, S. T. Ramos, L. P. Montemor, V. C. Tavares, L. D. Filho, K. Irino, and M. B. Martinez. 26 May 2008, posting date. Etiology of childhood diarrhea in the northeast of Brazil: significant emergent diarrheal pathogens. Diagn. Microbiol. Infect. Dis. doi: 10.1016/j.microbio.2008.03.017. [DOI] [PubMed]
- 53.Nakazato, G., C. Gyles, K. Ziebell, R. Keller, L. R. Trabulsi, T. A. Gomes, K. Irino, W. D. Da Silveira, and A. F. Pestana De Castro. 2004. Attaching and effacing Escherichia coli isolated from dogs in Brazil: characteristics and serotypic relationship to human enteropathogenic E. coli (EPEC). Vet. Microbiol. 101:269-277. [DOI] [PubMed] [Google Scholar]
- 54.Neves, B. C., R. K. Shaw, G. Frankel, and S. Knutton. 2003. Polymorphisms within EspA filaments of enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 71:2262-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen, R. N., L. S. Taylor, M. Tauschek, and R. M. Robins-Browne. 2006. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg. Infect. Dis. 12:597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Connell, C. B., E. A. Creasey, S. Knutton, S. Elliott, L. J. Crowther, W. Luo, M. J. Albert, J. B. Kaper, G. Frankel, and M. S. Donnenberg. 2004. SepL, a protein required for enteropathogenic Escherichia coli type III translocation, interacts with secretion component SepD. Mol. Microbiol. 52:1613-1625. [DOI] [PubMed] [Google Scholar]
- 57.Ørskov, F., and I. Ørskov. 1984. Serotyping of Escherichia coli. Methods Microbiol. 14:43-112. [Google Scholar]
- 58.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marches, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peixoto, J. C., S. Y. Bando, J. A. Ordonez, B. A. Botelho, L. R. Trabulsi, and C. A. Moreira-Filho. 2001. Genetic differences between Escherichia coli O26 strains isolated in Brazil and in other countries. FEMS Microbiol. Lett. 196:239-244. [DOI] [PubMed] [Google Scholar]
- 60.Penteado, A. S., L. A. Ugrinovich, J. Blanco, M. Blanco, J. E. Blanco, A. Mora, J. R. Andrade, S. S. Correa, and A. F. Pestana de Castro. 2002. Serobiotypes and virulence genes of Escherichia coli strains isolated from diarrheic and healthy rabbits in Brazil. Vet. Microbiol. 89:41-51. [DOI] [PubMed] [Google Scholar]
- 61.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 62.Pospischil, A., J. G. Mainil, G. Baljer, and H. W. Mo. 1987. Attaching and effacing bacteria in the intestines of calves and cats with diarrhoea. Vet. Pathol. 24:330-334. [DOI] [PubMed] [Google Scholar]
- 63.Prager, R., A. Fruth, U. Siewert, U. Strutz, and H. Tschäpe. 2009. Escherichia coli encoding Shiga toxin 2f as an emerging human pathogen. Int. J. Med. Microbiol. 299:343-353. [DOI] [PubMed] [Google Scholar]
- 64.Ribot, E. M., M. A. Fair, R. Gautom, D. N. Cameron, S. B. Hunter, B. Swaminathan, and T. J. Barrett. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella spp. and Shigella spp. for PulseNet. Foodborne Pathog. Dis. 3:59-67. [DOI] [PubMed] [Google Scholar]
- 65.Robins-Browne, R. M., A. M. Bordun, M. Tauschek, V. R. Bennett-Wood, J. Russell, F. Oppedisano, N. A. Lister, K. A. Bettelheim, C. K. Fairley, M. I. Sinclair, and M. E. Hellard. 2004. Escherichia coli and community-acquired gastroenteritis, Melbourne, Australia. Emerg. Infect. Dis. 10:1797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodrigues, J., I. C. Scaletsky, L. C. Campos, T. A. T. Gomes, T. S. Whittam, and L. R. Trabulsi. 1996. Clonal structure and virulence factors in strains of Escherichia coli of the classic serogroup O55. Infect. Immun. 64:2680-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodrigues, J., C. M. Thomazini, C. A. Lopes, and L. O. Dantas. 2004. Concurrent infection in a dog and colonization in a child with a human enteropathogenic Escherichia coli clone. J. Clin. Microbiol. 42:1388-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ronquist, F., and J. P. Huelsenbeck. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572-1574. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt, H., J. Scheef, S. Morabito, A. Caprioli, L. H. Wieler, and H. Karch. 2000. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 66:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trabulsi, L. R., R. Keller, and T. A. T. Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vettorato, M. P., L. Leomil, B. E. Guth, K. Irino, and A. F. Pestana de Castro. 2003. Properties of Shiga toxin-producing Escherichia coli (STEC) isolates from sheep in the State of Sao Paulo, Brazil. Vet. Microbiol. 95:103-109. [DOI] [PubMed] [Google Scholar]
- 73.Viljanen, M. K., T. Peltola, S. Y. Junnila, L. Olkkonen, H. Järvinen, M. Kuistila, and P. Huovinen. 1990. Outbreak of diarrhoea due to Escherichia coli O111:B4 in schoolchildren and adults: association of Vi antigen-like reactivity. Lancet 336:831-834. [DOI] [PubMed] [Google Scholar]
- 74.Whittam, T. S., and T. E. A. McGraw. 1996. Clonal analysis of EPEC serogroups. Proceedings of the 2nd International Symposium on Enteropathogenic Escherichia coli (EPEC), 1995. Rev. Microbiol. 27(Suppl. 1):7-16. [Google Scholar]
- 75.Wilgenbusch, J. C., and D. Swofford. 2003. Inferring evolutionary trees with PAUP*. Curr. Protoc. Bioinformatics 6:6.4. [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto, T., N. Wakisaka, F. Sato, and A. Kato. 1997. Comparison of the nucleotide sequence of enteroaggregative Escherichia coli heat-stable enterotoxin 1 genes among diarrhea-associated Escherichia coli. FEMS Microbiol. Lett. 147:89-95. [DOI] [PubMed] [Google Scholar]
- 77.Yatsuyanagi, J., S. Saito, Y. Miyajima, K. Amano, and K. Enomoto. 2003. Characterization of atypical enteropathogenic Escherichia coli strains harboring the astA gene that were associated with a waterborne outbreak of diarrhea in Japan. J. Clin. Microbiol. 41:2033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]