Abstract
C57BL/6 (B6) mice are genetically highly susceptible to chronic type II Toxoplasma gondii infections that invariably cause lethal toxoplasmic encephalitis. We examined the ability of an attenuated type I vaccine strain to elicit long-term immunity to lethal acute or chronic type II infections in susceptible B6 mice. Mice immunized with the type I cps1-1 vaccine strain were not susceptible to a lethal (100-cyst) challenge with the type II strain ME49. Immunized mice challenged with 10 ME49 cysts exhibited significant reductions in brain cyst and parasite burdens compared to naive mice, regardless of the route of challenge infection. Remarkably, cps1-1 strain-immunized B6 mice chronically infected with ME49 survived for at least 12 months without succumbing to the chronic infection. Potent immunity to type II challenge infections persisted for at least 10 months after vaccination. While the cps1-1 strain-elicited immunity did not prevent the establishment of a chronic infection or clear established brain cysts, cps1-1 strain-elicited CD8+ immune T cells significantly inhibited recrudescence of brain cysts during chronic ME49 infection. In addition, we show that uracil starvation of the cps1-1 strain induces early markers of bradyzoite differentiation. Collectively, these results suggest that more effective immune control of chronic type II infection in the genetically susceptible B6 background is established by vaccination with the nonreplicating type I uracil auxotroph cps1-1 strain.
Toxoplasma gondii is a common and significant obligate intracellular pathogen of humans and animals. There are three clonal types that exist which are also thought to be derived when T. gondii acquired oral infectivity (50). Virulence in mice is strain specific where type I clones are universally virulent, type II clones are of intermediate virulence, and type III clones are avirulent. Ingestion of contaminated food sources is the most common route of human infection, resulting in systemic disease that can be divided into two stages: the acute disseminating tachyzoite stage and the chronic encysted bradyzoite stage (12). Recrudescent infections arising from reactivation of preexisting chronic latent cyst stages are particularly severe in the context of immune deficiency such as AIDS (38), and improved treatments and the development of vaccines to reduce disease burden are important therapeutic objectives. Strategies with the potential to eradicate the latent cyst stages present in already-infected individuals could be helpful but, unfortunately, the biology of cyst development, as well as the immune control mechanisms of latent stages, are relatively poorly understood at this time. Clearly, CD8+ T cells and gamma interferon (IFN-γ) are significant effectors in mediating resistance to acute and chronic T. gondii infection (17, 19).
Numerous studies have evaluated responses to vaccines based on protein or DNA components of T. gondii with various degrees of success (1, 2, 4, 5, 9, 11, 22, 24, 25, 28, 36, 37, 40, 44, 46). Virulent parasite strains, as well as attenuated T. gondii strains, have been paramount in dissecting the immunobiology of host response in regard to understanding adaptive immune responses that may be helpful in vaccine design. Dense granule protein 6 (GRA6), GRA4, and rhoptry bulb protein 7 (ROP7) were recently identified as parasite antigens possessing a H-2Ld-restricted major histocompatibility complex class I (MHC-I) epitope that correlates with stage-specific expression and resistance to lethal chronic type II infections in the H-2Ld background (BALB/c). These data further define a potential molecular basis for genetic susceptibility to lethal type II chronic infections in the C57BL/6 H-2b MHC-I-restricted background (6, 16). Vaccine models using either live attenuated parasites, such as type I strain ts-4, or irradiated tachyzoites, have had the greatest success in providing complete protection against lethal type I challenges. These studies also report more significant reductions in type II cyst burdens than component vaccines or whole-dead parasite vaccines (42, 48, 53, 54). However, live parasite-based vaccines such as strain ts-4 are still slowly replicating and retain a significant potential for virulence in the immunocompromised host. Furthermore, immune protection elicited by strain ts-4 is not long-lasting and significantly decreases within months after immunization (27).
From the same parental RH strain that strain ts-4 was developed (45), our laboratory developed a fully attenuated nonreplicating type I cps1-1 strain that exhibits a severe uracil auxotrophy. The cps1-1 strain in a single immunization elicits complete immune protection and is able to clear high lethal dose virulent type I infection (14). Significantly, this highly attenuated strain is completely avirulent at extreme doses in immunocompromised hosts, such as in Tyk2−/− mice (49), which cannot control inflammation, and also in IFN-γ−/− mice (14, 20). The cps1-1 strain elicits potent Th1 immunity to lethal type I challenge infection after immunization of BALB/c, C57BL/6, Tyk2−/− (C57BL/6), or MyD88−/− (C57BL/6) mice (13, 14, 20, 49, 51, 56). Immunity to lethal type I challenge infection induced by the cps1-1 strain is dependent on CD8+ T cells (20), local production of IFN-γ (20), and interleukin-12 (IL-12) p70 (20, 51, 56). Remarkably, the potent immunity elicited by vaccination with the cps1-1 strain does not require systemic IFN-γ (20).
We show here that the immunity induced in C57BL/6 mice after vaccination with the cps1-1 strain provides a surprisingly effective and complete protection from a lethal oral or intraperitoneal (i.p.) challenge infection of type II cysts from the ME49 strain. We address the durability of cps1-1 strain-induced immunity to lethal type II cyst challenge infection by different routes and find that immunization with the cps1-1 strain provides long-term protective immunity to lethal type II challenge. Vaccination with the cps1-1 strain also markedly reduces the cyst burden and protects susceptible C57BL/6 mice from succumbing to chronic infection. CD8+ immune T cells elicited by vaccination with the cps1-1 strain prevent cyst recrudescence during chronic infection.
MATERIALS AND METHODS
Mice.
Adult 6- to 8-week-old C57BL/6 (B6) mice were obtained from the National Cancer Institute and mice were maintained in Tecniplast Seal Safe mouse cages on vent racks at the Dartmouth-Hitchcock Medical Center mouse facility. All mice were cared for and handled according to the Animal Care and Use Program of Dartmouth College using National Institutes of Health-approved institutional animal care and use committee guidelines.
Tachyzoite culture and immunization with the cps1-1 strain.
Tachyzoites of the attenuated cps1-1 strain were obtained by uracil supplemented tissue culture (14). Tachyzoites were isolated from freshly lysed human foreskin fibroblast (HFF) monolayers by filtration through 3.0-μm-pore-size Nucleopore membranes, washed with phosphate-buffered saline (PBS), centrifuged, and then resuspended in PBS at defined numbers after determination of the tachyzoite concentration using a hemacytometer. The viability of tachyzoite preparations was tested in plaque assays to confirm that 30 to 50% of tachyzoites were infectious. Mice were immunized with 106 freshly isolated tachyzoites of the cps1-1 strain twice 14 days apart. At 1 or 10 months after final immunization, the mice were infected i.p. or perorally (i.g.) via gavage needle with either 10 or 100 brain-derived cysts of the ME49 strain.
ME49 cyst maintenance, isolation, and enumeration.
Brain cysts of the strain ME49 were obtained by continuous passage every 4 to 5 weeks in B6 mice infected i.p. with 10 cysts. Brains from mice infected with ME49 were harvested and homogenized by using a Dounce homogenizer in 2 ml of sterile 1× PBS. Cysts were then counted by examining 10 μl of the brain homogenate under a coverslip using light microscopy under ×40 magnification. Total cyst numbers were determined per brain based on counting 5 to 50 slides prepared from each brain homogenate.
Adoptive transfer of total splenocytes and purified T-cell subsets.
Spleens from cps1-1 strain-immunized or naive B6 mice were harvested and splenocytes released by grinding the spleen through a 70-μm-pore-size nylon screen in 5 ml of Hanks balanced salt solution. Splenocytes were pelleted then subject to erythrocyte lysis for 3 min at room temperature with sterile ACK buffer prepared in house using 0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2 EDTA in distilled H2O. Splenocytes were then washed extensively in sterile PBS, and live cells were identified via trypan blue exclusion and enumerated by using a hemacytometer. Then, 5 × 107 whole naive strain or cps1-1 strain immune splenocytes were then transferred to chronically infected mice via tail vein injection. Separately, from naive or cps1-1 strain immune splenocytes, purified CD4+ and CD8+ T cells were isolated by using EasySep positive selection (Stem Cell Technologies). Either 9 × 106 purified CD4+ T cells, 4.5 × 106 purified CD8+ T cells, or a combination of 9 × 106 CD4+ and 4.5 × 106 CD8+ T cells were injected via tail vein injection into chronically infected recipient mice.
Parasite burden measured by quantitative real-time PCR.
Infected animals were euthanized via CO2 overdose, and brain tissue was harvested and flash frozen in liquid nitrogen. DNA was extracted from the entire organ by using a DNeasy tissue kit (Qiagen, Inc., Germantown, MD), and samples were pooled. Amplification of parasite DNA from 400 ng of purified tissue DNA was performed using primers specific for the T. gondii B1 gene at 10 pmol of each per reaction (29, 31) (Integrated DNA Technologies, Coralville, IA) and amplified by real-time PCR using SMartMix HM (Cepheid, Sunnyvale, CA) on a Cepheid Smart Cycler. Each reaction contained one lyophilized SMartMix HM bead and SYBR green I (Cambrex BioScience, Inc., Rockland, ME). Known parasite DNA equivalents were used to establish a standard curve, and then parasite numbers in tissue samples were calculated via extrapolation from the standard curve.
Immunohistochemistry of the cps1-1 tachyzoite-to-bradyzoite differentiation.
HFF monolayers were infected with the cps1-1 strain in the presence or absence of uracil for 2 days, and immunohistochemistry analysis of infected HFF monolayers was performed (15). Briefly, confluent HFF cultures on glass coverslips were infected with tachyzoites and then fixed with Histochoice tissue fixative (Amresco) as specified by the manufacturer. Fixed infected cells were treated in 0.2% Triton X-100 for 20 min and blocked with 3% bovine serum albumin overnight. Infected HFF cells on coverslips were incubated with appropriate dilutions of primary antibody or biotinylated Dolichos biflorus lectin (DBA; Vector Laboratories) for 90 min at 37°C, washed, and incubated with the secondary antibody or Strepavidin-Alexa 568, respectively. Monospecific polyclonal rabbit antibodies (immunoglobulin G) to SAG1 (43) were used and stained with anti-rabbit secondary antibody coupled to Alexa 488 (Molecular Probes). Biotinylated DBA was stained by using streptavidin-Alexa 568 (Molecular Probes) (32). Coverslips were mounted by using Vectashield (Vector Laboratories) or a SlowFade Light Antifade kit with DAPI (4′,6′-diamidino-2-phenylindole) for staining nucleic acid (Molecular Probes). Images were captured by using a Zeiss AxioPhot upright photomicroscope with a slow-scan, cooled charge-coupled device CH250 detector (Photometrics). The computer with this microscope uses IPLab software (Scanalytics), which provides control of excitation filters and image acquisition. The brightest pixels in an image were below saturation, and some images were contrast enhanced for display purposes but were qualitatively reflective of unprocessed data collected from the original image(s).
Statistical analysis.
The Kaplan-Meier product limit test was used to measure significant differences between survival curves (GraphPad Prism software). All other samples were subject to a Student t test and are represented as the means ± the standard errors of the mean (SEM).
RESULTS
Immunization with the type I cps1-1 strain elicits long-term immunity to lethal type II infection in genetically susceptible C57BL/6 mice.
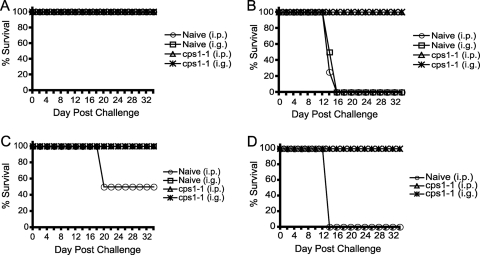
We measured the ability of the cps1-1 vaccine strain to elicit immune protection against lethal type II infection. One month after immunization, immunized and age-matched naive mice were infected with either a nonlethal (10-cyst) or lethal (100-cyst) dose of the type II strain ME49 by the i.p. or i.g. route, and survival was monitored. Mice immunized with the cps1-1 strain completely survived both doses of challenge regardless of route (Fig. 1A and B). As expected, naive mice infected with the nonlethal challenge of 10 cysts survived and naive mice infected with the lethal 100 cyst dose succumbed to infection (Fig. 1A and B).
FIG. 1.
Immunization with the cps1-1 vaccine elicits long-lasting immunity against a lethal type II challenge regardless of the immunization route. C57BL/6 mice were either not immunized or immunized i.p. with 106 cps1-1 tachyzoites given twice 14 days apart. One month later (A and B) or 10 months later (C and D) naive mice were challenged i.p. (○) or i.g. (□), and cps1-1 strain-immunized mice were challenged i.p. (▵) or i.g. (✳) with either 10 (A and C) or 100 (B and D) ME49 cysts. The percent survival was monitored for 5 weeks, at which time the experiment was stopped. The data represent the results of one experiment with six mice per group.
Due to the high potency of immunization with the cps1-1 vaccine in protecting mice against high-dose type I and type II lethal challenge infections and induction of long-lasting immunity to type I challenge (14, 20), we measured the durability of cps1-1 strain-induced protective immunity against type II challenge. Ten months after immunization, immunized and age-matched naive mice were infected with either a nonlethal (10-cyst) or lethal (100-cyst) dose of the type II strain ME49 by the i.p. or i.g. route, and survival was monitored. cps1-1 strain-immunized mice completely survived both doses of challenge regardless of the challenge route (Fig. 1C and D). In contrast, only 50% of nonimmunized mice that were age matched to those that had been immunized survived the 10-cyst challenge when given i.p., most likely due to the loss of immune function with age (Fig. 1C). Age-matched 10-month-old unimmunized mice did not survive the lethal 100-cyst challenge (Fig. 1C and D).
Vaccination with the cps1-1 strain significantly reduces the brain cyst and parasite burden after type II challenge infection but does not prevent establishment of chronic infection.
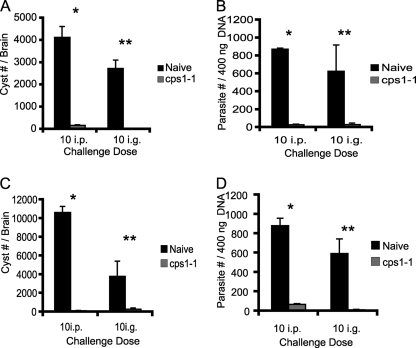
C57BL/6 mice are genetically highly susceptible to type II infections in that they acquire higher brain cyst burdens during acute infection, and these cysts ultimately recrudesce and cause lethal toxoplasmic encephalitis during chronic infection (3, 7, 10, 23, 41, 52). Thus, we measured cyst burdens and parasite burden in brains of cps1-1 strain-immunized or naive mice 5 weeks after ME49 challenge infection. Naive mice (1 month age-matched), challenged with 10 cysts i.p. or i.g., contained 4,130 ± 490 cysts and 2,730 ± 380 cysts per brain, respectively (Fig. 2A). Immunized mice, challenged with 10 cysts either i.p. or i.g., had significantly (P = 0.0001 and P = 0.0001) fewer cysts per brain compared to naive mice (170 ± 30 and 10 ± 6, respectively) (Fig. 2A). Immunized mice challenged at 10 months postimmunization showed similar reductions in cyst burden and exhibited brain cyst burdens of 106 ± 48.3 cysts (i.p.) and 275 ± 140 cysts (i.g.), respectively, compared to naive mice challenged with 10 cysts i.p. or i.g. that exhibited brain cyst burdens of 10,633 ± 638 and 3,800 ± 1625, respectively (Fig. 2C). Interestingly, cps1-1 strain-immunized mice challenged with a lethal high cyst dose (100 cysts) at either 1 month or 10 months postimmunization survived acute infection and exhibited a decrease in brain cysts similar to that observed with the 10-cyst dose challenges.
FIG. 2.
Short-term and long-term immunity induced by the cps1-1 strain prevents cyst formation. C57BL/6 mice were either not immunized or immunized i.p. with 106 cps1-1 tachyzoites twice 14 days apart. One month and ten months after cps1-1 vaccine administration, age-matched naive (▪) and immune (░⃞) mice were challenged with 10 cysts of ME49 i.p. or i.g. At 5 weeks after challenge infection, cyst enumeration was performed in the brains of the mice challenged 1 month (A) and 10 months (C) after final immunization. Brain parasite burdens were measured by real-time quantitative PCR of the T. gondii B1 gene in mice challenged 1 month (B) and 10 months (D) after final the immunization. The data represent the means ± the SEM from one experiment with six mice per group. Statistical differences were calculated by using the Student t test, and P < 0.001 was considered significant (* for the i.p.-challenged group and ** for the i.g.-challenged group).
During the course of our cyst enumeration, we observed brain cysts of various sizes under light microscopy. Consequently, a real-time PCR assay was used to determine absolute parasite burdens in brain tissue based on measurement of genomes or parasite equivalents (PE) (see Materials and Methods). As previously observed (20, 26), unchallenged cps1-1 strain-immunized mice exhibited no detectable PE in the blood, spleen, and brain, indicating that the cps1-1 strain does not replicate or develop mature cysts and is cleared before challenge is administered (data not shown). Naive mice contained 871.6 ± 13.7 or 626.9 ± 292.8 PE per 400 ng of total brain DNA when challenged at 1 month by either the i.p. or the i.g. route, respectively (Fig. 2B). Naive mice challenged at 10 months contained 881.9 ± 75.8 (i.p.) and 593.1 ± 151.2 (i.g.) PE, respectively, per 400 ng of brain DNA (Fig. 2D). In contrast, cps1-1 strain-immunized mice challenged 1 month after immunization contained 28.8 ± 6.2 (i.p.) PE (P = 0.001) and 30.8 ± 16.5 (i.g.) PE (P = 0.001) (Fig. 2B). Mice challenged 10 months after immunization contained 69.3 ± 7.3 (i.p.) PE (P = 0.001) and 12.4 ± 4.9 (i.g.) PE (P = 0.01) (Fig. 2D). Despite the significant immune protection afforded after immunization with cps1-1, these observations revealed that a population of ME49 parasites was still capable of trafficking to the brain and establishing a chronic infection with a reduced cyst burden.
Adoptive transfer of immune splenocytes to a type II T. gondii-infected mouse enhances control of chronic infection.
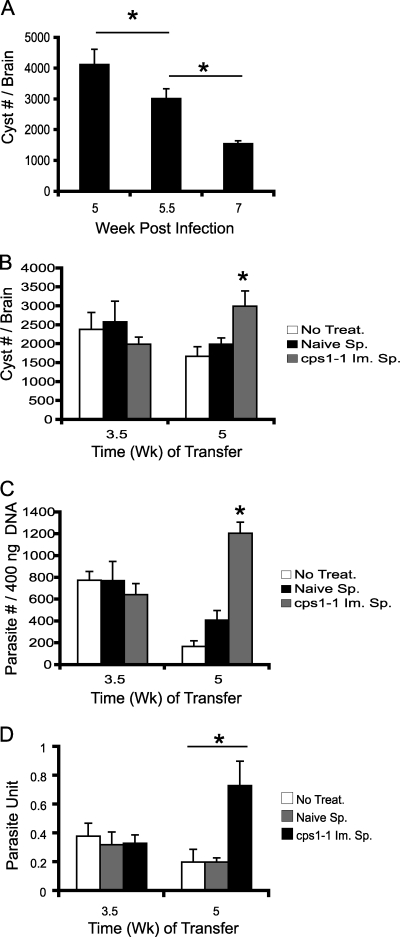
Vaccination with the cps1-1 strain is highly effective at promoting survival to acute infection, as well as reducing cyst burdens and parasite numbers in chronic infection in mice challenged with type II ME49 cysts. The immune control mechanisms elicited by cps1-1 strain vaccination could prevent tachyzoites from trafficking to brain, could promote bradyzoite or cyst development, could eradicate existing brain cysts, or could prevent parasite access to the brain. In type I RH challenge infection, immunity elicited by the cps1-1 strain shows a powerful effect and rapidly clears tachyzoites (20, 26). To establish a kinetic of parasite recrudescence, genetically susceptible B6 mice were infected with 10 ME49 cysts i.p., the brains were harvested, and the cysts were enumerated at weeks 5, 5.5, and 7 postinfection. As shown in Fig. 3A, cyst burdens decreased overtime and higher numbers were found at week 5 compared to week 7. To measure the ability of cps1-1 strain-induced immunity to target established brain cysts, total splenocytes were adoptively transferred into mice with established ME49 chronic infections. B6 mice were infected with 10 ME49 cysts i.p., and then at 3.5 or 5 weeks after infection mice received either no treatment, 5 × 107 whole naive splenocytes, or 5 × 107 whole cps1-1 strain-immune splenocytes. At 2 weeks after adoptive transfer of total splenocytes, the brains were harvested, and the cysts were enumerated. Treatments conducted at 3.5 weeks after ME49 infection showed no significant differences in the brain cyst burden (Fig. 3B) or parasite burden (Fig. 3C). In contrast, treatments conducted at 5 weeks after ME49 infection showed significant differences in brain cyst burden (Fig. 3B) and parasite burden (Fig. 3C). Mice receiving naive splenocytes or no treatment showed nearly identical cyst and parasite burden reductions compared to week 3.5 treatment groups. Remarkably, mice receiving cps1-1 strain-immune splenocytes exhibited significantly higher brain cyst burden (Fig. 3B), as well as higher parasite burdens compared to naive or no treatment groups (Fig. 3C). However, compared to the week 5 cyst burdens shown in the kinetic analysis (Fig. 3A), the cyst numbers were only slightly reduced. The parasite burden (Fig. 3C) correlated well with the cyst burden (Fig. 3B), but we observed an increase in the average cyst diameter in light microscopy (data not shown) and an increase in parasite units (Fig. 3D). These observations indicated that adoptive transfer of the cps1-1 strain-immune splenocytes could delay or prevent cyst recrudescence in chronic infection and cysts continue to develop.
FIG. 3.
Immune splenocytes from cps1-1 strain-immunized mice prevents cyst recrudescence. C57BL/6 mice were either not immunized or immunized i.p. with 106 cps1-1 tachyzoites twice given 14 days apart. Separately, susceptible C57BL/6 mice were infected i.p. with 10 cysts of ME49 to establish chronic infection. (A) Brains were harvested from chronically infected mice at weeks 5, 5.5, and 7 postinfection as indicated, and the cysts were enumerated. (B, C, and D) Chronically infected mice received no treatment (□), naive splenocytes (▪), or immune splenocytes (░⃞) from cps1-1 strain-immunized mice at 3.5 or 5 weeks after ME49 infection. At 2 weeks after adoptive transfer, the cysts were enumerated (B), the parasite burdens (PE) were calculated by using quantitative real-time PCR of the T. gondii B1 gene (C), and the parasite units were calculated as PE per 400 ng of brain tissue DNA per cyst (D). The data represent the means ± the SEM from a group of five animals; the experiment was repeated twice. Statistical differences were calculated by using the Student t test, and P < 0.05 (*) was considered significant.
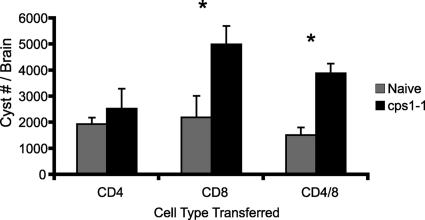
To define which cell type in cps1-1 strain-immune splenocytes was preventing recrudescence of brain cysts, purified CD4+, CD8+, or CD4+ and CD8+ T cells from cps1-1 strain-immunized or naive mice were adoptively transferred into mice infected with 10 ME49 cysts 5 weeks earlier, and then the brains were harvested 2 weeks later to enumerate the cyst burden. Mice receiving naive CD4+, CD8+, CD4+ CD8+, or immune CD4+ T cells were not statistically different in brain cyst burden of 1,945 ± 254, 2,200 ± 826, 1,519 ± 298, or 2,531 ± 767, respectively (Fig. 4). In contrast, mice receiving immune CD8+ T cells or a combination of immune CD4+ and CD8+ T cells had significantly higher cyst burdens (5,006 ± 705 and 3,891 ± 368, respectively) (Fig. 4) compared to naive controls. The cyst burdens in mice receiving immune CD8+ T cells were not different from those measured at week 5 as shown in Fig. 3A. These results suggest that cps1-1 strain-immune CD8+ T cells delay or prevent cyst recrudescence in chronic infection.
FIG. 4.
CD8 T cells from cps1-1 strain-immunized mice prevent cyst recrudescence. C57BL/6 mice were either not immunized or immunized i.p. with 106 cps1-1 tachyzoites given twice 14 days apart. Separately, genetically susceptible C57BL/6 mice were infected i.p. with 10 cysts of ME49 to establish chronic infection. Chronically infected mice received naive (░⃞) or immune (▪) CD4+, CD8+, or CD4+ CD8+ T cells isolated from cps1-1 strain-immunized mice at 5 weeks after ME49 infection. At 2 weeks after adoptive transfer, the cysts were enumerated. The data represent the means ± the SEM from a group of five animals; the experiment was repeated twice. Statistical differences were calculated by using the Student t test, and P < 0.05 (*) was considered significant.
C57BL/6 mice immunized with cps1-1 strain do not succumb to chronic type II ME49 infection.
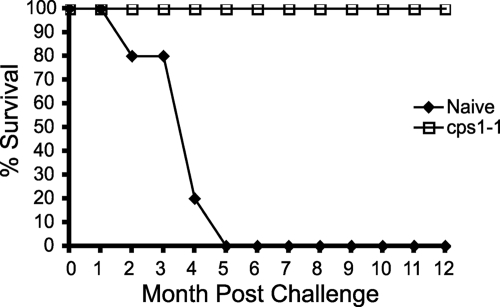
Type II strain T. gondii-infected C57BL/6 mice invariably succumb to complications associated with either recrudescent disease or the damage incurred during the acute stage of infection typically sometime after 12 weeks of infection (3, 18, 21, 34). Sensitivity to type II T. gondii infection is clearly dependent on the mouse genotype (3, 7, 10, 23, 41, 52) and, as recently proposed, may be specifically related to immunodominant H-2Ld-restricted MHC-I epitopes found within GRA6, GRA4, and ROP7 proteins (6, 16). We examined whether cps1-1 strain immunization would prolong the survival of susceptible C57BL/6 mice challenged long term with 10 cysts of ME49 i.p. The cps1-1 strain-immunized mice survived chronic infection for at least 12 months after ME49 infection. In contrast, naive mice rapidly succumbed to chronic ME49 infection (Fig. 5).
FIG. 5.
Immunization with the cps1-1 strain prevents lethality of chronic infection in the genetically sensitive C57BL/6 mouse strain. C57BL/6 mice were either not immunized or immunized i.p. with 106 cps1-1 tachyzoites given twice 14 days apart. At 1 month after immunization, naive mice (⧫) or cps1-1 strain-immunized mice (□) were challenged with 10 cysts of ME49 i.p., and the percent survival was monitored. The data represent the results of one experiment performed with 10 animals per group.
cps1-1 tachyzoites begin to differentiate into bradyzoites during uracil starvation.
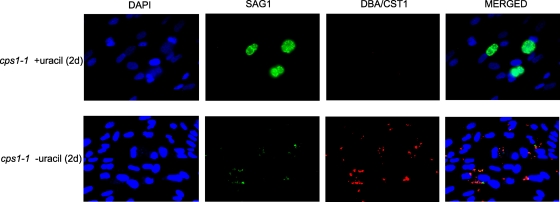
Recent data show that the nonreplicating cps1-1 strain elicits a strong local and systemic IL-12 p70 response that is more typical of a type II strain than a type I strain (20), and cytolytic CD8+ T cells develop with faster kinetics compared to infection with replicating parasites (26). Previous studies also suggest that T. gondii life-stage-specific CD8+ T-cell responses are important for control of the parasite (16, 30, 39). We examined the expression pattern of tachyzoite-stage marker (SAG1) and an early-bradyzoite-stage marker (CST1) during uracil starvation of the cps1-1 strain. While replicating cps1-1 parasites expressed SAG1 at high levels and did not express CST1 bradyzoite marker (Fig. 6, top panels), uracil starvation induced a nearly complete loss of expression of SAG1 and markedly upregulated the expression of CST1 (Fig. 6, bottom panels). These data suggest that an altered profile of tachyzoite and early bradyzoite antigens occurs within 2 days of initiating uracil starvation of the cps1-1 strain.
FIG. 6.
Uracil starvation of the cps1-1 strain induces the loss of tachyzoite-stage antigens and the expression of early-bradyzoite-stage antigens. Confluent HFF monolayers were infected with cps1-1 tachyzoites in the presence (top panels) or absence (bottom panels) of uracil for 2 days. Immunohistochemistry analysis was performed after fixation of infected HFF monolayers with DAPI (blue), anti-P30/SAG1 (green), and DBA/CST1 (red) as indicated. The images show the results with DAPI alone (left panels, top and bottom), anti-P30/SAG1 alone (left center panels, top and bottom), DBA/CST1 alone (right center panels, top and bottom), and merged DAPI/SAG1/DBA (right panels, top and bottom). The images contain the brightest pixels below saturation, are unprocessed, and were collected from the original image(s).
DISCUSSION
These studies extend a series of recent and significant reports that have examined the immune responses elicited by vaccination with the attenuated cps1-1 strain (13, 14, 20, 35, 49, 51, 56-59). To our knowledge, this is the first report that vaccination of genetically susceptible C57BL/B6 mice with a type I strain can prevent the development of lethal chronic infection.
A potent and long-lasting immunity to lethal type 1 infection is elicited by a single cps1-1 strain immunization of BALB/c, C57BL/6, Tyk2−/− (C57BL/6), or MyD88−/− (C57BL/6) mice (13, 14, 20, 49, 51, 56). Immunity to lethal type I challenge infection induced by the cps1-1 strain is dependent on CD8+ T cells (20) and the local production of IFN-γ (20) and IL-12 p70 (20, 51, 56) and, surprisingly, does not require significant production of systemic IFN-γ (20).
Previous studies of the durability of immunity elicited by immunization with type I strain ts-4 or by infection with type II parasite strains in susceptible B6 mice suggest that immunity significantly wanes over time (8, 18, 27, 33, 47). Protecting highly genetically susceptible B6 mice from lethal acute or lethal chronic type II infection by vaccination is problematic. If parasites traffic to the brain or other tissues before the immune response can prevent cyst formation, it is currently unknown whether any immune response can clear preexisting cysts. Many studies have documented that B6 mice (MHC-I-restricted H-2b background) are extremely susceptible to type II infections (10, 23, 52). If these mice survive acute type II infection, then they will invariably succumb from the chronic infection within a few months. Recently, the GRA6 protein of type II strains was shown to possess an immunodominant H-2Ld-restricted MHC-I cell epitope that appears to play a major role in protecting BALB/c mice from lethal chronic infection. In addition, GRA4 and ROP7 have been characterized as containing life-stage-specific dominant epitopes (16). Since these epitopes are not recognized in B6 mice, loss of immune response to these immunodominant epitopes may at least partly explain the increased susceptibility of B6 mice to lethal type II chronic infections (6).
Interestingly, the immunodominant H-2Ld-restricted MHC-I epitope HF10 within the GRA6 protein of type II strains is not present in type I strains such as RH from which the cps1-1 and ts-4 vaccine strains are derived (14, 45). Our results show that immunization of B6 mice with the cps1-1 strain completely protects mice from lethal type II cyst challenge (i.p. or i.g. route) at 1 month (short term) and equally well at 10 months (long term). These results suggest that adaptive immunity directed against the immunodominant H-2Ld-restricted MHC-I epitope within the GRA6 protein of type II strains is not necessary for control of acute lethal type II infections delivered by the i.p. or i.g. route in B6 mice.
Immune control of chronic infection in regard to whether mice succumb or survive is clearly multifaceted and highly dependent on both genetic factors and parasite genotype. Our results with immunization of genetically susceptible B6 mice with the cps1-1 strain show that mice challenged with a sublethal acute infection dose of ME49 cysts at 1 month or 10 months postimmunization exhibit remarkably reduced levels of brain cyst burden and brain parasite burden and that these chronically infected B6 mice survive significantly longer than chronically infected naive mice. Our results show that a sterilizing immunity to brain cyst formation is not generated in cps1-1 strain-immunized mice. The simplest explanation of these results is that type II infections can rapidly elicit new brain cysts early after oral infection and prior to immune destruction of tachyzoite stages. Several studies have suggested that cysts may develop early after infection since cysts containing only a few bradyzoites have been observed (55). Considering the potent immune responses elicited after cps1-1 strain immunization, it seems likely that parasites emerging from challenge cysts most likely traffic to the brain early during infection or possibly after each wave of cellular infection before immune control of tachyzoite-stage infection is fully reestablished by the recall response to cps1-1 strain immunity (49).
A significant fraction of cps1-1 strain-elicited immunity is clearly targeted to tachyzoite stages based on rapid clearance of tachyzoite challenge (20, 26). Our experiments do not specifically address whether cps1-1 strain-elicited immunity to type II infection is directed against tachyzoite- or bradyzoite-stage antigens. Several studies show that tachyzoite-stage antigens and bradyzoite-stage antigens induce potent stage-specific CD8+ T-cell responses important for the control of T. gondii infection (16, 30, 39). Our results suggest that antigens from both stages may play a role in the more potent immunity induced by the cps1-1 strain compared to the ts-4 strain. The cps1-1 strain vaccine is delivered in the tachyzoite stage but within 2 days of uracil starvation begins to differentiate to early bradyzoite stages with a corresponding loss of tachyzoite-stage antigens. Although our results show that the cps1-1 strain may elicit T-cell responses to both stages, our data do not discriminate whether early bradyzoite antigen expression or the loss of tachyzoite-stage antigens contributes to the potent protective immunity induced by the cps1-1 strain. In addition, our study did not determine whether cps1-1 strain-induced immunity is solely tachyzoite stage specific or both tachyzoite and bradyzoite stage specific. Because the cps1-1 strain is completely nonreplicating (compared to ts-4), the immune response elicited by cps1-1 strain vaccination may favor better control of type II infection. Recent results suggest there is markedly less inflammation induced by the cps1-1 vaccine model and the cytokine response to the cps1-1 strain more closely resembles that of a type II strain (20).
Autophagy could also play a role in the more potent immunity elicited by cps1-1 vaccination. Vaccination with the cps1-1 strain elicits potent autophagy responses in B6 mice (35, 57-59). Consequently, if the cps1-1 strain ultimately delivers “self-autophagic” responses and the nonreplicating cps1-1 parasite and its associated vacuole is digested in an autophagosome, a wider repertoire and/or increased presentation of parasite antigens may occur after immunization with cps1-1. Our data do not differentiate between these possibilities or other potential mechanisms that could explain the potent protection against type II infection elicited in C57BL/6 mice by the cps1-1 vaccine.
Interestingly, our results show that CD8+ T cells elicited by the cps1-1 vaccine have a direct effect on the outcome of chronic infection in the brain. cps1-1 strain-elicited immune CD8+ T cells prevent the recrudescence of already-established brain cysts in B6 mice. Cysts that were prevented from recrudescing by cps1-1 immunity continued to develop. These data do not distinguish whether these T cells recognize tachyzoite- or bradyzoite-stage antigens, and this mechanistic question will be addressed in future studies. Regardless of the mechanism of action, immune CD8+ T cells elicited by cps1-1 strain vaccination delay or prevent cyst recrudescence, and this clearly correlates with prolonged survival of chronically infected B6 mice. This immune control is independent of the H-2Ld-restricted GRA6, GRA4, and ROP7 derived epitopes and suggests that other factors and antigens are required to control chronic infection in cps1-1 strain-immunized B6 mice. Our data suggest this immune control may be due to bradyzoite-stage antigens expressed by the cps1-1 strain during uracil starvation, but we cannot yet rule out the possibility that the increased control of type II infection observed in our study is due to tachyzoite-stage antigens other than GRA6, GRA4, and ROP7.
In summary, we demonstrate that the cps1-1 vaccine is highly effective at inducing both short-term and long-term protection against type II parasite challenge by significantly inhibiting lethal acute and lethal chronic disease in genetically susceptible C57BL/6 mice. CD8+ T cells elicited by cps1-1 vaccination delay or prevent cyst recrudescence in chronically infected B6 mice. These studies further establish that the live attenuated cps1-1 vaccine strain is an excellent vaccine that confers significant protection against both type I and type II strains of T. gondii. The cps1-1 vaccination model represents a valuable tool in which to further dissect the complex biology of how control of chronic infection is won or lost in C57BL/6 and other genetic backgrounds.
Acknowledgments
This research was supported by NIH grants AI075931 and AI041930 (to D.J.B.).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 21 September 2009.
REFERENCES
- 1.Aline, F., D. Bout, S. Amigorena, P. Roingeard, and I. Dimier-Poisson. 2004. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 72:4127-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aline, F., D. Bout, and I. Dimier-Poisson. 2002. Dendritic cells as effector cells: gamma interferon activation of murine dendritic cells triggers oxygen-dependent inhibition of Toxoplasma gondii replication. Infect. Immun. 70:2368-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo, F. G., D. M. Williams, F. C. Grumet, and J. S. Remington. 1976. Strain-dependent differences in murine susceptibility to toxoplasma. Infect. Immun. 13:1528-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beghetto, E., H. V. Nielsen, P. Del Porto, W. Buffolano, S. Guglietta, F. Felici, E. Petersen, and N. Gargano. 2005. A combination of antigenic regions of Toxoplasma gondii microneme proteins induces protective immunity against oral infection with parasite cysts. J. Infect. Dis. 191:637-645. [DOI] [PubMed] [Google Scholar]
- 5.Bertaux, L., M. N. Mevelec, S. Dion, V. Suraud, M. Gregoire, P. Berthon, and I. Dimier-Poisson. 2008. Apoptotic pulsed dendritic cells induce a protective immune response against Toxoplasma gondii. Parasite Immunol. 30:620-629. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard, N., F. Gonzalez, M. Schaeffer, N. T. Joncker, T. Cheng, A. J. Shastri, E. A. Robey, and N. Shastri. 2008. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat. Immunol. 9:937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, C. R., and R. McLeod. 1990. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 145:3438-3441. [PubMed] [Google Scholar]
- 8.Casciotti, L., K. H. Ely, M. E. Williams, and I. A. Khan. 2002. CD8+-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect. Immun. 70:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cong, H., Q. M. Gu, H. E. Yin, J. W. Wang, Q. L. Zhao, H. Y. Zhou, Y. Li, and J. Q. Zhang. 2008. Multi-epitope DNA vaccine linked to the A2/B subunit of cholera toxin protect mice against Toxoplasma gondii. Vaccine 26:3913-3921. [DOI] [PubMed] [Google Scholar]
- 10.Deckert-Schluter, M., D. Schluter, D. Schmidt, G. Schwendemann, O. D. Wiestler, and H. Hof. 1994. Toxoplasma encephalitis in congenic B10 and BALB mice: impact of genetic factors on the immune response. Infect. Immun. 62:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desolme, B., M. N. Mevelec, D. Buzoni-Gatel, and D. Bout. 2000. Induction of protective immunity against toxoplasmosis in mice by DNA immunization with a plasmid encoding Toxoplasma gondii GRA4 gene. Vaccine 18:2512-2521. [DOI] [PubMed] [Google Scholar]
- 12.Dubey, J. P. 1998. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 28:1019-1024. [DOI] [PubMed] [Google Scholar]
- 13.Dzierszinski, F., M. Pepper, J. S. Stumhofer, D. F. LaRosa, E. H. Wilson, L. A. Turka, S. K. Halonen, C. A. Hunter, and D. S. Roos. 2007. Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen-presenting cells. Infect. Immun. 75:5200-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox, B. A., and D. J. Bzik. 2002. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415:926-929. [DOI] [PubMed] [Google Scholar]
- 15.Fox, B. A., J. P. Gigley, and D. J. Bzik. 2004. Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int. J. Parasitol. 34:323-331. [DOI] [PubMed] [Google Scholar]
- 16.Frickel, E. M., N. Sahoo, J. Hopp, M. J. Gubbels, M. P. Craver, L. J. Knoll, H. L. Ploegh, and G. M. Grotenbreg. 2008. Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T-cell epitopes. J. Infect. Dis. 198:1625-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazzinelli, R., Y. Xu, S. Hieny, A. Cheever, and A. Sher. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175-180. [PubMed] [Google Scholar]
- 18.Gazzinelli, R. T., I. Eltoum, T. A. Wynn, and A. Sher. 1993. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the downregulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J. Immunol. 151:3672-3681. [PubMed] [Google Scholar]
- 19.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-292. [PubMed] [Google Scholar]
- 20.Gigley, J. P., B. A. Fox, and D. J. Bzik. 2009. Cell-mediated immunity to Toxoplasma gondii develops primarily by local Th1 host immune responses in the absence of parasite replication. J. Immunol. 182:1069-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimesaat, M. M., S. Bereswill, A. Fischer, D. Fuchs, D. Struck, J. Niebergall, H. K. Jahn, I. R. Dunay, A. Moter, D. M. Gescher, R. R. Schumann, U. B. Gobel, and O. Liesenfeld. 2006. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 177:8785-8795. [DOI] [PubMed] [Google Scholar]
- 22.Igarashi, M., F. Kano, K. Tamekuni, R. Z. Machado, I. T. Navarro, O. Vidotto, M. C. Vidotto, and J. L. Garcia. 2008. Toxoplasma gondii: evaluation of an intranasal vaccine using recombinant proteins against brain cyst formation in BALB/c mice. Exp. Parasitol. 118:386-392. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, J. J., C. W. Roberts, C. Pope, F. Roberts, M. J. Kirisits, R. Estes, E. Mui, T. Krieger, C. R. Brown, J. Forman, and R. McLeod. 2002. In vitro correlates of Ld-restricted resistance to toxoplasmic encephalitis and their critical dependence on parasite strain. J. Immunol. 169:966-973. [DOI] [PubMed] [Google Scholar]
- 24.Jongert, E., V. Melkebeek, S. De Craeye, J. Dewit, D. Verhelst, and E. Cox. 2008. An enhanced GRA1-GRA7 cocktail DNA vaccine primes anti-Toxoplasma immune responses in pigs. Vaccine 26:1025-1031. [DOI] [PubMed] [Google Scholar]
- 25.Jongert, E., D. Verhelst, M. Abady, E. Petersen, and N. Gargano. 2008. Protective Th1 immune responses against chronic toxoplasmosis induced by a protein-protein vaccine combination but not by its DNA-protein counterpart. Vaccine 26:5289-5295. [DOI] [PubMed] [Google Scholar]
- 26.Jordan, K. A., E. H. Wilson, E. D. Tait, B. A. Fox, D. S. Roos, D. J. Bzik, F. Dzierszinski, and C. A. Hunter. 2009. Kinetics and phenotype of vaccine-induced CD8+ T-cell responses to Toxoplasma gondii. Infect. Immun. 77:3894-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan, I. A., and L. Casciotti. 1999. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J. Immunol. 163:4503-4509. [PubMed] [Google Scholar]
- 28.Khan, I. A., K. H. Ely, and L. H. Kasper. 1991. A purified parasite antigen (p30) mediates CD8+ T-cell immunity against fatal Toxoplasma gondii infection in mice. J. Immunol. 147:3501-3506. [PubMed] [Google Scholar]
- 29.Khan, I. A., P. M. Murphy, L. Casciotti, J. D. Schwartzman, J. Collins, J. L. Gao, and G. R. Yeaman. 2001. Mice lacking the chemokine receptor CCR1 show increased susceptibility to Toxoplasma gondii infection. J. Immunol. 166:1930-1937. [DOI] [PubMed] [Google Scholar]
- 30.Kim, S. K., and J. C. Boothroyd. 2005. Stage-specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J. Immunol. 174:8038-8048. [DOI] [PubMed] [Google Scholar]
- 31.Kirisits, M. J., E. Mui, and R. McLeod. 2000. Measurement of the efficacy of vaccines and antimicrobial therapy against infection with Toxoplasma gondii. Int. J. Parasitol. 30:149-155. [DOI] [PubMed] [Google Scholar]
- 32.Knoll, L. J., and J. C. Boothroyd. 1998. Molecular biology's lessons about toxoplasma development: stage-specific homologs. Parasitol. Today 14:490-493. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman, L. A., E. N. Villegas, and C. A. Hunter. 2004. Interleukin-15-deficient mice develop protective immunity to Toxoplasma gondii. Infect. Immun. 72:6729-6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liesenfeld, O., J. Kosek, J. S. Remington, and Y. Suzuki. 1996. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling, Y. M., M. H. Shaw, C. Ayala, I. Coppens, G. A. Taylor, D. J. Ferguson, and G. S. Yap. 2006. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J. Exp. Med. 203:2063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, S., L. Shi, Y. B. Cheng, G. X. Fan, H. X. Ren, and Y. K. Yuan. 2009. Evaluation of protective effect of multi-epitope DNA vaccine encoding six antigen segments of Toxoplasma gondii in mice. Parasitol. Res. 105:267-274. [DOI] [PubMed] [Google Scholar]
- 37.Lourenco, E. V., E. S. Bernardes, N. M. Silva, J. R. Mineo, A. Panunto-Castelo, and M. C. Roque-Barreira. 2006. Immunization with MIC1 and MIC4 induces protective immunity against Toxoplasma gondii. Microbes Infect. 8:1244-1251. [DOI] [PubMed] [Google Scholar]
- 38.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211-222. [DOI] [PubMed] [Google Scholar]
- 39.Lutjen, S., S. Soltek, S. Virna, M. Deckert, and D. Schluter. 2006. Organ- and disease-stage-specific regulation of Toxoplasma gondii-specific CD8-T-cell responses by CD4 T cells. Infect. Immun. 74:5790-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin, V., A. Supanitsky, P. C. Echeverria, S. Litwin, T. Tanos, A. R. De Roodt, E. A. Guarnera, and S. O. Angel. 2004. Recombinant GRA4 or ROP2 protein combined with alum or the gra4 gene provides partial protection in chronic murine models of toxoplasmosis. Clin. Diagn. Lab. Immunol. 11:704-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLeod, R., P. Eisenhauer, D. Mack, C. Brown, G. Filice, and G. Spitalny. 1989. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J. Immunol. 142:3247-3255. [PubMed] [Google Scholar]
- 42.McLeod, R., J. K. Frenkel, R. G. Estes, D. G. Mack, P. B. Eisenhauer, and G. Gibori. 1988. Subcutaneous and intestinal vaccination with tachyzoites of Toxoplasma gondii and acquisition of immunity to peroral and congenital toxoplasma challenge. J. Immunol. 140:1632-1637. [PubMed] [Google Scholar]
- 43.Mineo, J. R., R. McLeod, D. Mack, J. Smith, I. A. Khan, K. H. Ely, and L. H. Kasper. 1993. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J. Immunol. 150:3951-3964. [PubMed] [Google Scholar]
- 44.Parmley, S., T. Slifer, and F. Araujo. 2002. Protective effects of immunization with a recombinant cyst antigen in mouse models of infection with Toxoplasma gondii tissue cysts. J. Infect. Dis. 185(Suppl. 1):S90-S95. [DOI] [PubMed] [Google Scholar]
- 45.Pfefferkorn, E. R., and L. C. Pfefferkorn. 1976. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp. Parasitol. 39:365-376. [DOI] [PubMed] [Google Scholar]
- 46.Schaap, D., A. N. Vermeulen, C. W. Roberts, and J. Alexander. 2007. Vaccination against toxoplasmosis: current status and future prospects, p. 721-752. In L. M. Weiss and K. Kim (ed.), Toxoplasma gondii, the model apicomplexan: perspectives and methods. Academic Press, London, United Kingdom.
- 47.Scharton-Kersten, T. M., G. Yap, J. Magram, and A. Sher. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seah, S. K., and G. Hucal. 1975. The use of irradiated vaccine in immunization against experimental murine toxoplasmosis. Can. J. Microbiol. 21:1379-1385. [DOI] [PubMed] [Google Scholar]
- 49.Shaw, M. H., G. J. Freeman, M. F. Scott, B. A. Fox, D. J. Bzik, Y. Belkaid, and G. S. Yap. 2006. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-γ-dependent IL-10 reactivation. J. Immunol. 176:7263-7271. [DOI] [PubMed] [Google Scholar]
- 50.Su, C., D. Evans, R. H. Cole, J. C. Kissinger, J. W. Ajioka, and L. D. Sibley. 2003. Recent expansion of toxoplasma through enhanced oral transmission. Science 299:414-416. [DOI] [PubMed] [Google Scholar]
- 51.Sukhumavasi, W., C. E. Egan, A. L. Warren, G. A. Taylor, B. A. Fox, D. J. Bzik, and E. Y. Denkers. 2008. TLR adaptor MyD88 is essential for pathogen control during oral Toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J. Immunol. 181:3464-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki, Y., K. Joh, O. C. Kwon, Q. Yang, F. K. Conley, and J. S. Remington. 1994. MHC class I gene(s) in the D/L region but not the TNF-α gene determines development of toxoplasmic encephalitis in mice. J. Immunol. 153:4649-4654. [PubMed] [Google Scholar]
- 53.Waldeland, H., and J. K. Frenkel. 1983. Live and killed vaccines against toxoplasmosis in mice. J. Parasitol. 69:60-65. [PubMed] [Google Scholar]
- 54.Waldeland, H., E. R. Pfefferkorn, and J. K. Frenkel. 1983. Temperature-sensitive mutants of Toxoplasma gondii: pathogenicity and persistence in mice. J. Parasitol. 69:171-175. [PubMed] [Google Scholar]
- 55.Weiss, L. M., and K. Kim (ed.). 2007. Toxoplasma gondii, the model apicomplexan: perspectives and methods, p.341-361. [DOI] [PMC free article] [PubMed]
- 56.Wilson, D. C., S. Matthews, and G. S. Yap. 2008. IL-12 signaling drives CD8+ T-cell IFN-γ production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection. J. Immunol. 180:5935-5945. [DOI] [PubMed] [Google Scholar]
- 57.Yap, G. S., Y. Ling, and Y. Zhao. 2007. Autophagic elimination of intracellular parasites: convergent induction by IFN-γ and CD40 ligation? Autophagy 3:163-165. [DOI] [PubMed] [Google Scholar]
- 58.Zhao, Y., D. J. Ferguson, D. C. Wilson, J. C. Howard, L. D. Sibley, and G. S. Yap. 2009. Virulent Toxoplasma gondii evade immunity-related GTPase-mediated parasite vacuole disruption within primed macrophages. J. Immunol. 182:3775-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao, Y., D. Wilson, S. Matthews, and G. S. Yap. 2007. Rapid elimination of Toxoplasma gondii by gamma interferon-primed mouse macrophages is independent of CD40 signaling. Infect. Immun. 75:4799-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]