Abstract
Clostridium perfringens type C isolates cause enterotoxemias and enteritis in humans and livestock. While the major disease signs and lesions of type C disease are usually attributed to beta toxin (CPB), these bacteria typically produce several different lethal toxins. Since understanding of disease pathogenesis and development of improved vaccines is hindered by the lack of small animal models mimicking the lethality caused by type C isolates, in this study we developed two mouse models of C. perfringens type C-induced lethality. When inoculated into BALB/c mice by intragastric gavage, 7 of 14 type C isolates were lethal, whereas when inoculated intraduodenally, these strains were all lethal in these mice. Clinical signs in intragastrically and intraduodenally challenged mice were similar and included respiratory distress, abdominal distension, and neurological alterations. At necropsy, the small, and occasionally the large, intestine was dilated and gas filled in most mice developing a clinical response. Histological changes in the gut were relatively mild, consisting of attenuation of the mucosa with villus blunting. Inactivation of the CPB-encoding gene rendered the highly virulent type C strain CN3685 avirulent in the intragastric model and nearly nonlethal in the intraduodenal model. In contrast, inactivation of the genes encoding alpha toxin and perfringolysin O only slightly decreased the lethality of CN3685. Mice could be protected against lethality by intravenous passive immunization with a CPB antibody prior to intragastric challenge. This study proves that CPB is a major contributor to the systemic effects of type C infections and provides new mouse models for investigating the pathogenesis of type C-induced lethality.
Clostridium perfringens, an anaerobic, spore-forming, gram-positive rod, is a pathogen of humans and domestic or wild animals (9). The virulence of C. perfringens is mostly due to toxin secretion, which varies from strain to strain (9). This variability allows classification of C. perfringens isolates into five types (A to E), depending upon their production of four typing toxins (9, 11, 12). All five types produce alpha toxin (CPA), type B and C isolates produce beta toxin (CPB), type B and D isolates produce epsilon toxin (ETX), and type E isolates produce iota toxin (20).
C. perfringens type C isolates cause highly lethal diseases, mostly in newborn animals of many mammalian species (20). These diseases originate when type C isolates proliferate and produce toxins in the intestine. Although often involving intestinal damage, death in affected animals is thought to result primarily from a toxemia following absorption of toxins from the intestine into the circulation (17, 18). C. perfringens bacteremia is not usually observed in cases of type C infection (17). In humans, C. perfringens type C isolates cause enteritis necroticans (also known as Darmbrand or Pigbel) (13). Enteritis necroticans is a highly lethal and endemic disease throughout much of Southeast Asia, but particularly in Papua New Guinea, where this disease was the leading cause of mortality in children during the 1960s (3, 5). Less frequently, this disease also occurs in diabetic patients elsewhere (8).
Type C isolates produce CPB, which is a 35-kDa protein that forms pores in the membranes of susceptible cells, leading to swelling and lysis (10, 15, 19). CPB is lethal for mice, with a calculated 50% lethal dose of 310 ng per kg when administered intravenously (i.v.) (14). In addition, CPB has been shown to produce acute intestinal necrosis when inoculated into ligated intestinal loops of rabbits, the effects of which were inhibited when the toxin was mixed with a CPB monoclonal antibody (MAb) before inoculation (21). We recently constructed a series of C. perfringens type C toxin null mutants, which demonstrated that CPB, but not perfringolysin O (PFO) or CPA, is necessary and sufficient for the type C isolate CN3685 to cause intestinal damage in a rabbit ileal loop model (16). However, it was notable that none of the rabbits challenged with this potent type C isolate died during the 6-h course of those ileal loop studies (16).
Despite these recent advances, the systemic lethal effects of CPB or C. perfringens type C isolates remain poorly characterized. In part, this is due to the lack of a laboratory animal model that reproduces the lethality of natural C. perfringens type C enterotoxemia (16, 21). Mice have been used to study the lethal effects of i.v. administered C. perfringens type C vegetative culture supernatants or pure CPB (2). The mouse i.v. injection model is useful for studying the systemic lethal effects of CPB and indicates the sensitivity of this species to type C toxins. However, this model differs significantly from natural type C enterotoxemias in human and animals, where toxins are produced in the gastrointestinal tract, act locally, and are then absorbed into the circulation (20). We now present the development and application of infectious intragastric (i.g.) and intraduodenal (i.d.) challenge mouse models to investigate the lethal enterotoxemias induced by C. perfringens type C infections, including a virulence evaluation of C. perfringens type C toxin mutants.
MATERIALS AND METHODS
Animals.
BALB/c mice (20 to 25 g), purchased from Charles River Laboratories (Hollister, CA), were housed in a temperature- and light cycle-controlled room. All procedures involving animals were approved by the University of California, Davis, Committee for Animal Care and Use (permit 04-11593).
Growth of C. perfringens.
Fluid thioglycolate medium (Difco Laboratories) and TGY (3% tryptic soy broth, [Becton Dickinson], 2% glucose [Sigma Aldrich], 1% yeast extract [Becton Dickinson], 0.1% thioglycolate [Sigma Aldrich]) were used for growing broth cultures of C. perfringens type C isolates or their derivatives.
Wild-type bacterial strains.
The 14 wild-type C. perfringens type C isolates used in this study (Table 1) came from our laboratory collections and originated from animal or human infections (2). The type A control strain was purchased from ATCC (Table 1). Toxin genotypes of wild-type strains were previously determined by multiplex PCR (2).
TABLE 1.
Correlation between production of CPB, CPA, and PFO in vitro and lethality in the i.g. and i.d. mouse models using whole cultures of C. perfringens type C
| Straina | Toxin type | Lethality (%) |
In vitro toxin productionb |
|||
|---|---|---|---|---|---|---|
| i.d. | i.g. | CPB (μg/ml) | CPA (U/ml, 103) | PFO (log2 titer) | ||
| CN1797 | C | 92 | 75 | 18.9 | 5.5 | 3.9 |
| CN2109 | C | 100 | 83 | 12.8 | 9.2 | 3.1 |
| CN3685 | C | 100 | 93 | 13 | 6 | 2.9 |
| CN3686 | C | 92 | 0 | 6 | 1.2 | 1.9 |
| CN3717 | C | 83 | 67 | 1 | 0.5 | 2 |
| CN3758 | C | 42 | 0 | 14.8 | 5.2 | <1 |
| CN3955 | C | 100 | 50 | 30.9 | 9.3 | 3.6 |
| CN5383 | C | 100 | 0 | 23.5 | 3.6 | 1.6 |
| CN5388 | C | 58 | 0 | 3 | 0.9 | 1.8 |
| CN685 | C | 100 | 0 | 8.9 | 1.1 | 2.2 |
| CN885 | C | 100 | 58 | 23.6 | 6.3 | 2.6 |
| CN886 | C | 100 | 92 | 24.1 | 5.1 | 4.2 |
| JGS1071 | C | 58 | 0 | 2.5 | 0.9 | 3.1 |
| NCTC 10719 | C | 75 | 0 | 2.7 | 0.7 | <1 |
| ATCC 3624 | A | 0 | 0 | 0.8 | 1.7 | |
| Sterile TGY | 0 | 0 | ||||
Each mouse received approximately 3 × 108 CFU.
Determined for a previous study (2).
Quantification of toxin levels in vegetative culture supernatants of wild-type isolates.
In vitro toxin production levels among the wild-type isolates listed in Table 1 had been determined previously using cultures grown for 9 h in TGY (2). CPB protein levels and PFO or CPA activity levels in supernatants of those cultures were determined as described previously (2, 16).
Mutant bacterial strains.
All mutants used in these experiments were derived from C. perfringens type C isolate CN3685, which was isolated in 1954 from peritoneal fluid of a sheep with struck. CN3685 was chosen for mutant construction because it is transformable and is a relatively strong producer of CPA, PFO, and CPB (Table 1).
Isogenic CN3685 toxin null mutants BMC100, BMC101, BMC102, BMC103, BMC104, and BMC105 (Table 2) had been constructed and characterized previously (16).
TABLE 2.
Comparison of lethality in the i.d. and i.g. challenge mouse models inoculated with whole cultures of CN3685 and isogenic toxin mutants
| Isolatea | Description | Lethality (%)b |
|
|---|---|---|---|
| i.d. | i.g. | ||
| CN3685 | Wild type (cpb+cpa pfoA+tpeL+) | 100 | 93 |
| BMC100 | CN3685 Δcpbantisense | 25* | 0* |
| BMC101 | CN3685 Δcpa | 91 | 66 |
| BMC102 | CN3685 ΔpfoA | 83 | 66 |
| BMC103 | CN3685 Δcpa ΔpfoA | 76 | 50* |
| BMC104 | CN3685 Δcpbsense | 25* | 0* |
| BMC105 | CN3685 cpbsense(pJIR750cpbis) (complemented strain) | 50* | 0* |
| BMC107 | CN3685 Δplc ΔpfoA Δcpb | 8* | 0* |
Each mouse received approximately 3 × 108 CFU.
*, P < 0.05 relative to wild-type challenge, using the Fisher exact test.
Complementation of the CN3685 cpbsense null mutant.
Despite repeated attempts, it has not been possible to clone the cpb gene into a shuttle plasmid for complementation. Therefore, as described previously (16), CPB complementation of the cpbsense null mutant BMC104 has been achieved at the mRNA level by reintroducing the pJIR750cpbi-s plasmid, which (at 30°C) encodes the LtrA protein that can splice out the intron insertion from the disrupted cpb mRNA (16). This effect restores ∼30% of natural CPB production levels of the BMC105 complementing strain (16).
Construction of BMC107.
In the current study we constructed a new isogenic CN3685 triple toxin null mutant (CN3685 Δcpb Δcpa ΔpfoA) using the BMC103 cpa pfoA double mutant (16). The cpb gene in BMC103 was inactivated by inserting, in the sense orientation, a group II intron (∼900 bp) between nucleotides 182 and 183 of the cpb gene. The group II intron was delivered into BMC103 by electroporation of pJIR750cpbi-s (16), creating BMC107. Intron disruption of the cpb gene in BMC107 was confirmed by PCR and DNA sequencing. In addition, Southern blots (16) confirmed the presence of intron disruptions in the pfoA, cpa, and cpb genes. Using our standard techniques (16), Western immunoblotting was used to assessed CPB production by BMC107, while growth in the egg yolk agar plate or blood agar plate assay, respectively, was used to evaluate CPA and PFO production.
Purification of CPB protein.
CPB was purified from a culture of strain CN3685 as described before (21). The CPB concentration was estimated by the assay of Lowry et al., using bovine serum albumin as the standard (6). The final CPB purity, as assessed by densitometry of a 12% sodium dodecyl sulfate-polyacrylamide gel, was ∼95%.
Production of whole cultures, filtered culture supernatants, and washed cells for mouse inoculation.
Wild-type and mutant strains were grown overnight at 37°C in fluid thioglycolate medium. The BMC105 complementing strain was grown in the presence of 15 μg/ml of chloramphenicol to retain the LtrA-encoding plasmid (16). A 1-ml aliquot of each culture was then transferred into 9 ml of TGY and grown at 37°C for 8 h before transfer to 30°C and growth overnight. The cultures were adjusted to a cell concentration of approximately 108 CFU/ml and then used as a whole-culture inoculum for mice. In addition, the liquid phase of a strain CN2109 culture was centrifuged at 10,000 rpm for 25 min at 4°C and filtered through a 0.22-μm filter. The filtrate was used as filtered culture supernatant inoculum for mice. The centrifuged culture was resuspended in 100 ml of phosphate-buffered saline (pH 7.2) and centrifuged again as described above. The sediment was finally resuspended in phosphate-buffered saline, the concentration adjusted to approximately 108 CFU/ml, and this suspension was then used as washed-cell inoculum for mouse inoculation.
i.d. inoculation of purified CPB or C. perfringens type C whole cultures, washed cells, or filtered culture supernatants.
To determine if type C toxins (such as CPB) cause lethality when introduced into the mouse intestine and to evaluate whether this i.d. model reflects true infection or only intoxication, pilot studies where different doses of purified CPB (0.75 μg, 1.5 μg, 3 μg, 6 μg, 12 μg, or 25 μg) were inoculated into the duodenums of anesthetized mice (groups of four mice per experiment) were conducted. To determine further if the presence of C. perfringens type C or its products in the duodenum can produce disease in mice and if this disease is a true infection or an intoxication, C. perfringens type C (strain CN2109) whole cultures, washed cells, or filter-sterilized culture supernatants were inoculated directly into the mouse duodenum. Whole cultures of all of the other wild-type strains and the mutants were also inoculated i.d. into groups of four mice. Except where specified, all inocula were mixed with trypsin inhibitor (TI) (Sigma, St. Louis, MO; 1 μg/ml) before inoculation.
Mice were fasted for 24 h before inoculation but allowed access to water until 1 h before the start of the experiments. Their abdomens were disinfected with iodine solution (Betadine; Purdue Pharma LP, CT) immediately before surgery. The animals were anesthetized by intraperitoneal administration of Avertine (Winthrop Laboratories, NY). A midline laparotomy was performed, and 1 ml of either a C. perfringens type C culture, washed cells, filtered culture supernatant, or purified CPB was injected into the mouse duodenum immediately distal to the stomach. Controls included in this assay consisted of (i) replacement of the bacterial or toxin inoculum by sterile, nontoxic TGY; (ii) inoculation of the type A strain ATCC 3624 in place of the type C isolates; (iii) inoculation of purified CPB without TI; and (iv) inoculation of TI alone. The injections were performed by insertion of a 0.5-in., 27-gauge needle oblique to the intestinal lumen in a direction away from the stomach. After inoculation, the incision in the peritoneum, abdominal muscles, and skin was sutured in one plane using 3-0 Vicryl (Ethicon Inc., Somerville, NJ) or Super Glue (Henkel Corporation, Avon, OH). The surgical procedure lasted approximately 3 min per animal, and all mice were awake within 30 min after surgery. The animals were then monitored until the assay end point (see below).
i.g. inoculation of purified CPB or C. perfringens type C whole cultures, washed cells, or culture filtered supernatants.
Mice were fasted overnight but allowed access to water until 1 h before the start of the experiment. Groups of four mice were inoculated by i.g. gavage with 0.5 ml containing purified CPB (50, 100, or 200 μg) or whole cultures, washed cells, or filtered culture supernatants of strain CN2109. Similar experiments were carried out with the other wild-type strains and the mutants. Except when specified (see below for controls), all inocula were mixed with TI (Sigma; 1 μg/ml) before inoculation. Controls included in this assay consisted of (i) replacement of the bacterial or toxin inoculum by sterile, nontoxic TGY; (ii) inoculation of the type A strain ATCC 3624 in place of the type C isolates; and (iii) inoculation of TI alone. The mice were periodically observed until the assay end point (see below).
Determination of bacterial dose dependency in the i.g. challenge.
To determine if effects observed in i.g. challenged mice were bacterial dose dependent, groups of four mice were inoculated by gastric gavage with 2 × 103, 2 × 104, 2 × 105, 2 × 106, 2 × 107, or 2 × 108 CFU of strain CN2109 diluted in fresh TGY and mixed with TI as before. The mice were periodically observed until the assay end point (see below).
Determination of effects of pH and pepsin on CPB activity.
To understand why even high doses of purified CPB delivered via gastric gavage did not produce lethality, two hypotheses were tested: (i) CPB may be inactivated by the low pH of the stomach, or (ii) CPB may be inactivated by pepsin, a potent protease present in the stomach. For these experiments, groups of four mice received an i.v. injection containing 0.5 ml of a 10-μg/ml solution of purified CPB in peptone water at pH 2 or pH 7 that had been preincubated with pepsin (300 μg/ml) at 37°C for 30 min. Additional groups of four mice received similar inocula except that no pepsin was present during the preincubation.
Progression of lesions.
To study the progression of gross and histological lesions due to C. perfringens type C infection, whole cultures of CN2109 were used in an i.g. challenge of mice. These animals were monitored periodically, and two mice were euthanized every 2 h for the first 14 h and then at 24 h or 28 h after inoculation.
Antibody protection experiments.
Two groups of two mice each received a 0.1-ml i.v. injection containing anti-CPB or anti-CPA MAb (2 mg of immunoglobulin G per ml; provided by Paul Hauer, NVSL, USDA) plus 0.4 ml of normal saline solution, 1 hour before they received a 0.5-ml i.g. inoculation of a whole culture containing 3 × 108 CFU of strain CN2109. Other groups (two mice each) received an i.d. inoculation containing a mixture of 0.1 ml of the same anti-CPB or anti-CPA MAb plus 0.9 ml of a 25-μg/ml solution of CPB after that mixture had been preincubated for 30 min at room temperature. The mice were periodically observed until the assay end point (see below).
Assay end point and interpretation of results.
Assay end points for the i.d. or i.g. challenge experiments included spontaneous death, development of severe neurological or respiratory signs necessitating euthanasia, or 48 h of survival without clinical alterations. Since our experience indicates that mice developing severe neurological or respiratory signs after inoculation with C. perfringens type C never recover, animals euthanized after developing such signs were included in lethality calculations. Clinical signs necessitating euthanasia included severe respiratory distress, depression, incoordination, ataxia, and circling. Euthanasia was performed by inhalation of carbon dioxide.
Postmortem examinations.
Postmortem examinations were performed immediately after death on representative mice from each experiment. The gastrointestinal tract, brain, kidneys, lungs, heart, spleen, and liver were collected and fixed by immersion in 10% formalin at pH 7.2 for a minimum of 24 h before being processed routinely to obtain 4-μm-thick sections, which were stained with hematoxylin and eosin.
Statistical analysis.
Each experiment was repeated three times, usually using a total of 12 mice per experimental point. Data on mouse lethality and in vitro production of CPA, CPB, and PFO were analyzed by regression analysis to evaluate any correlation between lethality and toxin production in vitro, using Excel (Microsoft). Data on lethality of mice with and without TI were analyzed by Fisher's exact test using the Minitab program.
RESULTS
i.d. inoculations.
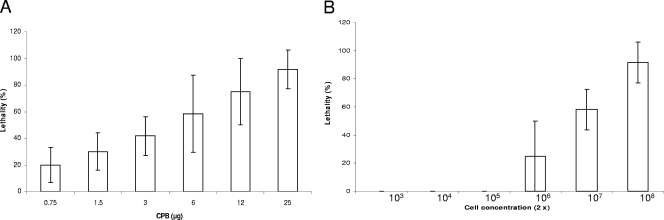
As a first step in mouse model development, we assessed whether the presence of type C toxins in the mouse gastrointestinal tract induces clinical signs. When purified CPB was administered into the mouse duodenum, a clear CPB dose-dependent lethality was observed (Fig. 1A). An i.d. dose containing 25 μg of CPB inoculated together with TI was sufficient to induce severe respiratory and/or neurologic signs or death in nearly all mice (Fig. 1A). Severe clinical signs and lethality occurred within 6 to 12 h of inoculation when mice received a high CPB dose via i.d. inoculation. When similarly inoculated i.d., whole cultures or washed cells of type C isolate CN2109 also produced 100% lethality, although filtered culture supernatants of the same cultures did not cause lethality (Table 3).
FIG. 1.
(A) Percent lethality in groups (four mice per group) of mice after i.d. inoculation with different doses of purified Clostridium perfringens CPB with TI. (B) Percent lethality in groups (four mice per group) of mice after i.g. inoculation with different concentrations of Clostridium perfringens type C (strain CN2109 in Table 1) plus TI. For each panel every experiment was performed independently three times; data shown are the means and standard errors.
TABLE 3.
Correlation between lethalities of C. perfringens type C (strain CN2109) in the i.g. and i.d. mouse models
| Inoculum | Lethality (%) |
|
|---|---|---|
| i.d. | i.g. | |
| Whole culturesa | 100 | 75 |
| Filtered culture supernatant | 0 | 0 |
| Washed cellsa | 100 | 50 |
Each mouse received approximately 3 × 108 CFU.
When mice received an i.d. challenge consisting of a whole culture from one of several different wild-type type C isolates, lethality varied considerably (Table 1). Clinical signs, in some cases followed by spontaneous death, developed at between 8 and 24 h postchallenge. Those signs consisted of a swollen abdomen, depression, inapetence, tachypnea with superficial abdominal breathing, and neurological signs, including circling and rolling. No clinical disease or lethality was observed in control mice receiving a similar inoculation of sterile TGY, the type A strain ATCC 3624, or TI alone.
i.g. inoculation.
Whole cultures of the type C isolates were also individually inoculated into the stomachs of mice by i.g. gavage (Table 1). In these animals, clinical alterations (similar to those described above for i.d. inoculation) usually developed at between 12 and 48 h postinoculation. Most animals showing clinical alterations were euthanized when they developed severe clinical signs, but death occurred spontaneously in a few animals before they could be euthanized. No clinical disease or mortality was observed in mice inoculated i.g. with any surveyed dose of purified CPB (up to 200 μg), with sterile TGY, with the type A strain ATCC 3624, or with TI alone. Whole cultures, washed cells and filtered culture supernatants of CN2109 inoculated i.g. into mice produced 75%, 50%, and 0% lethality, respectively (Table 3). When different concentrations of strain CN2109 (Table 1) were administered i.g. to mice (Fig. 1B), a clear dose-dependent lethality was observed.
The presence of TI is important for the mouse lethality caused by CPB or C. perfringens type C isolates.
Mice receiving an i.d. inoculation of CPB (25 μg) without TI exhibited only 17% mortality (Fig. 1A). The same CPB concentration inoculated with TI via the same route produced 92% lethality (P = 0.01). When whole cultures of strain CN2109 were inoculated i.d. or i.g. without TI, lethality was 50% and 33%, respectively, contrasting with the 100% (P = 0.01) or 83% (P = 0.04) lethality observed when the same isolate was inoculated i.d. or i.g. with TI.
Gross and microscopic pathology.
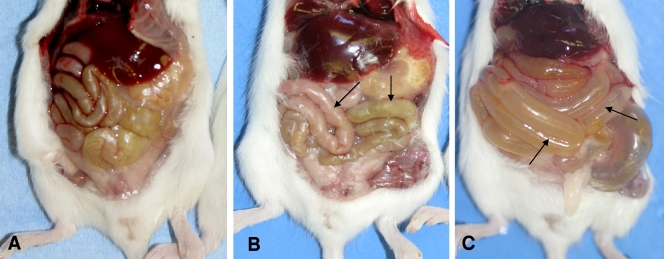
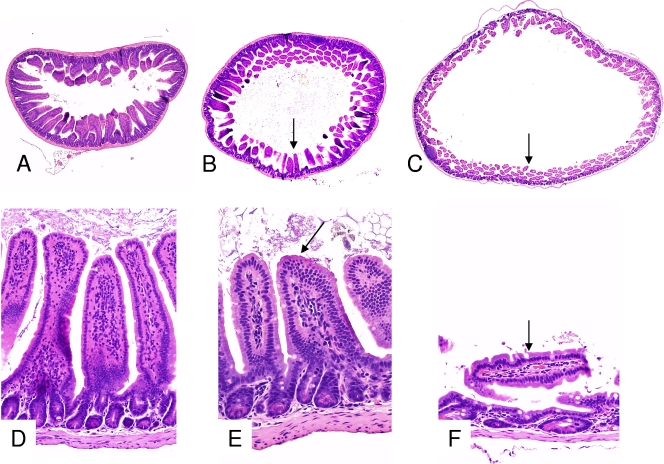
All mice that received an i.g. or i.d. inoculation of type C cultures and developed disease presented gross changes consisting of a distended abdomen with large amounts of gas, predominantly in the small bowel (Fig. 2), although 25% of the animals also showed gas distention of the cecum and colon. The distended bowel had very thin and almost transparent walls and was almost empty, except for a small amount of semifluid, dark red content. No other significant gross abnormalities were observed in these mice or in mice inoculated i.g. or i.d. with any CPB dose. Histologically, in all mice inoculated with type C isolates, the mucosa was severely attenuated, with villus blunting and mild degenerative changes on individual cells of the superficial epithelium (Fig. 3).
FIG. 2.
Abdominal cavities of mice euthanized at 12 h (B) and 24 h (C) after i.g. gavage with whole cultures of Clostridium perfringens type C (strain CN2019) plus TI. Note the moderate (B) and severe (C) dilation of the small intestine (arrows) in the mice inoculated with this microorganism. A normal control mouse inoculated with sterile, nontoxic TGY is shown for comparison (A).
FIG. 3.
Histological sections of the small intestines of mice that received an i.g. inoculation of TGY (A and D) or whole cultures of Clostridium perfringens type C (strain CN2019) plus TI and then were euthanized at 12 h (B and E) or 24 h (C and F) after inoculation. The sections from the control mouse (A and D) show normal long villi, while the sections from the mice inoculated with strain CN2019 and euthanized at 12 and 24 h after inoculation show progressive atrophy of the villi and dilation of the intestinal lumen (arrows). Note that in the section of the mouse euthanized 24 h after inoculation (C and F), the short villi are flattened. Hematoxylin-eosin staining was used. Magnifications, ×20 (A, B, and C) and ×250 (D, E, and F).
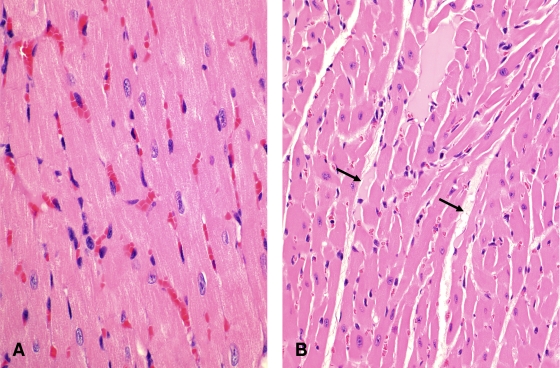
In mice dying >12 h after inoculation, interstitial edema of the myocardium was observed in 50% or 60% of mice receiving an i.g. or i.d., respectively, inoculation of a type C culture and in 90% of mice receiving an i.d. CPB inoculation (Fig. 4). No other significant histological abnormalities were observed in any mice in this study.
FIG. 4.
Histological section of the heart of a mouse inoculated i.g. with whole cultures of Clostridium perfringens type C (strain CN2109) plus TI (A), showing severe interstitial proteinaceous edema (arrows). A section of the normal heart of a mouse inoculated via the same route with sterile, nontoxic TGY is shown for comparison (B). Hematoxylin-eosin staining was used. Magnification, ×400.
Effect of pH and pepsin on CPB activity.
We next assessed whether low gastric pH or pepsin could explain why high CPB doses delivered via gastric gavage did not induce lethality. Those experiments showed that all mice receiving an i.v. injection of CPB, whether preincubated at pH 2 or pH 7, died within 24 h of inoculation; i.e., CPB activity is not sensitive to low pH. However, all mice receiving an i.v. injection of CPB preincubated with pepsin survived the 48-h study period and did not show any clinical abnormalities, demonstrating that pepsin can inactivate CPB. This protection occurred regardless of whether the CPB had been preincubated with pepsin in pH 2 or pH 7 buffer.
Progression of clinical alterations and lesions.
Mice receiving an i.g. inoculation of strain CN2109 were euthanized periodically over a 24-h period (no mice in this experiment survived >28 h). Animals started showing distended abdomens and depression at 12 h after inoculation. Respiratory and neurological signs, when observed, were present at between 18 and 28 h postinoculation. No significant gross abnormalities were observed at <12 h after inoculation. At this time, the small, and occasionally the large, intestine was moderately distended with gas, and the distention became progressively more severe until the end of the experiment (Fig. 2). Histologically, at 12 h there was moderate distention of the small intestine with villus blunting and flattening of the villi against the mucosa (Fig. 3B and E). These histological changes became progressively more severe until the experiment's end (Fig. 3C and F). Mice euthanized >12 h after inoculation showed diffuse interstitial edema of the myocardium (Fig. 4). No other significant gross or histological abnormalities were observed in mice during this experiment.
Application of mouse models to study type C pathogenesis.
Several experiments were performed to apply the new mouse models to explore type C pathogenicity.
(i) Antibody protection experiments.
Mice exhibited no lethality when challenged i.d. with purified CPB (22.5 μg) that had been preincubated with anti-CPB MAb. This neutralization was specific, since nearly 100% lethality occurred after i.d. administration of the same amount of purified CPB that had been preincubated either with anti-CPA MAb or without any MAb.
When mice received an i.v. injection of anti-CPB MAb prior to receiving an i.g. inoculation of a lethal dose of strain CN2109 (seroprotection), no mortality was observed. This seroprotection was specific, since mice receiving an anti-CPA MAb injection or an injection of Ringer's solution without any MAb showed nearly 100% lethality when similarly i.g. challenged with this type C isolate.
(ii) Comparison of in vivo lethality and in vitro toxin production levels of type C isolates.
When the lethalities of 14 type C isolates were compared (Table 1) with their in vitro toxin production levels, as determined previously (2), no strong correlation was observed between the amounts of CPB, PFO, or CPA produced by a type C isolate grown in vitro (2) and the lethality of that isolate in the new i.g. and i.d. mouse challenge models.
(iii) Evaluation of the lethality of C. perfringens toxin mutants.
As shown in Table 2, previously constructed single and double toxin null mutants (16), and a newly constructed triple toxin null mutant, of the type C strain CN3685 were tested for lethality in the i.d. and i.g. challenge models (Table 2). Inactivation of the cpb gene in two independently derived mutant strains (BMC 100 and BMC 104) completely eliminated their lethality in the i.g. challenge model and strongly attenuated their lethality in the i.d. challenge model. Complementation to partially restore CPB production (16) increased lethality in the i.d. model but not in the i.g. model.
These results suggested that CPB is the major toxin required for the lethality of type C isolates in both mouse models. However, like most type C isolates, CN3685 also produces PFO and CPA. Therefore, we assessed the effects on CN3685 lethality of inactivating the genes encoding those two toxins (Table 2), which revealed that single pfoA or cpa mutants (BMC 102 and BMC 101, respectively) both retained substantial lethal properties in the i.d. and i.g. challenge mouse models. A isogenic double mutant of CN3685 where both the pfoA and cpa genes are inactivated (BMC 103) showed, relative to the single pfoA and cpa mutants, a slight (but not statistically significant) further reduction in lethality in both type C lethality models (Table 2).
To assess whether other toxins besides CPB, CPA, and PFO might contribute to CN3685 lethality in the type C enterotoxemia models, a triple toxin null mutant (BMC107) that cannot produce CPB, PFO, or CPA was constructed. Southern blotting and sequencing confirmed that these introns were present in the pfoA, cpa, and cpb genes (not shown). Growth of BMC107 on egg yolk agar or blood agar (not shown) confirmed that the triple mutant is unable to produce CPA or PFO, respectively. Western blotting demonstrated that BMC107 does not produce CPB. When tested in the i.g. and i.d. challenge models (Table 2), BMC107 was nonlethal or had greatly reduced lethality, respectively, indicating that CPB, CPA, and PFO are responsible for most, if not all, of the lethal properties exhibited by CN3685.
DISCUSSION
Although C. perfringens type C infections are of veterinary and human significance, there has been no economical small animal infectious challenge model to study the lethality of such infections. In most natural hosts, type C disease originates in the intestine but often later involves systemic disease due to absorption of toxins into the general circulation (enterotoxemia) (18, 20). We recently used a rabbit intestinal loop model to study the local intestinal effects of CPB and type C isolates (21), but that model did not provide information regarding the lethality of type C disease.
We now show that mice can be utilized to study the systemic changes and lethality produced by C. perfringens type C isolates. In our mouse i.g. challenge model, lethality was related to the dose of the administered isolate, with maximum lethality developing in animals receiving the highest bacterial dose. Since no disease or lethality was observed in mice inoculated with similar amounts of C. perfringens type A, lethality in our mouse i.g. model was due to C. perfringens type C isolates and not simply the presence of large numbers of C. perfringens organisms in the gut. Our models challenged mice with type C vegetative cells for two reasons. First, despite an extensive search of the literature, no evidence indicating spore involvement in the pathogenesis of type C disease was found. Second, as spores do not produce toxins, disease must result from the presence of type C vegetative cells in the intestines.
The mouse models used here reproduced many, but not all, clinical signs reported during natural type C disease in livestock. Clinical alterations observed in both livestock and our mouse models include depression, swollen abdomen, and respiratory distress or neurological signs culminating in lethality. However, in livestock, C. perfringens type C strains also cause necrotizing enteritis, which is clinically characterized by severe bloody diarrhea and abdominal pain and distension (11, 17, 20); diarrhea was not observed in our mouse models. Also, while the mouse models reproduced severely distended small intestine, villus blunting and occasional degenerative changes in individual intestinal epithelial cells, they did not show the significant gross or histological abnormalities of the severe necrotizing enteritis characterizing type C disease in livestock (18, 20). The absence of severe gross and histological small intestinal lesions in mice might reflect a lack or lower abundance of CPB receptors in mouse enterocytes.
Death without severe histological lesions in the gut also occurs with other C. perfringens natural and experimental enterotoxemias, including type D disease in sheep (20) and mice (1). In both cases, lethality is considered a result of ETX absorbed from the intestines and then affecting extraintestinal tissues. The results of the present study, coupled with the severe histologic damage without death observed in rabbit ileal loops inoculated with CPB or type C isolates (16, 21), support type C lethality as also resulting primarily from enterotoxemia rather than from necrotizing enteritis.
While our mouse models do not reproduce all features of type C disease in natural hosts, they (unlike rabbit ileal loops) importantly mimic the systemic clinical alterations and lethality; therefore, these new models are valuable tools to study the pathogenesis of potentially lethal type C systemic disease and for vaccine efficacy trials.
Neurological alterations were observed in some mice receiving an i.g. or i.d. challenge with type C isolates or purified CPB, but no histological lesions were observed in their brains. These results are consistent with the absence of brain histological lesions in farm animals with type C enterotoxemia (18, 20). ETX can also affect the central nervous system without causing alterations visible by light microscopy (20), as is true for most forms of caprine type D enterotoxemia and a few cases of ovine type D disease. This may also be true for CPB-mediated disease (18, 20).
The large amounts of gas present in the intestines of our mice inoculated with type C isolates suggest that (once inoculated) the type C isolate multiplied actively within the small intestine. The observation of neurological signs in the type C-infected mice suggests that toxins were absorbed from the small intestine into the general circulation. This conclusion is further supported by the relatively large amount of time between oral challenge with these isolates and the onset of clinical signs (12 to 24 h), compared with the relatively short period elapsed between i.d. inoculation of CPB and development of clinical signs/death (6 to 12 h). The inability of sterile CN2109 culture supernatants to cause lethality in either the i.g. or i.d. mouse model, in contrast to the lethality induced by washed cells of this strain in both mouse models, further argues that in vivo growth is an important aspect of these models and that the mouse models are reproducing, at least in part, true type C infection rather than simple intoxications. These findings are similar to those from recent studies carried out on Clostridium difficile (7) and Clostridium septicum (4) infections. These three studies together emphasize the importance of using an in vivo infection model to study the role of toxins in clostridial disease processes involving true infections rather than intoxications.
CPB is easily inactivated by trypsin, and endogenous intestinal trypsin is an important innate defense mechanism against type C infection (9). Malnutrition or trypsin deficiency conditions, along with diets rich in TI, predispose people and animals to type C infections. For instance, among livestock, type C infections are seen mostly in newborn animals (18, 20), when the level of trypsin in the intestine is very low. This explains why attempts to reproduce type C disease in animal models by injecting CPB alone (without TI) have consistently failed (17) or have produced only minor lethality (this study). Our results using the new mouse models, and previous rabbit ileal loop models (16, 21), have demonstrated that addition of TI allows CPB to remain active.
While lethal quantities of purified CPB were absorbed from the gastrointestinal tract into the systemic circulation of mice receiving i.d. inoculations of this toxin plus TI, no clinical alterations were observed in the equivalent i.g. gavage experiment, even using 200 μg of CPB. This result strongly suggests that CPB can be inactivated in the stomach despite the presence of TI. An extensive literature search found no information on CPB sensitivity to low pH, but our experiments ruled out this possibility by showing that CPB was still active after a 30 min of incubation at pH 2. However, we found that CPB was easily inactivated by pepsin, a stomach protease. Therefore, pepsin-induced inactivation likely explains the lack of effect of purified CPB in the i.g. model and the lower sensitivity of the i.g. challenge model. These observations implicate pepsin as a potential innate defense mechanism against any CPB preformed in foods.
Type C isolates typically produce at least three potent toxins, CPA, PFO, and CPB (1, 2). While CPB has long been implicated in type C disease, its contribution to intestinal disease has only recently been demonstrated (16, 21); specifically, studies using isogenic toxin null mutants showed that CPB is necessary and sufficient to reproduce the intestinal pathology of the type C isolate CN3685 in rabbit ileal loops, while CPA or PFO did not produce significant effects in the loop model (16). However, those studies did not evaluate the contribution of CPB or other C. perfringens type C toxins to systemic alterations and/or lethality produced by this microorganism, because such effects were not observed in the ileal loop model. The current study exploited the availability of our new mouse lethality models to demonstrate, by two independent approaches, that CPB is also of major importance for the systemic lethality produced by C. perfringens type C. First, an i.v. injection of a CPB MAb was shown to protect mice against lethal challenge with the type C isolate CN2109 in the i.g. model. Second, using previously constructed mutants (16), inactivation of the cpb gene in the type C isolate CN3685 was shown to eliminate or significantly reduce lethality in both the i.d. and i.g. mouse challenge models. This lethality attenuation was partially reversed by complementation to restore (about 30% [see Materials and Methods]) CPB production in the cpb mutant. In addition to this lower CPB production, the inability of the complementing strain to cause full wild-type strain lethality likely further reflects the cessation of LtrA-mediated restoration of CPB production when the complementing strain encounters the warmer temperature of the mouse (16). This effect probably explains why the complementing strain was not lethal via i.g. challenge, where the stomach pepsin likely inactivated all CPB formed during in vitro culture at 30°C, i.e.; the complementing strain cannot produce CPB in vivo, so the i.g. administered complementing strain inoculum no longer contains active CPB and is avirulent by the time it reaches the pepsin-free intestines.
Our previous studies had shown that CPB production in vitro correlates with mouse lethality following i.v. injection of a sterile type C culture supernatant (2). However, when 14 isolates of C. perfringens type C were tested in our i.g. or i.d. challenge mouse model, no strong correlation was noted between their lethality and their previously determined (2) in vitro CPB, PFO, or CPA production levels. Similar conclusions were drawn previously using an oral mouse model for C. perfringens type D infection, in which there was no strong correlation between ETX production in vitro and lethality (1).
Several possibilities could explain the lack of correlation between type C isolate lethality and in vitro toxin production. First, type C toxin expression levels (particularly for CPB) might be regulated differently in the gastrointestinal tract than during in vitro growth. This possibility is supported by (i) our recent results (22) demonstrating that toxin expression differs when type C isolates are grown in vitro versus in the presence of cultured enterocyte-like cells and (ii) our current results indicating that in vivo toxin production during type C isolate infection is important to obtain lethality in the mouse challenge models. A second possibility is that in vivo growth rate differences might contribute to variations in type C in vivo virulence, with some type C isolates growing faster than others in vivo and thus producing higher intestinal toxin levels more quickly. A final possibility is that other C. perfringens type C virulence factors besides toxins may play an important role in lethality.
There is currently little or no information available regarding C. perfringens type C colonization, growth, or toxin production in vivo, in large part due to the absence of economical animal models. The new mouse i.d. and i.g. challenge models and our rabbit ileal loop model (16) should now allow these important pathogenesis questions to be addressed. Future studies will also assess whether this model (or variations thereof) can be used to study the virulence of other C. perfringens toxin types.
Acknowledgments
National Institute of Allergy and Infectious Diseases grant AI056177-06 supported this research. Research at Monash University was also supported by a grant to the ARC Centre of Excellence by the Australian Research Council.
We thank Richard D. Day of the University of Pittsburgh Biostatistics Consulting Service and P. Hauer for supplying monoclonal antibodies against CPB and CPA.
Editor: S. M. Payne
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Fernandez-Miyakawa, M. E., S. Sayeed, D. J. Fisher, R. Poon, V. Adams, J. I. Rood, B. A. McClane, J. Saputo, and F. A. Uzal. 2007. Development and application of a mouse oral challenge model for studying Clostridium perfringens type D infection. Infect. Immun. 75:4282-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher, D. J., M. E. Fernandez-Miyakawa, S. Sayeed, R. Poon, V. Adams, J. I. Rood, F. A. Uzal, and B. A. McClane. 2006. Dissecting the contributions of Clostridium perfringens type C toxins to lethality in the mouse intravenous injection model. Infect. Immun. 74:5200-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson, S., and D. N. Gerding. 1997. Enterotoxemic infections, p. 117-140. In J. I. Rood, B. A. McClane, J. G. Songer, and R. W. Titball (ed.), The clostridia. Molecular biology and pathogenesis. Academic Press, London, United Kingdom.
- 4.Kennedy, C. L., D. Lyras, L. M. Cordner, J. Melton-Witt, J. J. Emmins, R. K. Tweten, and J. I. Rood. 2009. Pore-forming activity of alpha-toxin is essential for Clostridium septicum-mediated myonecrosis. Infect. Immun. 77:943-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence, G., and R. Cooke. 1980. Experimental pigbel: the production and pathology of necrotizing enteritis due to Clostridium welchii type C in the guinea-pig. Br. J. Exp. Pathol. 61:261-271. [PMC free article] [PubMed] [Google Scholar]
- 6.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 7.Lyras, D., J. R. O'Connor, P. M. Howarth, S. P. Sambol, G. P. Carter, T. Phumoonna, R. Poon, V. Adams, G. Vedantam, S. Johnson, D. N. Gerding, and J. I. Rood. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda, T., Y. Okada, E. Inagi, Y. Tanabe, Y. Shimizu, K. Nagashima, J. Sakurai, M. Nagahama, and S. Tanaka. 2007. Enteritis necroticans ‘pigbel’ in a Japanese diabetic adult. Pathol. Int. 57:622-626. [DOI] [PubMed] [Google Scholar]
- 9.McClane, B. A., F. A. Uzal, M. Fernandez-Miyakawa, D. Lyerly, and T. D. Wilkins. 2004. The enterotoxigenic clostridia, p. 698-752. In S. F. M. Dworkin, E. Rosenburg, K. F. Schleifer, and E. Stackebrandt. (ed.), The prokaryotes, vol. 4. Springer-Verlag, New York, NY. [Google Scholar]
- 10.Nagahama, M., S. Hayashi, S. Morimitsu, and J. Sakurai. 2003. Biological activities and pore formation of Clostridium perfringens beta toxin in HL 60 cells. J. Biol. Chem. 278:36934-36941. [DOI] [PubMed] [Google Scholar]
- 11.Niilo, L. 1988. Clostridium perfringens type C enterotoxemia. Can. Vet. J. 29:658-664. [PMC free article] [PubMed] [Google Scholar]
- 12.Petit, L., M. Gibert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7:104-110. [DOI] [PubMed] [Google Scholar]
- 13.Petrillo, T. M., C. M. Beck-Sague, J. G. Songer, C. Abramowsky, J. D. Fortenberry, L. Meacham, A. G. Dean, H. Lee, D. M. Bueschel, and S. R. Nesheim. 2000. Enteritis necroticans (pigbel) in a diabetic child. N. Engl. J. Med. 342:1250-1253. [DOI] [PubMed] [Google Scholar]
- 14.Sakurai, J. 1995. Toxins of Clostridium perfringens. Rev. Med. Microbiol. 6:175-185. [Google Scholar]
- 15.Sakurai, J., and C. L. Duncan. 1978. Some properties of beta-toxin produced by Clostridium perfringens type C. Infect. Immun. 21:678-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayeed, S., F. A. Uzal, D. J. Fisher, J. Saputo, J. E. Vidal, Y. Chen, P. Gupta, J. I. Rood, and B. A. McClane. 2008. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 67:15-30. [DOI] [PubMed] [Google Scholar]
- 17.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Songer, J. G., and F. A. Uzal. 2005. Clostridial enteric infections in pigs. J. Vet. Diagn. Investig. 17:528-536. [DOI] [PubMed] [Google Scholar]
- 19.Steinthorsdottir, V., H. Halldorsson, and O. S. Andresson. 2000. Clostridium perfringens beta-toxin forms multimeric transmembrane pores in human endothelial cells. Microb. Pathog. 28:45-50. [DOI] [PubMed] [Google Scholar]
- 20.Uzal, F. A., and J. G. Songer. 2008. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. J. Vet. Diagn. Investig. 20:253-265. [DOI] [PubMed] [Google Scholar]
- 21.Vidal, J. E., B. A. McClane, J. Saputo, J. Parker, and F. A. Uzal. 2008. Effects of Clostridium perfringens beta-toxin on the rabbit small intestine and colon. Infect. Immun. 10:4396-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidal, J. E., K. Ohtani, T. Shimizu, and B. A. McClane. 2009. Contact with enterocyte-like Caco-2 cells induces rapid upregulation of toxin production by Clostridium perfringens type C isolates. Cell. Microbiol. 11:1306-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]