Abstract
Leprosy elimination has been a goal of the WHO for the past 15 years. Widespread BCG vaccination and multidrug therapy have dramatically reduced worldwide leprosy prevalence, but new case detection rates have remained relatively constant. These data suggest that additional control strategies, such as a subunit vaccine, are required to block transmission and to improve leprosy control. We recently identified several Mycobacterium leprae antigens that stimulate gamma interferon (IFN-γ) secretion upon incubation with blood from paucibacillary leprosy patients, a group who limit M. leprae growth and dissemination. In this study, we demonstrate that M. leprae-specific mouse T-cell lines recognize several of these antigens, with the ML0276 protein stimulating the most IFN-γ secretion. We then examined if the ML0276 protein could be used in a subunit vaccine to provide protection against experimental M. leprae infection. Our data demonstrate that combining ML0276 with either a Toll-like receptor 4 (TLR4) (EM005), TLR7 (imiquimod), or TLR9 (CpG DNA) agonist during immunization induces Th1 responses that limit local inflammation upon experimental M. leprae infection. Our data indicate that only the ML0276/EM005 regimen is able to elicit a response that is transferable to recipient mice. Despite the potent Th1 response induced by this regimen, it could not provide protection in terms of limiting bacterial growth. We conclude that EM005 is the most potent adjuvant for stimulating a Th1 response and indicate that while a subunit vaccine containing the ML0276 protein may be useful for the prevention of immune pathology during leprosy, it will not control bacterial burden and is therefore unlikely to interrupt disease transmission.
Leprosy, which is caused by infection with Mycobacterium leprae, can manifest across a wide spectrum of disease symptoms. Through the use of clinical, histopathological, and immunological diagnoses, five forms of leprosy have been characterized: lepromatous (LL), borderline lepromatous (BL), mid-borderline, borderline tuberculoid (BT), and tuberculoid (TT) leprosy (53, 56). Multibacillary (MB) patients, encompassing the BB, BL, and LL forms, are characterized as having multiple skin lesions largely devoid of functional lymphocytes. At the extreme MB pole, LL patients demonstrate high titers of anti-M. leprae antibodies but an absence of specific cell-mediated immunity (53). In the absence of a strong cellular immune response, LL patients do not control bacterial replication and have high bacterial indices (BI). It is still unclear why these patients do not mount effective cell-mediated immunity, but factors such as ineffective initial antigen presentation or priming of Treg cells may contribute. In marked contrast, paucibacillary (PB) leprosy patients, encompassing the BT and TT forms, are characterized as having one or few skin lesions and granulomatous dermatopathology with a low or absent BI. At the extreme PB pole, TT patients demonstrate specific cell-mediated immunity against M. leprae and have a low BI. Control of bacterial growth by PB patients indicates that these individuals mount an effective immune response against M. leprae infection. Identifying antigens that are the targets of this cell-mediated response and presenting them in a potent fashion with appropriate immune stimulation with adjuvants are likely to be the keys to effective vaccination against leprosy.
The WHO has promoted the widespread availability of drug cocktails for the standard care of patients diagnosed with leprosy. The multidrug therapy (MDT) program has been an overall success in reducing the prevalence of leprosy to the level of new cases detected annually, at ∼250,000 cases, from levels as high as 12 million cases per year only 20 years ago. Despite this success, complications can and do arise—treatments are long (6 to 24 months), relapse rates in some areas are unacceptably high (22), and drug resistance is emerging (11, 31, 37-39). While MDT remains effective in the majority of cases, the widespread emergence of drug-resistant M. leprae could have catastrophic consequences and undo the efforts of the last 20 years. These concerns and the now stagnant decline in new cases indicate that additional control strategies, such as a vaccine, are necessary to eliminate leprosy.
Vaccination to prevent infection with M. leprae has been performed. The most common vaccine strategy has been to immunize individuals with M. bovis BCG, conferring cross-protection against leprosy and tuberculosis. BCG vaccination to prevent leprosy has been efficacious, but the degree of protection has varied dramatically between studies (57). It is likely that, as with tuberculosis, protection afforded by BCG vaccination wanes over time. Many other whole bacteria have been examined for leprosy vaccine potential in mice, with several related mycobacteria conferring some degree of protection (46, 54, 59). The widespread use of a live bacterial vaccine is limited by concerns over safety and the inability to manufacture large, reproducible batches. The use of heat-killed M. leprae or crude M. leprae antigens, both of which confer protection in mice, is severely restricted by the inability to produce sufficient quantities of M. leprae to supply vaccine for a significant intervention campaign (23, 25, 26, 42-44). Some recombinant antigens have been shown to confer protection in mice, although many results are inconsistent. Most of these antigens, however, have not been evaluated in leprosy patients, and several are unlikely to be approved for use in humans due to their high homology with human proteins (9, 24, 40, 44, 45).
Following the recent completion of M. leprae and other mycobacterial genomes, molecular biology and bioinformatic tools have revealed M. leprae-specific antigens that may be used for leprosy diagnosis or vaccination (12, 20, 27, 49). Our own recent investigations identified several antigens that are recognized by immune cells of PB patients, suggesting that these antigens may be targets of an immune response associated with limited or localized disease (20). These antigens may be the key to producing an effective subunit vaccine against leprosy.
Of our recently identified antigens, ML0276 yielded the highest percentage of responders and the highest median gamma interferon (IFN-γ) response when evaluated against Brazilian PB leprosy patients (20). The current study was designed to explore the vaccine potential of the ML0276 protein. We compared the immune responses to this protein when various Toll-like receptor (TLR) ligands were used as adjuvants to enhance the response and examined the protective efficacy of the most potent vaccine regimen.
MATERIALS AND METHODS
Mice.
Female C57BL/6 (B6) mice were purchased from Charles River Laboratories (Wilmington, MA) and maintained under specific-pathogen-free conditions in the animal facilities of the Infectious Disease Research Institute (IDRI), Seattle, WA, or National Hansen's Disease Programs (NHDP), Baton Rouge, LA. All mice entered experiments at 6 to 8 weeks of age. All animal procedures were approved by the institutional animal care and use committee.
M. leprae inoculations.
Live M. leprae bacilli (Thai-53 strain) were extracted from the footpads of nu/nu mice at NHDP and were enumerated by direct microscopic counting of acid-fast bacilli according to the method of Shepard and McRae (58). Bacilli were used immediately at NHDP or shipped overnight on ice to IDRI for inoculations. The viability of all M. leprae used in these studies exceeded 80%, as judged by staining and radiorespirometry (36). Heat-killed M. leprae (HKML) was obtained by heating bacilli at 60°C for 30 min and then quenching them on ice. Mice were inoculated either by intradermal injection of 1 × 106 bacilli into the ear pinnae or by subcutaneous (s.c.) injection of 1 × 104 bacilli into the footpads.
Cell preparations.
Single-cell suspensions were prepared from the draining lymph nodes (DLN) (auricular), ears, and spleens. Spleens and LN were disrupted between frosted slides, and erythrocytes were removed by lysis in 1.66% NH4Cl solution. Mononuclear cells were enumerated using either a hemocytometer or a ViaCount assay with a PCA system (Guava Technologies, Hayward, CA).
Cell line stimulations.
Cell lines were generated from spleen cells of M. leprae-infected mice. In the first stimulation round, single-cell suspensions were prepared in RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum and 50,000 U penicillin-streptomycin (Invitrogen, Carlsbad, CA) and were seeded at 5 × 106 cells per well in 48-well plates. Cells were incubated in the presence of 2 μg/ml M. leprae cell sonicate (MLCS; kindly provided by John Spencer, Colorado State University, through NIH contract N01 AI-25469) for 5 days, and then cultures were supplemented with 2 ng/ml interleukin-2 (IL-2). After 10 to 12 days, cells were washed and counted and underwent one or two further stimulation rounds. In the restimulation rounds, spleen cells were prepared from uninfected mice and 2 × 106 cells incubated in 48-well plates overnight to permit binding of adherent cells. Nonadherent cells were removed by gentle pipetting, and 2 × 105 cells of each cell line were added to the adherent cells. Cells were incubated with 2 μg/ml MLCS, supplemented with 2 ng/ml IL-2 after 5 days and an additional 0.5 ng/ml IL-2 after 10 days. Cell lines were used to assess antigen specificity after 15 days.
To determine antigen specificity, spleen cells from uninfected mice were seeded at 5 × 105 cells per well in 96-well plates and allowed to adhere overnight. The next day, nonadherent cells were gently removed and cell lines were added at 5 × 104 cells per well with 10 μg/ml crude or recombinant antigen (MLCS, M. leprae membrane antigen, M. leprae cell wall antigen, or M. leprae secreted antigen; kindly provided by John Spencer, Colorado State University, through NIH contract N01 AI-25469). Culture supernatant was collected after 4 days, and IFN-γ content was determined by enzyme-linked immunosorbent assay (ELISA) following the manufacturer's instructions (eBioscience, San Diego, CA).
Immunizations and cell transfers.
Recombinant ML0276 protein was formulated with various TLR ligands to provide a final protein concentration of 100 μg/ml. Antigen was mixed with adjuvant to provide a final adjuvant concentration of either 250 μg/ml CpG ODN 1826 (CpG; Coley Pharmaceuticals, Ottawa, Ontario, Canada), 250 μg/ml imiquimod (IMQ; 3M Pharmaceuticals, Minneapolis, MN), or 200 μg/ml EM005 (IDRI) (7). All mice were immunized three times by s.c. injection of 0.1 ml of vaccine at the base of the tail at 2- to 3-week intervals.
To determine if immune cells could transfer protection, single-cell suspensions were prepared from the spleens of immunized or previously infected mice and 1 × 107 cells transferred to recipient mice by intravenous injection in the tail vein. One day after cell transfer, recipient mice were infected with 1 × 106 M. leprae cells in each ear, and DLN cell numbers were determined 15 weeks later.
Antibody analyses.
Individual mouse sera were analyzed by antibody capture ELISA. Briefly, ELISA plates (Nunc, Rochester, NY) were coated with 1 μg/ml recombinant antigen in 0.1 M bicarbonate buffer and blocked with 0.1% bovine serum albumin-phosphate-buffered saline. Then, in consecutive order and following washes in phosphate-buffered saline-Tween, serially diluted serum samples, anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (Southern Biotech, Birmingham, AL), and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)-H2O2 (ABTS-H2O2; Kirkegaard and Perry Laboratories, Gaithersburg, MD) were added to the plates. Plates were analyzed at 405 nm (ELX808; Bio-Tek Instruments Inc., Winooski, VT). The endpoint titer was determined as the last dilution to render a positive response, determined as 2 × the mean optical density derived from sera from unimmunized mice.
Antigen stimulation assays.
Single-cell suspensions from spleens were cultured at 2 × 105 cells per well in duplicate in a 96-well plate (Corning Incorporated, Corning, NY) in RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum and 50,000 units penicillin-streptomycin (Invitrogen). Cells were cultured in the presence of 10 μg/ml crude MLCS. Culture supernatants were harvested after 72 to 96 h, and cytokine content was assayed for IFN-γ production by ELISA performed according to the manufacturer's instructions (eBioscience, San Diego, CA).
Flow cytometry.
For the elucidation of intracellular cytokine expression, cells were cultured at 37°C for 12 to 16 h in the presence of 1 μg/ml phorbol myristate acetate-ionomycin (Sigma, St. Louis, MO) or 10 μg/ml recombinant antigen and Golgi Stop (BD Biosciences, San Diego, CA). Cells were fixed and permeabilized in Cytofix/Cytoperm (BD Biosciences, San Diego, CA). For staining, cells were first incubated with the anti-FcγII/IIIR antibody 2.4G2 to block nonspecific binding before the addition of a cocktail of fluorescently conjugated antibodies to identify activated antigen-experienced T helper cells (anti-CD4 [clone GK1.5], anti-CD3ɛ [clone 17A2], anti-CD44 [clone IM7], and anti-IFN-γ [clone XMG1.2] [all from eBioscience]). Flow cytometry was performed using LSR Vantage (BD Biosciences), and the data were analyzed with FlowJo software (Treestar, Ashland, OR).
Enumeration of M. leprae cells in mouse footpads.
The number of M. leprae cells in footpad homogenates was assessed by DNA amplification, using real-time PCR with previously described primers and probes (62). Suspensions of nude mouse-derived M. leprae were used as a standard to establish relative numbers of M. leprae in tissues.
Statistics.
P values were determined using Student's t test. Vaccine studies comparing acid-fast bacillus growth in mouse footpads were assessed using the Mann-Whitney rank sum test.
RESULTS
Suitability and selection of ML0276 as a vaccine candidate in mice.
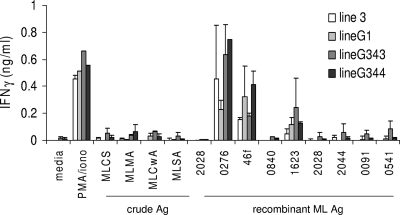
We recently described several recombinant M. leprae antigens that are recognized by PB leprosy patients (19). To examine the potential of these antigens as vaccine candidates, we first queried if these antigens were meaningful during experimental infection and could therefore be used in mice. M. leprae-reactive T-cell lines were generated by repeated stimulation of mouse spleen cells with a crude M. leprae antigen (MLCS). The antigen-specific responses of these T-cell lines were then evaluated by stimulation with the recombinant M. leprae antigens previously identified by IFN-γ secretion from PB patient cells (ML0276, ML0840, ML1623, ML2044, and 46f) (19). In agreement with our previous data generated with human samples, the antigens induced IFN-γ secretion from the M. leprae-specific mouse T-cell lines (Fig. 1). Among all four of the cell lines tested, ML0276 induced the highest levels of IFN-γ secretion (15- to 70-fold over control level). These data indicate that M. leprae-generated mouse T-cell lines are capable of recognizing similar antigens to those recognized by PB leprosy patients.
FIG. 1.
IFN-γ responses of MLCS-reactive T-cell lines following antigen (Ag) stimulation. Mice were infected with M. leprae in the ear, spleens were removed, and MLCS-reactive T-cell lines were derived in vitro. Cell lines were stimulated with 10 μg/ml recombinant antigen for 4 days, culture supernatants were collected, and IFN-γ content was assayed by ELISA. MLCS, M. leprae cell sonicate; MLMA, M. leprae membrane antigen; MLCwA, M. leprae cell wall antigen; MLSA, M. leprae secreted antigen; PMA, phorbol myristate acetate.
Immunization with ML0276/CpG induces Th1-like responses.
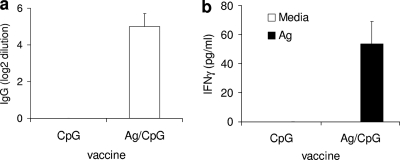
Since ML0276 was the most immunogenic protein tested against both patient cells and mouse T-cell lines, we hypothesized that this protein possessed the greatest vaccine potential. To examine this vaccine potential, mice were immunized with ML0276 mixed with CpG (a TLR9 agonist). Although immunization with ML0276/CpG induced an antigen-specific antibody response, there was only a fourfold increase in serum antibody titer compared with that for mice treated with CpG only (Fig. 2a). Spleen cells from immunized mice responded to antigen stimulation by secreting significant levels of IFN-γ, indicating the generation of a strong cellular response (Fig. 2b). These data indicate that immunization with ML0276/CpG promotes a Th1-like response that could be protective against M. leprae infection.
FIG. 2.
Immunization with ML0276 in the presence of CpG induces B- and T-cell responses. Mice were immunized by s.c. injection of ML0276 and CpG a total of three times, at biweekly intervals. One month after the last immunization, sera were collected and antigen-specific IgG titers were assayed by ELISA (a) or spleen cells were cultured with 10 μg/ml ML0276 and supernatants were assayed for IFN-γ production (b). Results are shown as means and standard errors (SE) (n = 5 per group). Data are representative of two independent experiments.
Immunization with ML0276/CpG reduces local inflammation but not bacterial burden.
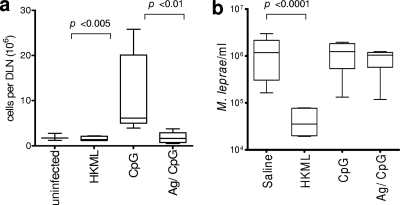
To investigate if the cellular response generated by immunization could reduce disease severity, mice were immunized with ML0276/CpG before being infected in the ear with M. leprae. While unimmunized, infected mice developed enlarged DLN with a characteristic increase in cell numbers, this was not observed in mice that received either control vaccine (HKML) or experimental vaccine (ML0276/CpG) (Fig. 3a). These data indicate that ML0276/CpG immunization can limit the local inflammatory reaction caused by M. leprae infection.
FIG. 3.
Immunization with ML0276 in the presence of CpG reduces lymphadenopathy but fails to decrease M. leprae burden. Mice were immunized by s.c. injection of ML0276 and CpG a total of three times, at biweekly intervals. One month after the last immunization, mice were infected. (a) Mice were infected with 1 × 106 M. leprae organisms in each ear, and DLN cell numbers were determined 15 weeks later. Results are shown as means and SE (n = 5 per group), and data are representative of three independent experiments. Student's t test was used to calculate P values between mice that were immunized with CpG only and those immunized with ML0276/CpG. (b) Mice were infected with 1 × 104 M. leprae organisms in each foot, and bacillus numbers were determined 24 weeks later. Results are shown as means and SE, and data are representative of two independent experiments. The Mann-Whitney test was used to calculate P values between infected and HKML-immunized mice for footpad infection (n = 12 per group).
To determine if the reduction in lymphadenopathy correlated with a reduction in bacterial number, we employed Shepard's mouse footpad model to evaluate M. leprae growth following vaccination. Mice were immunized and then infected in the footpad, and bacterial numbers were determined 24 weeks later. As expected, unimmunized mice demonstrated outgrowth of bacteria, whereas mice that were immunized with HKML had a reduced bacterial burden. Surprisingly, mice that were immunized with ML0276/CpG did not control bacterial numbers and exhibited burdens similar to those of unimmunized mice (Fig. 3b). These data indicate that despite limiting the local inflammatory reaction, vaccination with ML0276/CpG is not sufficient to limit bacterial growth.
Vaccination with ML0276 in conjunction with different TLR agonists alters the magnitude of the Th1-like response.
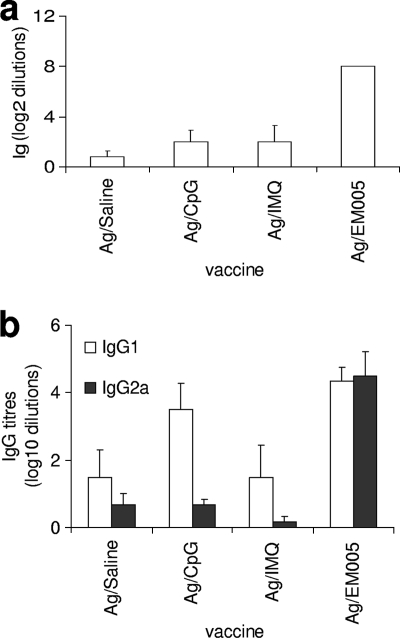
In an effort to enhance the antigen-specific immune response to provide a response sufficient to limit bacterial growth, we compared the responses induced by immunization with ML0276 in conjunction with a variety of adjuvants that are ligands of different TLRs. We first compared the effects of immunizing mice with ML0276 in the presence of different TLR ligands on the anti-ML0276 IgG response. Immunization with ML0276 in combination with a TLR7 ligand (IMQ) or a TLR9 ligand (CpG) induced comparable Ig titers (Fig. 4a). The use of a TLR4 ligand (EM005), however, enhanced the IgG response 10-fold over control (saline) levels. Further evaluation of the IgG subtypes indicated that immunizing with ML0276 and EM005 also induced a potent shift to a Th1-like profile, with an IgG2a/IgG1 ratio of 18.95 (Fig. 4b; data not shown). These results suggest that EM005 promotes a more potent Th1-like response against ML0276 than that obtained with either CpG or IMQ.
FIG. 4.
Immunization with ML0276 in conjunction with different TLR agonists affects serum Ig responses. Mice were injected s.c. with ML0276 in the presence of CpG, IMQ, or EM005 at biweekly intervals, for a total of three immunizations. (a) Sera were collected 1 month after the third immunization, and ML0276-specific IgG titers were determined by ELISA. (b) ML0276-specific IgG1 and IgG2a titers were determined. Results are shown as means and SE (n = 6 per group). Data are representative of three independent experiments.
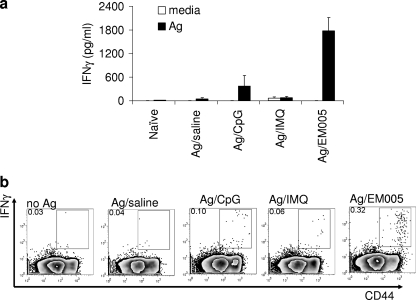
To further examine if ML0276/EM005 immunization promoted an antigen-specific Th1-like response, we evaluated the ability of spleen cells from immunized mice to produce IFN-γ when cultured with ML0276. As expected, spleen cells from unimmunized mice did not respond to the antigen, while cells from immunized mice secreted IFN-γ. Cells from mice immunized with ML0276/EM005 secreted the highest levels of IFN-γ (Fig. 5a). More detailed analyses of these responses by flow cytometry revealed that antigen-experienced CD44hi CD4+ T cells were the dominant source of IFN-γ (Fig. 5b). Mice immunized with ML0276/EM005 possessed significantly larger populations of IFN-γ-producing cells than did mice immunized with ML0276/CpG or ML0276/IMQ (P < 0.05). These results indicate that although all of the vaccine formulations stimulated a Th1-like response against ML0276, the strongest response was induced by ML0276/EM005. These data suggest that immunization with ML0276/EM005 represents the best opportunity to limit both pathology and bacterial burden during M. leprae infection.
FIG. 5.
Immunization with ML0276 in the presence of EM005 stimulates a strong Th1 response. Mice were injected s.c. with ML0276 in the presence of CpG, IMQ, or EM005 at biweekly intervals, for a total of three immunizations. Spleens were collected 1 month after the third immunization, and single-cell suspensions were prepared and cultured with 10 μg/ml ML0276. (a) Culture supernatants were collected and IFN-γ content determined by ELISA. Results are shown as means and SE (n = 3 per group). Data are representative of three independent experiments. (b) Alternatively, spleen cells were cultured with antigen and BD Golgi Stop (to prevent secretion) overnight and then fixed and stained for flow cytometry to determine the percentage of CD3+ CD4+ CD44hi IFN-γ cells. A representative dot plot for groups of three mice is shown, and data are representative of three independent experiments.
ML0276/EM005 immunization reduces disease pathology.
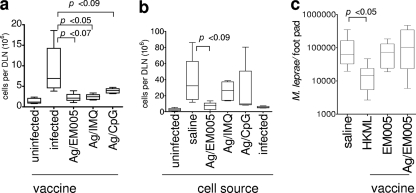
Having established the most immunogenic vaccine regimen for ML0276, we hypothesized that the ML0276/EM005 regimen would limit experimental infection. To fully examine the relationship of effective immunization to protection, mice were immunized with antigen in saline or antigen in conjunction with adjuvant directed to either TLR4, TLR7, or TLR9 before being infected with M. leprae. DLN cell numbers were significantly increased in unimmunized mice following experimental infection, indicating that local pathology was not controlled (Fig. 6a). In contrast, DLN cell numbers in mice immunized with ML0276/CpG, ML0276/IMQ, and ML0276/EM005 were not markedly increased following infection, suggesting that infection was controlled by vaccination of these mice, irrespective of the adjuvant used (Fig. 6a). These data suggest that when used for immunization with an appropriate adjuvant, ML0276 can limit local inflammation associated with M. leprae infection.
FIG. 6.
ML0276 immunization in the presence of EM005 provides potent and transferable protection. Mice were injected s.c. with ML0276 in the presence of EM005 at biweekly intervals, for a total of three immunizations. (a) One month after the final immunization, mice were infected with 1 × 106 M. leprae organisms in each ear, and DLN cell numbers were determined 15 weeks later. Results are shown as means and SE. Student's t test was used to calculate P values between infected controls and immunized mice (n = 5 per group). (b) Single-cell suspensions were prepared from the spleens of immunized mice and 1 × 107 cells transferred to recipient mice by intravenous injection in the tail vein. One day after cell transfer, recipient mice were infected with 1 × 106 M. leprae organisms in each ear, and DLN cell numbers were determined 15 weeks later. Results are shown as means and SE. Student's t test was used to calculate P values between infected controls and mice that received cells from ML0276/EM005-immunized mice (n = 5 per group). (c) Immunization with ML0276 and EM005 does not decrease M. leprae burden. One month after the final immunization, mice were infected with 1 × 104 M. leprae organisms in each foot, and bacterial burdens were determined 24 weeks after infection. Results are shown as means and SE, and data are representative of two independent experiments. The Mann-Whitney test was used to calculate P values between infected and HKML-immunized mice for footpad infection (n = 10 per group).
To determine if the cellular response promoted by immunization was responsible for the reduction in inflammation observed in these mice, we transferred spleen cells from immunized donor mice to unimmunized recipient mice. The recipient mice were infected with M. leprae within a day of cell transfer, and DLN cell numbers were determined 15 weeks later. In contrast to cells from unimmunized or ML0276/CpG- or ML0276/IMQ-immunized mice, cells from ML0276/EM005-immunized mice limited the lymphadenopathy induced by infection (Fig. 6b). These data indicate that ML0276/EM005 vaccination generated a transferable memory response that could limit the local inflammatory response during experimental M. leprae infection.
Immunization with ML0276/EM005 does not reduce bacterial burden.
Our results suggested that EM005 was a more appropriate adjuvant than the other tested adjuvants for elicitation of both ML0276-specific effector and memory cell generation and for reducing local inflammation during infection. To investigate if this adjuvant could limit bacterial growth, ML0276/EM005-immunized mice were infected with M. leprae in the footpad and bacillus numbers assessed 24 weeks later. Unexpectedly, even with a more potent vaccine regimen that can limit the local inflammatory response, and unlike immunization with HKML, ML0276 immunization did not decrease bacterial numbers compared with those in unimmunized mice (Fig. 6c). Taken together, our data indicate that while ML0276 immunization can limit the local inflammatory response during experimental M. leprae infection, additional antigens are required to limit bacterial growth.
DISCUSSION
Leprosy is a complex disease that manifests across a highly divergent range of pathological, bacteriologic, and immunologic outcomes. The immune responses range from strong cellular responses in TT patients to predominantly humoral responses in LL patients. The strong antigen-specific cellular responses of TT patients allow these individuals to control bacterial numbers and to limit dissemination. Experimental M. leprae infection of immunocompromised mice has demonstrated that T cells and IFN-γ participate in the control of bacterial growth (1, 2, 5, 15, 16, 34, 51). We recently described several recombinant M. leprae antigens that stimulate IFN-γ secretion when recognized by PB leprosy patients. In this study, we have extended those observations to demonstrate that mouse T cells can recognize and respond to these antigens and that immunization with the most immunogenic antigen (ML0276) can limit infection-induced inflammation in the mouse ear short-term infection model. Surprisingly, despite adjuvant refinement of ML0276-based vaccines to generate a potent and transferable Th1 response, vaccination with ML0276 did not limit bacterial growth in the long-term footpad model.
Due to its documented ability to induce potent Th1 responses against even weak antigens, we initially used CpG, a TLR9 agonist, to potentiate the response against ML0276 (10, 48). The adjuvant properties of CpG have been well characterized for a variety of species, and various CpG DNAs are being used in human clinical trials (8, 10, 30, 35, 60, 61). In our study, ML0276/CpG-immunized mice exhibited a modest Th1 response that reduced the local inflammatory response. Our previous characterization of the rapid ear infection model identified a correlation between live M. leprae infection and the magnitude of the local inflammatory response (21). It was therefore highly surprising that ML0276/CpG vaccination failed to control bacterial numbers. A similar phenotype has been described following M. bovis BCG vaccination of neonatal calves, where tuberculosis-associated pathology was reduced but bacterial colonization was not impacted (65). These data indicate that despite the inability to control bacterial burden, the local inflammatory response can be limited following vaccination. This outcome would be highly beneficial to individual leprosy patients, as a common complication is uncontrolled inflammatory reactions (type 1 and type 2) that cause significant distress and worsening of nerve damage and occasionally result in hospitalization. However, at a population level, this outcome would likely not impact M. leprae transmission and would not reduce new case numbers.
Our data prompted us to evaluate if adjuvants that engage other TLRs could improve the potency of an ML0276 vaccine and thereby stimulate stronger Th1 responses with more potential to limit M. leprae growth (41). Besides TLR9, agonists of TLR4 and TLR7/8 have been well characterized in experimental vaccine studies and are being employed in human clinical trials (10). To engage TLR4, we used a synthetic TLR4 agonist (EM005) (7) that is related to the powerful yet nontoxic adjuvant monophosphoryl lipid A (MPL) (13, 28). MPL has been used extensively as an adjuvant in clinical trials with parenterally administered vaccines (6, 13, 14, 28, 32, 47, 50, 63). To engage TLR7, we used IMQ (29). IMQ has demonstrated potent antiviral and antitumor properties in animal models and stimulates Th1 and cytotoxic T-lymphocyte responses (3, 10, 64). In humans, it has been used widely as a topical cream to treat skin diseases (Aldara; 3M Pharmaceuticals), and the immunomodulatory properties are retained when IMQ is administered either orally, intraperitoneally, or intravenously (3, 4, 52). Our data demonstrate that when administered with ML0276, IMQ induced similar serum IgG responses to those achieved when CpG was coadministered with the antigen. In contrast, EM005 potentiated the anti-ML0276 response to levels well above those achieved when either IMQ or CpG was used as adjuvant. In addition to stimulating greater levels of ML0276-specific IgG, EM005 promoted a strong shift in the antibody response to a Th1 profile, as demonstrated by an enhancement of the IgG2a/IgG1 ratio. This Th1-biased antibody profile was supported by an increased population of antigen-experienced, IFN-γ-producing CD4 T cells in mice immunized with ML0276/EM005. ML0276/EM005 not only stimulated a greater Th1 response than the other vaccines, but unlike the other vaccines, it also generated a response that could limit inflammation when transferred to recipient mice. Together, these data demonstrate that EM005 was able to generate strong and transferable antigen-specific immunity.
We were extremely surprised to find that despite ML0276/EM005 inducing strong and transferable Th1 responses that could limit local inflammation, this vaccine regimen still did not reduce the bacterial burden when assessed in a long-term growth model. It is unclear why ML0276 immunization could not decrease the bacterial burden. ML0276 is transcribed during M. leprae infection in humans and mice and is clearly recognized by immune cells of leprosy patients and MLCS-reactive T-cell lines of mice (19; D. Williams, personal communication). The functional classification of ML0276 is as a conserved hypothetical protein, and protein expression levels during infection are unknown. It may be that the ML0276 protein is not expressed consistently or is presented in insufficient amounts that do not permit identification of M. leprae-infected cells. These data indicate that additional antigens are required to provide a vaccine that can reduce bacterial burden. We are currently striving to identify additional antigens that are recognized by leprosy patients and will evaluate these as vaccine candidates in mice to determine if they can limit both local inflammation and bacterial burden.
Some clinicians and researchers fear that immunization to boost inflammatory responses will lead to reversal reactions in leprosy patients. It is common practice in some countries, however, to reimmunize leprosy patients and their close contacts with BCG, even though the efficacy of this BCG reimmunization regimen is debated (17, 18, 57). In addition, direct injection of IL-2 and IFN-γ into leprosy lesions does not induce type 1 reactions, although prolonged intradermal IFN-γ treatment of LL patients does increase the risk of type 2 reactions (erythema nodosum leprosum) (33, 55). These results highlight the importance of preclinical evaluation of leprosy vaccines in animal models that present with inflammation at the infection site. Events seen in the immunocompetent mouse footpad and ear model systems following infection with M. leprae appear to be similar to those in indeterminate leprosy (16a, 21). Indeterminate leprosy in humans is a disease manifestation characterized by small numbers of T-cell and other mononuclear cell infiltrates that partially control bacterial growth. Clinical descriptions of this form of leprosy indicate that approximately 50% of indeterminate lesions self-heal and clear infection. Therefore, infection of mice with live M. leprae provides legitimate models of immune-mediated inhibition of bacterial growth that can be boosted by immunization prior to challenge. Given these outcomes, the mouse model appears to be appropriate for leprosy vaccine assessments. Our data, obtained with mouse models, suggest that inducing inflammatory anti-M. leprae responses in uninfected individuals will be safe and will limit local inflammatory responses. Vaccination of individuals with subclinical infection or even of patients with low BI is also likely to be safe, but vaccination of MB patients, particularly LL patients, may not be wise. Our data suggest that immunization could reduce the likelihood of developing reversal reactions, and this merits analysis of how already-infected animals respond to immune challenge. Mice do not develop nerve damage during experimental M. leprae infection, and it may be that vaccine testing in armadillos, which can develop nerve damage following M. leprae infection, is required to resolve these safety concerns. Examining how MDT affects immunization would also appear merited.
The ideal vaccine against leprosy would induce a strong, long-lasting T-cell response directed against M. leprae that would both prevent disease and reduce bacterial transmission. The most common vaccine strategy has been to immunize individuals with BCG, conferring cross-protection against leprosy and tuberculosis. BCG vaccination to prevent leprosy has been efficacious, but the degree of protection has varied dramatically between studies (57). It is also likely that, as with tuberculosis, protection afforded by BCG vaccination against leprosy wanes over time. A defined subunit vaccine would appear well suited to provide a second, long-lasting line of protection. A potent adjuvant that facilitates a strong inflammatory response against the antigenic components of a subunit vaccine, such as EM005, is also likely to be critical for protection against intracellular pathogens such as M. leprae.
Our data suggest that vaccination with ML0276, by limiting infection-induced inflammation, may be beneficial to individuals but would not impact M. leprae transmission. We are currently identifying additional proteins that are recognized by PB leprosy patients and evaluating the potential of these antigens to limit bacterial growth.
Acknowledgments
This work was supported by the American Leprosy Missions.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 28 September 2009.
REFERENCES
- 1.Adams, L. B., T. P. Gillis, D. H. Hwang, and J. L. Krahenbuhl. 1997. Effects of essential fatty acid deficiency on prostaglandin E2 production and cell-mediated immunity in a mouse model of leprosy. Infect. Immun. 65:1152-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, L. B., D. M. Scollard, N. A. Ray, A. M. Cooper, A. A. Frank, I. M. Orme, and J. L. Krahenbuhl. 2002. The study of Mycobacterium leprae infection in interferon-gamma gene-disrupted mice as a model to explore the immunopathologic spectrum of leprosy. J. Infect. Dis. 185(Suppl. 1):S1-S8. [DOI] [PubMed] [Google Scholar]
- 3.Ahonen, C. L., C. L. Doxsee, S. M. McGurran, T. R. Riter, W. F. Wade, R. J. Barth, J. P. Vasilakos, R. J. Noelle, and R. M. Kedl. 2004. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 199:775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahonen, C. L., A. Wasiuk, S. Fuse, M. J. Turk, M. S. Ernstoff, A. A. Suriawinata, J. D. Gorham, R. M. Kedl, E. J. Usherwood, and R. J. Noelle. 2008. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines. Blood 111:3116-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azouaou, N., R. H. Gelber, K. Abel, D. T. Sasaki, L. P. Murray, R. M. Locksley, and N. Mohagheghpour. 1993. Reconstitution of Mycobacterium leprae immunity in severe combined immunodeficient mice using a T-cell line. Int. J. Lepr. Other Mycobact. Dis. 61:398-405. [PubMed] [Google Scholar]
- 6.Baldridge, J. R., and R. T. Crane. 1999. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods 19:103-107. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin, S. L., N. Shaverdian, Y. Goto, M. S. Duthie, V. Raman, T. Evers, F. Mompoint, T. Vedvick, S. Bertholet, R. N. Coler, and S. G. Reed. 2009. Enhanced humoral and type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine 27:5956-5963. [DOI] [PubMed] [Google Scholar]
- 8.Ballas, Z. K., A. M. Krieg, T. Warren, W. Rasmussen, H. L. Davis, M. Waldschmidt, and G. J. Weiner. 2001. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J. Immunol. 167:4878-4886. [DOI] [PubMed] [Google Scholar]
- 9.Baumgart, K. W., K. R. McKenzie, A. J. Radford, I. Ramshaw, and W. J. Britton. 1996. Immunogenicity and protection studies with recombinant mycobacteria and vaccinia vectors coexpressing the 18-kilodalton protein of Mycobacterium leprae. Infect. Immun. 64:2274-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdin, N., B. Guy, and P. Moingeon. 2004. Immunological foundations to the quest for new vaccine adjuvants. BioDrugs 18:79-93. [DOI] [PubMed] [Google Scholar]
- 11.Cambau, E., P. Bonnafous, E. Perani, W. Sougakoff, B. Ji, and V. Jarlier. 2002. Molecular detection of rifampin and ofloxacin resistance for patients who experience relapse of multibacillary leprosy. Clin. Infect. Dis. 34:39-45. [DOI] [PubMed] [Google Scholar]
- 12.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 13.Coler, R. N., Y. Goto, L. Bogatzki, V. Raman, and S. G. Reed. 2007. Leish-111f, a recombinant polyprotein vaccine that protects against visceral leishmaniasis by elicitation of CD4+ T cells. Infect. Immun. 75:4648-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coler, R. N., and S. G. Reed. 2005. Second-generation vaccines against leishmaniasis. Trends Parasitol. 21:244-249. [DOI] [PubMed] [Google Scholar]
- 15.Colston, M. J., and G. R. Hilson. 1976. Growth of Mycobacterium leprae and M. marinum in congenitally athymic (nude) mice. Nature 262:399-401. [DOI] [PubMed] [Google Scholar]
- 16.Converse, P. J., V. L. Haines, A. Wondimu, L. E. Craig, and W. M. Meyers. 1995. Infection of SCID mice with Mycobacterium leprae and control with antigen-activated “immune” human peripheral blood mononuclear cells. Infect. Immun. 63:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Cooper, A. M., L. B. Adams, D. K. Dalton, R. Appelberg, and S. Ehlers. 2002. IFN-gamma and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol 10:221-226. [DOI] [PubMed] [Google Scholar]
- 17.Cunha, S. S., N. Alexander, M. L. Barreto, E. S. Pereira, I. Dourado, M. de Fatima Maroja, Y. Ichihara, S. Brito, S. Pereira, and L. C. Rodrigues. 2008. BCG revaccination does not protect against leprosy in the Brazilian Amazon: a cluster randomised trial. PLoS Negl. Trop. Dis. 2:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duppre, N. C., L. A. Camacho, S. S. da Cunha, C. J. Struchiner, A. M. Sales, J. A. Nery, and E. N. Sarno. 2008. Effectiveness of BCG vaccination among leprosy contacts: a cohort study. Trans. R. Soc. Trop. Med. Hyg. 102:631-638. [DOI] [PubMed] [Google Scholar]
- 19.Duthie, M. S., W. Goto, G. C. Ireton, S. T. Reece, L. H. Sampaio, A. B. Grassi, A. L. Sousa, C. M. Martelli, M. M. Stefani, and S. G. Reed. 2008. Antigen-specific T-cell responses of leprosy patients. Clin. Vaccine Immunol. 15:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duthie, M. S., G. C. Ireton, G. V. Kanaujia, W. Goto, H. Liang, A. Bhatia, J. M. Busceti, M. Macdonald, K. D. Neupane, C. Ranjit, B. R. Sapkota, M. Balagon, J. Esfandiari, D. Carter, and S. G. Reed. 2008. Selection of antigens and development of prototype tests for point-of-care leprosy diagnosis. Clin. Vaccine Immunol. 15:1590-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duthie, M. S., S. T. Reece, R. Lahiri, W. Goto, V. S. Raman, J. Kaplan, G. C. Ireton, S. Bertholet, T. P. Gillis, J. L. Krahenbuhl, and S. G. Reed. 2007. Antigen-specific cellular and humoral responses are induced by intradermal Mycobacterium leprae infection of the mouse ear. Infect. Immun. 75:5290-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelber, R. H., V. F. Balagon, and R. V. Cellona. 2004. The relapse rate in MB leprosy patients treated with 2-years of WHO-MDT is not low. Int. J. Lepr. Other Mycobact. Dis. 72:493-500. [DOI] [PubMed] [Google Scholar]
- 23.Gelber, R. H., P. J. Brennan, S. W. Hunter, M. W. Munn, J. M. Monson, L. P. Murray, P. Siu, M. Tsang, E. G. Engleman, and N. Mohagheghpour. 1990. Effective vaccination of mice against leprosy bacilli with subunits of Mycobacterium leprae. Infect. Immun. 58:711-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelber, R. H., S. W. Hunter, L. P. Murray, P. Siu, M. Tsang, and P. J. Brennan. 1994. Effective vaccination of mice against Mycobacterium leprae with density-gradient subfractions of soluble M. leprae proteins: clues to effective protein epitopes. Lepr. Rev. 65:175-180. [PubMed] [Google Scholar]
- 25.Gelber, R. H., V. Mehra, B. Bloom, L. P. Murray, P. Siu, M. Tsang, and P. J. Brennan. 1994. Vaccination with pure Mycobacterium leprae proteins inhibits M. leprae multiplication in mouse footpads. Infect. Immun. 62:4250-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelber, R. H., L. Murray, P. Siu, and M. Tsang. 1992. Vaccination of mice with a soluble protein fraction of Mycobacterium leprae provides consistent and long-term protection against M. leprae infection. Infect. Immun. 60:1840-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geluk, A., and T. H. Ottenhoff. 2006. HLA and leprosy in the pre and postgenomic eras. Hum. Immunol. 67:439-445. [DOI] [PubMed] [Google Scholar]
- 28.Goto, Y., L. Y. Bogatzki, S. Bertholet, R. N. Coler, and S. G. Reed. 2007. Protective immunization against visceral leishmaniasis using Leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine 25:7450-7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 30.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388-3393. [DOI] [PubMed] [Google Scholar]
- 31.Ji, B., P. Jamet, S. Sow, E. G. Perani, I. Traore, and J. H. Grosset. 1997. High relapse rate among lepromatous leprosy patients treated with rifampin plus ofloxacin daily for 4 weeks. Antimicrob. Agents Chemother. 41:1953-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, A. G., M. A. Tomai, Y. F. Chen, and M. Odean. 1991. A comparison of the immunomodulating properties of two forms of monophosphoryl lipid A analogues. J. Immunother. 10:398-404. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan, G., W. J. Britton, G. E. Hancock, W. J. Theuvenet, K. A. Smith, C. K. Job, P. W. Roche, A. Molloy, R. Burkhardt, J. Barker, et al. 1991. The systemic influence of recombinant interleukin 2 on the manifestations of lepromatous leprosy. J. Exp. Med. 173:993-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krahenbuhl, J., and L. B. Adams. 2000. Exploitation of gene knockout mice models to study the pathogenesis of leprosy. Lepr. Rev. 71(Suppl.):S170-S175. [DOI] [PubMed] [Google Scholar]
- 35.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 36.Lahiri, R., B. Randhawa, and J. Krahenbuhl. 2005. Application of a viability-staining method for Mycobacterium leprae derived from the athymic (nu/nu) mouse foot pad. J. Med. Microbiol. 54:235-242. [DOI] [PubMed] [Google Scholar]
- 37.Maeda, S., M. Matsuoka, N. Nakata, M. Kai, Y. Maeda, K. Hashimoto, H. Kimura, K. Kobayashi, and Y. Kashiwabara. 2001. Multidrug-resistant Mycobacterium leprae from patients with leprosy. Antimicrob. Agents Chemother. 45:3635-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuoka, M., Y. Kashiwabara, Z. Liangfen, M. Goto, and S. Kitajima. 2003. A second case of multidrug-resistant Mycobacterium leprae isolated from a Japanese patient with relapsed lepromatous leprosy. Int. J. Lepr. Other Mycobact. Dis. 71:240-243. [DOI] [PubMed] [Google Scholar]
- 39.Matsuoka, M., Y. Kashiwabara, and M. Namisato. 2000. A Mycobacterium leprae isolate resistant to dapsone, rifampin, ofloxacin and sparfloxacin. Int. J. Lepr. Other Mycobact. Dis. 68:452-455. [PubMed] [Google Scholar]
- 40.Matsuoka, M., H. Nomaguchi, H. Yukitake, N. Ohara, S. Matsumoto, K. Mise, and T. Yamada. 1997. Inhibition of multiplication of Mycobacterium leprae in mouse foot pads by immunization with ribosomal fraction and culture filtrate from Mycobacterium bovis BCG. Vaccine 15:1214-1217. [DOI] [PubMed] [Google Scholar]
- 41.McCluskie, M. J., and R. D. Weeratna. 2001. Novel adjuvant systems. Curr. Drug Targets Infect. Disord. 1:263-271. [DOI] [PubMed] [Google Scholar]
- 42.Ngamying, M., L. Levy, and P. J. Brennan. 1999. Vaccination of mice against the leprosy bacillus with skin-test antigens. Int. J. Lepr. Other Mycobact. Dis. 67:305-307. [PubMed] [Google Scholar]
- 43.Ngamying, M., P. Sawanpanyalert, R. Butraporn, J. Nikasri, S. N. Cho, L. Levy, and P. J. Brennan. 2003. Effect of vaccination with refined components of the organism on infection of mice with Mycobacterium leprae. Infect. Immun. 71:1596-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ngamying, M., P. Varachit, P. Phaknilrat, L. Levy, P. J. Brennan, and S. N. Cho. 2001. Effects of vaccination with several mycobacterial proteins and lipoproteins on Mycobacterium leprae infection of the mouse. Int. J. Lepr. Other Mycobact. Dis. 69:43-45. [PubMed] [Google Scholar]
- 45.Nomaguchi, H., T. Mukai, F. Takeshita, M. Matsuoka, Y. Maeda, T. M. Aye, N. Jahan, Y. Yogi, M. Endo, and Y. Sato. 2002. Effect of hsp65 DNA vaccination carrying immunostimulatory DNA sequences (CpG motifs) against Mycobacterium leprae multiplication in mice. Int. J. Lepr. Other Mycobact. Dis. 70:182-190. [PubMed] [Google Scholar]
- 46.Ohara, N., M. Matsuoka, H. Nomaguchi, M. Naito, and T. Yamada. 2000. Inhibition of multiplication of Mycobacterium leprae in mouse foot pads by recombinant bacillus Calmette-Guerin (BCG). Vaccine 18:1294-1297. [DOI] [PubMed] [Google Scholar]
- 47.Persing, D. H., R. N. Coler, M. J. Lacy, D. A. Johnson, J. R. Baldridge, R. M. Hershberg, and S. G. Reed. 2002. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10:S32-S37. [DOI] [PubMed] [Google Scholar]
- 48.Qian, F., K. M. Rausch, O. Muratova, H. Zhou, G. Song, A. Diouf, L. Lambert, D. L. Narum, Y. Wu, A. Saul, L. H. Miller, C. A. Long, and G. E. Mullen. 2008. Addition of CpG ODN to recombinant Pseudomonas aeruginosa ExoProtein A conjugates of AMA1 and Pfs25 greatly increases the number of responders. Vaccine 26:2521-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reece, S. T., G. Ireton, R. Mohamath, J. Guderian, W. Goto, R. Gelber, N. Groathouse, J. Spencer, P. Brennan, and S. G. Reed. 2006. ML0405 and ML2331 are antigens of Mycobacterium leprae with potential for diagnosis of leprosy. Clin. Vaccine Immunol. 13:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed, S. G., R. N. Coler, and A. Campos-Neto. 2003. Development of a leishmaniasis vaccine: the importance of MPL. Expert Rev. Vaccines 2:239-252. [DOI] [PubMed] [Google Scholar]
- 51.Rees, R. J., M. F. Waters, A. G. Weddell, and E. Palmer. 1967. Experimental lepromatous leprosy. Nature 215:599-602. [DOI] [PubMed] [Google Scholar]
- 52.Reiter, M. J., T. L. Testerman, R. L. Miller, C. E. Weeks, and M. A. Tomai. 1994. Cytokine induction in mice by the immunomodulator imiquimod. J. Leukoc. Biol. 55:234-240. [DOI] [PubMed] [Google Scholar]
- 53.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255-273. [PubMed] [Google Scholar]
- 54.Roche, P. W., K. D. Neupane, S. S. Failbus, A. Kamath, and W. J. Britton. 2001. Vaccination with DNA of the Mycobacterium tuberculosis 85B antigen protects mouse foot pad against infection with M. leprae. Int. J. Lepr. Other Mycobact. Dis. 69:93-98. [PubMed] [Google Scholar]
- 55.Sampaio, E. P., A. L. Moreira, E. N. Sarno, A. M. Malta, and G. Kaplan. 1992. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J. Exp. Med. 175:1729-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scollard, D. M. 2004. Classification of leprosy: a full color spectrum, or black and white? Int. J. Lepr. Other Mycobact. Dis. 72:166-168. [DOI] [PubMed] [Google Scholar]
- 57.Setia, M. S., C. Steinmaus, C. S. Ho, and G. W. Rutherford. 2006. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect. Dis. 6:162-170. [DOI] [PubMed] [Google Scholar]
- 58.Shepard, C. C., and D. H. McRae. 1968. A method for counting acid-fast bacteria. Int. J. Lepr. Other Mycobact. Dis. 36:78-82. [PubMed] [Google Scholar]
- 59.Skeiky, Y. A., R. N. Coler, M. Brannon, E. Stromberg, K. Greeson, R. T. Crane, J. R. Webb, A. Campos-Neto, and S. G. Reed. 2002. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine 20:3292-3303. [DOI] [PubMed] [Google Scholar]
- 60.Sugai, T., M. Mori, M. Nakazawa, M. Ichino, T. Naruto, N. Kobayashi, Y. Kobayashi, M. Minami, and S. Yokota. 2005. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria-tetanus-pertussis vaccine. Vaccine 23:5450-5456. [DOI] [PubMed] [Google Scholar]
- 61.Tritel, M., A. M. Stoddard, B. J. Flynn, P. A. Darrah, C. Y. Wu, U. Wille, J. A. Shah, Y. Huang, L. Xu, M. R. Betts, G. J. Nabel, and R. A. Seder. 2003. Prime-boost vaccination with HIV-1 Gag protein and cytosine phosphate guanosine oligodeoxynucleotide, followed by adenovirus, induces sustained and robust humoral and cellular immune responses. J. Immunol. 171:2538-2547. [DOI] [PubMed] [Google Scholar]
- 62.Truman, R. W., P. K. Andrews, N. Y. Robbins, L. B. Adams, J. L. Krahenbuhl, and T. P. Gillis. 2008. Enumeration of Mycobacterium leprae using real-time PCR. PLoS Negl. Trop. Dis. 2:e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulrich, J. T., and K. R. Myers. 1995. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm. Biotechnol. 6:495-524. [PubMed] [Google Scholar]
- 64.Wagner, T. L., C. L. Ahonen, A. M. Couture, S. J. Gibson, R. L. Miller, R. M. Smith, M. J. Reiter, J. P. Vasilakos, and M. A. Tomai. 1999. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol. 191:10-19. [DOI] [PubMed] [Google Scholar]
- 65.Waters, W. R., M. V. Palmer, B. J. Nonnecke, T. C. Thacker, C. F. Scherer, D. M. Estes, W. R. Jacobs, Jr., A. Glatman-Freedman, and M. H. Larsen. 2007. Failure of a Mycobacterium tuberculosis DeltaRD1 DeltapanCD double deletion mutant in a neonatal calf aerosol M. bovis challenge model: comparisons to responses elicited by M. bovis bacille Calmette Guerin. Vaccine 25:7832-7840. [DOI] [PubMed] [Google Scholar]