Abstract
We have identified new malaria vaccine candidates through the combination of bioinformatics prediction of stable protein domains in the Plasmodium falciparum genome, chemical synthesis of polypeptides, in vitro biological functional assays, and association of an antigen-specific antibody response with protection against clinical malaria. Within the predicted open reading frame of P. falciparum hypothetical protein PFF0165c, several segments with low hydrophobic amino acid content, which are likely to be intrinsically unstructured, were identified. The synthetic peptide corresponding to one such segment (P27A) was well recognized by sera and peripheral blood mononuclear cells of adults living in different regions where malaria is endemic. High antibody titers were induced in different strains of mice and in rabbits immunized with the polypeptide formulated with different adjuvants. These antibodies recognized native epitopes in P. falciparum-infected erythrocytes, formed distinct bands in Western blots, and were inhibitory in an in vitro antibody-dependent cellular inhibition parasite-growth assay. The immunological properties of P27A, together with its low polymorphism and association with clinical protection from malaria in humans, warrant its further development as a malaria vaccine candidate.
Attempts to develop an effective malaria vaccine have focused on only a few malaria proteins out of the possible 5,400 predicted in the Plasmodium falciparum genome (36). The publication of the genome (16) is, however, expected to stimulate the development of new vaccines, drugs, and other interventions for the control of malaria infection.
In our earlier published work (34), we described a new approach for the identification of novel malaria vaccine candidates through a genome-wide search for proteins with predicted α-helical coiled-coil motifs. These protein segments are known to be stable as isolated fragments, and some of the malaria vaccine candidates under development contain such segments (34). The synthetic polypeptides were recognized by sera of adults living in different regions where malaria is endemic, and affinity-purified human antibodies specific for some of them were found to be active in antibody-dependent cellular inhibition (ADCI) assays. Some of them also induced high antibody titers in mice, and both the peptide-specific purified human antibodies and mouse sera recognized native epitopes present in P. falciparum 3D7-infected erythrocytes. One of the most promising peptides identified was peptide 27 (P27), which is derived from the hypothetical protein PFF0165c that consists of 1,103 amino acids (16). Since P27 contains only 27 amino acids and activated only 30% of peripheral blood mononuclear cells (PBMC) from human semi-immune donors (unpublished data), it is probable that coverage of the general population will not be obtained when it is used as a vaccine. Thus, we proceeded to screen the entire PFF0165c protein for intrinsically unstructured protein domains which could be easily mimicked and rapidly obtained through peptide synthesis for evaluation of their vaccine potential.
It was previously assumed that the functions of proteins are closely linked to their three-dimensional structure. However, in the post-genome era, many gene sequences appear to code for proteins or long stretches of amino acids that are likely to be unfolded (nonglobular) in solution (14, 37). They are identified by their low content of bulky hydrophobic amino acids, which normally form the core of folded globular proteins. Two malaria vaccine candidates currently being evaluated (MSP2 and MSP3) have been found to contain such segments (26, 38, 39), and they have both been shown to be safe and immunogenic in human trials (4, 17).
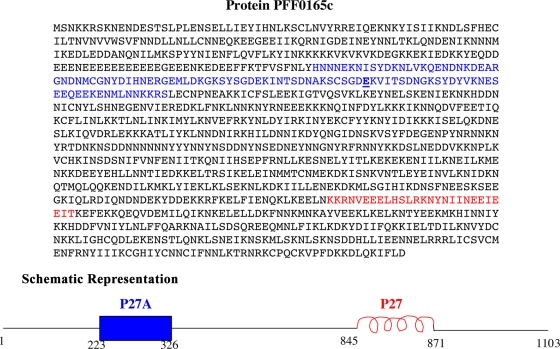
In the case of the PFF165c protein, it was found that it contains at least three segments that are low in hydrophobic amino acid content and high in hydrophilic amino acid content and thus are likely to be intrinsically unstructured. The work presented here focuses on one of these unstructured domains, the 104-residue-long peptide P27A, which was the largest one and did not contain long stretches of identical amino acids (poly-Glu or poly-Asn; Fig. 1).
FIG. 1.
Protein sequence and schematic representation of the PFF0165c protein. The protein sequence is 1,103 amino acids in length. Amino acids in blue form the unstructured region that was synthesized, shown by the blue block in the schematic representation. Residue 297, where a n E/G mutation occurs, is underlined. Amino acids in red form the α-helical coiled-coil region, shown in red in the schematic representation.
MATERIALS AND METHODS
Peptide synthesis.
The synthetic P27A polypeptide sequence with 104 amino acids was produced by solid-phase 9-fluorenylmethoxycarbonyl chemistry according to the method of Atherton and Sheppard (3) as modified by Verdini et al. (33), using Applied Biosystems 431A and 433A synthesizers (Foster City, CA). The final product was cleaved from p-alkoxybenzylalcohol resin and then reverse-phase high-pressure liquid chromatography (HPLC) purified. The purity was assessed by analytic C18 HPLC and matrix-assisted laser desorption ionization-time of flight mass spectrometry (Applied Biosystem) and was found to be higher than 85%. All the reagents used were purchased from Fluka (Buchs, Switzerland) and Novabiochem (Laufelfingen, Switzerland).
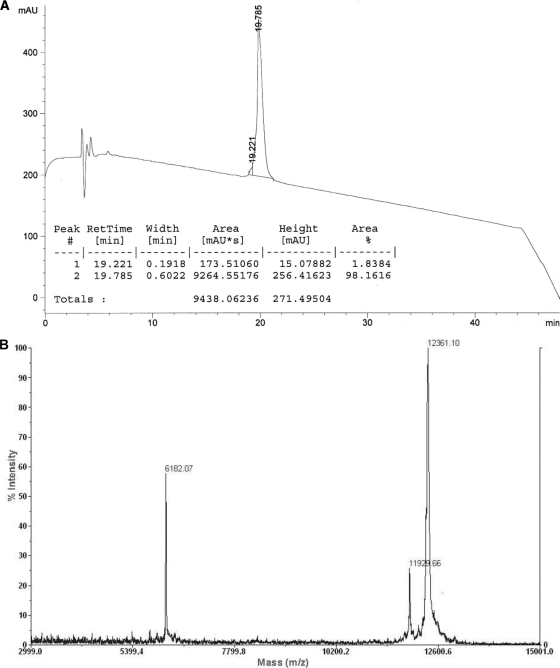
A pilot industrial synthesis of the construct was later prepared by Almac Sciences, Craigavon, Northern Ireland. The purity of the product was 98% as judged by analytical HPLC (Fig. 2A and B).
FIG. 2.
(A) Analytical HPLC of P27A. mAU, milli-absorbance unit, #, number. (B) Mass spectrum of P27A. Peaks at 12,361.10 and 6,182.07 represent the cytochrome c mass plus 1 or 2 hydrogen ions, respectively, taken as the standard. The peak at 11,929.66 represents the mass of P27A.
Circular dichroism (CD) spectroscopy.
The CD spectrum of P27A was evaluated on a Jasco J-810 CD spectrometer. The measurements were taken over a wavelength range of 190 to 250 nm at 20°C and pH 7.3 with a 1-mm-path-length cuvette. The polypeptide was dissolved in water or phosphate-buffered saline (PBS) at a concentration of 150 μg/ml.
Human sera.
Serum samples were collected from the regions of Burkina Faso and Tanzania, where malaria is endemic, and were the same samples used in our previous study (34). Briefly, the samples from Burkina Faso were collected from donors living in the village of Goundry in the province of Oubritenga (n = 37). Ethical approval was obtained from the Ministry of Health, Burkina Faso. The Tanzanian samples were obtained from donors in Kikwawila village in the Morogoro region (n = 42). Ethical approval was obtained from the Tanzanian Commission for Science and Technology. Negative control samples were Swiss adult donors with no history of malaria and no previous travel to areas where malaria is endemic (n = 6). Additional serum samples obtained from Papua New Guinea were collected in the Maprik District of the East Sepik Province during a cross-sectional survey in July 1992 within the framework of the Malaria Vaccine Epidemiology and Evaluation Project (MVEEP), supported by the United States Agency for International Development. Malaria is highly endemic in the area. Ethical clearance for MVEEP was obtained from the Papua New Guinea Medical Research Advisory Committee. Blood samples were obtained by venipuncture into tubes containing EDTA. Sera of 20 of these subjects were pooled and used as the positive control for enzyme-linked immunosorbent assays (ELISAs).
Human PBMC.
PBMC were obtained from adult donors living in Lagos, southwest Nigeria, where malaria transmission is high all year, with seasonal peaks during the rainy season (n = 17). Ethical approval was obtained from the Lagos State University Teaching Hospital (LASUTH) ethical review committee.
Affinity-purified human antibodies.
For the antigen-Sepharose conjugate preparation, 2 to 5 mg of antigen was dissolved in 1 ml of coupling buffer (0.1 M NaHCO3 containing 0.5 M NaCl, pH 8.0). The CNBr-Sepharose 4B (Amersham Bioscience AB, Uppsala, Sweden) was swollen in 1 mM HCl and then washed with coupling buffer. The antigen solution was added to the gel, and the mixture was stirred for 1 h at room temperature (RT). After the coupling reaction, excess antigen was washed away with coupling buffer. The remaining activated groups were blocked by treatment with ethanolamine (0.25 M, pH 8.0) for 30 min at RT. The gel was then washed with sodium acetate buffer (0.1 M, pH 4.0), followed by coupling buffer. The antigen-Sepharose beads were either used immediately or stored at 4°C in PBS (1×) containing 1 mM azide.
For the isolation of specific antibody, pooled human or rabbit sera were diluted five times with PBS (1×) containing 0.5 M sodium chloride and mixed with the antigen-Sepharose conjugate. This mixture was then stirred gently on a wheel overnight at 4°C. After centrifugation, the supernatant was collected and stored at −20°C for further use. The antigen-Sepharose beads were washed first with 5 ml of Trizma base Tris (20 mM containing 0.5 M NaCl, pH 8.0) and then with 5 ml of Tris (20 mM, pH 8.0). The elution of bound antibody was achieved with glycine (0.1 M, pH 2.5). The fractions obtained were instantly neutralized with Tris (1 M, pH 8.0) and dialyzed against phosphate buffer (0.1 M, pH 7.0), and the antibody concentration was determined by the absorbance of the solution at 280 nm.
ELISA.
Antigenic recognition of P27A by human, mouse, and rabbit sera was assessed as previously described (34). Briefly, each 96-well microtiter plate (Maxisorb F96; Nunc, Roskilde, Denmark) was coated with 1 μg/ml of polypeptide solution and incubated overnight in a humid chamber at 4°C. Serum solutions (50 μl/well) were added (1:200 dilution for human samples; 1 to 3 serial dilutions starting from 1:100 for mouse/rabbit sera) and incubated for 1 h. The secondary antibodies used were anti-human immunoglobulin G (IgG), anti-mouse polyclonal Ig, or anti-rabbit polyclonal Ig conjugated to alkaline phosphatase (Sigma, St. Louis, MO). Optical density (OD) was measured at 405 nm with a Thermo Labsystems Multiskan Ascent. A sample was considered positive when the OD was higher than the mean OD of the negative controls plus 3 standard deviations (SD). Negative controls were sera obtained from the naïve animals prior to immunization. For human IgG1 and IgG3 isotypes, anti-human Ig1 (Skybio) and IgG3 (Immunotech) were used at dilutions of 1:2,000 and 1:10,000, respectively. A third horseradish peroxidase-labeled goat anti-mouse antibody was used at 1:4,000, and the OD was read at 492 nm after color development. For mouse IgG isotypes, secondary antibodies were used at dilutions of 1:1,000 (IgG1), 1:5,000 (IgG2a), and 1:2,000 (IgG2b). The color development was stopped with 1 M H2SO4, and the OD was read at 492 nm.
T-cell proliferation.
PBMC were isolated from venous blood of 17 healthy adult Nigerians by Ficoll gradient centrifugation and stored in liquid nitrogen until use. Five replicates containing 2 × 105 cells per well were put in culture with 3 μM of P27A solution. The complete culture medium used consisted of RPMI medium (Sigma) supplemented with glutamine, 100 IU/ml penicillin-streptomycin, 100 μM nonessential amino acids, 100 μg/ml kanamycin (Invitrogen, Paisley, United Kingdom), 2 mM sodium pyruvate (Invitrogen), and 8% human AB serum (Blutspendendienst SRK, Bern, Switzerland). The positive control was a mixture of tetanus toxoid (Pasteur Merieux, Lyon, France), purified protein derivative (SSI, Copenhagen, Denmark), and Candida albicans extract (NIBSC, London, United Kingdom).
On day 5, the cultures were pulsed with 1 μCi of [3H]thymidine dissolved in culture medium. The cells were harvested 24 h later, and the tracer incorporation rate was measured by beta counting. The stimulation index (SI) was calculated with the formula (average count/minutes in culture with antigen)/(average count/minutes in culture without antigens). An SI value of ≥2 was considered positive.
Immunizations of mice and rabbits.
ICR (outbred) and C3H (H-2k) mice were injected three times with P27A (20 μg/mouse) formulated with Alhydrogel (500 μg/mouse) or EM005 (20 μg/mouse), a newly developed adjuvant (IDRI, Seattle, WA), on days 0, 21, and 42. Each mouse was injected with 500 μl of the formulation intraperitoneally (Alhydrogel) or 100 μl subcutaneously (EM005). Blood samples were collected from the base of the tail 10 days after second and third injections. Rabbits (four per group) were immunized intramuscularly with P27A (100 μg) with Alhydrogel or EM005 three times at 3-week intervals and bled at baseline and 10 days after each injection. The rabbit experiments were carried out by Eurogentec, Seraing, Belgium. Negative control animals used for ELISA analysis were unimmunized animals.
Indirect immunofluorescence antibody test.
For indirect immunofluorescence microscopy, infected red blood cells (iRBCs) were fixed in paraformaldehyde-glutaraldehyde as described previously (30) or smeared onto glass slides and fixed in ice-cold acetone/methanol (1:1 [vol/vol]) for 10 min. Slides were blocked for 1 h with 3% bovine serum albumin-PBS and probed with one of the following primary or secondary antibodies: mouse anti-P27A (1:200), rabbit anti-P27A (1:1,000), human affinity-purified antibodies specific for P27A (1:1,000), Cy3-labeled anti-mouse IgG (1:500), fluorescein isothiocyanate-labeled anti-human IgG (1:500; Jackson ImmunoResearch, Suffolk, United Kingdom), and Alexa Fluor 488-conjugated anti-rabbit IgG (1:200; Invitrogen). The slides were mounted in Vectashield (Vector Laboratories, Burlingame, CA) supplemented with 4′,6′-diamidino-2-phenylindole (DAPI) for nucleus staining. Fluorescence microscopy was performed on a Leica DM-5000B using a 60× oil immersion objective lens and documented with a Leica DC200 digital camera system.
ADCI assay.
The inhibition of P. falciparum (3D7 strain) growth in vitro in the presence of human monocytes (MN) and antigen-specific antibodies was carried out using methods described elsewhere (7). In brief, PBMC isolated from healthy (malaria naïve) blood donors were separated by Ficoll-Hypaque gradient, and MN were further separated by adherence to plastic surfaces as described previously (1 h at 37°C). Mature schizonts from a synchronized parasite culture were diluted at a starting parasitemia of 0.5% in human type AB+ RBCs from healthy donors, and the hematocrit was adjusted to 2% with RPMI 1640 culture medium. Duplicate assays were set up in preheated 96-well flat-bottom sterile plastic plates (TPP, Trasadingen, Switzerland) containing 2 × 105 MN/well with the addition of 50 μl of parasite culture mixed with 50 μl of RPMI and various dilutions of each pool of sera at final concentrations of 0.5% to 10%. Control wells with parasite culture and RPMI medium were done in parallel. The plates were incubated in a candle jar at 37°C in a 5% CO2 incubator for 96 h. Thin blood smears for each well were fixed in methanol and stained in eosin and methylene blue. The parasitemia was determined by microscopic examination and counting of at least 5,000 RBCs in duplicate. The specific growth inhibitory index (SGI), which estimates the parasite growth inhibition due to the effect of test antibodies cooperating with MN, was calculated as follows: SGI = 100 × [1 − (% parasitemia with MN and test antibodies/% parasitemia with test antibodies)/(% parasitemia with MN and IgGs from healthy donors/% parasitemia with IgGs from healthy donors)].
For each antibody tested, duplicate wells included the following controls: (i) for nonspecific monocytic inhibition, both MN plus parasite and MN plus IgGs from healthy donors plus parasites, and (ii) for direct inhibition by control or test IgGs, both IgGs from healthy donors plus parasites and test antibodies plus parasites. A pool of IgGs from sera of hyperimmune African donors and IgGs from healthy donors were used at a final concentration of 1 mg/ml as positive and negative controls, respectively. Immunopurified test human antibodies were used at 15 μg/ml, while mouse sera were used at different dilutions.
Parasite culture.
The culture strains were grown in 10-cm petri dishes and cultured by standard methods in an atmosphere of 93% N2, 4% CO2, 3% O2 at 37°C as described previously (31). The culture medium was 10.44 g/liter RPMI 1640 supplemented with 5.94 g/liter HEPES, 5 g/liter Albumax II, 50 mg/liter hypoxanthine, 2.1 g/liter sodium bicarbonate, and 100 mg/liter neomycin.
Preparation of parasite protein extracts.
The protein extracts of late-stage parasites, trophozoites and schizonts, were obtained from P. falciparum 3D7-infected erythrocytes in a 30-ml petri dish (5% hematocrit, 6% parasitemia) which was enriched using a magnetic cell sorter (Miltenyi Biotec, Bergisch Gladbach, Germany). The enriched infected erythrocytes were lysed in a 200-ml volume of PBS, 0.03% saponin (Fluka) in the presence of proteinase inhibitors (Roche Diagnostics, Basel, Switzerland) for 5 min at 4°C. The parasites were pelleted by centrifugation at 4,000 × g for 10 min, the supernatant was removed, and an equal volume of 2× Laemmli sample buffer was added. The parasite pellet was resuspended in 0.1 M Tris, pH 6.8, and an equal volume of 2× Laemmli sample buffer.
Western blot analysis.
Protein extracts were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to nitrocellulose (0.2 mm; Whatman Schleicher + Schuell, Florham Park, NJ) under cooling conditions for 1 h at 80 V and an additional hour at 100 V. The membrane was blocked for 1 h in PBS-3% milk powder at RT, and the primary antibody was diluted 1:500 in PBS and 1.5% milk powder. The membrane was incubated in the primary antibody solution overnight at 4°C. It was then washed 8× in PBS, 0.05% Tween 20 and incubated with a peroxidase-conjugated goat anti-mouse IgG antibody (1:3,000, Sigma). Bound secondary antibodies were visualized using Western Lightning (PerkinElmer Life Sciences, Schwerzenbach, Switzerland).
Polymorphism study of P. falciparum in vitro culture strains and field samples.
The genetic diversity of the P27A sequence was assessed in 13 P. falciparum in vitro culture strains (3D7, W2mef, HB3, ITG2F6, IFA18, FVO, 7G8, K1, RO33, MAD20, FCR3, RFCR3, and FC27) and malaria-positive field samples from Tanzania and Papua New Guinea. Sixty-three blood samples were obtained from 1- to 5-year-old children from Ifakara, Tanzania, with uncomplicated malaria (18). Nineteen samples were from asymptomatic donors living in Papua New Guinea (29). Genomic DNA was isolated by phenol-chloroform extraction or with a QIAamp DNA blood mini kit 250 (Qiagen, Hombrechtikon, Switzerland). Forward primer 5′-ACACTTTGCACAGTTCCTATCTTCTCTTCTA-3′ and reverse primer 5′-AGAAGGAGAAGAAGAAAATAAAGAGGATGAAG-3′ were used to amplify the P27A region from genomic parasite DNA. PCR conditions consisted of denaturation at 94°C for 5 min followed by 35 cycles of denaturation (94°C for 30 s), annealing (58°C for 1 min), and extension (72°C for 1 min). The reaction products were incubated at 72°C for 10 min to ensure complete DNA extension. The PCR products were directly sequenced on both strands and aligned with Auto Assembler software to screen for single nucleotide polymorphisms (SNPs) and length polymorphisms within the sequence corresponding to P27A.
RESULTS AND DISCUSSION
Only a few malaria antigens are currently being evaluated as candidate vaccines (23, 25). The published P. falciparum genomic data (16) provide ample opportunities for the discovery of new malaria antigens. Our group previously explored this publicly available data to identify novel malaria antigens through the combination of chemical peptide synthesis, natural human immunity, and biological functional assays (10, 34). Exploration of the structural domains present within one of the hypothetical proteins, PFF0165c, identified by the α-helical coiled-coil P27 (34) revealed several segments with low hydrophobic amino acid content. Such segments have been found to be functional and to fold on binding to their targets (13). The P. falciparum genome is predicted to contain numerous such unstructured proteins and segments (15). Similar predicted unstructured segments (or full-length proteins) have been found in the genomes of other organisms (37), and the frequency likely increases with increasing complexity of organisms.
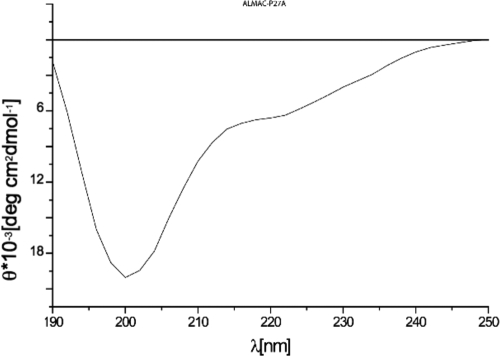
We chemically synthesized one such segment (P27A) that consists of amino acid residues 222 to 326 of PFF0165c and explored its malaria vaccine potential. This choice was dictated by several factors which took into account the feasibility of chemical synthesis of the fragment; the necessity to contain a maximum number of T- and B-cell epitopes in order to be immunogenic in the majority of the target population; and the lack of stretches containing poly-glutamic acid or poly-asparagine residues, potentially dangerous when present in a vaccine (Fig. 1). The advantage of choosing unstructured regions of proteins is that no folding procedure is required to mimic the native conformation. As expected, the CD pattern of P27A solution in water or PBS was indicative of random coil structure (Fig. 3), with a single deep minimum at around 200 nm, thus confirming the bioinformatics prediction (32, 39).
FIG. 3.
The CD spectrum of P27A was assessed over a 190- to 250-nm-wavelength range at 20°C, pH 7.3, in a 150-μg/ml solution, using a Jasco J-810 spectrometer. There is a single dip at around 200 nm, which is indicative of random coil conformation.
Antigenic recognition by human sera and PBMC.
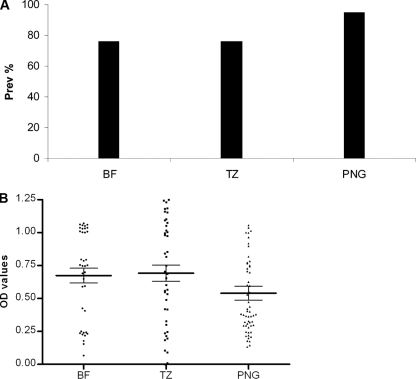
The antigenic recognition of P27A was assessed with serum samples from adults living in the regions of Burkina Faso, Tanzania, and Papua New Guinea, where malaria is endemic. The sera of the majority of donors living in these geographically diverse regions recognized P27A (Table 1 and Fig. 4A). The donor samples were obtained from adults living in Burkina Faso and Tanzania and from children living in Papua New Guinea. The prevalence of anti-P27A antibodies among these populations ranged from 76% (Burkina Faso and Tanzania) to 95% (Papua New Guinea). The average OD values for all donor samples were generally high (0.67, 0.68, and 0.54, respectively), while those for positive samples were 1.29, 1.18, and 0.56, respectively (Fig. 4B). The average OD was lowest among samples obtained from children living in Papua New Guinea, as expected. This may suggest that there is age-related (i.e., exposure related) acquisition of anti-P27A antibodies, as is observed with other malaria antigens. The majority of these donors also have P27A-specific antibodies of the IgG1 and IgG3 cytophilic classes (Table 1), which have been shown to be necessary for protection against intracellular pathogens, especially malaria (8, 9, 28). Such a distribution of IgG isotypes specific for P27A may suggest that it is an important part of the natural protection against malaria infection (or disease) that is observed in adults living in regions where malaria is endemic.
TABLE 1.
Prevalence of total IgG and cytophilic IgG1/IgG3 among adult donors living in regions where malaria is endemic
| Ig class | No. (%) of donors with indicated Iga from: |
||
|---|---|---|---|
| Burkina Faso (n = 37) | Tanzania (n = 42) | Papua New Guinea (n = 56) | |
| Total IgG | >28 (76) | 32 (76) | 53 (95) |
| IgG1 | 25 (68) | 30 (71) | 50 (89) |
| IgG3 | 28 (76) | 31 (73) | 52 (93) |
Positive samples were those with OD values greater than the average of the OD values of negative controls (Swiss adults with no history of malaria) plus 3 SD.
FIG. 4.
(A) Prevalence of recognition of P27A by sera of adults living in regions of Burkina Faso (BF, n = 37), Tanzania (TZ, n = 42), and Papua New Guinea (PNG, n = 56) where malaria is endemic. A serum sample was considered positive when the OD is greater than the average OD of naïve sera plus 3 SD. (B) Average OD values of donor samples with significant anti-P27A antibodies (i.e., serum samples with OD values greater than the average OD of negative controls plus 3 SD). BF, Burkina Faso (n = 28/37); TZ, Tanzania (n = 32/42); PNG, Papua New Guinea (n = 53/56). Bars represent mean OD values, while whikers indicate ±SD.
Affinity-purified human antibodies specific for P27A recognized P. falciparum 3D7-infected erythrocytes (Fig. 5 and 6), as did mouse and rabbit antibodies (details below).
FIG. 5.
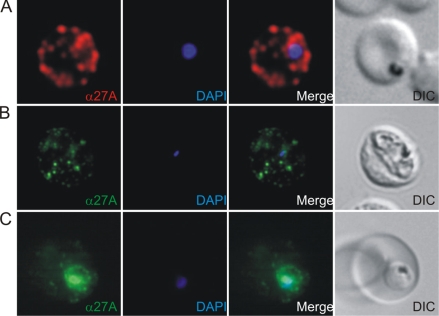
Immunofluorescence staining of malaria-infected erythrocytes. (A) Trophozoite-stage parasites were stained with mouse antibodies against P27A (red). Mouse antibodies recognize the parasite and structures of the parasite in the iRBC cytosol. (B, C) Polyclonal rabbit sera (B) and human affinity-purified sera (C) specific to P27A recognize identical structures, suggesting that all the sera recognize the same target (green), the parasite-derived PFF0165c protein. Nuclei are stained with DAPI (in blue). Right panels, transmission light microscopy images of the iRBC (differential interference contrast). α, anti.
FIG. 6.
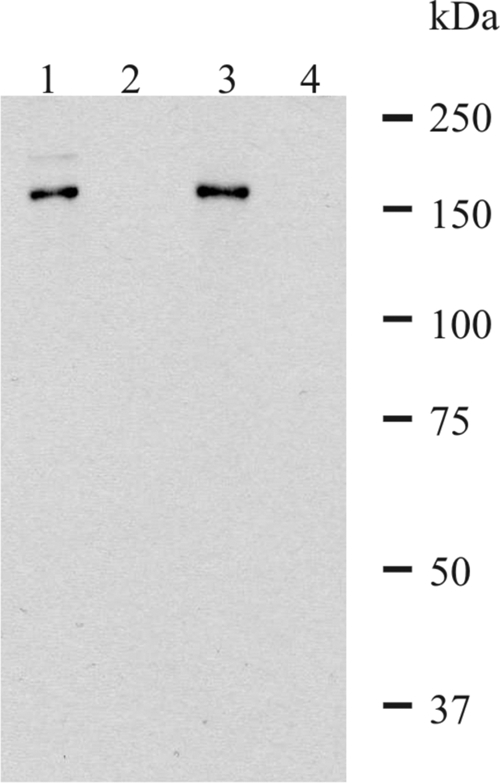
Western blot analysis of 3D7 parasite lysate with mouse sera specific for P27A and affinity-purified rabbit sera specific for P27A. Late stages (trophozoites/schizonts) were enriched using a magnetic cell sorter. The enriched infected erythrocytes were fractionated into pellet fraction (lanes 1 and 3) and supernatant fraction (lanes 2 and 4) by saponin lysis in a 200-μl volume. P27A-specific rabbit sera (lanes 1 and 2) and P27A-specific mouse sera (lanes 3 and 4) recognize the protein at 160 kDa in the pellet fraction.
Prevalence of recognition by PBMC and sera of Nigerian adults.
Antigenic recognition of P27A by PBMC of adults exposed to malaria was assessed by T-cell proliferation assay, and SI values equal to or higher than 2 were considered positive. Over half (9/17, 53%) of the PBMC samples from adult donors from Nigeria proliferated when stimulated with a 3 μM solution of P27A. The average SI of the positive samples was 3.6, with a range of 2.1 to 5.3. Recognition of P27A by sera was assessed by ELISA, and a test sample was considered positive when the OD value was greater than the average of the OD values of naïve controls (adult Europeans with no prior exposure to malaria) plus 3 SD. Of sera of the same 17 donors, 14 (82%) recognized P27A, and the average OD value was 0.31 (P = 0.0668); these results are comparable to the SI results. All donor PBMC proliferated in the presence of tetanus toxoid, purified protein derivative, and C. albicans extract (positive control).
The high prevalence of recognition of P27A by both sera and T cells of semi-immune adults, together with its immunogenicity in several strains of mice and rabbits (see below), suggests that P27A contains multiple B- and T-cell epitopes and that the T-cell epitopes are recognized by a wide range of major histocompatibility complex molecules present in the general population. These are important characteristics to be considered for a potential malaria vaccine.
Immunogenicity in mice and rabbits.
The immunogenicity of P27A formulated with Alhydrogel was studied in several strains of mice. All the C3H (H-2k) mice immunized with this formulation had antibody titers of over 1:1,000 after the third injection, with an average of 160 × 103 (Table 2). The prevalent IgG isotypes were the cytophilic IgG1 and IgG2a. However, lower titers were obtained in outbred ICR mice. Similar immunogenicity results were also obtained with the Montanide ISA 720 formulation (data not shown). This prompted us to assess P27A immunogenicity with the recently developed adjuvant EM005 (IDRI, Seattle, WA).
TABLE 2.
Geometric mean antibody titers of sera of mice and rabbits after three injections with P27A formulated with alum or EM005
| Type of animal | Ig classb | Aluma |
EM005a |
||||
|---|---|---|---|---|---|---|---|
| GMT (log10) | SD (log10) | No. of responders | GMT (log10) | SD (log10) | No. of responders | ||
| Rabbits | 3.74 | 1.62 | 3/4 | 4.32 | 0.87 | 4/4 | |
| ICR mice | 2.2 | 0.48 | 1/8 | 3.73 | 1.03 | 7/8 | |
| C3H mice | 5.23 | 0.4 | 5/5 | 5.9 | 0.4 | 5/5 | |
| IgG1 | 5.04 | 0.43 | 5/5 | 5.57 | 0.40 | 5/5 | |
| IgG2a | 4.31 | 1.37 | 4/5 | 5.97 | 0.87 | 5/5 | |
| IgG2b | 3.64 | 0.80 | 4/5 | 5.52 | 0.72 | 5/5 | |
GMT, geometric mean antibody titer. Antibody titer is the serum dilution at which the OD is greater than the average OD of sera of naïve mice/rabbits plus 3 SD. Responders are mice/rabbits with titers of >1:1,000.
Values obtained from C3H mice.
EM005 is an oil-in-water stable emulsion containing a lipid A-like synthetic compound (2, 6). Lipid A is a component of lipopolysaccharide, which is responsible for the toxicity associated with gram-negative bacteria. Lipid A is known to be a potent Toll-like receptor 4 agonist that activates antigen-presenting cells, leading to the induction of high antibody titers and Th1 response (1, 5, 11, 19, 21, 24, 27, 35). Unlike the currently available lipid A-like adjuvant MPL, which is naturally derived and thus heterogeneous in composition, with a predisposition to batch-to-batch variations, EM005 is a homogeneous synthetic product with potentially higher potency and a better batch-to-batch consistency. It has been found to be safe in several animal models, including nonhuman primates (S. Bertholet, personal communication).
In outbred ICR mice, seven of the eight mice injected with the EM005 formulation had antibody titers of over 1:1,000, with an average of 57 × 103, compared to only one out of eight with the Alhydrogel formulation (Table 2). The average antibody titers induced in C3H mice were also higher with the EM005 formulation than with the Alhydrogel formulation (1,094 × 103 and 160 × 103, respectively). Very high titers of the cytophilic IgG1 and IgG2a were induced (Table 2). These isotypes are functionally similar to the predominant, naturally induced cytophilic antibodies found in human sera (IgG1 and IgG3).
The mouse antibodies recognized native proteins in erythrocytes infected with different strains of P. falciparum (Fig. 5 shows results for 3D7; results for others are not shown) at dilutions of up to 1:8,000. This is consistent with the finding that the sequence of P27A is conserved, with only a single mutation among the 90 P. falciparum field and laboratory isolates tested (see below).
High antibody titers were also induced in rabbits (four per group) injected with P27A formulated with EM005 and, to a lower degree, Alhydrogel.
The antigen specificity of the antibodies obtained with the formulations used was determined by using sera from mice and rabbits immunized with two MSP2 long synthetic fragments in Alhydrogel and EM005. These sera did not recognize P27A (data not shown).
Mouse polyclonal antibodies and affinity-purified rabbit antibodies specific for P27A detected the corresponding protein in Western blot analysis of lysate of late-stage parasites (trophozoites/schizonts) (Fig. 7). Affinity-purified rabbit antibodies specific for P27A mainly recognized a protein at 160 kDa, exclusively in the pellet fraction (Fig. 6, lane 1). This suggests that the protein associates with membranes (Maurer's clefts, RBC membrane) after export out of the parasite. Similar to the affinity-purified rabbit sera, the polyclonal mouse sera recognized a protein at 160 kDa (lane 3), suggesting identical specificities of mouse and rabbit sera. The full-length PFF0165c protein has an expected size of 132 kDa, which apparently runs at a higher molecular mass, probably due to a low content of hydrophobic amino acids.
FIG. 7.
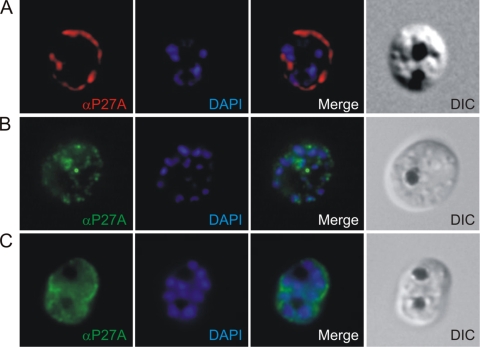
Immunofluorescence staining of malaria-infected erythrocytes. (A) Schizont-stage parasites were stained with mouse antibodies against P27A (red). Polyclonal mouse antibodies recognize structures in very close proximity to or at the RBC membrane. (B, C) Affinity-purified rabbit sera (B) and human affinity-purified sera (C) specific to P27A recognize identical structures (green). Nuclei are stained with DAPI (blue). Right panels, transmission light microscopy images of the iRBC (differential interference contrast). α, anti.
Genetic diversity of the sequence of P27A.
The genetic diversity of P27A was assessed by PCR amplification and direct sequencing in order to detect SNPs and length polymorphisms. P27A shows limited polymorphism with essentially a single SNP at nucleotide position 875 (T→C) that results in an amino acid change from glutamic acid to glycine at position 292 of the protein (E292G) (Fig. 1). The prevalence varies, with allelic frequencies of 0.31 (6/19) in samples from Papua New Guinea, 0.6 (38/63) in samples from Tanzania, and 0.44 (4/11) in worldwide isolates published in the SNP database available at PlasmoDB. Two additional SNPs at nucleotide positions 880 (C→T; V294I) and 798 (C→T; M266I) were only observed in one isolate from PlasmoDB by microarray, a technique prone to false positives (22).
Cytological localization of P27A.
In indirect immunofluorescence assays, P. falciparum-infected erythrocytes (3D7 strain) were stained with murine antibodies specific to P27A and analyzed by immunofluorescence microscopy. With trophozoites, P27A mouse sera recognized the parasites in the iRBC and parasite-derived structures in the cytoplasm of the iRBC (Fig. 5A). The polyclonal rabbit sera (Fig. 5B) and the human affinity-purified sera specific for P27A (Fig. 5C) localized the PFF0165c protein to identical structures. With schizonts, the polyclonal mouse sera recognized structures in close proximity to the RBC membrane or the corresponding protein at the RBC membrane. Similar structures are recognized by the affinity-purified rabbit (Fig. 6B) and human sera (Fig. 6C), suggesting identical specificities of the antibodies generated.
Biological activity in ADCI assay of mouse and human purified antibodies.
One of the principal mechanisms that mediates clinical malaria in humans has been shown to be the cooperation between cytophilic antibodies and MN (7, 20). The in vitro ADCI assay, based on this mechanism, has been used to assess the capacity of purified human and murine antibodies to inhibit malaria parasite growth (Fig. 8). This mechanism has also been shown to be induced following immunization with a malaria vaccine candidate (12).
FIG. 8.
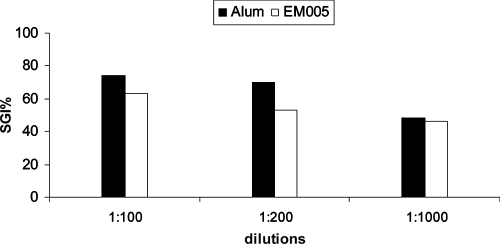
Results of ADCI assays with murine antibodies. Pooled sera of C3H (H-2k) mice immunized with P27A formulated with Alhydrogel and EM005. The percent SGI is calculated with the formula 100 × [1 − (% parasitemia with MN and test antibodies/% parasitemia with test antibodies)/(% parasitemia with MN and IgGs from healthy donors/parasitemia with IgGs from healthy donors)].
The ability of the human Fc receptors to bind cytophilic murine isotypes (IgG2a and IgG2b) enables the ADCI mechanism. Significant levels of inhibition of parasite growth were obtained with pooled sera of C3H mice immunized with P27A formulated with alum and EM005 (Fig. 8). This was as high as 74% SGI at 1:100 dilutions and still significant at 46% at 1:1,000 dilutions. The degree of inhibition obtained with affinity-purified human antibodies was also very high and comparable to that of pooled Igs of adults living in areas of Africa where malaria is endemic (7, 12, 20). These results, obtained with two different adjuvants in animal models and with naturally induced antibodies from humans, suggest that the primary mechanism of protection employed by P27A antibodies is likely to be in cooperation with MN.
Concluding remarks.
P27A, a synthetic polypeptide that is derived from a recently identified unstructured segment present in the malaria protein PFF0165c (2), meets the principal requirements that are vital for a malaria vaccine candidate. In fact, it is recognized by sera of a majority of naturally exposed individuals, is highly immunogenic with different adjuvants, and has an extremely well conserved sequence. Both human-specific antibodies and antibodies induced in mice actively inhibit parasite growth and recognize native proteins in immunofluorescence assays. In addition, human P27A-specific antibodies are associated with clinical protection against malaria in humans in two cohorts of semi-immune adults (P. Druilhe, C. Roussilhon, A. Jafarshad, A. Kajava, S. Olugbile, A. Tall, C. Sokhna, C. Kulangara, I. Felger, and G. Corradin, unpublished data). Taken together, these data support further preclinical and clinical development of P27A, which is scheduled to begin soon.
Acknowledgments
This work was supported by Swiss National Science Foundation grant no. 310000-112244 and by grants from the Swiss Secretary for Education and Research (no. 0536) in the context of Commission of the European Communities, Sixth Framework Programme, contract LSHP-CT-2003-503240, Mucosal Vaccines for Poverty-Related Diseases (MUVAPRED), and the European Malaria Vaccine Initiative.
We would like to thank S. Reed and T. Vedvick for their support in providing EM005, which was developed under grant no. 42387 from the Bill & Melinda Gates Foundation.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 28 September 2009.
REFERENCES
- 1.Alderson, M. R., P. McGowan, J. R. Baldridge, and P. Probst. 2006. TLR4 agonists as immunomodulatory agents. J. Endotoxin Res. 12:313-319. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, R. C., C. B. Fox, T. S. Dutill, N. Shaverdian, T. Evers, G. R. Poshusta, et al. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf. B, in press. [DOI] [PubMed]
- 3.Atherton, E., and R. C. Sheppard. 1989. Solid phase synthesis; a practical approach. Oxford University Press, Oxford, United Kingdom.
- 4.Audran, R., M. Cachat, F. Lurati, S. Soe, O. Leroy, G. Corradin, P. Druilhe, and F. Spertini. 2005. Phase 1 malaria vaccine trial with a long synthetic peptide derived from merozoite surface protein 3. Infect. Immun. 3:8017-8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldridge, J. R., P. McGowan, J. T. Evans, C. Cluff, S. Mossman, D. Johnson, and D. Persing. 2004. Taking a toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and immunotherapeutic agents. Expert Opin. Biol. Ther. 4:1129-1138. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin, S., S. Bertholet, M. Kahn, I. Zharkikh, G. Ireton, T. Vedvick, S. Reed, and R. Coler. 2009. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine 27:3063-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouharoun-Tayoun, H., and P. Druilhe. 1992. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 60:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouharoun-Tayoun, H., and P. Druilhe. 1992. Antibodies in falciparum malaria: what matters most, quantity or quality? Mem. Inst. Oswaldo Cruz 87:229-234. [DOI] [PubMed] [Google Scholar]
- 10.Corradin, G., V. Villard, and A. Kajava. 2007. Protein structure based strategies for antigen discovery and vaccine development against malaria and other pathogens. Endocr. Metab. Immune Disord. Drug Targets 7:259-265. [DOI] [PubMed] [Google Scholar]
- 11.De Becker, G., V. Moulin, B. Pajak, C. Bruck, M. Francotte, C. Thiriart, J. Urbain, and M. Moser. 2000. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int. Immunol. 12:807-815. [DOI] [PubMed] [Google Scholar]
- 12.Druilhe, P., F. Spertini, D. Soesoe, G. Corradin, P. Mejia, S. Singh, R. Audran, A. Bouzidi, C. Oeuvray, and C. Roussilhon. 2005. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLoS Med. 2:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyson, H. J., and P. E. Wright. 2002. Coupling of folding and binding for unstructured proteins. Curr. Opin. Struct. Biol. 12:54-60. [DOI] [PubMed] [Google Scholar]
- 14.Dyson, H. J., and P. E. Wright. 2005. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 6:197-208. [DOI] [PubMed] [Google Scholar]
- 15.Feng, Z. P., X. Zhang, P. Han, N. Arora, R. F. Anders, and R. S. Norton. 2006. Abundance of intrinsically unstructured proteins in P. falciparum and other apicomplexan parasite proteomes. Mol. Biochem. Parasitol. 150:256-267. [DOI] [PubMed] [Google Scholar]
- 16.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, et al. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genton, B., I. Betuela, I. Felger, F. Al-Yaman, R. F. Anders, A. Saul, L. Rare, M. Baisor, K. Lorry, G. V. Brown, et al. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 185:820-827. [DOI] [PubMed] [Google Scholar]
- 18.Irion, A., I. Felger, S. Abdulla, T. Smith, R. Mull, M. Tanner, C. Hatz, and H. P. Beck. 1998. Distinction of recrudescences from new infections by PCR-RFLP analysis in a comparative trial of CGP 56 697 and chloroquine in Tanzanian children. Trop. Med. Int. Health. 3:490-497. [DOI] [PubMed] [Google Scholar]
- 19.Ismaili, J., J. Rennesson, E. Aksoy, J. Vekemans, B. Vincart, Z. Amraoui, F. Van Laethem, M. Goldman, and P. M. Dubois. 2002. Monophosphoryl lipid A activates both human dendritic cells and T cells. J. Immunol. 168:926-932. [DOI] [PubMed] [Google Scholar]
- 20.Jafarshad, A., M. H. Dziegiel, R. Lundquist, L. K. Nielsen, S. Singh, and P. L. Druilhe. 2007. A novel antibody-dependent cellular cytotoxicity mechanism involved in defence against malaria requires costimulation of monocytes FcγRII and FcγRIII. J. Immunol. 78:3099-3106. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, A. G., and M. A. Tomai. 1990. A study of the cellular and molecular mediators of the adjuvant action of a non-toxic monophosphoryl lipid A. Adv. Exp. Med. Biol. 256:567-579. [DOI] [PubMed] [Google Scholar]
- 22.Kidgell, C., S. K. Volkman, J. Daily, J. O. Borevitz, D. Plouffe, Y. Zhou, J. R. Johnson, K. Le Roch, O. Sarr, O. Ndir, et al. 2006. A systematic map of genetic variation in Plasmodium falciparum. PLoS Pathog. 2:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malkin, E., F. Dubovsky, and M. Moree. 2006. Progress towards the development of malaria vaccines. Trends Parasitol. 22:292-295. [DOI] [PubMed] [Google Scholar]
- 24.Martin, M., S. M. Michalek, and J. Katz. 2003. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect. Immun. 71:2498-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matuschewski, K. 2006. Vaccine development against malaria. Curr. Opin. Immunol. 18:449-457. [DOI] [PubMed] [Google Scholar]
- 26.McColl, D. J., and R. S. Anders. 1997. Conservation of structural motifs and antigenic diversity in the Plasmodium falciparum merozoite surface protein-3 (MSP3). Mol. Biochem. Parasitol. 90:21-31. [DOI] [PubMed] [Google Scholar]
- 27.Puggioni, F., S. R. Durham, and J. N. Francis. 2005. Monophosphoryl lipid A (MPL) promotes allergen-induced immune deviation in favour of Th1 responses. Allergy 60:678-684. [DOI] [PubMed] [Google Scholar]
- 28.Roussilhon, C., C. Oeuvray, C. Müller-Graf, A. Tall, C. Rogier, J. F. Trape, M. Theisen, A. Balde, J. L. Pérignon, and P. Druilhe. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 4:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoepflin, S., J. Marfurt, M. Goroti, M. Baisor, I. Mueller, and I. Felger. 2008. Heterogeneous distribution of Plasmodium falciparum drug resistance haplotypes in subsets of the host population. Malar. J. 7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonkin, C. J., G. G. van Dooren, T. P. Spurk, N. S. Struck, R. T. Good, E. Handman, A. F. Cowman, and G. I. McFadden. 2004. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol. Biochem. Parasitol. 137:13-21. [DOI] [PubMed] [Google Scholar]
- 31.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 32.Uversky, V. N. 2002. What does it mean to be natively unfolded? Eur. J. Biochem. 269:2-12. [DOI] [PubMed] [Google Scholar]
- 33.Verdini, A., S. Terenzi, V. Brossard, M. Roggero, and G. Corradin. 2008. Oxidative folding of synthetic polypeptides S-protected as tert-butylthio derivatives. J. Pept. Sci. 14:1271-1282. [DOI] [PubMed] [Google Scholar]
- 34.Villard, V., G. W. Agak, G. Frank, A. Jafarshad, C. Servis, I. Nébié, S. B. Sirima, I. Felger, M. Arevalo-Herrera, S. Herrera, F. Heitz, et al. 2007. Rapid identification of malaria vaccine candidates based on alpha-helical coiled coil protein motif. PLoS ONE 2:e645.r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler, A. W., J. S. Marshall, and J. T. Ulrich. 2001. A Th1 adjuvant, MPL, enhances antibody profiles in experimental animals, suggesting it has the potential to improve the efficacy of allergy vaccines. Int. Arch. Allergy Immunol. 126:135-139. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. April 2005. New vaccines against infectious diseases: research and development status, updated February 2006. Initiative for Vaccine Research, WHO, Geneva, Switzerland. http://www.who.int/vaccine_research/documents/en/Status_Table.pdf.
- 37.Wright, P. E., and J. H. Dyson. 1999. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J. Mol. Biol. 293:321-331. [DOI] [PubMed] [Google Scholar]
- 38.Yang, X., C. G. Adda, D. W. Keizer, V. J. Murphy, M. M. Rizkalla, M. A. Perugini, D. C. Jackson, R. F. Anders, and R. S. Norton. 2007. A partially structured region of a largely unstructured protein, Plasmodium falciparum merozoite surface protein 2 (MSP2), forms amyloid-like fibrils. J. Pept. Sci. 13:839-848. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, X., M. A. Perugini, S. Yao, C. G. Adda, V. J. Murphy, A. Low, R. F. Anders, and R. S. Norton. 2008. Solution conformation, backbone dynamics and lipid interactions of the intrinsically unstructured malaria surface protein MSP2. J. Mol. Biol. 379:105-121. [DOI] [PMC free article] [PubMed] [Google Scholar]