Abstract
Changes in airway dynamics have been reported in the rat model of pulmonary cryptococcosis. However, it is not known if Cryptococcus neoformans-induced changes in lung functions are related to the immunophenotype that develops in response to cryptococcal infection in the lungs. In this study we performed a parallel analysis of the immunophenotype and airway resistance (standard resistance of the airways [SRAW]) in BALB/c mice infected with highly virulent C. neoformans strain H99 and moderately virulent strain 52D. H99 infection evoked a Th2 response and was associated with increased SRAW, while the SRAW for 52D infection, which resulted in a predominantly Th1-skewed response, did not differ from the SRAW for uninfected mice. We found that an altered SRAW in mice did not positively or negatively correlate with the pulmonary fungal burden, the magnitude of inflammatory response, the numbers of T cells, eosinophils or eosinophil subsets, neutrophils, or monocytes/macrophages, or the levels of cytokines (interleukin-4 [IL-4], IL-10, gamma interferon, or IL-13) produced by lung leukocytes. However, the level of a systemic Th2 marker, serum immunoglobulin E (IgE), correlated significantly with SRAW, indicating that the changes in lung functions were proportional to the level of Th2 skewing in this model. These data also imply that IgE may contribute to the altered SRAW observed in H99-infected mice. Lung histological analysis revealed severe allergic bronchopulmonary mycosis pathology in H99-infected mice and evidence of protective responses in 52D-infected mice with well-marginalized lesions. Taken together, the data show that C. neoformans can significantly affect airflow physiology, particularly in the context of a Th2 immune response with possible involvement of IgE as an important factor.
Cryptococcus neoformans is an encapsulated yeast and is one of the leading fungal opportunistic pathogens worldwide, with increasing potential to affect both immunocompromised and noncompromised individuals (22, 35). The primary site of C. neoformans infection is the respiratory tract, where the infection is either cleared (predominantly by Th1 immune responses) or persists in the absence of protective responses. Th1 immune responses in C. neoformans infection models are characterized by recruitment of CD4+ and CD8+ lymphocytes, production of Th1 cytokines (tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], and interleukin-12 [IL-12]), and formation of tight granulomas containing classically activated macrophages, followed by clearance of the infection and resolution (4, 7, 26, 39). In contrast, Th2 immune responses are nonprotective. These responses are characterized by production of Th2 cytokines (IL-4, IL-5, and IL-13), pulmonary eosinophilia, alternative activation of macrophages (YM crystal formation), elevation of serum immunoglobulin E (IgE) levels, and chronic infection with severe lung pathology (3, 8, 17, 33, 34, 40).
The Th2-driven pulmonary diseases are allergic diseases and include asthma, hypersensitivity pneumonitis, and allergic bronchopulmonary mycosis (ABPM). These disorders can result in severe limitations of airflow that result from direct changes in baseline lung functions (i.e., increased baseline airway resistance) and/or airway hyperresponsiveness characterized by increased sensitivity to spasmogens, such as methacholine (MCh) (15). Goldman et al. (13) demonstrated using a rat model that pulmonary cryptococcosis can modify allergic responses. Increased airway responsiveness to MCh was associated with an aggravated allergic reaction to ovalbumin challenge following cryptococcal infection. This reaction was associated with allergic inflammation and Th2 cytokine production (13). However, the requirement of the Th2 response and its various parameters for changes in lung functions during C. neoformans were not clarified in this study.
Pulmonary eosinophilia is an important component of allergic airway inflammation and is thought to contribute to the changes in airway responses (9, 32, 37, 38). Th2 cytokines induce eosinophil transmigration through the endothelial and epithelial layers into airways and alveoli; this process results in damage to the respiratory tract epithelium, which in turn could contribute to bronchospasm and airway hyperresponsiveness (12). Upregulation of CD44 and CD48 cell surface antigens on pulmonary eosinophils has been shown to play a role in allergic airway inflammation and changes in lung functions (21, 31). CD44 binds to hyaluronic acid on vascular endothelium to promote extravasation of lymphocytes and eosinophils to inflamed tissues (23, 25), while CD48 is a signaling protein with a broad immunologic role. While a number of studies have looked into the role of eosinophils in the context of a cryptococcal infection (10, 11, 19), no study has directly assessed the possible contribution of eosinophils to the changes in airway responses during a C. neoformans infection.
Another hallmark of the Th2 response phenotype is IgE class antibody accumulation due to the Th2 cytokine-mediated class switch in antibody-producing B cells and plasma cells. The increase in the IgE level not only is a systemic marker of Th2 response but also plays an important role in induction of allergic symptoms. IgE may contribute to the allergic airway response by binding to Fcɛ receptors on the mast cells and basophiles (36). Antigen-specific cross-linking of Fcɛ receptors leads to mast cell-basophil deregulation and release of allergic mediators (histamine, leukotrienes) that trigger a variety of allergic symptoms, including bronchospasm (5, 16).
In this study we sought to determine if the immunophenotype that develops in mice with pulmonary cryptococcosis is related to changes in lung functions. We utilized BALB/c mice in which Th1- and Th2-type immune responses were induced by infection with C. neoformans strains 52D and H99, respectively. In both infection models, airway resistance was measured prior to and following MCh challenge, and comparative immunological and pathological analyses were performed, followed by evaluation of how different components of the immune response correlated with the changes in airway responses.
MATERIALS AND METHODS
Mice.
All experimental procedures were approved by the VA Institutional Animal Care and Use and Committee. Female wild-type BALB/c mice used in these studies were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were raised in specific-pathogen-free conditions at the Ann Arbor VA Medical Center using sterilized cages covered with a filter top and were given sterile food and water ad libitum. Mice were 6 to 8 weeks old at the time of infection. At the time of data collection mice were humanely euthanized by CO2 inhalation.
Cultures of C. neoformans.
C. neoformans wild-type strain H99 (= ATCC 208821) and C. neoformans strain 52D (= ATCC 24067) were recovered from frozen (−70°C) glycerol stocks and grown to stationary phase in Sabouraud dextrose broth (1% neopeptone, 2% dextrose; Difco, Detroit, MI) on a shaker for 72 h at 37°C. Cultures were washed twice with saline, cells were counted with a hemocytometer, and cultures were diluted to obtain a concentration of 3.3 × 105 yeast cells/ml in nonpyrogenic saline. Final dilutions used for infection were serially diluted and plated on Sabouraud dextrose agar to confirm the number of viable CFU in each inoculum.
Experimental design.
Mice were divided into three groups; one group was infected with C. neoformans H99, one group was infected with C. neoformans 52D, and the control group received saline (all by the intratracheal route). Following infection, the fungal lung burden was assessed at weeks 1, 2, 3, and 4. Measurement of lung function followed by detailed immunological analysis was performed at week 3 postinfection, a time point consistent with a fully polarized adaptive immune response to C. neoformans.
Intratracheal inoculation of C. neoformans.
Mice were anesthetized by intraperitoneal injection of a ketamine-xylazine solution. Anesthetized mice were fixed on surgical boards, and a small incision was made through the skin covering the trachea. The underlying salivary glands and muscle were separated to expose the trachea. A 1-ml syringe filled with the appropriate concentration of C. neoformans was attached to a 30-gauge needle, which was inserted into the trachea. Delivery of 30 μl of inoculum (104 C. neoformans cells) into the trachea and lungs was confirmed by observing inhalation of the droplet; then the needle was removed and the skin was closed with a cyanoacrylate adhesive.
Measurement of lung functions.
At week 3 postinfection, noninvasive airway resistance (standard resistance of the airways [SRAW]) was measured using a dual-chamber plethysomograph and Buxco system before and after administration of aerosolized MCh. BALB/c mice were fitted with a collar around the neck to prevent backflow of air out of the chamber and to ensure accurate measurement. Airway resistance following exposure to MCh was measured at MCh concentrations of 0, 2.5, 5, 10, 20, and 40 mg/ml or until the SRAW was 200% of the baseline value. The measurements were based on the physiological readout of lung functions obtained utilizing a computerized Buxco system coupled with a dual-chamber plethysmograph that measured the changes in tidal volume and pressure in the lungs.
Collection and processing of the infected lungs.
Mice were euthanized by CO2 asphyxiation. To exsanguinate the animals, the abdominal vena cava was severed to remove circulating blood cells from the lungs prior to excision. Individual lungs were excised, washed in phosphate-buffered saline, and minced with scissors. The minced lung tissue was enzymatically digested for 30 min in 15 ml of digestion buffer (RPMI medium containing 5% fetal calf serum, penicillin, streptomycin, 1 mg/ml collagenase, and 0.25 ml/mouse of DNase) at 37°C. The resulting cell suspension was dispersed further using the bore of a 10-ml syringe 20 times.
Lung CFU assay.
For determination of the numbers of CFU in lung tissue, small aliquots of digested lung suspensions were collected. Series of 10-fold dilutions of the lung samples were plated on Sabouraud dextrose agar plates using duplicate 10-μl aliquots and incubated at room temperature for 3 days. Following incubation, C. neoformans colonies were counted, and the number of CFU was determined on a per-organ basis.
Isolation and enumeration of lung leukocytes.
Following removal of the enzymatic digestion solution by centrifugation, the erythrocyte pellets were lysed by addition of 3 ml of NH4Cl buffer for 3 min, followed by addition of 10 ml of RPMI medium to return the solution to isotonicity. Resuspended cells were filtered through a 70-μm sterile nylon screen and centrifuged for 25 min at 3,000 rpm in the presence of 20% Percoll to separate leukocytes from cell debris and epithelial cells. The total number of viable cells of an isolate was calculated by microscopic counting with a hemocytometer using a trypan blue exclusion assay. The total number of leukocytes was obtained by multiplying the total number of cells by the frequency of CD45+ cells in each sample obtained in a subsequent flow cytometry analysis.
Leukocyte subset analysis.
Macrophages, neutrophils, eosinophils, monocytes, and lymphocytes were visually counted using Wright-Giemsa-stained samples of lung cell suspensions cytospun onto glass slides. Slides were stained by fixing them for 2 min with a one-step methanol-based Wright-Giemsa stain, followed by steps 2 and 3 of the Diff-Quik whole-blood stain kit procedure. For each sample 300 cells from a randomly chosen field were then counted using a high-power objective with a microscope. The percentage for a leukocyte subset was multiplied by the total number of leukocytes to obtain the absolute number of cells in the specific leukocyte subset in the sample.
Antibody staining and flow cytometry analysis.
All staining reactions were performed as described previously (7). Data were collected with a FACS LSR2 flow cytometer using DIVA software and were analyzed using FlowJo software (Tree Star Inc., San Carlos, CA). A minimum of 30,000 cells per sample were analyzed. Initial gates were set based on light scatter characteristics to exclude debris, unlysed red blood cells, and cell clusters. In cell suspensions, leukocytes were stained with Pacific blue-labeled anti-CD45. The lymphocyte subsets of CD45+ cells were determined by using phycoerythrin-labeled anti-CD8 (for Tc cells), fluorescein isothiocyanate-labeled anti-CD4 antibodies (for Th cells), and peridinin chlorophyll protein-Cy5.5-labeled anti-CD19 (for B cells). Eosinophil populations were recognized as SSChi/FSClow expressing CCR3. Anti-CD44 and anti-CD48 antibodies and associated isotype controls were purchased from eBioscience (San Diego, CA). Anti-CCR3 antibody was purchased from R&D Systems (Minneapolis, MN). All other monoclonal antibody reagents were purchased from PharMingen (San Diego, CA). The absolute number of cells in each leukocyte subset in a sample was obtained by multiplying the percentage for the type of subset by the total number of leukocytes.
Lung leukocyte culture.
The concentration of isolated lung leukocytes was adjusted to 5 × 106 cells/ml, and the leukocytes were cultured in 24-well plates in 2 ml of complete RPMI medium at 37°C with 5% CO2 for 24 h. Plates were centrifuged to spin down the leukocytes, and cell-free supernatants were collected for subsequent enzyme-linked immunosorbent assays (ELISA).
Cytokine protein measurement.
The cytokines IFN-γ, IL-4, IL-13, and IL-10 were quantified by ELISA using DuoSet kits (R&D Systems, Minneapolis, MN). Assays were performed in 96-well ELISA plates (Costar) by following the manufacturer's instructions. The optical density was read with a microplate reader (VersaMax; Molecular Devices), and the cytokine concentration was interpolated from each sample's optical density compared with the appropriate standard curve. Standard four-parameter curves were generated, and all calculations were performed using the Softmax analysis and curve-fitting program (Molecular Devices).
Real-time PCR.
Total RNA from isolated lung leukocytes was prepared using an RNeasy Plus mini kit (Qiagen, United States), and first-strand cDNA was synthesized using SuperScript III (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Cytokine mRNA was quantified with SYBR green-based detection using an MX 3000P system (Stratagene, La Jolla, CA) according to the manufacturer's protocols. Forty cycles of PCR (94°C for 15 s followed by 60°C for 30s and 72°C for 30s) were performed using a cDNA template. The mRNA level for each sample was compared to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression level and was expressed as a percentage of the GAPDH level (relative expression).
Total serum IgE.
Serum was obtained from the blood samples collected by severing the vena cava of the mice before lung excision. Blood samples were then allowed to clot and were spun to separate serum. Serum samples were diluted 100-fold and assayed for total IgE levels using the standard ELISA protocol (see above) and a mouse IgE sandwich ELISA kit (Opti IA; BD Pharmingen).
Histological analysis.
Lungs were fixed by inflation with 1 ml of 10% neutral buffered formalin. After processing and paraffin embedding, 5-μm sections were cut and stained with hematoxylin and eosin. In addition, periodic acid-Schiff (PAS) staining was performed to identify mucus-secreting cells (goblet cells). Sections were analyzed by light microscopy, and microphotographs were taken using a digital microphotography system (DFX1200) with ACT-1 software (Nikon Co, Tokyo, Japan).
Calculations and statistics.
Statistical significance was calculated using Student's t test for individual paired comparisons or one-way analysis of variance whenever multiple groups were compared. For individual comparisons of multiple groups, Student-Newman-Keuls post hoc test was used to calculate P values. Data from separate matched experiments were pooled whenever these experiments showed no statistical difference. Means with P values of <0.05 were considered significantly different. All values are expressed below as means ± standard errors of the means (SEM). Correlations between each of the measured host response parameters and the airway resistance were evaluated by plotting pairs of SRAW values (obtained at baseline and with each MCh concentration) against the specific immune parameter for individual animals. For each of these data sets r2 and P values were calculated. A positive slope was an indicator of a positive correlation, while a negative slope was an indicator of an inverse correlation. All statistical calculations were performed using Primer of Biostatistics (McGraw-Hill, NY).
RESULTS
Moderately virulent strain 52D promotes a protective immune response, whereas infection with highly virulent strain H99 results in a nonprotective immune response.
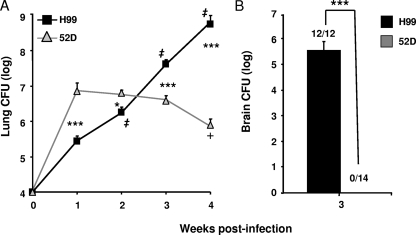
Strains 52D and H99 are the clinical isolates of C. neoformans that are most commonly used in translational research; however, the host responses to these strains have not been compared side by side in a uniform model system. Our first goal was to compare the pulmonary growth and clearance of C. neoformans 52D and H99 in a BALB/c mice inhalational infection model. Mice were intratracheally inoculated with 104 cells of one of the strains, and the pulmonary fungal burden was determined at weeks 1 through 4 postinfection. By week 1, strain 52D had rapidly proliferated (1,000-fold increase compared with the original inoculum), but its subsequent growth was inhibited (weeks 2 and 3) and significant clearance (1 log) was observed at week 4 compared to the peak level at week 1 (Fig. 1A). In contrast, strain H99 exhibited progressive, logarithmic growth in the lungs through all 4 weeks of infection, and the burden was significantly greater at each subsequent time point (Fig. 1A). At the early time points of infection (week 1 to 2), the differences in growth and clearance dynamics resulted in significantly higher fungal loads in 52D-infected lungs than in H99-infected lungs. However, H99 infection surpassed 52D infection in terms of the pulmonary fungal burden by week 3 postinfection (a time point consistent with the polarized adaptive immune response), and the difference continued to increase, reaching 3 orders of magnitude at week 4 postinfection (Fig. 1A). In addition, extrapulmonary cryptococcal dissemination was evaluated at week 3 for both infection groups. Our results show that H99-infected mice exhibited severe C. neoformans dissemination in the central nervous system. All of the H99-infected mice showed a fungal burden in the brain (5.58 ± 0.34 log CFU; n = 12). In contrast, none of the 14 52D-infected mice examined contained detectable numbers of CFU in the brain (Fig. 1B). The uncontrolled growth of C. neoformans in the lungs and brains of H99-infected mice was also associated with increasing mortality, which peaked between weeks 3 and 4 (10.5% [2 of 19] mice died in week 3, and 61.0% [11 of 18] mice died in week 4), while 100% of the 52D-infected mice survived to the end of this study. Thus, 52D-infected mice had a high level of protection against cryptococcal growth, whereas in H99-infected mice there was uncontrolled growth of C. neoformans in both lungs and brains.
FIG. 1.
Pulmonary and cerebral growth and clearance of C. neoformans in BALB/c mice. Mice were inoculated intratracheally with 104 cells of either C. neoformans 52D or H99. (A) The pulmonary fungal burden was evaluated at weekly intervals. 52D-infected mice, n = 12 (week 1), n = 12 (week 2), n = 19 (week 3), and n = 10 (week 4); H99-infected mice, n = 9 (week 1), n = 15 (week 2), n = 17 (week 3), and n = 7 (week 4). (B) Cerebral fungal burden was evaluated at week 3 postinfection. 52D-infected mice, n = 14; H99-infected mice, n = 12. Data, pooled from three separate matched experiments, are shown as mean and SEM numbers of CFU per organ. *, P < 0.05, and ***, P < 0.001, between the H99 and 52D groups at the matching time points; +, P < 0.05 within the 52D-infected group compared to week 1; ‡, P < 0.001 within the H99-infected group compared to the fungal burden 1 week earlier.
Inflammatory responses in C. neoformans 52D- and H99-infected lungs demonstrate that there are significant differences in cellular composition.
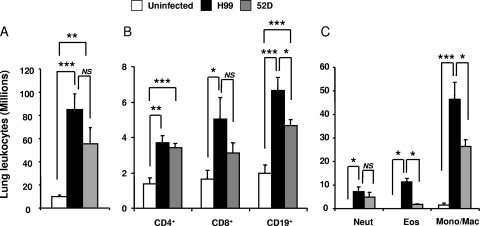
To determine if the differences in pulmonary control of C. neoformans were caused by differences in the magnitude and composition of the inflammatory response, we analyzed leukocyte populations in H99- and 52D-infected lungs at week 3 postinfection. Both H99- and 52D-infected mice developed significant inflammatory responses in their lungs, marked by great increases in the leukocyte number compared to the leukocyte number in uninfected mice (Fig. 2A). Although the values were not significantly different, a strong trend toward an increased inflammatory response was observed in H99-infected lungs (82.95 × 106 ± 14.1 × 106 cells) compared to 52D-infected lungs (52.21 × 106 ± 14.1 × 106 cells) (Fig. 2A), indicating that the uncontrolled pulmonary growth of strain H99 was not due to the absence of an inflammatory response or a diminished inflammatory response.
FIG. 2.
Pulmonary inflammatory response in mice infected with either C. neoformans 52D or H99. Lungs were collected from uninfected and H99- or 52D-infected BALB/c mice at week 3 postinfection. (A) Total numbers of recruited pulmonary leukocytes were enumerated from individual mice following enzymatic dispersion of whole lungs. Uninfected mice, n = 4; H99-infected mice, n = 12; 52D-infected mice, n = 28. (B) Numbers of lymphocyte subsets were determined by staining samples of leukocyte suspensions from infected mice with fluorochrome-labeled antibodies specific for CD4+, CD8+, and CD19+ lymphocytes and analyzed by flow cytometry. Uninfected mice, n = 4; H99-infected mice, n = 11; 52D-infected mice, n = 10. (C) Differential myeloid subset recruitment was determined by microscopic enumeration of cytospun-leukocyte isolates. Uninfected mice, n = 4; H99-infected mice, n = 12; 52D-infected mice, n = 8. Data, pooled from three separate matched experiments (A) and two separate matched experiments (B and C), are expressed as means and SEM. Neut, neutrophils; Eos, eosinophils; Mono/Mac, monocytes/macrophages. NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Previous studies demonstrated that C. neoformans clearance depends on CD4+ and CD8+ T-cell-mediated responses (6, 20, 27, 28) and a possible contribution by B cells (1). These lymphocyte subsets were enumerated by flow cytometry. Both groups of infected mice contained significantly higher numbers of CD4+ T cells than uninfected controls contained; however, there was no difference in the number of CD4+ T cells between the infected groups (Fig. 2B). In addition, both infected groups showed trends toward increased numbers of CD8+ T cells compared to uninfected controls, but only H99-infected mice showed a significant increase compared with uninfected mice (Fig. 2B). Furthermore, mice in both infection groups contained significantly higher numbers of B cells than uninfected mice contained; H99-infected mice also contained significantly higher numbers of B cells than 52D-infected mice contained (Fig. 2B).
We also analyzed if H99- and 52D-infected mice differed in the number of myeloid subsets by using differential microscope counts of pulmonary leukocytes. While there was no difference in the numbers of neutrophils in the lungs, the numbers of pulmonary eosinophils were significantly elevated in H99-infected mice compared to 52D-infected mice (Fig. 2C). Furthermore, the numbers of monocytes/macrophages were significantly higher in H99-infected lungs (Fig. 2C). Thus, both H99- and 52D-infected mice recruited adequate numbers of leukocytes into the lungs, but the compositions of the inflammatory infiltrates in 52D- and H99-infected lungs suggest that each microbe evoked a different type of the immune response.
H99-infected lungs show a strong Th2 cytokine bias, whereas 52D-infected lungs have a predominantly Th1 cytokine profile.
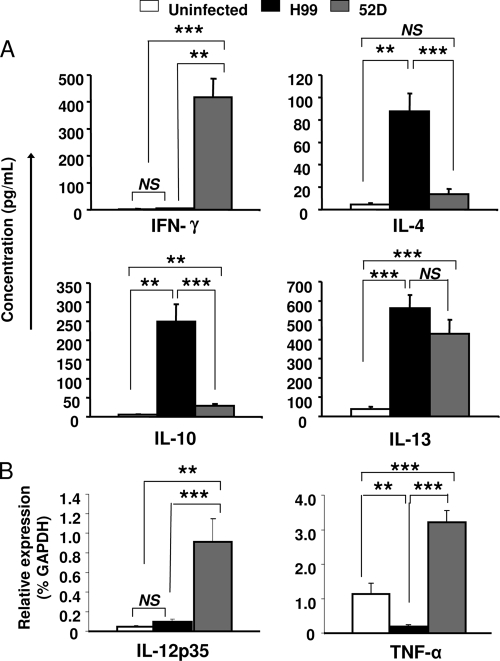
Having determined that the inflammatory cell infiltrates had significantly different compositions in H99- and 52D- infected lungs, we analyzed cytokine production to evaluate if the immune responses were differentially polarized. The secreted cytokine levels for IFN-γ, IL-4, IL-10, and IL-13 were evaluated using supernatants from 24-h lung leukocyte cultures isolated at week 3 postinfection. H99-infected mice produced predominantly Th2 cytokines (IL-4, IL-10, and IL-13) and negligible amounts of the Th1 cytokine IFN-γ. In contrast, 52D-infected mice produced predominantly IFN-γ and negligible amounts of IL-4 and showed a small, albeit statistically detectable, increase in the amount of IL-10, which was significantly less than the amount in H99-infected mice (Fig. 3A). Interestingly, comparable and significant increases in the IL-13 level were observed in both groups of infected mice (Fig. 3A). In addition to the protein evaluation, the expression of mRNA for selected cytokines important for cryptococcal clearance was analyzed. The levels of mRNA expression for IL-12p35 and TNF-α were quantified using real-time PCR. Consistent with the nonprotective Th2 phenotype, H99-infected mice showed baseline levels of IL-12p35 and downregulation of TNF-α compared to uninfected control mice (Fig. 3B). Consistent with the protective Th1 phenotype, 52D-infected mice showed significant upregulation of IL-12p35 and TNF-α compared to both uninfected and H99-infected mice (Fig. 3B). Thus, H99 infection induced a Th2 cytokine profile, while 52D-infected mice favored Th1 cytokines, with the exception of IL-13, which was induced at the same level in both infection models at week 3 postinfection.
FIG. 3.
Cytokine production from C. neoformans-infected BALB/c mice. Lung leukocytes were isolated from infected and uninfected mice at week 3 postinfection. (A) Isolated leukocytes (5 × 106 cells/ml) were cultured for 24 h, and supernatant was collected from the cell culture and analyzed by ELISA for the cytokines IL-4, IL-10, IFN-γ, and IL-13. Uninfected mice, n = 7; H99-infected mice, n = 30; 52D-infected mice, n = 27. (B) Total RNA from 5 × 106 isolated lung leukocytes was prepared, and first-strand cDNA was synthesized. The mRNA levels for cytokine IL-12p35 and TNF-α in each sample were compared to the GAPDH mRNA expression level and are expressed as percentages of the GAPDH level (relative expression). Uninfected mice, n = 6; H99-infected mice, n = 22; 52D-infected mice, n = 22. Data, pooled from four separate matched experiments, are expressed as means and SEM. NS, not significant; **, P < 0.01; ***, P < 0.001.
Elevated serum IgE production in H99-infected mice.
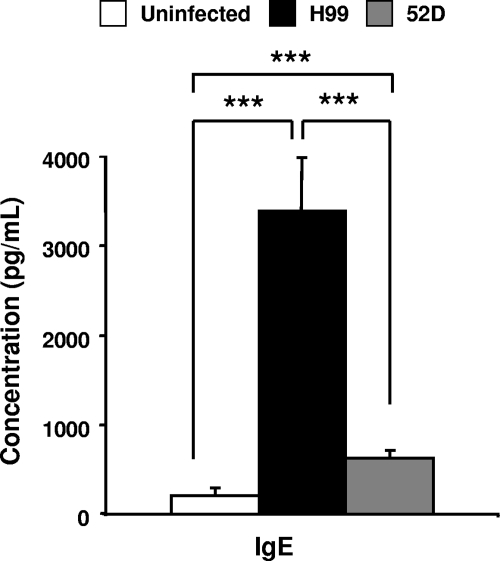
To determine if H99- and 52D-infected mice differed in terms of systemic immune polarization, we evaluated the levels of serum IgE, a hallmark of systemic Th2 polarization. The serum IgE level in 52D-infected mice was slightly but significantly elevated compared to the level in uninfected mice at week 3 postinfection (Fig. 4). However, H99-infected mice showed a dramatic increase in the serum IgE level compared to both uninfected and 52D-infected mice (Fig. 4), indicating that H99-infected mice show a strong systemic Th2 bias. Thus, H99-infected mice but not 52D-infected mice exhibit strong systemic accumulation of serum IgE.
FIG. 4.
Production of serum IgE from C. neoformans-infected BALB/c mice. Blood was collected from uninfected and infected BALB/c mice at week 3 postinfection. Sera were evaluated to determine the concentration of total IgE using the ELISA. Uninfected mice, n = 7; H99-infected mice, n = 17; 52D-infected mice, n = 30. Data, pooled from three separate matched experiments, are expressed as means and SEM. ***, P < 0.001.
Histological analysis of alveolar lung sections of mice infected with strains H99 and 52D.
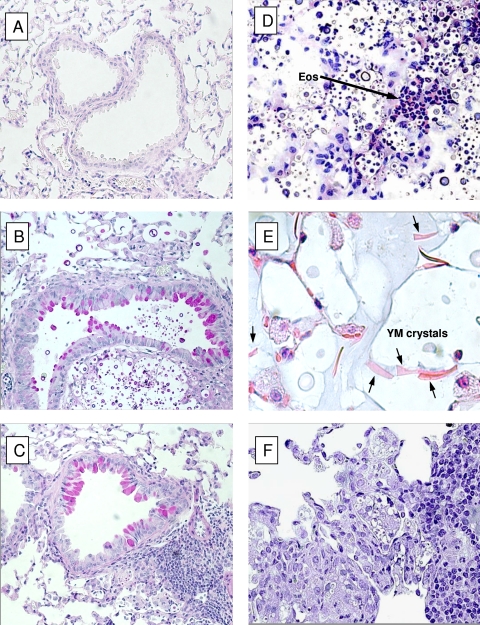
The development of a Th2 but not Th1 response in the H99-infected mice leads to the development of pathological lesions characteristic of ABPM (3, 8, 15-17). Our next goal was to determine if H99-infected BALB/c mice developed ABPM pathology and if 52D-infected mice were protected from this pathological outcome. At week 3 postinfection, we analyzed histological lung sections to compare the lung pathologies of the two infection groups. We observed significant recruitment of inflammatory cells, with the formation of infiltrates, in both 52D- and H99-infected lungs compared to uninfected lungs (compare Fig. 5A to Fig. 5B to F). However, high numbers of cryptococcal organisms were observed in H99-infected lungs (Fig. 5B and D), consistent with the uncontrolled growth of this microbe in the lungs. In contrast, the growth and spread of C. neoformans were effectively contained in 52D-infected lungs, and there was a clear demarcation between infected and noninfected portions of lung tissue (Fig. 5C and F). Examination using high-power magnification revealed additional features of ABPM in H99-infected mice, such as eosinophil infiltrates (Fig. 5D), the presence of crystals composed of YM1 and YM2 proteins (3), and proliferating yeasts within macrophages (Fig. 5E), which are indicative of alternative activation of macrophages. In contrast, macrophages in 52D-infected lungs displayed morphology more consistent with their classical activation. Lungs infected with strain 52D were characterized by mononuclear infiltrates surrounding cryptococci, and these infiltrates largely accumulated within or in proximity to bronchovascular bundles (Fig. 5F). We further analyzed changes in airway morphology, which is another feature of ABPM. Interestingly, H99- and 52D-infected mice displayed equal but significant goblet cell metaplasia visualized by PAS staining and apparent thickening of the airway walls (Fig. 5A, B, and C). Thus, histological analysis confirmed that there was fully developed ABPM pathology in the lungs of BALB/c mice infected with strain H99 and that there was significant protection from these lesions in 52D-infected mice.
FIG. 5.
Morphological patterns of pulmonary inflammation and pathological lesions in C. neoformans-infected lungs: photomicrographs of lung histology for BALB/c mice infected with either C. neoformans 52D or H99. Lungs were collected from infected mice at week 3 postinfection, fixed, processed for histology, and either stained with PAS stain and photographed with a ×20 objective (A to C) or stained with hematoxylin and eosin and photographed with a ×40 objective (D and F) or a ×100 objective (E). (A) Lung of uninfected control mouse. (B) Lung of a C. neoformans H99-infected mouse. Note the uncontrolled and widespread dissemination of cryptococcal cells in the absence of inflammation. (C) Lung of a C. neoformans 52D-infected mouse. Note the tight leukocyte infiltrate forming a granuloma around the yeast cells and clear segregation between uninfected and infected tissue. Note the presence of PAS stain-positive goblet cells and mucus accumulation in both groups of mice. (D and E) Lungs of a C. neoformans H99-infected mouse, showing pulmonary eosinophilia (Eos) (D) and evidence of alternative activation macrophages (E). YM crystals in the alveolar macrophages are indicated by arrows. (F) Lung of a C. neoformans 52D-infected mouse showing containment of infection within a tight mononuclear infiltrate (Th1 granuloma).
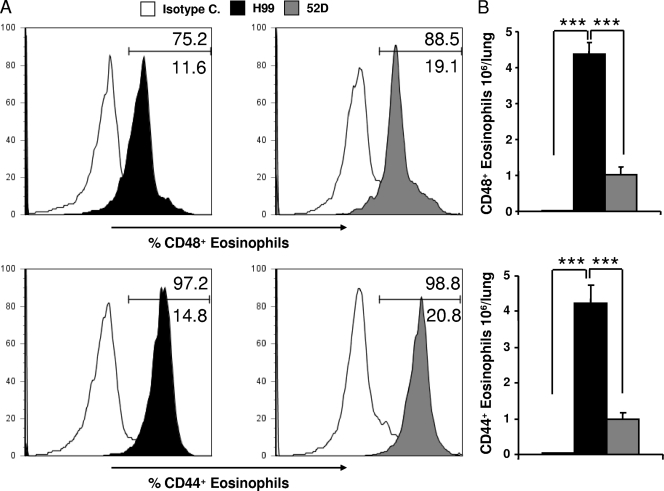
CD44 and CD48 expression on pulmonary eosinophils is similar in H99- and 52D-infected mice.
The function of eosinophils in allergic airway responses is controversial; however, CD44 and CD48 expression on pulmonary eosinophils has been shown to be critical in allergic eosinophilic airway inflammation (21, 31, 38). We analyzed eosinophil populations and their expression of CD44 and CD48 to determine if eosinophil phenotypes were different in our Th1 and Th2 infection models. Eosinophil populations were identified by CCR3 expression and the side and forward scatter characteristics (SSChi and FSClow). Subsequently, we analyzed eosinophil CD44 and CD48 surface expression to determine if strain H99 and 52D infections resulted in differential activation of these cells (Fig. 6). The numbers of eosinophils in H99-infected mice were significantly higher than the numbers in 52D-infected mice, consistent with data obtained by cytological evaluation (Fig. 2C). However, the frequencies of CD44+ and CD48+ cells in an eosinophil gate were similarly high in both H99- and 52D-infected lungs (Fig. 6A). The total number of CD44+ and CD48+ eosinophils was higher in H99-infected lungs than in 52D-infected lungs (Fig. 6B), but the difference was attributed to a higher number of eosinophils rather than to increased frequency of these activation markers. Furthermore, the GR1 staining intensity, which is also considered a hallmark of eosinophil activation, was similar in the two groups (data not shown). Thus, significant pulmonary eosinophilia was prevalent only in H99-infected lungs; however, the frequencies of activation marker expression by eosinophils recruited into H99- and 52D-infected lungs were not significantly different.
FIG. 6.
Eosinophil phenotypes of C. neoformans-infected BALB/c mice. Eosinophils from lung digest samples isolated from C. neoformans-infected BALB/c mice were analyzed by flow cytometry at week 3 postinfection. (A) Eosinophil populations were identified by CCR3 expression and the side and forward scatter characteristics (CCR3+ SSChi FSClow). The overlaid histograms show the frequency of CD48+ CD44+ eosinophils in either H99-infected mice (black histograms) or 52D-infected mice (gray histograms) compared with the results for the isotype control (white histograms). The percentages of CD48+ and CD44+ are indicated above the gate, and the percentages of autofluorescent cells are indicated below the gate. Note that there was no significant difference in the expression frequency of either surface marker in either strain when the value for the isotype control was subtracted. (B) Total numbers of activated CD44+ and CD48+ eosinophils. Uninfected mice, n = 1; H99-infected mice, n = 5; 52D-infected mice, n = 5. Data are shown as means and SEM. ***, P < 0.001.
Effect of C. neoformans 52D and H99 infection on lung functions.
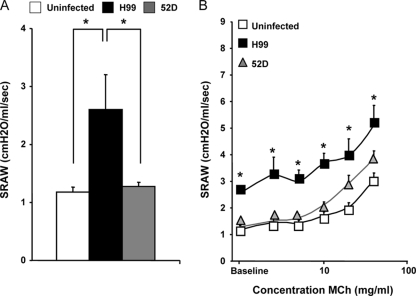
Cryptococcal infection has been demonstrated to affect lung physiology in animal models; however, the underlying immunological bases of the changes were not defined (13, 29). Our goal was to determine if changes in lung functions and airway responses during C. neoformans infections were linked to specific types of immune responses and pathologies in our mouse models. We first compared the SRAW of uninfected mice with the SRAW of 52D- and H99-infected mice at week 3 postinfection. While 52D-infected mice (1.27 ± 0.08 cm H2O/ml/s) had an SRAW similar to that of uninfected controls (1.18 ± 0.09 cm H2O/ml/s), H99-infected mice exhibited a significant increase in the SRAW (2.60 ± 0.61 cm H2O/ml/s) (Fig. 7A), suggesting that changes in lung functions may be associated with the increased microbial burden and/or the Th2 immune bias in these mice.
FIG. 7.
Effect of C. neoformans 52D and H99 infection on lung functions. At 3 weeks postinfection, measurements of lung functions in mice were made using a dual-chamber plethysomograph and Buxco system at baseline and after administration of increasing doses of aerosolized MCh (0, 2.5, 5, 10, 20, 40, and 80 mg/ml) as described in Materials and Methods. (A) Baseline airway resistance. Uninfected mice, n = 8; H99-infected mice, n = 15; 52D-infected mice, n = 18. (B) Airway responsiveness of mice to MCh. Uninfected mice, n = 12; H99-infected mice, n = 12; 52D-infected mice, n = 20. Data, pooled from three separate matched experiments, are expressed as means and SEM. *, P < 0.05 in comparison with the uninfected control.
We next evaluated the effect of C. neoformans on airway responsiveness by assessing changes in SRAW in response to different doses of aerosolized MCh (0, 2.5, 5, 10, 20, and 40 mg/ml MCh or until the SRAW increased to 200% of the baseline value). At each MCh dose, the SRAW in H99-infected mice was significantly higher than that in control mice (Fig. 7B). Furthermore, at each MCh dose, the SRAW in 52D-infected mice was not significantly different from the SRAW in uninfected mice (Fig. 7B); however, the SRAW in H99-infected mice was not significantly higher than that in 52D-infected mice at MCh doses of ≥20 mg. This could suggest that 52D-infected mice showed some increase in airway responsiveness to MCh; however, linear regression analysis of SRAW data showed that the linear slopes of dose-response curves were not different for H99-infected, 52D-infected, and uninfected mice (Fig. 7B). Thus, the baseline lung airway resistance was highly elevated in H99-infected mice; however, the dynamics of airway responses to MCh were not dramatically altered in either H99- or 52D-infected animals.
Correlations of evaluated Th2 parameters with airway resistance.
Our data demonstrate that there were an increased microbial load and skewing to a Th2 response in H99-infected animals that were accompanied by increased baseline airway resistance. Our next goal was to examine whether the increased airway resistance was related to the microbial burden or if it was linked with specific elements of the Th2 response. The readouts for airway resistance at the baseline for each individual mouse were plotted against the values for specific immune parameters from the same animal to determine which of the immune parameters correlated with SRAW. Interestingly, no significant correlation was detected between the SRAW and the number of either lung and brain CFU or the total number of pulmonary leukocytes, indicating that the magnitude of the microbial load or inflammation was not related to increased airway resistance.
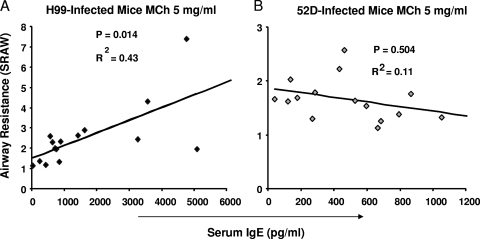
To determine if eosinophilic inflammation contributed to increased airway resistance, we evaluated if the number of pulmonary eosinophils or the numbers of CD44+ and CD48+ eosinophils correlated with SRAW. The total numbers of eosinophils or their CD44+ and CD48+ subsets did not correlate at all with SRAW, suggesting that eosinophilia and changes in lung functions are not related in C. neoformans infection. Furthermore, no positive or inverse correlation was found between SRAW and IL-4, IL-10, IL-13, or IFN-γ production by cells from mice infected with either strain (data not shown). However, a significant correlation was observed between serum IgE levels and SRAW measured at the baseline and at all MCh doses tested in H99-infected mice, and the strongest correlation (r2 = 0.43; P = 0.014) was at an MCh concentration of 5 mg/ml (Fig. 8A); in contrast, no significant correlation was observed at any dose of MCh for mice infected with strain 52D (Fig. 8B). Thus, alterations in airway dynamics were related to a systemic Th2 marker, the level of IgE in serum of the H99-infected mice, but did not correlate with the microbial burden, the total number of inflammatory cells, or acute cytokine production.
FIG. 8.
Correlations between the serum IgE level and airway resistance in infected mice at week 3 postinfection. (A) Correlation for H99-infected mice at an MCh concentration of 5 mg/ml. (B) Correlation for 52D-infected mice at an MCh concentration of 5 mg/ml. H99-infected mice, n = 13; 52D-infected mice, n = 15. Data were pooled from three parallel experiments.
DISCUSSION
The goal of our study was to determine if the immunophenotype of the host response to C. neoformans affects lung functions in an infected host. Using the BALB/c pulmonary C. neoformans infection model, we observed that (i) H99-infected mice develop a nonprotective, Th2-skewed immune response, while 52D-infected mice develop a protective, Th1-skewed immune response; (ii) H99-infected mice had altered lung functions characterized by significantly increased baseline airway resistance, while 52D-infected mice were similar to uninfected controls; and (iii) changes in SRAW did not correlate with the microbial burden, the magnitude of the inflammatory response, cytokine production, pulmonary eosinophilia, or the numbers of CD44+- or CD48+-expressing eosinophils but showed a high-level correlation with the level of serum IgE.
Our model allowed simultaneous assessment of immunopathology and changes in lung functions caused by highly virulent C. neoformans strain H99 and moderately virulent strain 52D in the same mouse strain. H99-infected mice exhibited a Th2 immune response that included Th2 cytokine production (IL-4, IL-13, and IL-10) and the absence of IFN-γ, systemic IgE production, mucus production or thickening of the airways, uncontrolled expansion of fungus in the lungs, morphological evidence of alternative activation of macrophages, and pulmonary eosinophilia. These features fully reproduced cryptococcal ABPM described previously (3, 17-19). In contrast, infection of BALB/c mice with strain 52D resulted in a protective Th1-type immune response that included IFN-γ induction, low levels of IL-4, IL-10, and systemic IgE, containment or clearance of C. neoformans by inflammatory cells, and morphological evidence of classical activation of macrophages.
Subsequent analysis of lung functions revealed that only H99-infected mice had altered airway resistance at the baseline. At week 3 postinfection, a time point when there was a significantly polarized immune response, the H99-infected mice had significantly higher baseline airway resistance than the 52D-infected mice and the uninfected mice. In contrast, the protective Th1 response in 52D-infected mice did not result in a change in the baseline lung function readout. These data demonstrate that cryptococcal infection per se does not result in major changes in lung functions unless it is accompanied by the Th2 response.
We next investigated possible causes of changes in lung function seen in H99-infected mice. H99-infected mice had an elevated fungal burden and severe lung pathology at gross anatomical and histological levels (widespread cryptococcal presence throughout the lungs and in some airways) (Fig. 5). Significant accumulation of the pathogen could contribute to narrowing of the airway lumen and therefore increased SRAW. However, we did not observe any correlation between the cryptococcal load and SRAW at the baseline or at any concentration of MCh. Similarly, a significant inflammatory response could result in altered lung functions; however, no correlation was found between the number of total leukocytes and SRAW, indicating that the observed changes in SRAW were specifically associated with a Th2 response rather than with the magnitude of inflammation in the respiratory tract.
The number and activation status of pulmonary eosinophils were elements of the Th2 response that could potentially affect SRAW in the mice (38). Our studies evaluated the correlation between the total numbers of pulmonary eosinophils and the changes that we detected in lung functions. We found that the number of eosinophils did not correlate with SRAW, indicating that the magnitude of eosinophil recruitment was not linked with changes in lung functions in our pulmonary cryptococcal disease model. CD44 expression and CD48 expression (hallmarks of eosinophil activation) on pulmonary eosinophils have been shown to be critical in allergic airway inflammation and changes in lung functions (21, 31). We further explored if the eosinophil activation status rather than the number of eosinophils affected lung functions and airway pathology. We evaluated expression of CD44+ and CD48+ markers in both 52D- and H99-infected lungs. Interestingly, there was no difference in the expression of either surface molecule when the two groups of infected mice were compared. Although H99-infected mice had higher total numbers of both CD44+ and CD48+ cells (Fig. 6) in their lungs, these subsets also did not correlate with airway resistance at the baseline or at any concentration of MCh. Collectively, these data suggest that eosinophil recruitment or the eosinophil activation status was not associated with changes in lung functions.
The induction of Th2 cytokines (in particular IL-4 and IL-13) and antibody class switching with resultant accumulation of serum IgE are implicated in pathogenesis of allergic airway disease (5, 16). Consistent with this, Goldman et al. observed that rats treated with ovalbumin and infected with C. neoformans showed synergistic increases in the IL-10, IL-13, and TNF-α levels and the total serum-IgE levels compared with groups treated with either ovalbumin or C. neoformans alone (13). Although no significant correlation between airway resistance and pulmonary cytokine level was observed, it is possible that the systemic cytokine level could affect airway responses. Further studies are needed to assess the extrapulmonary cytokines and their role in the development of changes in airway dynamics.
In contrast to the cytokine analysis that revealed that neither Th1 nor Th2 cytokines correlated with changes in lung functions, we found a highly significant positive correlation between the level of serum IgE and SRAW. The strong correlation between the level of serum IgE and SRAW is not surprising, as IgE has been shown to directly contribute to changes in airway responses in allergic asthma and experimental models of this disease. However, the present study provided the first demonstration that IgE induced in response to cryptococcal infection may be an important contributor to changes in lung functions. Furthermore, apart from a possible direct role in changes in airway tone, IgE is a more stable factor than dynamic levels of cytokines for assessment of the Th2 bias. Therefore, the lack of correlation between Th2 cytokine production and lung function, although intriguing, can be explained by the relatively short life of cytokines and the fluctuation of their levels in infected animals. Conversely, the IgE level represents good cumulative readout of systemic Th2 bias. Thus, our findings point to a major role of the Th2 response in driving the changes in airway dynamics.
In addition to our studies that dissected the possible roles of different aspects of the immune response to C. neoformans infection in changes in lung function, we performed a comparative analysis of lung histopathology in the 52D and H99 infection models. Lung areas infected by strain 52D were surrounded by a tight inflammatory infiltrate that showed the clear demarcation between healthy and infected parts of the lungs. This suggests that recruited leukocytes effectively prevented the spread of the fungus to the airways and adjacent alveoli. These observations, along with the classical activation of macrophage morphology of macrophages found in the infected areas, are consistent with Th1-type immune responses. In contrast, strain H99 infection resulted in severe Th2 lung pathology characterized by uncontrolled fungal dissemination and wide spread of the pathogen within the lungs. In many instances the pathogen was not accompanied by inflammatory cells, showing that there was unopposed spread to unaffected areas of lungs. This is consistent with the inefficient immune response and mismatch in localization of the inflammatory response in the areas of expanding microbial growth. Furthermore, cryptococcal growth was visible within the macrophages, showing characteristics of alternative activation of macrophage morphology and YM crystal formation consistent with the alternative activation of macrophage polarization previously reported in a Th2-type immune response.
Interestingly, not all of the immunopathological features of H99 infection were distinct from those found in 52D-infected lungs. First, H99-infected mice recruited adequate numbers of lymphocytes (CD4+ CD8+ T cells and B cells) and macrophages, which are critical for clearance of C. neoformans. Second, regardless of the predominant Th1 bias, some aspects of Th2 responses were found in the lungs of 52D-infected animals. These aspects included goblet cell metaplasia and thickening of the airway walls, which were seen in 52D-infected lungs and were similar to goblet cell metaplasia and thickening of the airway walls in H99-infected lungs. Finally, the induction of IL-13 was similar at week 3 postinfection in the two infected groups. It is likely that the findings for airway morphology and the induction of IL-13 were linked, since IL-13 is a known inducer of goblet cell metaplasia in allergic airway inflammation (14, 24, 30). However, IL-13 is also induced in chronic inflammation as a repair cytokine, and the overlapping functions of this molecule could explain its presence in both 52D and H99 infections (2).
Our studies focused on the time point when the immune response is fully polarized, and our data provided evidence that Th2 polarization was largely responsible for changes in lung functions at this time. It is possible that changes in lung functions in H99 infection could also correlate with an additional mechanism(s) at earlier time points. Additional studies are needed to carefully address this question.
In summary, cryptococcal infection can alter lung functions when there is a Th2 immune bias; however, lung functions remain largely unaffected by the Th1 immune response. Th2 immune parameters, such as cytokine levels and pulmonary eosinophilia, do not seem to play a direct role in altering lung functions, and the serum IgE level shows a strong positive correlation with airway resistance. These results provide a link between the Th2 response, C. neoformans-induced ABPM, and changes in airway dynamics.
Acknowledgments
We acknowledge the help of Joanne Stonstein and Jami Milam, as well as the technical assistance of Jana Jacobs, Gari Martinovski, Stuart Zeltzer, Kristin Tompkins, Fuyuan Wang, Lauren Berent, and Andrew Michalsky.
This work was supported by merit review awards (M.A.O. and G.B.T.) from the Department of Veterans Affairs and by grants R01-AI-059201 (G.B.H.) and R01-HL51082 (G.B.T.). We thank the Undergraduate Research Opportunity Program at the University of Michigan for supporting undergraduate students working in our laboratory, including a Summer Biomedical Fellowship for A.V.J.
Editor: A. Casadevall
Footnotes
Published ahead of print on 14 September 2009.
REFERENCES
- 1.Aguirre, K. M., and L. L. Johnson. 1997. A role for B cells in resistance to Cryptococcus neoformans in mice. Infect. Immun. 65:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allahverdian, S., N. Harada, G. K. Singhera, D. A. Knight, and D. R. Dorscheid. 2008. Secretion of IL-13 by airway epithelial cells enhances epithelial repair via HB-EGF. Am. J. Respir. Cell Mol. Biol. 38:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 174:6346-6356. [DOI] [PubMed] [Google Scholar]
- 4.Arora, S., R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2006. Effect of a CD4-depleting antibody on the development of Cryptococcus neoformans-induced allergic bronchopulmonary mycosis in mice. Infect. Immun. 74:4339-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes, P. J. 2000. Anti-IgE therapy in asthma: rationale and therapeutic potential. Int. Arch. Allergy Immunol. 123:196-204. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan, K. L., and H. A. Doyle. 2000. Requirement for CD4+ T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infect. Immun. 68:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, G. H., D. A. McNamara, Y. Hernandez, G. B. Huffnagle, G. B. Toews, and M. A. Olszewski. 2008. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect. Immun. 76:2379-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, G. H., M. A. Olszewski, R. A. McDonald, J. C. Wells, R. Paine III, G. B. Huffnagle, and G. B. Toews. 2007. Role of granulocyte macrophage colony-stimulating factor in host defense against pulmonary Cryptococcus neoformans infection during murine allergic bronchopulmonary mycosis. Am. J. Pathol. 170:1028-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cieslewicz, G., A. Tomkinson, A. Adler, C. Duez, J. Schwarze, K. Takeda, K. A. Larson, J. J. Lee, C. G. Irvin, and E. W. Gelfand. 1999. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J. Clin. Investig. 104:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmesser, M., A. Casadevall, Y. Kress, G. Spira, and A. Orlofsky. 1997. Eosinophil-Cryptococcus neoformans interactions in vivo and in vitro. Infect. Immun. 65:1899-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmesser, M., Y. Kress, and A. Casadevall. 1998. Effect of antibody to capsular polysaccharide on eosinophilic pneumonia in murine infection with Cryptococcus neoformans. J. Infect. Dis. 177:1639-1646. [DOI] [PubMed] [Google Scholar]
- 12.Foster, P. S., M. Martinez-Moczygemba, D. P. Huston, and D. B. Corry. 2002. Interleukins-4, -5, and -13: emerging therapeutic targets in allergic disease. Pharmacol. Ther. 94:253-264. [DOI] [PubMed] [Google Scholar]
- 13.Goldman, D. L., J. Davis, F. Bommarito, X. Shao, and A. Casadevall. 2006. Enhanced allergic inflammation and airway responsiveness in rats with chronic Cryptococcus neoformans infection: potential role for fungal pulmonary infection in the pathogenesis of asthma. J. Infect. Dis. 193:1178-1186. [DOI] [PubMed] [Google Scholar]
- 14.Grunig, G., M. Warnock, A. E. Wakil, R. Venkayya, F. Brombacher, D. M. Rennick, D. Sheppard, M. Mohrs, D. D. Donaldson, R. M. Locksley, and D. B. Corry. 1998. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282:2261-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gueders, M. M., G. Paulissen, C. Crahay, F. Quesada-Calvo, J. Hacha, C. Van Hove, K. Tournoy, R. Louis, J. M. Foidart, A. Noel, and D. D. Cataldo. 2009. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm. Res. [Epub ahead of print.] doi: 10.1007/s00011-009-0054-2. [DOI] [PubMed]
- 16.Haczku, A., K. F. Chung, J. Sun, P. J. Barnes, A. B. Kay, and R. Moqbel. 1995. Airway hyperresponsiveness, elevation of serum-specific IgE and activation of T cells following allergen exposure in sensitized Brown-Norway rats. Immunology 85:598-603. [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez, Y., S. Arora, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J. Immunol. 174:1027-1036. [DOI] [PubMed] [Google Scholar]
- 18.Hoag, K. A., N. E. Street, G. B. Huffnagle, and M. F. Lipscomb. 1995. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 13:487-495. [DOI] [PubMed] [Google Scholar]
- 19.Huffnagle, G. B., M. B. Boyd, N. E. Street, and M. F. Lipscomb. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 160:2393-2400. [PubMed] [Google Scholar]
- 20.Huffnagle, G. B., M. F. Lipscomb, J. A. Lovchik, K. A. Hoag, and N. E. Street. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 55:35-42. [DOI] [PubMed] [Google Scholar]
- 21.Katoh, S., N. Matsumoto, K. Kawakita, A. Tominaga, P. W. Kincade, and S. Matsukura. 2003. A role for CD44 in an antigen-induced murine model of pulmonary eosinophilia. J. Clin. Investig. 111:1563-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacs, J. A., A. A. Kovacs, M. Polis, W. C. Wright, V. J. Gill, C. U. Tuazon, E. P. Gelmann, H. C. Lane, R. Longfield, G. Overturf, et al. 1985. Cryptococcosis in the acquired immunodeficiency syndrome. Ann. Intern. Med. 103:533-538. [DOI] [PubMed] [Google Scholar]
- 23.Lee, N. A., E. W. Gelfand, and J. J. Lee. 2001. Pulmonary T cells and eosinophils: coconspirators or independent triggers of allergic respiratory pathology? J. Allergy Clin. Immunol. 107:945-957. [DOI] [PubMed] [Google Scholar]
- 24.Leigh, R., R. Ellis, J. N. Wattie, J. A. Hirota, K. I. Matthaei, P. S. Foster, P. M. O'Byrne, and M. D. Inman. 2004. Type 2 cytokines in the pathogenesis of sustained airway dysfunction and airway remodeling in mice. Am. J. Respir. Crit. Care Med. 169:860-867. [DOI] [PubMed] [Google Scholar]
- 25.Lesley, J., R. Hyman, and P. W. Kincade. 1993. CD44 and its interaction with extracellular matrix. Adv. Immunol. 54:271-335. [DOI] [PubMed] [Google Scholar]
- 26.Milam, J. E., A. C. Herring-Palmer, R. Pandrangi, R. A. McDonald, G. B. Huffnagle, and G. B. Toews. 2007. Modulation of the pulmonary type 2 T-cell response to Cryptococcus neoformans by intratracheal delivery of a tumor necrosis factor alpha-expressing adenoviral vector. Infect. Immun. 75:4951-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mody, C. H., G. H. Chen, C. Jackson, J. L. Curtis, and G. B. Toews. 1994. In vivo depletion of murine CD8 positive T cells impairs survival during infection with a highly virulent strain of Cryptococcus neoformans. Mycopathologia 125:7-17. [DOI] [PubMed] [Google Scholar]
- 28.Mody, C. H., M. F. Lipscomb, N. E. Street, and G. B. Toews. 1990. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J. Immunol. 144:1472-1477. [PubMed] [Google Scholar]
- 29.Muller, U., W. Stenzel, G. Kohler, T. Polte, M. Blessing, A. Mann, D. Piehler, F. Brombacher, and G. Alber. 2008. A gene-dosage effect for interleukin-4 receptor alpha-chain expression has an impact on Th2-mediated allergic inflammation during bronchopulmonary mycosis. J. Infect. Dis. 198:1714-1721. [DOI] [PubMed] [Google Scholar]
- 30.Muller, U., W. Stenzel, G. Kohler, C. Werner, T. Polte, G. Hansen, N. Schutze, R. K. Straubinger, M. Blessing, A. N. McKenzie, F. Brombacher, and G. Alber. 2007. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 179:5367-5377. [DOI] [PubMed] [Google Scholar]
- 31.Munitz, A., I. Bachelet, F. D. Finkelman, M. E. Rothenberg, and F. Levi-Schaffer. 2007. CD48 is critically involved in allergic eosinophilic airway inflammation. Am. J. Respir. Crit. Care Med. 175:911-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochkur, S. I., E. A. Jacobsen, C. A. Protheroe, T. L. Biechele, R. S. Pero, M. P. McGarry, H. Wang, K. R. O'Neill, D. C. Colbert, T. V. Colby, H. Shen, M. R. Blackburn, C. C. Irvin, J. J. Lee, and N. A. Lee. 2007. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J. Immunol. 178:7879-7889. [DOI] [PubMed] [Google Scholar]
- 33.Olszewski, M. A., G. B. Huffnagle, R. A. McDonald, D. M. Lindell, B. B. Moore, D. N. Cook, and G. B. Toews. 2000. The role of macrophage inflammatory protein-1 alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J. Immunol. 165:6429-6436. [DOI] [PubMed] [Google Scholar]
- 34.Olszewski, M. A., G. B. Huffnagle, T. R. Traynor, R. A. McDonald, D. N. Cook, and G. B. Toews. 2001. Regulatory effects of macrophage inflammatory protein 1α/CCL3 on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infect. Immun. 69:6256-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pappas, P. G., J. R. Perfect, G. A. Cloud, R. A. Larsen, G. A. Pankey, D. J. Lancaster, H. Henderson, C. A. Kauffman, D. W. Haas, M. Saccente, R. J. Hamill, M. S. Holloway, R. M. Warren, and W. E. Dismukes. 2001. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin. Infect. Dis. 33:690-699. [DOI] [PubMed] [Google Scholar]
- 36.Redhu, N. S., A. Saleh, L. Shan, W. T. Gerthoffer, S. K. Kung, A. J. Halayko, B. Lamkhioued, and A. S. Gounni. 2009. Proinflammatory and Th2 cytokines regulate the high affinity IgE receptor (FcɛRI) and IgE-dependent activation of human airway smooth muscle cells. PLoS One 4:e6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen, H. H., S. I. Ochkur, M. P. McGarry, J. R. Crosby, E. M. Hines, M. T. Borchers, H. Wang, T. L. Biechelle, K. R. O'Neill, T. L. Ansay, D. C. Colbert, S. A. Cormier, J. P. Justice, N. A. Lee, and J. J. Lee. 2003. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J. Immunol. 170:3296-3305. [DOI] [PubMed] [Google Scholar]
- 38.Sorkness, R., W. Castleman, J. Clough, M. Ritter, D. McCarthy, and R. F. Lemanske, Jr. 1993. Association of mononuclear cells and eosinophils with airway resistance and responsiveness in rat pulmonary inflammatory responses. Pediatr. Allergy Immunol. 4:144-151. [DOI] [PubMed] [Google Scholar]
- 39.Stenzel, W., U. Muller, G. Kohler, F. L. Heppner, M. Blessing, A. N. McKenzie, F. Brombacher, and G. Alber. 2009. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am. J. Pathol. 174:486-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traynor, T. R., W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2000. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 164:2021-2027. [DOI] [PubMed] [Google Scholar]