Abstract
The course and outcome of infection with mycobacteria are determined by a complex interplay between the immune system of the host and the survival mechanisms developed by the bacilli. Recent data suggest a regulatory role of histamine not only in the innate but also in the adaptive immune response. We used a model of pulmonary Mycobacterium tuberculosis infection in histamine-deficient mice lacking histidine decarboxylase (HDC−/−), the histamine-synthesizing enzyme. To confirm that mycobacterial infection induced histamine production, we exposed mice to M. tuberculosis and compared responses in C57BL/6 (wild-type) and HDC−/− mice. Histamine levels increased around fivefold above baseline in infected C57BL/6 mice at day 28 of infection, whereas only small amounts were detected in the lungs of infected HDC−/− mice. Blocking histamine production decreased both neutrophil influx into lung tissue and the release of proinflammatory mediators, such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α), in the acute phase of infection. However, the accumulation and activation of CD4+ T cells were augmented in the lungs of infected HDC−/− mice and correlated with a distinct granuloma formation that contained abundant lymphocytic infiltration and reduced numbers of mycobacteria 28 days after infection. Furthermore, the production of IL-12, gamma interferon, and nitric oxide, as well as CD11c+ cell influx into the lungs of infected HDC−/− mice, was increased. These findings indicate that histamine produced after M. tuberculosis infection may play a regulatory role not only by enhancing the pulmonary neutrophilia and production of IL-6 and TNF-α but also by impairing the protective Th1 response, which ultimately restricts mycobacterial growth.
Mycobacterium tuberculosis, the causative agent of tuberculosis, is an intracellular bacterium that is capable of surviving and persisting within host cells. The host response against tubercle bacilli is dominated by the interaction of innate and adaptive immunity (47). Some previous studies revealed the recognition and activation of innate immune cells, such as macrophages and mast cells, during the recognition of the pathogen by the immune system (7, 29). The adaptive immune response is initiated during the early phase of infection, and generation of an efficient T-cell response is an essential feature of protective host immunity (3). Host control of M. tuberculosis infection in both humans and mouse models has been shown to be associated with the production of gamma interferon (IFN-γ) by CD4+ and CD8+ T cells (39). Interleukin-12 (IL-12) has a central role in priming T cells to produce IFN-γ in response to M. tuberculosis (33, 46). In addition, several other cytokines are important to the general response to M. tuberculosis. For example, studies of the infection of mice treated with cytokines or cytokine-blocking antibodies and, more recently, of knockout mice have identified tumor necrosis factor alpha (TNF-α), IL-6, and IL-1 as essential for a protective immune response to M. tuberculosis (17, 18, 26, 32). Transforming growth factor beta and IL-6 lead to a pathway of T-cell differentiation that results in the generation of antigen-specific T cells that produce IL-17 (Th17). Upon activation, this population upregulates IL-23 receptor expression and increases its responsiveness to IL-23. During mycobacterial infection, IL-23 drives the establishment of a sustained CD4+ T-cell population that produces IL-17, IL-6, and TNF-α (30). Th17 cells mediate neutrophil recruitment (16, 27) and are negatively regulated by IFN-γ (21, 40).
A study carried out by our group demonstrated that the treatment of M. tuberculosis-infected mice with compound 48/80, a pharmacological agent able to activate and induce the release of preformed granules by mast cells, negatively modulates acute inflammation and delays the host defense (7). Histamine, which is formed by the decarboxylation of histidine, catalyzed by the histidine decarboxylase (HDC) enzyme, is a major component of these mast cell granules and possesses many biological and immunological activities (22). In mast cells and basophils, HDC is constitutively expressed (31). In contrast, in macrophages (42), T cells (2), and neutrophils (43), HDC is induced by inflammatory stimuli, resulting in continuous production of the enzyme (4). The effects of histamine are mediated through four types of membrane histamine receptors: H1R, H2R, H3R, and H4R, all of which are heptahelical G-protein-coupled receptors (1).
Recent investigations have generated much interest in the immune regulatory mechanisms triggered by histamine. However, diverse experimental systems have yielded conflicting data indicating either proinflammatory or anti-inflammatory effects of histamine, depending upon the predominance of a certain type of histamine receptor (28). Furthermore, it has been difficult to assess the effects of histamine on infectious diseases in vivo, and most studies have used either histamine receptor antagonists or agonists. Using these types of pharmacological tools, histamine signaling through H2R reportedly was required for the early stages of controlling Yersinia enterocolitica colonization within the Peyer's patches and mesenteric lymph nodes of mice (20). H1R and H2R blockers decreased lung edema and pneumonia severity in mycoplasma infection, suggesting that histamine is proinflammatory in this model (49). However, the use of these agents comes with several drawbacks, including unknown specificity and selectivity and a short half-life. In order to avoid these problems, we used HDC gene knockout (HDC−/−) mice that have been developed by Ohtsu et al. (38). In experimental bacterial peritonitis induced by Escherichia coli in HDC−/− mice, histamine impaired bacterial clearance, presumably through the inhibition of phagocyte recruitment (24). In our model, HDC−/− mice displayed controlled granulomatous inflammation in the lung with an intense influx of activated CD4+ T cells and dendritic cells (DCs); inhibition of IL-17, IL-6, and TNF-α cytokines; augmentation of Th1 cytokine production; and inducible nitric oxide (NO) synthase (iNOS) expression, all of which led to a more effective control of M. tuberculosis infection.
MATERIALS AND METHODS
Mice.
HDC−/− mice were backcrossed to C57BL/6 mice and bred at the Centre National de la Recherche Scientifique (CNRS). HDC−/− mice were produced by the routine homologous recombination method, by replacement of the region of the HDC gene from intron 6 to exon 9, which contains the coenzyme pyridoxal 5′-phosphate binding site (Lys), with a phosphoglycerate kinase promoter-driven neomycin resistance gene (37). The HDC−/− mice have a normal phenotype under steady-state conditions and were fertile. The histamine contents of various organs of HDC−/− mice decreased extensively but were not null, though HDC activity was undetectable. Mice were maintained in a temperature-controlled (23°C) facility with a strict 12-h light-dark cycle and given free access to food and water. All protocols complied with the French government's ethical and animal experiment regulations.
Bacteria and infection.
Pulmonary infections with M. tuberculosis H37Rv (Pasteur) were performed in isolators by delivering 100 bacteria into both nasal cavities (20 μl each) under xylazine-ketamine anesthesia as described previously (17). The bacterial load in the lung was determined at day 1 postinfection. Two independent experiments were conducted, a short-term study over 28 days (acute phase) and a long-term study over 112 days (chronic phase) (n = 5 or 6 per group).
Colony enumeration assay.
Bacterial loads in the lungs of infected mice were evaluated at day 28 after infection with M. tuberculosis H37Rv. Lungs were weighed, and defined aliquots were homogenized in 0.04% Tween 80 saline, by using closed disposable homogenization tubes (Dispomix; Medictool, Switzerland). Tenfold serial dilutions of organ homogenates were plated in duplicate onto Middlebrook 7H10 agar plates containing 10% oleic acid-albumin-dextrose-catalase and incubated at 37°C for 19 to 21 days. Colonies on plates were enumerated, and the results were expressed as CFU per lung.
Measurement of cytokines and histamine in lung tissue.
For cytokine determinations, lungs were removed after 28 days of infection, homogenized in Dispomix closed homogenization tubes (Medictool, Switzerland) in 2 ml of RPMI 1640 (Sigma, St. Louis, MO), and centrifuged at 10,000 × g for 15 min, and the supernatants were stored at −70°C until the assay was performed. Levels of TNF-α, IL-12, IL-6, IFN-γ, IL-10, IL-17, and IL-4 were determined in the supernatants by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (BD PharMingen, San Diego, CA). Sensitivities were >10 pg/ml. The histamine concentration was quantified using commercial specific ELISA kits according to the manufacturer's instructions (IBL, Hamburg, Germany).
Fluorescence-activated cell sorting analysis of infiltrating cells from infected lung.
Whole lungs were removed from infected mice at different time points and were homogenized in 1 ml of 0.04% Tween 80 saline, and supernatants were collected after low-speed centrifugation, aliquoted, and frozen at −80°C. Isolated lung cells were obtained by collagenase and DNase treatment. The cells were counted and incubated with antibodies against CD3 (anti-CD3 phycoerythrin [PE], clone 145.2C11), CD4 (anti-CD4 fluorescein isothiocyanate [FITC], clone H129.19), CD8 (anti-CD8 FITC, clone 53-6.7), CD44 (anti-CD44 PE, clone IM7), CD11c (anti-CD11c FITC, clone HL3), I-A/I-E (anti-I-A/I-E PE, clone M5/114.15.2), or CD16/32 (clone 2.4G2) or isotype controls. All staining procedures were performed in phosphate-buffered saline containing 0.1% bovine serum albumin and 0.1% sodium azide (fluorescence-activated cell sorting buffer) for 20 min at 4°C. All antibodies were used at 0.2 μg/106 cells and obtained from BD PharMingen (San Diego, CA). Cells were fixed with 4% paraformaldehyde for at least 1 h and analyzed by flow cytometry. Cells were gated on the lymphocyte or DC population by forward and side scatter, and the data were analyzed using CellQuest software (BD Systems, San Jose, CA).
Antigen-specific IFN-γ production.
T-cell priming was assessed by the production of IFN-γ upon antigen restimulation ex vivo. Single-cell suspensions of T cells were prepared from the lungs of infected wild-type and HDC−/− mice 4 weeks after infection with 102 CFU of M. tuberculosis H37Rv. These cells were cultured at 5 × 105 cells/ml in RPMI 1640 with glutamine, 5% fetal calf serum, and antibiotics and stimulated with 2.5 μg/ml concanavalin A (ConA; Sigma-Aldrich) or a lyophilized soluble fraction from Mycobacterium bovis BCG culture (10 μg/ml; Pasteur Institute) for 2 days at 37°C. IFN-γ production in the supernatant was quantified by ELISA (Duoset; R&D Systems). Alternatively, lung cells from M. tuberculosis-infected mice restimulated as described above were analyzed for intracellular IFN-γ staining of CD4+ or CD8+ T cells: after 72 h of antigen restimulation, addition of GolgiStop to block protein transport, and incubation for an additional 6 h, cells were labeled with anti-CD4-peridinin chlorophyll protein (clone RM4-5) or anti-CD8-allophycocyanin (APC) (clone 53-6.7) antibodies, fixed, and permeabilized, and intracellular IFN-γ was stained with antibodies to IFN-γ-FITC (clone XMG1.2; according to BD Cytofix/Cytoperm kit manual; all reagents from BD Biosciences) (19).
Histological analysis.
The lungs were fixed in 4% phosphate-buffered formalin, and fragments from the upper lobes were embedded in paraffin. Five 5-μm-thick sections were obtained and stained with hematoxylin and eosin (H&E). Some sections were submitted to Ziehl-Neelsen staining specific for mycobacteria. For the immunostaining, formalin-fixed paraffin-embedded sections were deparaffinized, rehydrated, and stained with rabbit anti-mouse antibody (courtesy of J. Pfeilschifter, University of Frankfurt) specific for iNOS as described above. The sections were then washed in phosphate-buffered saline and incubated for 30 min at room temperature with the biotinylated secondary antibody. The sections were incubated with avidin-biotin complexes (ABC vector kit) for 30 min, washed, and incubated with diaminobenzidine substrate (Dako, Glostrup, Denmark).
Morphometric analysis of the neutrophil influx in the lungs.
Morphometric analysis was performed with a Leica DMR microscope (Leica Microsystems, Wetzlar, Germany). The analysis was evaluated by a researcher blinded to the protocol design. The number of neutrophils present in the lung tissue stained with H&E was counted in five randomly noncoincident fields, at a magnification of ×1,000. The means were calculated, and the values expressed as number of neutrophils per field.
Quantification of NO.
NO production was assessed by measuring the amount of nitrite in lung homogenates, obtained as described using the Griess reagent (45). Values were determined using a standard curve with serial dilutions of NaNO2 (Sigma, St. Louis, MO).
Statistical analysis.
Each experiment was performed twice. The results of the experiments are expressed as means ± standard errors of the means (SEM). Statistical variations were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey test and Student's t test using the Prism 4.0 statistical program (GraphPad Software, San Diego, CA). The level of statistical significance was set at P < 0.05.
RESULTS
Histamine modulates acute inflammation associated with IL-17, IL-6, and TNF-α production during M. tuberculosis infection.
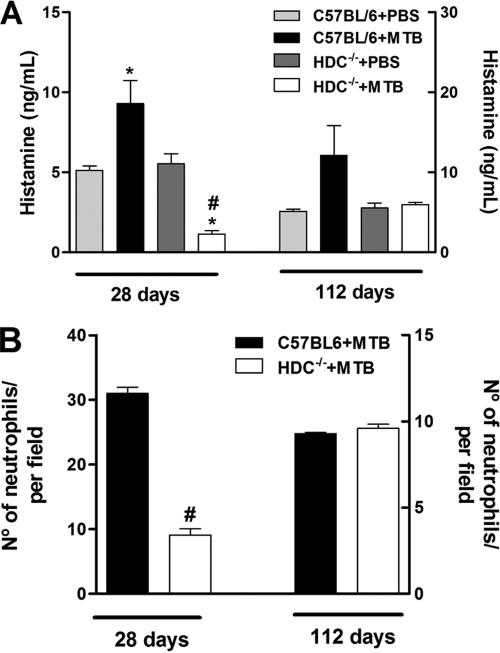
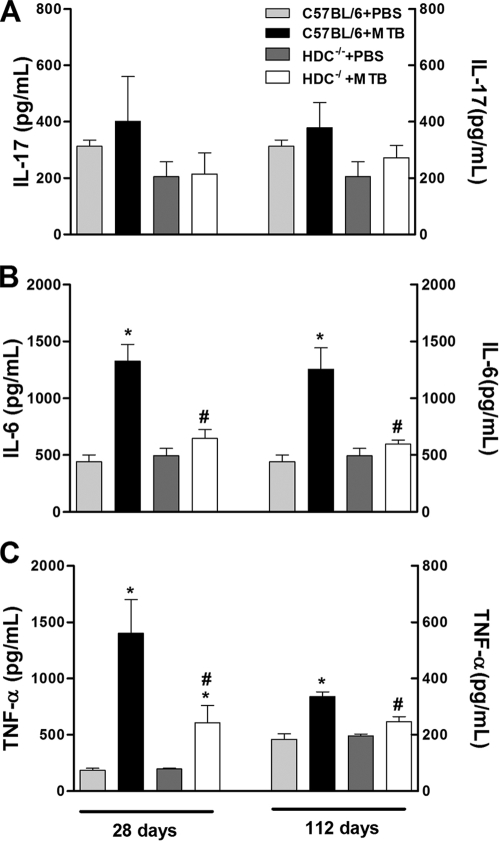
HDC−/− mice infected intranasally with 102 CFU of virulent M. tuberculosis (H37Rv) showed normal weight development and survived the 112-day observation period (data not shown). Histamine concentrations in the lung were significantly increased at 28 days after M. tuberculosis infection and persisted up to 112 days in C57BL/6 mice (wild type). In contrast, histamine levels were consistently very low in lung homogenate from infected HDC−/− mice at both time points analyzed relative to those of infected C57BL/6 mice (Fig. 1A). In addition, HDC−/− mice had significantly fewer pulmonary neutrophils at day 28, but not at day 112, than did infected C57BL/6 mice (Fig. 1B). Further, we also assayed the IL-17, IL-6, and TNF-α proinflammatory cytokine levels in lung tissue. Pulmonary IL-17 levels were diminished in infected HDC−/− mice compared to those in infected C57BL/6 mice, but the reduction was not statistically significant (Fig. 2A). The levels of IL-6 and TNF-α in the lungs of infected C57BL/6 mice were much higher after both 28 and 112 days of infection than those of uninfected mice. However, levels of these cytokines were significantly lower in infected HDC−/− mice (Fig. 2B and C). These results suggest that endogenous histamine released in response to M. tuberculosis infection may contribute to pulmonary neutrophilic inflammation via IL-17, IL-6, and TNF-α production.
FIG. 1.
HDC−/− mice displayed marked histamine inhibition and decreased neutrophil number in response to M. tuberculosis infection. (A) C57BL/6 and HDC−/− mice were infected with M. tuberculosis H37Rv (1 × 102 bacilli/mouse) by an intranasal route (black and white bars, respectively) or not infected (light gray and dark gray bars, respectively). Histamine concentrations were measured in lung tissue from uninfected and infected C57BL/6 or HDC−/− mice at the same time points. (B) Quantification of neutrophils in lung tissue from C57BL/6 or HDC−/− (black and white bars, respectively) mice after 28 and 112 days of infection. The results are expressed as means ± SEM from at least five animals in experiments repeated twice. Statistical testing found a significant difference (P < 0.05) relative to uninfected (*) or M. tuberculosis-infected (#) C57BL/6 mice. Statistical variations were analyzed by ANOVA followed by Tukey posttest. PBS, phosphate-buffered saline.
FIG. 2.
HDC−/− mice revealed lower pulmonary levels of Th17-related proinflammatory cytokines upon M. tuberculosis infection. C57BL/6 and HDC−/− mice were infected with M. tuberculosis H37Rv (1 × 102 bacilli/mouse) by an intranasal route (black and white bars, respectively) or not infected (light gray and dark gray bars, respectively). Levels of IL-17 (A), IL-6 (B), and TNF-α (C) were measured in lung homogenate by an ELISA at 28 and 112 days after M. tuberculosis H37Rv infection. The results are expressed as means ± SEM from at least five animals in experiments repeated twice. Statistical testing found a significant difference (P < 0.05) relative to uninfected (*) or M. tuberculosis-infected (#) C57BL/6 mice. Statistical variations were analyzed by ANOVA followed by Tukey posttest. PBS, phosphate-buffered saline.
Histamine attenuates Th1 cytokine production and is associated with a heightened Th2 response upon M. tuberculosis infection.
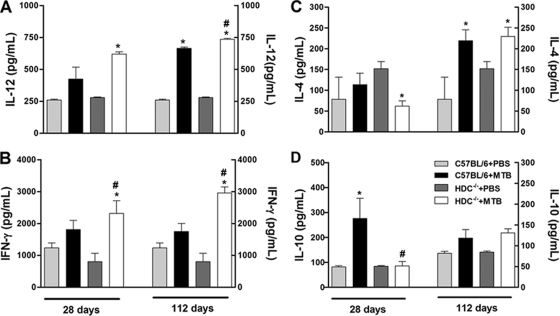
To evaluate the potential mechanisms underlying the different inflammatory responses observed in C57BL/6 and HDC−/− mice, we next characterized the local Th1 and Th2 cytokine responses in the lung tissue during infection by M. tuberculosis. The proinflammatory cytokines IL-12 and IFN-γ are both critical for a protective immune response against mycobacterial infection (9, 44). As expected, pulmonary IL-12 and IFN-γ levels were augmented upon M. tuberculosis infection, but importantly, they were higher in infected HDC−/− mice than in infected C57BL/6 mice at 28 and 112 days of infection (Fig. 3A and B). To investigate whether the elevation of Th1 cytokines correlated with a downregulation of Th2 cytokine response, we measured IL-4 and IL-10 levels in lung tissues. The IL-4 levels were not affected by histamine deficiency after M. tuberculosis infection (Fig. 3C). In contrast, we found that pulmonary IL-10 levels were augmented in M. tuberculosis-infected C57BL/6 mice at day 28 but significantly reduced in infected HDC−/− mice (Fig. 3D). These observations support the notion that histamine released mainly in the acute phase of M. tuberculosis infection downregulates the Th1 response, such as IL-12 and IFN-γ production, while it may upregulate the Th2 response, such as IL-10 production.
FIG. 3.
HDC−/− mice showed higher pulmonary levels of Th1 cytokines and lower levels of Th2 cytokines upon M. tuberculosis infection. C57BL/6 and HDC−/− mice were infected with M. tuberculosis H37Rv (1 × 102 bacilli/mouse) by an intranasal route (black and white bars, respectively) or not infected (light gray and dark gray bars, respectively). Levels of IL-12 (A), IFN-γ (B), IL-4 (C), and IL-10 (D) were measured in lung homogenate by an ELISA at 28 and 112 days after M. tuberculosis H37Rv infection. The results are expressed as means ± SEM from at least five animals in experiments repeated twice. Statistical testing found a significant difference (P < 0.05) relative to uninfected (*) or M. tuberculosis-infected (#) C57BL/6 mice. Statistical variations were analyzed by ANOVA followed by Tukey posttest. PBS, phosphate-buffered saline.
Endogenous histamine may dampen the protective granulomatous response to M. tuberculosis and inhibit mycobacterial clearance.
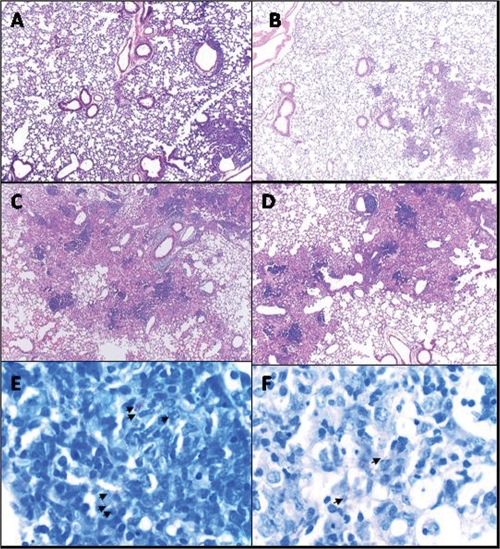
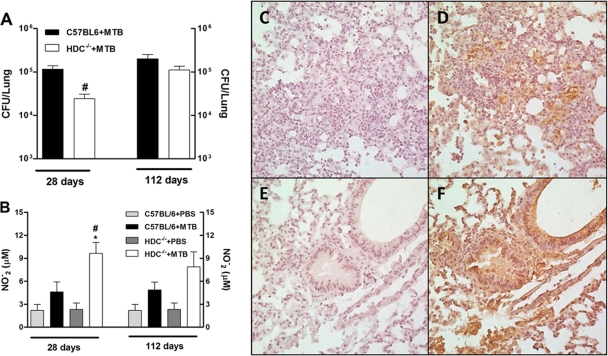
The lungs of infected C57BL/6 and HDC−/− mice were examined histologically to assess the profile of cellular influx and to follow the progression and severity of disease. At day 28 of infection, the lungs of infected C57BL/6 mice exhibited diffuse inflammation, with lymphocyte infiltrations and high numbers of foamy macrophages and neutrophils (Fig. 4A). After 112 days of infection, these mice exhibited progressive granulomatous lesions and tissue damage (Fig. 4C). In contrast, after 28 days of infection, infected HDC−/− mice displayed less-extensive and transient lung inflammation, with a cell infiltrate containing a predominance of lymphocytes and macrophages with a characteristic granulomatous structure (Fig. 4B). Overall, during the chronic phase (day 112 of infection), HDC−/− mice developed a generalized inflammatory response similar to that of infected C57BL/6 mice (Fig. 4D). Further, a reduction of bacillus numbers was evident at day 28 in acid-fast-stained lung sections of infected HDC−/− lungs compared to C57BL/6 lungs (Fig. 4E and F). Later, the mycobacterial burdens were comparable in the two types of mice (data not shown). These data imply that endogenous histamine appears to be involved in susceptibility to early M. tuberculosis infection but not to chronic infection, due to impaired induction of well-defined granuloma formation that is responsible for the control of bacterial replication.
FIG. 4.
HDC−/− mice exhibited a protective granulomatous response with a predominance of lymphocytic infiltrate upon M. tuberculosis infection. H&E-stained lung tissue from C57BL/6 (A and C) and HDC−/− (B and D) mice was analyzed at 28 or 112 days after M. tuberculosis H37Rv infection, respectively. Formalin-fixed, paraffin-embedded lung tissue sections from mouse groups were also stained with the Ziehl-Neelsen acid-fast stain to detect mycobacteria (E and F). Arrows point to acid-fast bacilli. Sections shown are representative of the lungs of at least five animals per group. Original magnifications, ×50 (A to D) and ×640 (E and F).
Impaired recruitment of activated CD11c+ and CD4+ T cells into the lungs mediated by histamine upon M. tuberculosis infection.
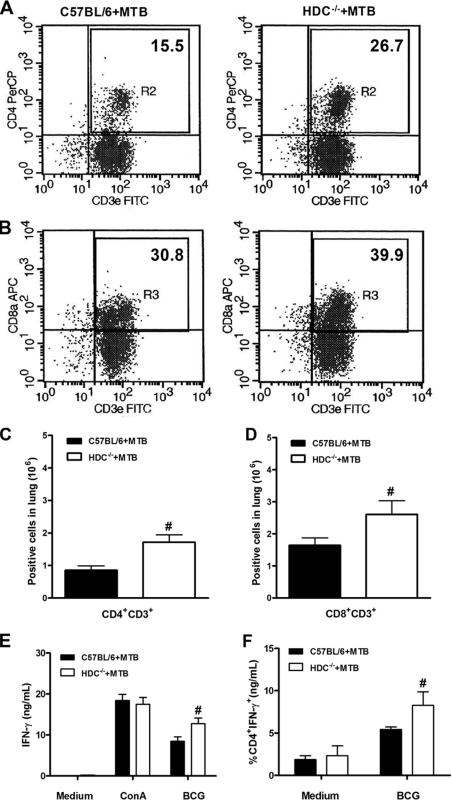
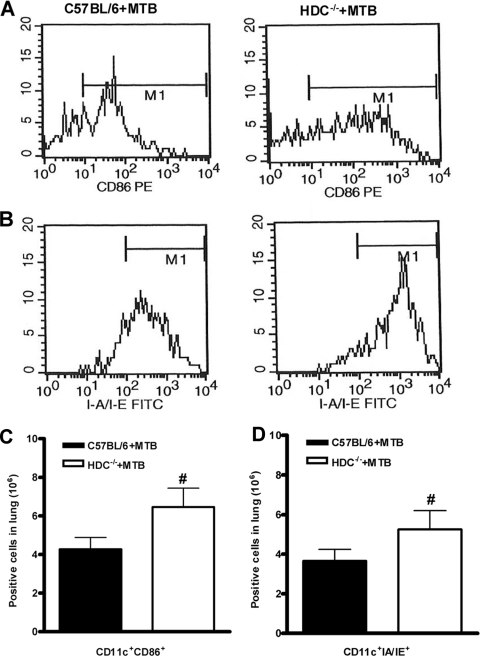
In view of the controlled granulomatous response observed in infected HDC−/− mice, we investigated whether histamine interferes with cellular influx into the lungs in response to M. tuberculosis infection. The percentage of CD4+ T cells in the lungs of infected HDC−/− mice was about twofold higher than that of C57BL/6 mice, while CD8+ T cells were slightly increased (Fig. 5A and B). In addition, absolute numbers of CD4+ and CD8+ T cells in the lungs of HDC−/− mice were significantly increased (Fig. 5C and D). This was accompanied by significantly enhanced expression of the CD44 activation marker on CD4+ T cells in the lungs of infected HDC−/− mice compared to C57BL/6 mice (691.8 ± 35.1 and 530.5 ± 66.8, respectively [expressed as mean fluorescence intensity]). After that, we tested the antigen-specific response in vitro by restimulation of lung T cells from wild-type and HDC−/− animals infected with M. tuberculosis at 4 weeks. Levels of IFN-γ secretion induced by ConA in HDC−/− and wild-type mouse T cells were not different, but the IFN-γ levels induced induced by T cells from HDC−/− mice restimulated with soluble BCG antigens were much higher (Fig. 5E). In order to further demonstrate the T-cell origin of the IFN-γ response, the intracellular expression of IFN-γ in pulmonary T cells was analyzed. Upon M. tuberculosis infection, HDC−/− mice exhibited a higher percentage of IFN-γ-producing CD4+ T cells than did C57BL/6 mice (5.42% and 8.27%, respectively) upon restimulation with BCG soluble antigens (Fig. 5F) while CD8 T cells expressed very low levels (data not shown). Of interest, we detected an elevated expression of CD86 and major histocompatibility complex (MHC) class II molecules in CD11c+ cells obtained from the lungs of infected HDC−/− mice (Fig. 6A and B). In addition, higher numbers of activated CD11c+ cells were recruited into the lungs of HDC−/− mice after 28 days of infection (Fig. 6C and D). These data suggest that histamine might dampen CD4+ and CD8+ T-cell activation and recruitment by limiting activated APC infiltration into the lungs following M. tuberculosis infection.
FIG. 5.
HDC−/− mice showed an augmented CD4+ T-cell influx in the lungs upon M. tuberculosis infection. Lung tissue was removed at 28 days after M. tuberculosis H37Rv infection (102 CFU intranasally) and lung cells from M. tuberculosis-infected C57BL/6 (black bars in panels C to F) or HDC−/− (white bars in panels C to F) mice were analyzed by flow cytometry for the percentage of CD4+ CD3+ (A) and CD8+ CD3+ (B) T cells. Results are expressed as the absolute number of positive cells per lung (C and D). Further, isolated lung cells were restimulated with BCG or ConA for 48 h, and IFN-γ production was assessed in supernatant by ELISA (E) and in T cells stained for intracellular IFN-γ as a percentage of CD4 T cells expressing IFN-γ (F). Means ± SEM from at least five mice per group in one independent experiment repeated twice are shown. Numeral signs represent a significant difference (P < 0.05) relative to M. tuberculosis-infected C57BL/6 mice. Statistical variations were analyzed by Student's t test. PerCP, peridinin chlorophyll protein.
FIG. 6.
HDC−/− mice demonstrated enhanced activation and frequency of APCs in the lungs upon M. tuberculosis infection. Lung tissue was removed at 28 days after M. tuberculosis H37Rv infection (102 CFU intranasally), and lung cells from M. tuberculosis-infected C57BL/6 (black bars in panels C and D) or HDC−/− (white bars in panels C and D) mice were analyzed by flow cytometry. Representative histograms of the expression of CD86 (A) and MHC class II (B) in a gated CD11c-positive cell population are shown. The presence of CD11c+ CD86+ or CD11c+ IA IE cells is expressed as absolute number of positive cells (C and D). The means ± SEM from at least five mice per group in one independent experiment repeated twice are shown. Numeral signs represent a significant difference (P < 0.05) relative to M. tuberculosis-infected C57BL/6 mice. Statistical variations were analyzed by Student's t test.
Histamine favors bacterial replication and diminishes iNOS and NO expression upon M. tuberculosis infection.
To quantify the reduction of bacilli in infected HDC−/− mice as observed in lung tissue sections, we determined the bacterial burden by counting viable bacilli in a CFU assay. In agreement with our microscopic findings, the bacterial burden in HDC−/− mouse lungs was lower than that in infected C57BL/6 mice only at 28 days after infection (Fig. 7A). In accordance with these results, infected HDC−/− mice also displayed approximately 60% higher nitrite levels in lung homogenates (Fig. 7B). We also investigated the extent of pulmonary macrophage activation in infected HDC−/− and C57BL/6 mice by iNOS immunostaining. We found a distinct increase of iNOS expression in infected HDC−/− mouse lungs over that in the lungs of C57BL/6 mice (Fig. 7D and F). Both the viable bacillus count and nitrite levels in the lung were not different at 112 days after M. tuberculosis infection. Thus, these data illustrate that histamine inhibition augmented microbicidal activity associated with iNOS expression and NO production, which resulted in enhanced bacterial clearance at least 4 weeks after M. tuberculosis inoculation.
FIG. 7.
Accelerated clearance associated with profound iNOS and NO expression in the lungs of HDC−/− mice was observed after M. tuberculosis infection. (A) The mycobacterial burden was enumerated by counting CFU at 28 and 112 days after M. tuberculosis H37Rv infection (102 CFU intranasally). (B) Nitrite production was quantified by Griess reaction in the lung homogenates recovered at the same time points. (C to F) Expression of iNOS in lung tissue of infected C57BL/6 (D) or HDC−/− (F) mice was analyzed by immunostaining on day 28 after infection. Brown staining shows iNOS-positive cells. iNOS staining without primary antibody of infected C57BL/6 (C) or HDC−/− (E) mice was used as a background control. Results are expressed as means ± SEM from at least five animals per group in one independent experiment repeated twice. In panels A and B, significant differences (P < 0.05) relative to uninfected (*) or M. tuberculosis-infected (#) C57BL/6 mice are shown. PBS, phosphate-buffered saline.
DISCUSSION
We demonstrate here a critical role of histamine in pulmonary inflammation and host immune responses during the acute phase of mycobacterial infection. M. tuberculosis has been shown to interact with mast cells, triggering the release of several preformed mediators, such as histamine and β-hexosaminidase, as well as de novo-synthesized cytokines like TNF-α and IL-6 (37). Furthermore, we show that in vivo infection with M. tuberculosis induced the production of histamine in the lung. In the absence of the key synthetic enzyme HDC, the histamine concentration was dramatically reduced upon M. tuberculosis infection. Our results also show that infected HDC−/− mice had significantly fewer pulmonary neutrophils at day 28, but not at day 112, than did wild-type mice. In contrast, other studies have already demonstrated a suppressive effect of endogenous histamine on neutrophil infiltration, as suggested by exaggerated neutrophilia in a skin pouch model of acute inflammation (23) or by experimental bacterial peritonitis (24) in mice lacking HDC. The difference may be due in part due to a lower bacterial burden developing in mice lacking HDC at day 28 of M. tuberculosis infection and not be due to a direct effect on neutrophil influx by histamine. In agreement with this hypothesis, at the later time point (day 112 of infection), when mycobacterial burdens became similar in the two types of mice, neutrophil numbers in the lung tissues were comparable.
Although not statistically significant, there was a trend for lower IL-17 levels in the lungs of infected HDC−/− mice than in the lungs of C57BL/6 mice. IL-17 is known to stimulate fibroblasts, endothelial cells, macrophages, and epithelial cells to secrete multiple proinflammatory mediators, like IL-1, IL-6, TNF-α, and chemokines (15). In addition to IL-17, we previously found that the production of IL-6 and TNF-α was significantly decreased in the lungs of infected HDC−/− mice compared to those of C57BL/6 mice, suggesting that neutrophil recruitment was inhibited by the reduction of these proinflammatory cytokines. In this context, a previous study showed that the production of the inflammatory cytokines IL-6 and IL-17 by T cells in vitro was suppressed in the absence of H4R signaling (12). IFN-γ- and IL-17-producing CD4+ T cells are expanded by M. tuberculosis-infected DCs and are induced in vivo during M. tuberculosis infection. Although both cell types are generated during primary M. tuberculosis infection, higher IFN-γ levels negatively regulate the induction and expansion of Th17 cells (10). In further support of this notion, lung IFN-γ levels were significantly increased while IL-17 levels were reduced in the absence of HDC. Thus, we suggest that histamine deficiency enhances IFN-γ production and, as a consequence, probably dampens TNF-α/IL-6/IL-17 production during M. tuberculosis infection. However, additional studies are required to test this hypothesis.
M. tuberculosis bacilli are internalized by alveolar macrophages and DCs, which present antigens and release IL-12 and other cytokines, stimulating the adaptive immune response (11, 14). Our data showed a significant increase in IL-12 levels in lung tissue from M. tuberculosis-infected HDC−/− mice. Therefore, we suggest that histamine interferes with the activation and release of IL-12 by APCs in this experimental model. The presence of IL-12 in inflamed tissues allows for the differentiation of CD4+ T cells with production of IL-2 and IFN-γ, important cytokines for the course and outcome of M. tuberculosis infection. As previously described, histamine inhibition augments pulmonary IFN-γ levels, but it does not influence IL-2 production (data not shown). Consistent with this observation is the fact that massive histamine release by mast cell degranulation with compound 48/80 blocks the generation of specific Th1 effector cells in the draining lymph nodes (35). Because mast cells have the capacity to process and present antigens to both CD4+ and CD8+ T cells in vitro, it was speculated that they might play an important role in activating and polarizing T cells in vivo (34, 41). We also investigated the T-cell response of lung cells to test the IFN-γ production in supernatant upon in vitro restimulation with BCG antigens. As expected, an increased IFN-γ response was found in HDC−/− mice. In addition, upon M. tuberculosis infection, HDC−/− mice exhibited a higher percentage of IFN-γ-producing CD4+ T cells than did C57BL/6 mice upon restimulation with BCG soluble antigens. Both mast cells and immature DCs are located in the periphery, often in close proximity to each other, indicating that mast cell-derived histamine might influence T-cell polarization by acting on DCs. Histamine may suppress IL-12, but it also stimulates IL-10 via histamine type 2 receptors (H2R), which shift the Th1/Th2 balance toward a Th2 response (13, 48). Of interest, levels of IL-10 were reduced in the lung tissue from infected HDC−/− mice in comparison to that from C57BL/6 mice.
After inoculation with virulent M. tuberculosis, C57BL/6 mice were able to generate a stronger Th1 immune response in the lung. The lungs of these animals are remarkable for a multifocal inflammatory cell influx, mostly containing macrophages and neutrophils but also some lymphocytes. Surprisingly, we noted less-diffuse inflammation with a dense lymphocytic infiltrate and a lower mycobacterial load in infected HDC−/− mice after 28 days of infection. Later (at day 112 of infection), greater inflammation and progressive tissue damage were observed in infected C57BL/6 and HDC−/− mice. We propose that histamine deficiency confers the ability to stimulate the immune system in an efficient manner that results in bacillus reduction and lung preservation during acute M. tuberculosis infection. These effects could be due to cytokines present in the pulmonary parenchyma, as higher IFN-γ and reduced IL-10 levels were detected in infected HDC−/− mice. In this context, IL-10-deficient mice revealed a vigorous granulomatous response and augmented lymphocyte recruitment to intravenous challenge with BCG (25). To summarize, the histamine inhibition in M. tuberculosis-infected mice caused a reduction in the lung mycobacterial burden that corresponded with a heightened Th1 responsiveness, increased immune activation, and reduced immunopathology.
In an attempt to verify whether the histamine deficiency during M. tuberculosis infection conferred protective immunity by inducing a favorable lymphocyte activation and influx, we carried out a phenotypic analysis of the cells infiltrating the lung. Flow cytometry analysis revealed increased recruitment of CD4+ T lymphocytes and activated APCs in the lungs of mice lacking HDC. In correlation with higher IL-12 production, greater expression of CD86 and MHC class II was detected on APCs from infected HDC−/− mice than on those from C57BL/6 mice. In this view, expression of costimulatory molecules such as CD80, CD86, and MHC class II and several chemokines was enhanced by histamine in DCs via the H1 and H2 receptors (8). According to our results, by limiting the expression of CD86 and MHC class II as well as IL-12 production, histamine may curb Th1 immunity within the lungs, consequently allowing more mycobacteria to persist. NO, an important immune system metabolite with bactericidal activity in vitro and in vivo (36), can be synthesized by iNOS in murine macrophages when induced by proinflammatory cytokines like TNF-α and IFN-γ (6). Together with augmented numbers of CD4+ T lymphocytes and levels of IL-12 and IFN-γ, iNOS expression and NO levels were increased in the lungs of infected HDC−/− mice. In addition, we also confirmed the presence of a decreased bacterial load through counting CFU in the lung tissue of mice lacking HDC at the early time point after infection (28 days) but not at the late time point (112 days). Along these lines, inhibition of histamine-mediated signaling confers significant protection against severe malaria, as HDC−/− mice are more resistant because they develop a blood-stage infection with only low levels of parasitemia (5). In summary, histamine synthesis is induced in the lung upon in vivo M. tuberculosis infection, and genetic ablation of the enzyme HDC suppressed the production of this mediator. Histamine appears to be required for the maximal production of TNF-α and IL-6, and possibly IL-17, following M. tuberculosis infection. Histamine inhibition augmented the recruitment of activated APCs and CD4+ T and CD8+ T cells associated with higher IL-12, IFN-γ, and NO levels that could explain the heightened resistance to M. tuberculosis infection. Therefore, histamine is induced upon M. tuberculosis infection and may dampen protective Th1 immunity against acute M. tuberculosis infection and augment inflammatory pathology.
Acknowledgments
This study was supported by a grant from the CAPES and FAPESP (no. 03/12885-5) and grants from EC (TB REACT contract no. 028190).
We thank Elaine Medevies Floriano from the Laboratório de Histologia, Departamento de Patologia, Faculdade de Medicina de Ribeirão Preto, for her technical assistance with histological material. We also thank Carlos Artério Sorgi for his technical assistance with the cytokine assay.
We have no conflicts of interest.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Akdis, C. A., and F. E. Simons. 2006. Histamine receptors are hot in immunopharmacology. Eur. J. Pharmacol. 533:69-76. [DOI] [PubMed] [Google Scholar]
- 2.Aoi, R., I. Nakashima, Y. Kitamura, H. Asai, and K. Nakano. 1989. Histamine synthesis by mouse T lymphocytes through induced histidine decarboxylase. Immunology 66:219-223. [PMC free article] [PubMed] [Google Scholar]
- 3.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 4.Beer, D. J., S. M. Matloff, and R. E. Rocklin. 1984. The influence of histamine on immune and inflammatory responses. Adv. Immunol. 35:209-268. [DOI] [PubMed] [Google Scholar]
- 5.Beghdadi, W., A. Porcherie, B. S. Schneider, D. Dubayle, R. Peronet, M. Huerre, T. Watanabe, H. Ohtsu, J. Louis, and S. Mecheri. 2008. Inhibition of histamine-mediated signaling confers significant protection against severe malaria in mouse models of disease. J. Exp. Med. 205:395-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benbernou, N., S. Esnault, H. C. Shin, H. Fekkar, and M. Guenounou. 1997. Differential regulation of IFN-gamma, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunology 91:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlos, D., D. A. de Souza Junior, L. de Paula, M. C. Jamur, C. Oliver, S. G. Ramos, C. L. Silva, and L. H. Faccioli. 2007. Mast cells modulate pulmonary acute inflammation and host defense in a murine model of tuberculosis. J. Infect. Dis. 196:1361-1368. [DOI] [PubMed] [Google Scholar]
- 8.Caron, G., Y. Delneste, E. Roelandts, C. Duez, N. Herbault, G. Magistrelli, J. Y. Bonnefoy, J. Pestel, and P. Jeannin. 2001. Histamine induces CD86 expression and chemokine production by human immature dendritic cells. J. Immunol. 166:6000-6006. [DOI] [PubMed] [Google Scholar]
- 9.Chackerian, A. A., T. V. Perera, and S. M. Behar. 2001. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 69:2666-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz, A., S. A. Khader, E. Torrado, A. Fraga, J. E. Pearl, J. Pedrosa, A. M. Cooper, and A. G. Castro. 2006. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 177:1416-1420. [DOI] [PubMed] [Google Scholar]
- 11.Doherty, T. M., and P. Andersen. 2005. Vaccines for tuberculosis: novel concepts and recent progress. Clin. Microbiol. Rev. 18:687-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunford, P. J., N. O'Donnell, J. P. Riley, K. N. Williams, L. Karlsson, and R. L. Thurmond. 2006. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J. Immunol. 176:7062-7070. [DOI] [PubMed] [Google Scholar]
- 13.Elenkov, I. J., E. Webster, D. A. Papanicolaou, T. A. Fleisher, G. P. Chrousos, and R. L. Wilder. 1998. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J. Immunol. 161:2586-2593. [PubMed] [Google Scholar]
- 14.Flynn, J. L. 2004. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinburgh) 84:93-101. [DOI] [PubMed] [Google Scholar]
- 15.Fossiez, F., J. Banchereau, R. Murray, C. Van Kooten, P. Garrone, and S. Lebecque. 1998. Interleukin-17. Int. Rev. Immunol. 16:541-551. [DOI] [PubMed] [Google Scholar]
- 16.Fossiez, F., O. Djossou, P. Chomarat, L. Flores-Romo, S. Ait-Yahia, C. Maat, J. J. Pin, P. Garrone, E. Garcia, S. Saeland, D. Blanchard, C. Gaillard, B. Das Mahapatra, E. Rouvier, P. Golstein, J. Banchereau, and S. Lebecque. 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fremond, C., N. Allie, I. Dambuza, S. I. Grivennikov, V. Yeremeev, V. F. Quesniaux, M. Jacobs, and B. Ryffel. 2005. Membrane TNF confers protection to acute mycobacterial infection. Respir. Res. 6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fremond, C. M., D. Togbe, E. Doz, S. Rose, V. Vasseur, I. Maillet, M. Jacobs, B. Ryffel, and V. F. Quesniaux. 2007. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 179:1178-1189. [DOI] [PubMed] [Google Scholar]
- 19.Fremond, C. M., V. Yeremeev, D. M. Nicolle, M. Jacobs, V. F. Quesniaux, and B. Ryffel. 2004. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J. Clin. Investig. 114:1790-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handley, S. A., P. H. Dube, and V. L. Miller. 2006. Histamine signaling through the H(2) receptor in the Peyer's patch is important for controlling Yersinia enterocolitica infection. Proc. Natl. Acad. Sci. USA 103:9268-9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrington, L. E., R. D. Hatton, P. R. Mangan, H. Turner, T. L. Murphy, K. M. Murphy, and C. T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123-1132. [DOI] [PubMed] [Google Scholar]
- 22.Hill, S. J. 1990. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol. Rev. 42:45-83. [PubMed] [Google Scholar]
- 23.Hirasawa, N., H. Ohtsu, T. Watanabe, and K. Ohuchi. 2002. Enhancement of neutrophil infiltration in histidine decarboxylase-deficient mice. Immunology 107:217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hori, Y., Y. Nihei, Y. Kurokawa, A. Kuramasu, Y. Makabe-Kobayashi, T. Terui, H. Doi, S. Satomi, E. Sakurai, A. Nagy, T. Watanabe, and H. Ohtsu. 2002. Accelerated clearance of Escherichia coli in experimental peritonitis of histamine-deficient mice. J. Immunol. 169:1978-1983. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs, M., L. Fick, N. Allie, N. Brown, and B. Ryffel. 2002. Enhanced immune response in Mycobacterium bovis bacille calmette guerin (BCG)-infected IL-10-deficient mice. Clin. Chem. Lab. Med. 40:893-902. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs, M., A. Samarina, S. Grivennikov, T. Botha, N. Allie, C. Fremond, D. Togbe, V. Vasseur, S. Rose, F. Erard, A. Monteiro, V. Quesniaux, and B. Ryffel. 2007. Reactivation of tuberculosis by tumor necrosis factor neutralization. Eur. Cytokine Netw. 18:5-13. [DOI] [PubMed] [Google Scholar]
- 27.Jones, C. E., and K. Chan. 2002. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 26:748-753. [DOI] [PubMed] [Google Scholar]
- 28.Jutel, M., T. Watanabe, M. Akdis, K. Blaser, and C. A. Akdis. 2002. Immune regulation by histamine. Curr. Opin. Immunol. 14:735-740. [DOI] [PubMed] [Google Scholar]
- 29.Kahnert, A., P. Seiler, M. Stein, S. Bandermann, K. Hahnke, H. Mollenkopf, and S. H. Kaufmann. 2006. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur. J. Immunol. 36:631-647. [DOI] [PubMed] [Google Scholar]
- 30.Khader, S. A., J. E. Pearl, K. Sakamoto, L. Gilmartin, G. K. Bell, D. M. Jelley-Gibbs, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 175:788-795. [DOI] [PubMed] [Google Scholar]
- 31.Kuramasu, A., H. Saito, S. Suzuki, T. Watanabe, and H. Ohtsu. 1998. Mast cell-/basophil-specific transcriptional regulation of human L-histidine decarboxylase gene by CpG methylation in the promoter region. J. Biol. Chem. 273:31607-31614. [DOI] [PubMed] [Google Scholar]
- 32.Ladel, C. H., C. Blum, A. Dreher, K. Reifenberg, M. Kopf, and S. H. Kaufmann. 1997. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect. Immun. 65:4843-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazarevic, V., D. Nolt, and J. L. Flynn. 2005. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. J. Immunol. 175:1107-1117. [DOI] [PubMed] [Google Scholar]
- 34.Malaviya, R., N. J. Twesten, E. A. Ross, S. N. Abraham, and J. D. Pfeifer. 1996. Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to T cells. J. Immunol. 156:1490-1496. [PubMed] [Google Scholar]
- 35.Mazzoni, A., R. P. Siraganian, C. A. Leifer, and D. M. Segal. 2006. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. J. Immunol. 177:3577-3581. [DOI] [PubMed] [Google Scholar]
- 36.Miles, P. R., L. Bowman, A. Rengasamy, and L. Huffman. 1998. Constitutive nitric oxide production by rat alveolar macrophages. Am. J. Physiol. 274:L360-L368. [DOI] [PubMed] [Google Scholar]
- 37.Munoz, S., R. Hernandez-Pando, S. N. Abraham, and J. A. Enciso. 2003. Mast cell activation by Mycobacterium tuberculosis: mediator release and role of CD48. J. Immunol. 170:5590-5596. [DOI] [PubMed] [Google Scholar]
- 38.Ohtsu, H., S. Tanaka, T. Terui, Y. Hori, Y. Makabe-Kobayashi, G. Pejler, E. Tchougounova, L. Hellman, M. Gertsenstein, N. Hirasawa, E. Sakurai, E. Buzas, P. Kovacs, G. Csaba, A. Kittel, M. Okada, M. Hara, L. Mar, K. Numayama-Tsuruta, S. Ishigaki-Suzuki, K. Ohuchi, A. Ichikawa, A. Falus, T. Watanabe, and A. Nagy. 2001. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 502:53-56. [DOI] [PubMed] [Google Scholar]
- 39.Ottenhoff, T. H., T. de Boer, C. E. Verhagen, F. A. Verreck, and J. T. van Dissel. 2000. Human deficiencies in type 1 cytokine receptors reveal the essential role of type 1 cytokines in immunity to intracellular bacteria. Microbes Infect. 2:1559-1566. [DOI] [PubMed] [Google Scholar]
- 40.Park, H., Z. Li, X. O. Yang, S. H. Chang, R. Nurieva, Y. H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poncet, P., M. Arock, and B. David. 1999. MHC class II-dependent activation of CD4+ T cell hybridomas by human mast cells through superantigen presentation. J. Leukoc. Biol. 66:105-112. [DOI] [PubMed] [Google Scholar]
- 42.Shiraishi, M., N. Hirasawa, Y. Kobayashi, S. Oikawa, A. Murakami, and K. Ohuchi. 2000. Participation of mitogen-activated protein kinase in thapsigargin- and TPA-induced histamine production in murine macrophage RAW 264.7 cells. Br. J. Pharmacol. 129:515-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiraishi, M., N. Hirasawa, S. Oikawa, Y. Kobayashi, and K. Ohuchi. 2000. Analysis of histamine-producing cells at the late phase of allergic inflammation in rats. Immunology 99:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, S., D. Liggitt, E. Jeromsky, X. Tan, S. J. Skerrett, and C. B. Wilson. 2002. Local role for tumor necrosis factor alpha in the pulmonary inflammatory response to Mycobacterium tuberculosis infection. Infect. Immun. 70:2082-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuehr, D. J., and C. F. Nathan. 1989. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 169:1543-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 47.Tufariello, J. M., J. Chan, and J. L. Flynn. 2003. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect. Dis. 3:578-590. [DOI] [PubMed] [Google Scholar]
- 48.van der Pouw Kraan, T. C., A. Snijders, L. C. Boeije, E. R. de Groot, A. E. Alewijnse, R. Leurs, and L. A. Aarden. 1998. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J. Clin. Investig. 102:1866-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, X., D. Zhang, H. Zhang, P. J. Wolters, N. P. Killeen, B. M. Sullivan, R. M. Locksley, C. A. Lowell, and G. H. Caughey. 2006. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J. Exp. Med. 203:2907-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]