Abstract
Cestodes are unable to synthesize de novo most of their own membrane lipids, including cholesterol, and have to take them up from the host during an infection. The underlying molecular mechanisms are so far unknown. Here we report the identification and characterization of a novel gene, Emabp, which is expressed by larval stages and adults of the fox tapeworm Echinococcus multilocularis. The encoded protein, EmABP, displays significant homologies to apolipoprotein A-I binding protein (AI-BP) of mammalian origin and to metazoan YjeF_N domain proteins. Like mammalian AI-BP, EmABP carries an export-directing signal sequence which is absent in predicted AI-BP orthologs from the related flatworms Schistosoma japonicum and Schmidtea mediterranea. Using a specific antibody and immunoprecipitation techniques, we demonstrate that EmABP is secreted into the extraparasitic environment and into the hydatid fluid of in vitro-cultivated metacestode vesicles. Furthermore, we show that apolipoprotein A-I (apoA-I), a major constituent of cholesterol-transporting high-density lipoproteins, is present in hydatid fluid. By pulldown experiments, we demonstrate that recombinantly expressed, purified EmABP interacts with purified human apoA-I and is able to precipitate apoA-I from human serum. On the basis of these features and the suggested function of AI-BP in cholesterol transport in higher eukaryotes, we propose a role for EmABP in cholesterol and lipid uptake mechanisms of larval E. multilocularis.
The metacestode larval stage of the fox tapeworm Echinococcus multilocularis is the causative agent of alveolar echinococcosis (AE) in humans, one of the most serious and life-threatening parasitoses of the Northern Hemisphere (16). The E. multilocularis life cycle involves an adult stage which dwells in the intestines of definitive hosts, such as foxes or dogs, and produces infective eggs that contain the parasite's oncosphere larva. Upon oral ingestion of the eggs by intermediate hosts (rodents and, occasionally, humans), the oncosphere is activated, hatches, and penetrates the intestinal barrier. Within the liver of the intermediate host, the oncosphere undergoes a metamorphosis toward the bladder-like metacestode stage which grows infiltratively, like a malignant tumor, into the surrounding host tissue. At a later stage of the infection, numerous protoscoleces are formed from the parasite's germinal tissue, which are passed onto the definitive host when it takes the prey (6-8, 16, 49). Human E. multilocularis infections are relatively rare but pose serious problems to surgical and/or chemotherapeutic treatment (28). A very similar life cycle is displayed by the closely related dog tapeworm Echinococcus granulosus, the causative agent of cystic echinococcosis (CE), with several modifications concerning the spectrum of host species (domestic animals), metacestode morphology (unilocular versus multilocular), and organ tropism (the lung, kidney, and brain in addition to the liver) (6, 16).
Although E. multilocularis and E. granulosus contain complex mixtures of lipids, including cholesterol, in their membranes, they are unable to synthesize most of these molecules de novo and share this trait with other cestodes (35). As a consequence, they have to take up host-derived lipids during an infection. Particularly in the case of cholesterol, Frayha (19, 20) already demonstrated that this compound cannot be synthesized by both E. multilocularis and E. granulosus and that at least E. granulosus incorporates radioactively labeled, host-derived cholesterol during experimental infection of mice. Although several Echinococcus proteins with fatty acid and hydrophobic ligand binding properties have been reported (12, 25), none of these displayed cholesterol binding activities nor has, as yet, any cestode molecule been identified that interacts with components of the host's cholesterol transport machinery.
Mammalian cells acquire exogenous cholesterol mainly from low-density lipoprotein (LDL) particles via the LDL receptor pathway. During this process, the LDL receptor specifically interacts with the major protein component of LDL particles, apolipoprotein B-100 (apoB-100), resulting in the formation of clathrin-coated vesicles which are processed via the classical endocytic pathway. Upon fusion of the vesicles with lysosomes, the entire LDL particle is disassembled by enzymatic hydrolysis, releasing cholesterol and lipids for cellular metabolism (9, 36, 41). The majority of LDL receptors expressed in mammals are on the surfaces of liver cells, although a certain level of LDL receptor expression also occurs in the peripheral tissue (9). The LDL/LDL receptor lipid transport system appears to be evolutionarily conserved, since apoB-100-like cholesterol binding proteins (vitellogenins) have already been identified in yolk of invertebrates, such as Caenorhabditis elegans and Drosophila melanogaster (29, 34, 45). Furthermore, surface receptors of the LDL receptor family have been reported to be expressed by invertebrates (34).
In addition to exogenous uptake of cholesterol, nearly all mammalian cells are able to also synthesize cholesterol de novo. In cells of peripheral tissues, excess cholesterol needs to be removed and transported to the liver for reutilization and excretion. The underlying mechanism of “reverse cholesterol transport” is mediated by high-density lipoprotein (HDL) particles, the major component of which is apolipoprotein A-I (apoA-I) (38). Lipid-free apoA-I is secreted predominantly by the liver and intestine and acquires phospholipids and cholesterol via cellular efflux from peripheral tissue cells and macrophages, giving rise to nascent HDL. Once mature, HDL particles are transported to the liver, adrenal glands, and steroidogenic tissue where they are recognized by the HDL receptor, scavenger receptor type B class I, upon which the process of “selective lipid uptake” by the target cell is induced, which fundamentally differs from receptor-mediated endocytosis (9, 36, 38, 39). During “selective lipid uptake,” cholesterol and phospholipids are effectively transferred to target cells, releasing extracellular, lipid-depleted HDL particles which can reenter circulation. Although LDL particles are the major carriers of cholesterol in human blood, sera from rodents and ungulates typically contain much higher levels of HDL components than LDL components (10). Another difference concerns the extracellular transfer of cholesteryl esters from HDL particles to other lipoproteins (e.g., LDL) for further transport, which can be observed only in humans and not in rodents (9). Although as in the case of LDL receptors, the scavenger receptor type B family appears to be evolutionarily conserved and occurs also in invertebrates (15), soluble apolipoproteins, such as apoA-I or apoE, are probably deuterostome specific and may have first appeared around 450 million years ago in an Ordovician vertebrate (27).
As yet, only two parasitic flatworm proteins which can interact with the cholesterol and lipid transport machinery of mammalian hosts have been reported and both derive from trematodes, an LDL receptor-like very low-density lipoprotein binding protein from Schistosoma japonicum (17) and a CD36-like class B scavenger receptor from Schistosoma mansoni (15) which interacts with modified host LDL at the tegumental surface. As a first step toward the characterization of cestode molecules that are involved in cholesterol uptake during infections of the intermediate host, we herein report the identification and characterization of a secreted E. multilocularis protein, EmABP, which interacts with human apoA-I, the major component of HDL particles for reverse cholesterol transport. The role of EmABP in Echinococcus cholesterol uptake mechanisms is discussed in the background of what is known on the function of the homologous apolipoprotein A-I binding protein (AI-BP) (26, 42, 44) in humans.
MATERIALS AND METHODS
Organisms and culture methods.
All experiments were performed with the natural Echinococcus multilocularis isolate H95 which was propagated in Mongolian jirds or Mongolian gerbils (Meriones unguiculatus) as described previously (50). In vitro cultivation of metacestode vesicles under axenic conditions was performed according to an established protocol (47), and protoscoleces were isolated from in vivo-cultivated parasite material as described previously (5). Pepsin activation of protoscoleces was performed as described previously (48) by incubation of the larvae in pepsin and low pH (0.5 mg/ml pepsin in Hanks' solution, pH 2.0, 37°C) for 3 h. Egg-free, adult E. multilocularis worms were isolated from experimentally infected dogs essentially as previously described (14) and kept on RNAlater RNA stabilization reagent (Qiagen) prior to RNA isolation.
RT-PCR analyses.
For RNA isolation from in vitro-cultivated metacestode vesicles, protoscoleces, and adult worms, the RNeasy kit (Qiagen) was utilized according to the manufacturer's instructions. Three to five metacestode vesicles of 5 mm in diameter or 30 to 50 protoscoleces were used per isolation step, and the RNA was eluted in 30 μl diethyl pyrocarbonate-treated water. For cDNA synthesis, total RNA was reverse transcribed using the Omniscript RT kit (Qiagen) according to a previously established protocol (5). Reverse transcription-PCR (RT-PCR) for the Emabp and elp genes (3) was performed on cDNA preparations from metacestode, invaginated protoscolex, and activated protoscolex using the primers LA1BP-5′mod (5′-GTG CAG GTC GCT CCG TGC G-3′) and LABPUP2 (5′-GAG TGA GAT CAA GCA ATC TG-3′) as well as ELP-DW and ELP-UP (5), respectively. PCR products were separated on a 1% agarose gel and stained with ethidium bromide.
Heterologous expression and purification of Emabp in Escherichia coli.
For heterologous expression in E. coli, the pBAD/TOPO-ThioFusion system (Invitrogen) was used. The Emabp reading frame was PCR amplified from E. multilocularis cDNA using the primers APOBPDWY (5′-CTC AGT CAG GAG GAG GCG-3′) and LA1BP-3′ (5′-CTT ATT GGG TGT CTG GAG G-3′), and the resulting fragment was cloned into pBAD/Thio via TA cloning, yielding plasmid pPB-ExABP. In pPB-ExABP, Emabp was translationally fused to an N-terminal thioredoxin moiety and carried the V5 antibody epitope (Invitrogen) as well as a hexahistidine tag at the C terminus (thio-EmABP; 43 kDa). Recombinant protein expression was induced through the addition of arabinose to E. coli cultures as previously described (46), and purification of thio-EmABP under native conditions was performed according to established protocols (22, 48). For a control, we recombinantly expressed a fusion protein that consisted of only the thioredoxin moiety fused to the V5 epitope and the hexahistidine tags (thio-V5-His6; 16 kDa) from plasmid pBAD/Thio (Invitrogen) and purified it under identical conditions as thio-EmABP.
Antibodies and Western blot analyses.
For the detection of EmABP in culture supernatant and hydatid fluid, a rabbit anti-human AI-BP polyclonal antiserum (42) was used. Detection of the heterologously expressed thio-EmABP and thio-V5-His6 was done using the monoclonal (mouse) anti-V5 antibody (Invitrogen). For immunoprecipitation and detection of apoA-I, a goat anti-human apoA-I antiserum (Acris Antibodies GmbH; catalogue no. R1029P) was used, and Echinococcus antigen B was detected by employing the monoclonal antibody EB7 (23). For Western blot detection, lysates or immunoprecipitation complexes were separated on 12% acrylamide gels and transferred to nitrocellulose membranes (Schleicher and Schuell GmbH). Detection was performed with the above-mentioned antibodies using peroxidase-conjugated anti-mouse immunoglobulin G or M (IgG/M), anti-rabbit IgG (both Jackson Immuno Research Laboratories Inc.), or anti-goat/sheep IgG antibodies (Sigma) as secondary antibodies according to the manufacturer's instructions.
Immunoprecipitation of ApoA-I and EmABP from hydatid fluid and medium.
Thirty microliters of protein G-agarose (Upstate) was equilibrated in binding buffer (1% bovine serum albumin and 1% Triton X-100 in phosphate-buffered saline [PBS]). The anti-AI-BP (diluted 1:100) (41) or anti-apoA-I (1:100; Acris) antibodies were bound to the agarose beads overnight at 4°C in binding buffer. In vitro-cultivated metacestode vesicles (∼1 cm in diameter) were washed with prewarmed PBS and transferred to an incubation tube. The vesicles were pinched with a syringe, and the cellular fraction was pelleted by centrifugation (1,000 rpm, 3 min) at 4°C. Hydatid fluid (supernatant) was transferred to a fresh tube and kept on ice. After the protein G-antibody complexes were washed with binding buffer, 2 ml of hydatid fluid was added and incubated under agitation overnight at 4°C. The protein G-antibody complexes were pelleted by centrifugation for 1 min at 14,000 rpm, and the supernatant was removed. The pellet was subsequently washed three times with ice-cold washing buffer (1% Triton X-100 in PBS). Finally, 30 μl of 2× sodium dodecyl sulfate sample buffer containing β-mercaptoethanol was added to the agarose beads, followed by boiling for 5 min prior to acrylamide gel electrophoresis and Western blot detection as described above.
Pulldown assays.
To test the interaction between purified thio-EmABP and apoA-I, agarose G beads (Upstate) (30 μl) were equilibrated in binding buffer. The anti-ApoA-I antibody (1:100) and human apoA-I (15 μl) (1.32 mg/ml; Acris) were bound to the beads overnight at 4°C in binding buffer. After the protein G-antibody/antigen complexes had been washed in washing buffer, 30 μl of purified thio-EmABP (110 μg/μl) was added and incubated for 2 h at 4°C. The antibody/antigen complexes were pelleted by centrifugation for 1 min at 14,000 rpm, and the pellet was washed three times with cold washing buffer prior to sample preparation for gel electrophoresis as described above. To precipitate apoA-I from human serum, 50 μl of protein A Sepharose (Sigma) was equilibrated in binding buffer. The anti-AI-BP antibody (1:100) (42) and purified EmABP (15 μl) (110 μg/μl) were bound to the beads overnight at 4°C in binding buffer. After the protein G-antibody/antigen complex was washed with washing buffer, 1 ml of human serum was added. After overnight incubation at 4°C, the antibody/antigen complexes were further processed essentially as described above. Each assay was performed at least twice.
Computer-based analyses.
Sequence alignments and comparisons were performed using the Basic Local Alignment Search Tool (BLAST) software on the SWISSPROT and nr-aa database collections available at http://blast.genome.jp, the Schmidtea mediterranea genome database (http://smedgd.neuro.utah.edu/), and the E. multilocularis genome project database (http://www.sanger.ac.uk/Projects/Echinococcus/). Predictions of export-directing signal sequences, transmembrane regions, and domains were done using the iPSORT algorithm, available at http://hc.ims.u-tokyo.ac.jp/iPSORT/ (2) and the Simple Modular Architecture ResearchTools (SMART), available at http://smart.embl-heidelberg.de/ (30). Secondary structure predictions were made using the Jpred 3 algorithm (http://www.compbio.dundee.ac.uk/www-jpred/index.html) as described previously (13).
Nucleotide sequence accession number.
The complete Emabp cDNA sequence reported in this paper was deposited in the GenBank database under the accession number FM958505.
RESULTS
Characterization of the Emabp cDNA and genomic locus.
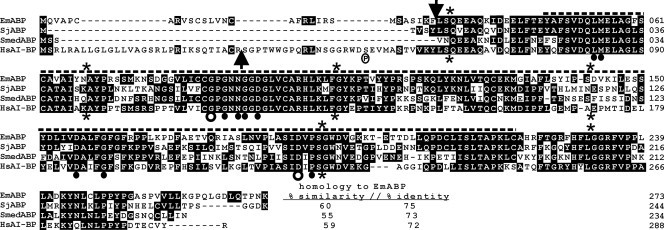
During previous studies of the trans-splicing mechanism in Echinococcus multilocularis, we have established cDNA libraries for trans-spliced mRNAs, isolated from in vitro-cultivated metacestode vesicles and protoscoleces (4). Approximately 300 cDNAs from each library were sequenced and subjected to BLAST sequence analyses using the SWISSPROT and nr-aa databases. One of the characterized clones, designated Z2-45, which was derived from the metacestode cDNA library, encoded a protein with significant homology to human AI-BP (42) and was further analyzed. Due to this homology and to the apoA-I binding activity of the encoded protein (see below), this gene was named Emabp (for Echinococcus multilocularis apoA-I binding protein), encoding the protein EmABP. The full-length Emabp cDNA comprised 950 nucleotides [excluding the spliced leader (SL) and the poly(A) tail] and contained a putative polyadenylation signal (AATAAA), 17 nucleotides upstream of the poly(A) tail. Immediately following the SL, there was an open reading frame which coded for a 273-amino-acid protein (theoretical molecular mass of 30 kDa) which displayed significant homologies (up to 60% identical and 72% similar residues) to various mammalian AI-BP orthologs (Fig. 1) and somewhat lower homologies to YjeF_N domain-containing proteins of invertebrates and bacteria (data not shown). Using SMART analyses, a YjeF_N domain was identified in EmABP between residues Y47 and C220, and all residues that are known to be invariant or highly conserved in YjeF_N domains were also present in EmABP (Fig. 1). Furthermore, using the computer-based program iPSORT, an export-directing signal sequence of 30 amino acids was identified at the N terminus of EmABP (Fig. 1), leading to a mature protein of 26.6 kDa after cleavage. Secondary structure predictions were performed using the Jpred 3 algorithm (13) for EmABP and human AI-BP and were compared with crystallographic data previously obtained for murine AI-BP (26). In all three cases, a very similar distribution of α-helices and β-strands was observed, indicating that all three proteins are capable of adopting a Rossmann-like fold in the YjeF_N domain (data not shown). Homology searches in expressed sequence tag databases of the related parasitic tapeworm Schistosoma japonicum (31) and the free-living planarian Schmidtea mediterranea (43) revealed the presence of EmABP orthologs in both organisms, which displayed similarity values to the Echinococcus protein in the range of values observed between EmABP and human AI-BP. Interestingly, neither in the trematode nor in the planarian ortholog was a signal sequence present (Fig. 1). Between the N-terminal signal sequence and the YjeF_N domain, mammalian AI-BP orthologs typically carry a stretch of 25 amino acids (Fig. 1) which contains a serine residue that, during murine sperm capacitation, is phosphorylated by protein kinase A (26). A similar sequence stretch was absent in EmABP and was also missing upstream of the YjeF_N domain in the Schistosoma and Schmidtea orthologs (Fig. 1).
FIG. 1.
Structural features and sequence homologies of EmABP. Displayed is a CLUSTAL V alignment (MEGALIGN) between E. multilocularis EmABP (this work), human AI-BP (Homo sapiens AI-BP [HsAI-BP]) (GenBank accession no. AJ315849) (42) as well as EmABP orthologs from S. japonicum (SjABP) (GenBank accession no. AY815871) (31) and S. mediterranea (SmedABP) (mk4.005551.03; http://smedgd.neuro.utah.edu/) (43). Amino acid positions are numbered at the right margin. Residues that are conserved in at least two of the sequences are printed in white on black background. Homologies of the aligned proteins to EmABP are indicated at the end of the alignment (percent similarity and percent identity). A thick dashed overline indicates the YjeF_N domain, as predicted by the SMART algorithm (30). Arrows above and below the alignment indicate predicted signal peptidase cleavage sites in EmABP and human AI-BP (42), respectively. Asterisks above and below the alignment mark the positions of introns in the coding regions for EmABP and human AI-BP (44), respectively. Open circles below the alignment indicate invariant residues, and closed circles indicate highly conserved residues within YjeF_N domains (26). A circled “P” below the alignment indicates the serine residue which, in murine AI-BP, is phosphorylated by protein kinase A (26). Gaps introduced to maximize alignment are indicated by dashes in the sequences.
Fernandez et al. (18) have previously generated SL- and oligonucleotide-capped cDNA libraries from E. granulosus which are available under ftp://ftp.sanger.ac.uk/pub/pathogens/Echinococcus/. Homology searches in these databases revealed the presence of two full-length clones coding for proteins with 98% (EGPSPsl-14g07.q1k) and 97% (EGPSPsl-10b12.q1k) identity to EmABP. Both derived from a SL library made from protoscolex mRNA and contained the E. granulosus SL at the 5′ end. Compared to EmABP, four amino acid exchanges were observed in the sequence from EGPSPsl-14g07.q1k (Q2L, P5L, N182K, and K198E) and two additional exchanges in the EGPSPsl-10b12.q1k sequence (K38R and Y47H).
The E. multilocularis genome is currently being sequenced, and sequence information representing fourfold coverage is available under http://www.sanger.ac.uk/Projects/Echinococcus/. When the cDNA sequence of Emabp was compared with the available data, all exons were identified on three adjacent contigs and the reading frame was shown to be interrupted by six introns (Fig. 1) which all contained canonical GT and AG residues at the 5′ and 3′ ends, respectively. The human gene encoding AI-BP contains five introns (44) which all map to identical positions as the first five introns of Emabp (Fig. 1), indicating that both genes share a common genetic ancestor. On the genome sequence available at this time, no further gene encoding a YjeF_N domain protein was identified. Hence, Emabp is most probably a single-copy gene.
Expression of Emabp in Echinococcus larval stages and adult worms.
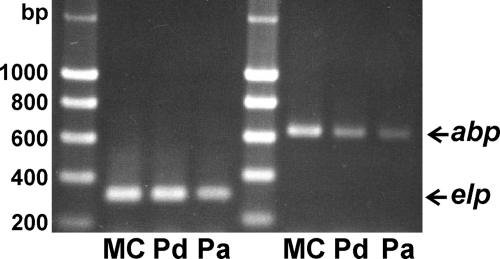
The expression of Emabp in larval stages was analyzed by semiquantitative RT-PCR. As shown in Fig. 2, Emabp transcripts were detected in metacestode vesicles, in resting protoscoleces, and to a somewhat lower extent, in the protoscolex after activation through pepsin/low-pH treatment. We also isolated RNA from adult E. multilocularis worms (free of eggs) and clearly identified Emabp in respective cDNA preparations by RT-PCR (data not shown). Taken together, these data indicated that Emabp is constitutively expressed throughout the parasite's life cycle.
FIG. 2.
Emabp expression patterns. Total RNA has been isolated from in vitro-cultivated metacestode vesicles (MC) as well as from dormant (Pd) and low-pH/pepsin-activated (Pa) protoscoleces and was reverse transcribed into cDNA. RT-PCR was performed using primers specific for Emabp (abp) and the constitutively expressed control gene elp (3, 5). PCR products were separated on a 1% agarose gel and stained with ethidium bromide.
Recombinant expression and purification of EmABP.
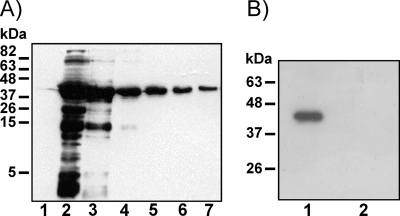
The Emabp reading frame, excluding the codons for the signal sequence, was translationally fused to thiredoxin (N-terminal) as well as a hexahistidine tag and the V5 antibody epitope (both C-terminal) using the pBAD/Thio-TOPO system (Invitrogen). The induced fusion protein (thio-EmABP) was subsequently purified under native conditions (Fig. 3A). An antibody against human AI-BP has previously been produced by immunization of a rabbit with the peptide V263PPALEKKYQLNLPPYPDTE282 (42). Since EmABP contained a very similar sequence (V236PPLLADKYNLCLPPYPGAS255 [identical residues underlined]), we tested whether the anti-AI-BP antibody also detected EmABP, which was indeed the case (Fig. 3B). On the other hand, the anti-AI-BP antibody did not interact with the thio-V5-His6 control protein (data not shown).
FIG. 3.
Heterologous expression, purification, and detection of EmABP. (A) Heterologous expression in E. coli and purification. The Emabp reading frame was cloned in the pBAD/TOPO ThioFusion expression system (Invitrogen) in E. coli. Lysates of arabinose-induced (lane 2) and noninduced (lane 1) E. coli were separated on an 12% acrylamide gel, transferred to a nitrocellulose membrane, and analyzed using the anti-V5 antibody (Invitrogen). Heterologously expressed thio-EmABP was purified under native conditions, and samples of elution steps 1 to 5 were applied to lanes 3 to 7, respectively. Separation of the samples and protein detection was performed as described above. The positions of molecular size markers (in kilodaltons) are indicated to the left of the gel. (B) Detection of thio-EmABP using an anti-AI-BP antibody. Lysates of recombinant E. coli carrying plasmids for the thio-EmABP fusion protein were prepared after arabinose induction (lane 1) or without arabinose induction (lane 2), separated on an 12% acrylamide gel, transferred to a nitrocellulose membrane, and detected by using an anti-AI-BP (human) antibody (42).
Secretion of EmABP and detection of apoA-I in hydatid fluid.
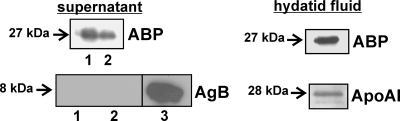
Since an export-directing signal sequence was identified in the deduced amino acid sequence of EmABP, we investigated whether the protein is secreted into the extraparasitic environment by the E. multilocularis metacestode. In vitro-cultivated metacestode vesicles (47, 50) were taken out of culture, thoroughly washed, and placed into minimal essential medium without protein supplements for 12 and 24 h. Western blot analyses of the supernatant with the anti-AI-BP antibody did not reveal bands, which was most probably due to a low concentration of secreted factors. We therefore employed the anti-AI-BP antibody in immunoprecipitation experiments and could successfully precipitate a single protein of the expected size (Fig. 4), indicating that EmABP is secreted by the E. multilocularis metacestode. For a control, we performed immunoprecipitation with an antibody against antigen B, one of the most abundant proteins in hydatid fluid (33). However, we never obtained bands despite the fact that antigen B was easily detected in hydatid fluid using the same antibody. These results showed that the metacestode vesicles in our assays were structurally intact and that the supernatant did not contain hydatid fluid contaminants.
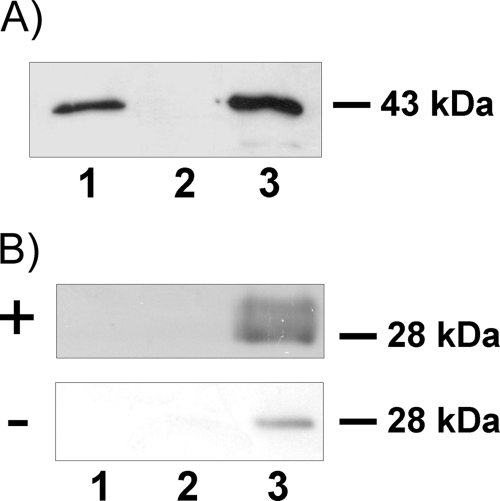
FIG. 4.
Detection of EmABP in hydatid fluid and culture supernatant. (Left, top) Metacestode vesicles from in vitro culture were thoroughly washed and kept for 12 h (lane 1) or 24 h (lane 2) in serum-free culture medium. Immunoprecipitation was subsequently carried out using the anti-AI-BP antibody (42). The precipitates were separated on a 12% acrylamide gel and analyzed using the anti-AI-BP antibody (ABP). (Bottom) To assess vesicle integrity, vesicle supernatant after 12 h (lane 1) and 24 h (lane 2) of incubation was subjected to immunoprecipitation using the monoclonal EB7 antibody, directed against Echinococcus antigen B (AgB) (23). Size separation and Western blot detection were performed as described above for EmABP, using the EB7 antibody instead of the anti-AI-BP antibody. For a control, hydatid fluid from in vitro-cultivated vesicles was size separated, and Western blot detection was performed using the EB7 antibody (lane 3). (Right) Hydatid fluid was isolated from in vitro-cultivated metacestode vesicles and subjected to immunoprecipitation using the anti-AI-BP antibody (top) or a commercial anti-apoA-I antibody (bottom). The precipitates were separated on a 12% acrylamide gel and transferred to a nitrocellulose membrane, and detection was carried out using the antibodies that had been used for immunoprecipitation. The sizes of the precipitated proteins (in kilodaltons) are indicated to the left of the gels.
Next, we investigated whether EmABP is also present in hydatid fluid. In Western blot analyses using the anti-AI-BP antibody on freshly isolated hydatid fluid, only a very faint band at the expected size was obtained (data not shown). Immunoprecipitation was therefore performed as described above with hydatid fluid from in vitro-cultured metacestode vesicles instead of supernatant, and as shown in Fig. 4, a protein of the expected size was clearly present. Since we never detected mammalian AI-BP in the conditioned medium that was used to cultivate metacestode vesicles in vitro (data not shown), we concluded that the immunoreactive protein in hydatid fluid was indeed EmABP and not host-derived AI-BP that had been transported across the parasite's laminated and germinal layers. Using a commercial antibody against apoA-I, we also tried to immunoprecipitate the mammalian apolipoprotein from hydatid fluid and were successful (Fig. 4). Since apoA-I orthologs are usually found only in higher metazoans (27) and since we could not find indications that the parasite's genome contains a gene for such an ortholog, we concluded that this immunoreactive protein indeed derived from the host and not from E. multilocularis. Taken together, these data indicated that EmABP is secreted by the metacestode both into the interior lumen (hydatid fluid) and into the exterior. Furthermore, similar to other host serum factors like albumin or immunoglobulins (11), apoA-I is present in hydatid fluid.
Interaction of EmABP with human apoA-I.
Human AI-BP has originally been identified in a screen for proteins that interact with apoA-I (42). To investigate similar activities for EmABP, immunoprecipitation and pulldown experiments were carried out. First, we tested whether the purified thio-EmABP fusion protein was able to interact with purified human apoA-I in a pulldown assay. As shown in Fig. 5A, thio-EmABP clearly interacted with apoA-I. On the other hand, we never observed interaction between the control protein thio-V5-His6 and apoA-I (data not shown). Second, we tested whether purified thio-EmABP was able to precipitate apoA-I from human serum and used sera from healthy donors and patients with active AE. In both cases, thio-EmABP precipitated a protein of the expected size which was immunoreactive with the anti-apoA-I antibody, while no precipitation was observed for the control protein thio-V5-His6. Taken together, the above experiments clearly indicated that EmABP, like its human ortholog AI-BP, is able to interact with human apoA-I.
FIG. 5.
Interaction between EmABP and human apoA-I. (A) Interaction of purified EmABP and apoA-I. Purified, recombinant apoA-I was bound to protein G-agarose beads using the anti-apoA-I antibody. The recombinantly expressed, purified thio-EmABP fusion protein was added, and after 2 h of incubation, agarose G beads were pelleted and thoroughly washed. The protein complexes were then separated on a 12% acrylamide gel, and Western blot detection using the anti-V5 antibody (Invitrogen) was carried out. In lane 1, the anti-apoA-I antibody, apoA-I, and thio-EmABP were applied. Conditions in lane 2 were identical to lane 1 except that apoA-I was omitted. In lane 3, the original input of thio-EmABP is shown. (B) Precipitation of apoA-I from human serum. The anti-V5 antibody was bound to Sepharose A beads (lanes 1 to 3), followed by the addition of the thio-EmABP fusion protein (lane 3) or the thio-V5-His6 control protein (lane 2) (in lane 1, no fusion protein was added). The antibody protein complexes were incubated overnight with serum from AE patients (+) or healthy donors (−). The Sepharose A beads were then pelleted and thoroughly washed. Protein complexes were separated on an 12% acrylamide gel, transferred to a nitrocellulose membrane, and detected using an anti-apoA-I antibody.
EmABP is not antigenic.
Since EmABP is apparently secreted by the E. multilocularis metacestode into the surrounding (host) medium, we finally investigated whether patients suffering from AE and CE produce antibodies against the parasite protein. In established enzyme-linked immunosorbent assays which utilize the parasite-derived protein Elp (also called EM10) for serological detection of AE and CE (24, 40), we consistently measured significantly higher levels of IgG and IgE antibodies against Elp in sera from patients with confirmed AE and CE compared to sera from healthy donors. When using thio-EmABP as an antigen, on the other hand, no elevated antibody levels were detected in patient sera. Furthermore, anti-EmABP antibody levels in AE patients were consistently as low as anti-Elp antibody levels in healthy donors (data not shown). Taken together, we conclude from these experiments that EmABP is not acting as a parasite antigen during active infections, which is most likely a result of its overall high homology to human AI-BP.
DISCUSSION
In the present study, we have identified a novel protein, EmABP, which is secreted by the Echinococcus multilocularis metacestode into the surrounding host medium (as well as into hydatid fluid; for a diagrammatic representation of the E. multilocularis metacestode, please see reference 8) and interacts with host-derived apoA-I. Due to the crucial function of apoA-I in mammalian lipid and cholesterol transport processes mediated through HDL particles, this feature suggests a potential role of EmABP in interfering with host lipid and cholesterol transport.
The fact that E. multilocularis and E. granulosus, like other parasitic flatworms, cannot de novo synthesize cholesterol and the majority of other lipid components, has already been firmly established by previous studies (19, 20, 35). This is supported by our own analyses of the first draft version of the E. multilocularis genome, which showed that genes for the majority of enzymes that are involved in cholesterol synthesis in other organisms (51) are absent in the cestode (data not shown). In the case of the E. granulosus metacestode, an uptake of radioactively labeled, host-derived cholesterol during an infection of laboratory animals has already been demonstrated (20), and it is reasonable to assume that E. multilocularis employs cholesterol uptake mechanisms similar to those of the closely related dog tapeworm. Hence, in addition, or as an alternative, to interfering with lipid/cholesterol transport of the host, EmABP might be actively involved in cholesterol and lipid uptake by the parasite.
Like its mammalian ortholog AI-BP, EmABP belongs to the widespread YjeF_N protein family, members of which are present in eukaryotes, bacteria, and archaea (1). Unfortunately, neither the general biochemical properties nor the cellular functions of YjeF_N proteins are known at this time, the protein family being in the top 10 list of highly attractive targets for functional characterization (21). YjeF_N domains either occur as single proteins, like in the case of EmABP and AI-BP, or as fusions with other domains and are commonly associated with enzymes (1). Structurally, the YjeF_N domain displays a Rossman-like fold, a structural motif found in proteins that bind nucleotides, especially the cofactor NAD (32), and it is generally thought that the domain exerts an unknown enzymatic activity, such as dephosphorylation, demethylation, and phosphoester or glycosyl bond hydrolysis (1). Although the precise biochemical and cell biological functions of AI-BP are unknown at this time, several lines of evidence indicate that this protein plays a role in cellular cholesterol and lipid transport mechanisms in a variety of organs in higher eukaryotes. First, AI-BP is expressed in a wide variety of mammalian tissues, and apart from its binding capacity to apoA-I, it localizes in domains in sperm in which cholesterol is known to be concentrated (26). Second, AI-BP is released into the medium during sperm capacitation, a process that is accompanied by cholesterol release (26). Third, immunohistochemical analyses of human testes and ovaries identified AI-BP in various cell types coexpressed with ABCA1 (ATP binding cassette transporter A1), the main determinant for plasma HDL, which is involved in cellular lipid transport and steroid hormone synthesis and which also interacts with apoA-I (44). Apart from its function in spermiogenesis and oogenesis, AI-BP seems to also play an important role in hepatocyte metabolism, since it is highly expressed in liver tissue as well as in HepG2 cells (42). Interestingly, although sera from healthy donors do not contain AI-BP, increased plasma levels were observed in patients suffering from systemic inflammation and sepsis, which could be linked to general changes in plasma lipoprotein metabolism that occur during inflammatory processes (35, 42). Hence, once released by the E. multilocularis metacestode at the primary site of infection (the liver), EmABP could indeed affect both the physiology of hepatocytes and the local immune response. It has to be kept in mind, however, that apoA-I and other lipid transporters are among the most abundant components of human serum (38) and that probably very large amounts of EmABP would have to be secreted in order to significantly interfere with this equilibrium. Hence, as further outlined below, we clearly favor a role for EmABP in cholesterol uptake mechanisms of the parasitic metacestode over a function in altering the host's immune response.
Apart from Emabp expression in the metacestode, we also found the gene transcribed in adult worms. Due to the absence of apoA-I and apoA-I-dependent cholesterol transport activities in the gut of the definitive host, we do not consider it likely that the suggested role of the protein in cholesterol uptake is shared between metacestode vesicles and adult worms. Since AI-BP orthologs are apparently also expressed by adult schistosomes and (free-living) planarians, they most probably exert additional cellular functions that still have to be determined. Due to the location of human AI-BP in the testes and ovaries (42), flatworm EmABP orthologs might be specifically involved in spermatogenesis and oogenesis, which does, however, clearly require additional experimentation.
One striking difference between mammalian AI-BP orthologs and YjeF_N family members from bacteria, lower eukaryotes, or invertebrates is the presence of an export-directing signal sequence at the N termini of the mammalian proteins (42, 44). Interestingly, Rudolph et al. (44) identified two additional YjeF_N proteins in humans which lack a signal sequence, are not secreted, and fail to interact with apoA-I. On the basis of immunolocalization studies of testes and ovaries, these authors concluded that AI-BP and both additional mammalian YjeF_N proteins are involved in mechanisms of cholesterol processing and steroid hormone metabolism and that the specific function of AI-BP is to link these functions to the HDL pathway (44). Since apoA-I and HDL transport mechanisms have arisen relatively late in animal evolution (27), it is reasonable to assume that this was accompanied by the acquisition of a signal sequence and thus, secretion of AI-BP into the extracellular environment. In this context, it is interesting to note that EmABP contains a signal sequence which is absent in the AI-BP orthologs of the related flatworms S. japonicum (parasitic) and S. mediterranea (free-living) and which has apparently been acquired during cestode evolution through genomic rearrangements (as indicated by the presence of an intron at the respective position). The fact that this signal sequence is indeed active is clearly indicated by our immunoprecipitation experiments which detected EmABP in the extralarval medium and in hydatid fluid. Hence, like in the case of mammalian AI-BPs, EmABP might have acquired a signal sequence to specifically link the host's HDL transport mechanisms to cholesterol and lipid uptake and metabolism by the parasite.
It is well established that host serum components, such as albumin or immunoglobulins, are present in hydatid fluid (11), and in this study, we have identified apoA-I as another host serum factor which is apparently transported from the surrounding host medium into the metacestode lumen. Unfortunately, the underlying transport mechanisms for any serum component are completely unknown at present. Likewise, little is known on protein secretion mechanisms in Echinococcus. Therefore, at this time, we cannot tell whether EmABP is simultaneously secreted by the metacestode into the hydatid lumen and into the exterior medium or whether the protein is first secreted into the surrounding medium and subsequently transported across the germinal layer into hydatid fluid. The fact that we measured somewhat smaller amounts of secreted EmABP in two different samples of in vitro-cultured metacestode vesicles after 24 h compared to 12 h might indeed indicate that the protein is first secreted and then transported into the parasite lumen once a critical external concentration is built up. This is supported by results from our Western blot analyses showing that EmABP is not one of the major constituents of hydatid fluid but rather is present in smaller amounts. However, although we have used comparable numbers of metacestode vesicles in both experimental settings, these might have had slight physiological differences, so this hypothesis surely awaits further experimentation. To this end, we are currently trying to use recombinantly expressed, purified EmABP together with apoA-I and in vitro-reconstituted HDL particles (37) to study whether the parasite protein might be directly involved in the transport host components into in vitro-cultivated metacestode vesicles. In any case, the fact that both apoA-I and EmABP are present in hydatid fluid indicates that the parasite protein plays a role in parasite-specific physiological processes. On the basis of the above outlined functional properties of the human ortholog AI-BP, we suggest that this function involves cholesterol metabolism and uptake by E. multilocularis metacestode vesicles.
Acknowledgments
This work was supported by grants SFB479 and IRTG 1522 (both to K.B.) from the Deutsche Forschungsgemeinschaft.
We are indebted to Gualberto Gonzalez-Sapienza (Montevideo, Uruguay) and Mara Rosenzvit (Buenos Aires, Argentina) for providing the monoclonal EB7 antibody. We also thank Dirk Radloff and Monika Bergmann for excellent technical assistance. Special thanks are addressed to Peter D. Olson (Natural History Museum, London, United Kingdom) for critically reading the manuscript. Sequence data to screen the E. multilocularis genome for EmABP orthologs have been produced by the Parasite Sequencing Group at the Sanger Institute and can be obtained from ftp://ftp.sanger.ac.uk/pub/pathogens/Echinococcus/.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Anantharaman, V., and L. Aravind. 2004. Novel conserved domains in proteins with predicted roles in eukaryotic cell-cycle regulation, decapping and RNA stability. BMC Genomics 5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannai, H., Y. Tamada, O. Marumyama, K. Nakai, and S. Miyano. 2002. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 18:298-305. [DOI] [PubMed] [Google Scholar]
- 3.Brehm, K., K. Jensen, P. Frosch, and M. Frosch. 1999. Characterization of the genomic locus expressing the ERM-like protein of Echinococcus multilocularis. Mol. Biochem. Parasitol. 100:147-152. [DOI] [PubMed] [Google Scholar]
- 4.Brehm, K., K. Jensen, and M. Frosch. 2000. mRNA trans-splicing in the human parasitic cestode Echinococcus multilocularis. J. Biol. Chem. 275:38311-38318. [DOI] [PubMed] [Google Scholar]
- 5.Brehm, K., M. Wolf, H. Beland, A. Kroner, and M. Frosch. 2003. Analysis of differential gene expression in Echinococcus multilocularis larval stages by means of spliced leader differential display. Int. J. Parasitol. 33:1145-1159. [DOI] [PubMed] [Google Scholar]
- 6.Brehm, K., M. Spiliotis, R. Zavala-Góngora, C. Konrad, and M. Frosch. 2006. The molecular mechanisms of larval cestode development: first steps into an unknown world. Parasitol. Int. 55:S15-S21. [DOI] [PubMed] [Google Scholar]
- 7.Brehm, K., and M. Spiliotis. 2008. The influence of host hormones and cytokines on Echinococcus multilocularis signalling and development. Parasite 15:286-290. [DOI] [PubMed] [Google Scholar]
- 8.Brehm, K., and M. Spiliotis. 2008. Recent advances in the in vitro cultivation and genetic manipulation of Echinococcus multilocularis metacestodes and germinal cells. Exp. Parasitol. 119:506-515. [DOI] [PubMed] [Google Scholar]
- 9.Chang, T. Y., C. C. Y. Chang, N. Ohgami, and Y. Yamauchi. 2006. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22:129-157. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, M. J. 1980. Animal lipoproteins: chemistry, structure, and comparative aspects. J. Lipid Res. 21:789-853. [PubMed] [Google Scholar]
- 11.Chemale, G., A. J. van Rossum, J. R. Jefferies, J. Barrett, P. M. Brophy, H. B. Ferreira, and A. Zaha. 2003. Proteomic analysis of the larval stage of the parasite Echinococcus granulosus: causative agent of cystic hydatid disease. Proteomics 3:1633-1636. [DOI] [PubMed] [Google Scholar]
- 12.Chemale, G., H. B. Ferreira, J. Barrett, P. M. Brophy, and A. Zaha. 2005. Echinococcus granulosus antigen B hydrophobic ligand binding proteins. Biochim. Biophys. Acta 1747:189-194. [DOI] [PubMed] [Google Scholar]
- 13.Cole, C., J. D. Barber, and G. J. Barton. 2008. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36:W197-W201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deplazes, P., P. Alther, I. Tanner, R. C. Thompson, and J. Eckert. 1999. Echinococcus multilocularis coproantigen detection by enzyme-linked immunosorbent assay in fox, dog, and cat populations. J. Parasitol. 85:115-121. [PubMed] [Google Scholar]
- 15.Dinguirard, N., and T. P. Yoshino. 2006. Potential role of a CD36-like class B scavenger receptor in the binding of modified low-density lipoprotein (acLDL) to the tegumental surface of Schistosoma mansoni sporocysts. Mol. Biochem. Parasitol. 146:219-230. [DOI] [PubMed] [Google Scholar]
- 16.Eckert, J., and P. Deplazes. 2004. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 17:107-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan, J., X. Gan, W. Yang, L. Shen, D. P. McManus, and P. J. Brindley. 2003. A Schistosoma japonicum very low-density lipoprotein-binding protein. Int. J. Biochem. Cell Biol. 35:1436-1451. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez, C., W. F. Gregory, P. Loke, and R. M. Maizels. 2002. Full-length-enriched cDNA libraries from Echinococcus granulosus contain separate populations of oligo-capped and trans-spliced transcripts and a high level of predicted signal sequences. Mol. Biochem. Parasitol. 122:171-180. [DOI] [PubMed] [Google Scholar]
- 19.Frayha, G. J. 1968. A study on the synthesis and absorption of cholesterol in hydatid cysts (Echinococcus granulosus). Comp. Biochem. Physiol. 27:875-878. [DOI] [PubMed] [Google Scholar]
- 20.Frayha, G. J. 1971. Comparative metabolism of acetate in the taeniid tapeworms Echinococcus granulosus, E. multilocularis and Taenia hydatigena. Comp. Biochem. Physiol. 39B:167-170. [DOI] [PubMed] [Google Scholar]
- 21.Galperin, M. Y., and E. V. Koonin. 2004. Conserved hypothetical proteins: prioritization of targets for experimental study. Nucleic Acids Res. 32:5452-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelmedin, V., R. Caballero-Gamiz, and K. Brehm. 2008. Characterization and inhibition of a p38-like mitogen activated protein kinase (MAPK) from Echinococcus multilocularis: antiparasitic activities of p38 MAPK inhibitors. Biochem. Pharmacol. 76:1068-1081. [DOI] [PubMed] [Google Scholar]
- 23.González-Sapienza, G., and R. E. Cachau. 2003. Identification of critical residues of an immunodominant region of Echinococcus granulosus antigen B. J. Biol. Chem. 278:20179-20184. [DOI] [PubMed] [Google Scholar]
- 24.Helbig, M., P. Frosch, P. Kern, and M. Frosch. 1993. Serological differentiation between cystic and alveolar echinococcosis by use of recombinant larval antigens. J. Clin. Microbiol. 31:3211-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakobsson, E., G. Alvite, T. Bergfors, A. Esteves, and G. J. Kleywegt. 2003. The crystal structure of Echinococcus granulosus fatty-acid-binding protein 1. Biochim. Biophys. Acta 1649:40-50. [DOI] [PubMed] [Google Scholar]
- 26.Jha, K. N., I. A. Shumilin, L. C. Digilio, O. Chertihin, H. Zheng, G. Schmitz, P. E. Visconti, C. J. Flickinger, W. Minor, and J. C. Herr. 2008. Biochemical and structural characterization of apolipoprotein A-I binding protein, a novel phosphoprotein with a potential role in sperm capacitation. Endocrinology 149:2108-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasap, M., A. Sazci, G. Akpinar, and E. Ergul. 2008. Apolipoprotein E phylogeny and evolution. Cell Biochem. Funct. 26:43-50. [DOI] [PubMed] [Google Scholar]
- 28.Kern, P., H. Wen, N. Sato, D. A. Vuitton, B. Gruener, Y. Shao, E. Dalabrousse, W. Kratzer, and S. Bresson-Hadni. 2006. WHO classification of alveolar echinococcosis: principles and application. Parasitol. Int. 55:S283-S287. [DOI] [PubMed] [Google Scholar]
- 29.Kurzchalia, T. V., and S. Ward. 2003. Why do worms need cholesterol? Nat. Cell Biol. 5:684-688. [DOI] [PubMed] [Google Scholar]
- 30.Letunic, I., T. Doerks, and P. Bork. 2009. SMART 6: recent updates and new developments. Nucleic Acids Res. 37:D229-D232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, F., J. Lu, W. Hu, S. Y. Wang, S. J. Cui, M. Chi, Q. Yan, X. R. Wang, H. D. Song, N. X. Xu, et al. 2006. New perspectives on host-parasite interplay by comparative transcriptomic and proteomic analyses of Schistosoma japonicum. PLoS Pathog. 2:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, B. G., L. Chen, H. F. Ji, Z. H. Chen, F. R. Yang, L. Wang, G. Qu, Y. Y. Jiang, C. Ji, and H. Y. Zhang. 2008. Characters of very ancient proteins. Biochem. Biophys. Res. Commun. 366:607-611. [DOI] [PubMed] [Google Scholar]
- 33.Mamuti, W., Y. Sako, M. Nakao, N. Xiao, K. Nakaya, Y. Ishikawa, H. Yamasaki, M. W. Lightowlers, and A. Ito. 2006. Recent advances in characterization of Echinococcus antigen B. Parasitol. Int. 55:S57-S62. [DOI] [PubMed] [Google Scholar]
- 34.Matyash, V., C. Geier, A. Henske, S. Mukherjee, D. Hirsch, C. Thiele, B. Grant, F. R. Maxfield, and T. V. Kurzchalia. 2001. Distribution and transport of cholesterol in Caenorhabditis elegans. Mol. Biol. Cell 12:1725-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McManus, D. P., and C. Bryant. 1986. Biochemistry and physiology of Echinococcus, p. 114-136. In R. C. A. Thompson (ed.), The biology of Echinococcus and hydatid disease. George Allen and Unwin Ltd., London, United Kingdom.
- 36.Olofsson, S. O., O. Wiklund, and J. Borén. 2007. Apolipoprotein A-I and B: biosynthesis, role in the development of atherosclerosis and targets for intervention against cardiovascular disease. Vasc. Health Risk Manag. 3:491-502. [PMC free article] [PubMed] [Google Scholar]
- 37.Pilon, A., O. Briand, S. Lestavel, C. Copin, Z. Majd, J. C. Fruchart, G. Castro, and V. Clavey. 2000. Apolipoprotein AII enrichment of HDL enhances their affinity for class B type I scavenger receptor but inhibits specific cholesteryl ester uptake. Arterioscler. Thromb. Vasc. Biol. 20:1074-1081. [DOI] [PubMed] [Google Scholar]
- 38.Pownall, H. J., and C. Ehnholm. 2006. The unique role of apolipoprotein A-I in HDL remodelling and metabolism. Curr. Opin. Lipidol. 17:209-213. [DOI] [PubMed] [Google Scholar]
- 39.Rader, D. J. 2006. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Investig. 116:3090-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiter-Owona, I., B. Grüner, M. Frosch, A. Hoerauf, P. Kern, and D. Tappe. 2009. Serological confirmatory testing of alveolar and cystic echinococcosis in clinical practice: results of a comparative study with commercialized and in-house assays. Clin. Lab. 55:41-48. [PubMed] [Google Scholar]
- 41.Rhainds, D., and L. Brissette. 1999. Low density lipoprotein uptake: holoparticle and cholesteryl ester selective uptake. Int. J. Biochem. Cell Biol. 31:915-931. [DOI] [PubMed] [Google Scholar]
- 42.Ritter, M., C. Buechler, A. Boettcher, S. Barlage, A. Schmitz-Madry, E. Orsó, S. M. Bared, G. Schmiedeknecht, C. H. Baehr, G. Fricker, and G. Schmitz. 2002. Cloning and characterization of a novel apolipoprotein A-I binding protein, AI-BP, secreted by cells of the kidney proximal tubules in response to HDL or apoA-I. Genomics 79:693-702. [DOI] [PubMed] [Google Scholar]
- 43.Robb, S. M. C., E. Ross, and A. Sánchez-Alvarado. 2008. SmedGD: the Schmidtea mediterranea genome database. Nucleic Acids Res. 36:D599-D606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudolph, C., A. Sigruener, A. Hartmann, E. Orso, M. Bals-Pratsch, W. Gronwald, B. Seifert, H. R. Kalbitzer, I. Verdorfer, C. M. Luetjens, O. Ortmann, S. R. Bornstein, and G. Schmitz. 2007. ApoA-I-binding protein (AI-BP) and its homologues hYjeF_N2 and hYjeF_N3 comprise the YjeF_N domain protein family in humans with a role in spermiogenesis and oogenesis. Horm. Metab. Res. 39:322-335. [DOI] [PubMed] [Google Scholar]
- 45.Smolenaars, M. M. W., O. Madsen, K. W. Rodenburg, and D. J. Van der Horst. 2007. Molecular diversity and evolution of the large lipid transfer protein superfamily. J. Lipid Res. 48:489-502. [DOI] [PubMed] [Google Scholar]
- 46.Spiliotis, M., and K. Brehm. 2004. Echinococcus multilocularis: identification and molecular characterization of a Ral-like small GTP-binding protein. Exp. Parasitol. 107:163-172. [DOI] [PubMed] [Google Scholar]
- 47.Spiliotis, M., D. Tappe, L. Sesterhenn, and K. Brehm. 2004. Long-term in vitro cultivation of Echinococcus multilocularis metacestodes under axenic conditions. Parasitol. Res. 92:430-432. [DOI] [PubMed] [Google Scholar]
- 48.Spiliotis, M., C. Konrad, V. Gelmedin, D. Tappe, S. Brückner, H. U. Mösch, and K. Brehm. 2006. Characterization of EmMPK1, an ERK-like MAP kinase from Echinococcus multilocularis which is activated in response to human epidermal growth factor. Int. J. Parasitol. 36:1097-1112. [DOI] [PubMed] [Google Scholar]
- 49.Spiliotis, M., S. Lechner, D. Tappe, C. Scheller, G. Krohne, and K. Brehm. 2008. Transient transfection of Echinococcus multilocularis primary cells and complete in vitro regeneration of metacestode vesicles. Int. J. Parasitol. 38:1025-1039. [DOI] [PubMed] [Google Scholar]
- 50.Spiliotis, M., and K. Brehm. 2009. Axenic in vitro cultivation of Echinoccoccus multilocularis metacestode vesicles and the generation of primary cell cultures. Methods Mol. Biol. 470:245-262. [DOI] [PubMed] [Google Scholar]
- 51.Vinci, G., X. Xia, and R. A. Veitia. 2008. Preservation of genes involved in sterol metabolism in cholesterol auxotrophs: facts and hypotheses. PLoS One 3:e2883. [DOI] [PMC free article] [PubMed] [Google Scholar]