Abstract
Cry11Aa and Cyt1Aa of Bacillus thuringiensis are active against mosquitoes and show synergism. Cyt1Aa functions as a membrane receptor inducing Cry11Aa oligomerization. Here we characterized Cry11Aa helix α-3 mutants impaired in oligomerization and toxicity against Aedes aegypti, indicating that oligomerization of Cry11Aa is important for toxin action. Cyt1Aa did not recover the insecticidal activity of Cry11Aa mutants.
Bacillus thuringiensis subsp. israelensis has been used worldwide for the control of different mosquitoes that are vectors of several human diseases (10, 11). This bacterium produces different toxins that individually show activity against mosquitoes, i.e., Cry4Aa, Cry4Ba, Cry11Aa, and Cyt1Aa (2). The toxicity of Cry11Aa and Cry4 toxins against Aedes aegypti is greatly increased in the presence of sublethal concentrations of Cyt1Aa (14). Also, Cyt1Aa overcomes the resistance of the Culex quinquefasciatus population to Cry11Aa (12, 13). Cyt1Aa synergizes the toxic activity of Cry11Aa by functioning as a Cry11Aa receptor, facilitating the oligomerization of Cry11Aa and its pore formation activity (7, 8). Oligomerization is a complex event that involves interaction with a toxin receptor and further proteolysis of helix α-1 (3). In the case of the Cry1Ab toxin, helix α-3 of domain I contains coiled-coil structures that are important for oligomerization (4). Some point mutations in helix α-3 do not affect interaction with receptors but severely affected oligomerization, influencing pore formation and toxicity against Manduca sexta larvae (4).
Since binding with Cyt1Aa facilitates Cry11Aa oligomerization, we hypothesize that Cry11Aa mutants unable to oligomerize would be affected in synergism with Cyt1Aa and in toxicity. In this report, we analyzed the effect of point mutations in helix α-3 of Cry11Aa on oligomerization, synergism with Cyt1Aa, and toxicity against A. aegypti larvae.
Helix α-3 of Cry11Aa potentially forms coiled-coil structures, as determined by the program COILS, which calculates the probability that a sequence will adopt a coiled-coil conformation (6). The coiled-coil structures are characterized by heptads of residues (abcdefg), where positions a and d are occupied mostly by apolar residues and g and e by charged residues. Here we mutagenized some residues located at positions g and a of the predicted coiled-coil (Fig. 1). Substitutions R90E, E97A, Y98E, V104E, and S105E were produced by site-directed mutagenesis (Quick Change; Stratagene, La Jolla, CA) using the pCG6 plasmid (1) as a template and appropriate mutagenic oligonucleotides. Point mutations were verified by automated DNA sequencing at Instituto de Biotecnología-UNAM and transformed into the acrystalliferous B. thuringiensis 407 strain. B. thuringiensis strains were grown in solid nutrient broth sporulation medium supplemented with 10 μg/ml erythromycin (5). Crystal inclusions were purified as described previously (8) and solubilized in 100 mM NaOH for 1 h at 4°C. After solubilization, the Cry11Aa protoxins were dialyzed for 12 h against 50 mM Na2CO3, pH 10.5. The pH was equilibrated at pH 8.6 with equal volumes of 1 M Tris-HCl, pH 8, and protoxins were activated with trypsin (1:50, wt/wt) for 2 h at 25°C. All mutants, with the exception of the V104E mutant, which was not analyzed further, produced crystal inclusions similar to those for the wild-type toxin, composed of a 70-kDa protoxin (Fig. 2A). After trypsin activation, all mutants produced two polypeptides of 32 and 36 kDa, similarly to the Cry11Aa toxin, suggesting that these mutations did not cause a major structural disturbance (Fig. 2B). The Cry11Aa and mutant activated toxins were analyzed by circular dichroism spectroscopy (Fig. 2C). The activated toxins were dialyzed against 10 mM Na2HPO4, 50 mM NaF, pH 9, and then purified by anion-exchange chromatography with HiTrap Q-Sepharose (Pharmacia LKB Biotechnology) in the same buffer, using a linear NaF gradient from 50 to 400 mM. The similarities among the curves indicate that the mutant toxins have a structure similar to that of the wild-type toxin.
FIG. 1.
Schematic representation of the coiled-coil structures of the α-3 helices of Cry1Ab and Cry11Aa toxins. The positions of residues a, b, c, d, e, f, and g of the heptads are presented. The mutated residues in both toxins that affected oligomerization and toxicity are shown in boldface type (reference 4 and this work).
FIG. 2.
SDS-PAGE analysis and circular dichroism spectra of Cry11Aa mutant toxins. (A) The Cry11Aa protoxins were solubilized at pH 10.5 and analyzed by SDS-PAGE (15% acrylamide). (B) SDS-PAGE analysis (15% acrylamide) of the activated toxins with trypsin. Both SDS-polyacrylamide gels were stained with Coomassie blue. Lanes 1, Cry11Aa; lanes 2, E97A mutant; lanes 3, Y98E mutant; lanes 4, R90E mutant; lanes 5, S105E mutant. (C) Analysis of the secondary-structure compositions of the mutants and Cry11Aa activated toxins. Circular dichroism spectra were recorded with a Jasco model J-715 spectropolarimeter equipped with a Peltier temperature control supplied by Jasco. Spectra were collected from 190 to 250 nm. Eight replicate spectra were collected for each sample to improve the signal-to-noise ratios. The final purified-protein concentration was 0.3 mg/ml, and spectra were collected in a 0.1-cm-pathlength cell. The secondary-structure prediction was performed using the CDSSTR algorithm (1a, 11a). Solid black line, Cry11Aa; dotted black line, E97A mutant; dashed black line, Y98E mutant; solid gray line, R90E mutant; dotted gray line, S105E mutant; MRE, mean residue ellipticity; [θ], ellipticity.
The toxicity of spore/crystal suspensions of Cry11Aa or the individual mutants (75 to 10,000 ng/ml) was analyzed with bioassays against 10 fourth-instar A. aegypti larvae reared at 28°C, 87% humidity, and 12:12 light-dark conditions in 100 ml dechlorinated water, and mortality was scored after 24 h (four independent assays). The Cry11Aa toxin showed a mean lethal concentration of 355 ng/ml, with 95% confidence limits of 265 to 446 (Probit analysis using Polo-PC LeOra Software). In contrast, the R90E, E97A, Y98E, and S105E mutants were severely affected in toxicity against A. aegypti larvae, since no mortality was observed at the highest concentration used (10,000 ng/ml).
We then analyzed the oligomerization of Cry11Aa toxins as previously described (8). Small unilamelar vesicles (SUV), composed of egg yolk phosphatidyl choline, cholesterol (Avanti Polar Lipids, Alabaster, AL), and stearylamine (Sigma, St. Louis, MO) at a 10:3:1 proportion, respectively, were used (8). Cyt1Aa was purified from the 4Q7/pWF45 strain (14) grown as described above. Cyt1Aa inclusions were purified by sucrose gradients, solubilized in 50 mM Na2CO3, 10 mM dithiothreitol, pH 10.5 (2 h at 30°C), and activated with 1:100 proteinase K (Sigma-Aldrich Co.), wt/wt, for 20 min at 30°C.
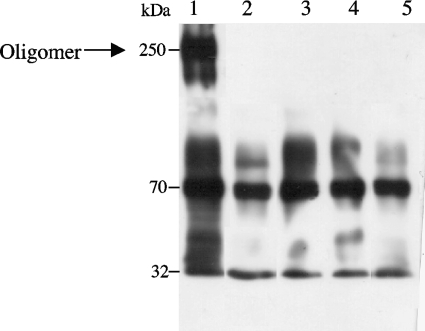
For oligomerization assays, 2.5 μg soluble Cry11Aa or mutant protoxin was incubated for 2 h at 37°C in a 100-μl final volume of 50 mM Na2CO3, pH 10.5, with 200 μM SUV, 1:50 trypsin (wt/wt), and 0.5 μg Cyt1Aa activated toxin. After 2 h of incubation, 1 mM phenylmethylsulfonyl fluoride was added to stop the reaction, and the membrane fraction was separated by centrifugation (1 h at 100,000 × g). The pellet was suspended in the same buffer solution. Oligomeric structures of Cry toxins are highly stable after boiling as well as after urea denaturation (9). The suspension was boiled for 4 min, analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8% acrylamide), and electrotransferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA). The oligomeric and monomeric structures of Cry11Aa were detected using polyclonal anti-Cry11Aa antibody (1/15,000; 1 h) and a secondary antibody coupled with horseradish peroxidase (Sigma, St. Louis, MO) (1/5,000; 1 h) followed by luminol (ECL; Amersham Pharmacia Biotech) as described by the manufacturers. Figure 3 shows that only the Cry11Aa wild-type toxin was able to oligomerize, while the mutants were severely impaired in oligomerization.
FIG. 3.
Analysis of Cry11Aa oligomer formation. Soluble Cry11Aa protoxin was activated with trypsin for 2 h at 37°C in the presence of SUV and Cyt1Aa activated toxin. The membrane fraction was separated by ultracentrifugation, and the Cry11Aa protein was analyzed by Western blotting of the membrane pellet with polyclonal anti-Cry11A antibody. The sizes of the proteins were estimated from a molecular prestained plus standard, all blue (Bio-Rad). Lane 1, Cry11Aa; lane 2, R90E mutant; lane 3, Y98E mutant; lane 4, E97A mutant; lane 5, S105E mutant.
Finally, the synergistic activity between Cyt1Aa and Cry11Aa was analyzed. A concentration of Cyt1Aa that produced 10% mortality was assayed in the presence of a protein concentration of wild-type Cry11A that produced 20% mortality. Larvae were examined 24 h after treatment, in three repetitions. This particular protein mixture produced a synergism factor of 8. Under these conditions, mortality was more than 80%, due to the synergistic activities of both toxins. Similar experiments were performed with the mutant toxins, using the same concentration of Cyt1Aa toxin and different concentrations (up to 6,000 ng/ml) of the mutant toxins. Cyt1A did not increase the toxicity of the Cry11Aa mutants, since only 10% mortality was observed, even at the highest concentration of the mutant toxins.
Previously, helix α-3 of a lepidopteran-specific toxin (Cry1Ab) was subjected to mutagenesis. The R99E and Y107E mutants of the Cry1Ab toxin were severely impaired in oligomerization and toxicity, showing that oligomer formation is a necessary step to kill the larvae (4). The data presented here indicate that oligomer formation is also an essential step in the mechanism of toxicity of the mosquitocidal Cry11Aa toxin and that helix α-3 is involved in this process.
Acknowledgments
We thank L. Cabrera for her technical assistance in the laboratory.
Support was provided by CONACyT 84648 and 81639 and by DGAPA IN210208 and IN222308-3.
Footnotes
Published ahead of print on 9 October 2009.
REFERENCES
- 1.Chang, C., Y. M. Yu, S. M. Dai, S. K. Law, and S. S. Gill. 1993. High-level cryIVD and cytA gene expression in Bacillus thuringiensis does not require the 20-kilodalton protein, and the coexpressed gene products are synergistic in their toxicity to mosquitoes. Appl. Environ. Microbiol. 59:815-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Compton, L. A., and W. C. Johnson, Jr. 1986. Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal. Biochem. 155:155-167. [DOI] [PubMed] [Google Scholar]
- 2.Crickmore, N., E. J. Bone, J. A. Williams, and D. J. Ellar. 1995. Contribution of the individual components of the δ-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp israelensis. FEMS Microbiol. Lett. 131:249-254. [Google Scholar]
- 3.Gómez, I., J. Sánchez, R. Miranda, A. Bravo, and M. Soberón. 2002. Cadherin-like receptor binding facilitates proteolytic cleavage of helix α-1 in domain I and oligomer pre-pore formation of Bacillus thuringiensis Cry1Ab toxin. FEBS Lett. 513:242-246. [DOI] [PubMed] [Google Scholar]
- 4.Jiménez-Juárez, N., C. Muñoz-Garay, I. Gómez, G. Saab-Rincon, J. Y. Damian-Alamazo, S. S. Gill, M. Soberón, and A. Bravo. 2007. Bacillus thuringiensis Cry1Ab mutants affecting oligomer formation are non toxic to Manduca sexta larvae. J. Biol. Chem. 282:21222-21229. [DOI] [PubMed] [Google Scholar]
- 5.Lereclus, D., H. Agaisse, M. Gominet, and J. Chaufaux. 1995. Overproduction of encapsulated insecticidal crystal proteins in a Bacillus thuringiensis spoOA mutant. Bio/Technology 13:67-71. [DOI] [PubMed] [Google Scholar]
- 6.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 7.Pérez, C., L. E. Fernandez, J. Sun, J. L. Folch, S. S. Gill, M. Soberón, and A. Bravo. 2005. Bti Cry11Aa and Cyt1Aa toxins interactions support the synergism-model that Cyt1Aa functions as membrane-bound receptor. Proc. Natl. Acad. Sci. USA 102:18303-18308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez, C., C. Muñoz-Garay, L. C. Portugal, J. Sánchez, S. S. Gill, M. Soberón, and A. Bravo. 2007. Bacillus thuringiensis subsp. israelensis Cyt1Aa enhances activity of Cry11Aa toxin by facilitating the formation of a pre-pore oligomeric structure. Cell. Microbiol. 9:2931-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rausell, C., L. Pardo-López, J. Sánchez, C. Muñoz-Garay, C. Morera, M. Soberón, and A. Bravo. 2004. Unfolding events in the water-soluble monomeric Cry1Ab toxin during transition to oligomeric pre-pore and membrane inserted pore channel. J. Biol. Chem. 279:55168-55175. [DOI] [PubMed] [Google Scholar]
- 10.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soberón, M., L. E. Fernández, C. Pérez, S. S. Gill, and A. Bravo. 2007. Mode of action of mosquitocidal Bacillus thuringiensis toxins. Toxicon 49:597-600. [DOI] [PubMed] [Google Scholar]
- 11a.Sreerama, N., and R. W. Woody. 2000. Estimation of protein secondary structure from CD spectra: comparison of CONTIN, SELCON and CDSSTR methods with an expanded reference set. Anal. Biochem. 287:252-260. [DOI] [PubMed] [Google Scholar]
- 12.Wirth, M. C., G. P. Georghiou, and B. A. Federici. 1997. CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito, Culex quinquefasciatus. Proc. Natl. Acad. Sci. USA 94:10536-10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirth, M. C., W. E. Walton, and B. A. Federici. 2000. Cyt1Aa from Bacillus thuringiensis restores toxicity of Bacillus sphaericus against resistant Culex quinquefasciatus (Diptera:Culicidae). J. Med. Entomol. 37:401-407. [PubMed] [Google Scholar]
- 14.Wu, D., J. J. Johnson, and B. A. Federici. 1994. Synergism of mosquitocidal toxicity between CytA and CryIV proteins using inclusions produced from cloned genes of Bacillus thuringiensis subsp. israelensis. Mol. Microbiol. 13:965-972. [DOI] [PubMed] [Google Scholar]