Abstract
Q fever is a zoonotic disease of worldwide significance caused by the obligate intracellular bacterium Coxiella burnetii. Humans with Q fever may experience an acute flu-like illness and pneumonia and/or chronic hepatitis or endocarditis. Various markers demonstrate significant phylogenetic separation between and clustering among isolates from acute and chronic human disease. The clinical and pathological responses to infection with phase I C. burnetii isolates from the following four genomic groups were evaluated in immunocompetent and immunocompromised mice and in guinea pig infection models: group I (Nine Mile, African, and Ohio), group IV (Priscilla and P), group V (G and S), and group VI (Dugway). Isolates from all of the groups produced disease in the SCID mouse model, and genogroup-consistent trends were noted in cytokine production in response to infection in the immunocompetent-mouse model. Guinea pigs developed severe acute disease when aerosol challenged with group I isolates, mild to moderate acute disease in response to group V isolates, and no acute disease when infected with group IV and VI isolates. C. burnetii isolates have a range of disease potentials; isolates within the same genomic group cause similar pathological responses, and there is a clear distinction in strain virulence between these genomic groups.
Coxiella burnetii, the etiologic agent of acute and chronic Q fever, is an obligate intracellular bacterium with worldwide distribution and a diverse host range. Livestock serve as the organism's primary reservoir and may be asymptomatic carriers or exhibit reproductive disorders. Ticks are important in the maintenance of the disease in nature and have been shown to transmit the infection transovarially (37). Humans are most often infected through inhalation of the bacterium in fine-particle aerosols, though transmission may also occur through ingestion of the organism from contaminated, unpasteurized dairy products (22, 27). Although a high percentage of infections may result in subclinical or asymptomatic infection, humans can become ill from exposure to as few as 10 organisms (6) and may display signs of (i) an acute flu-like illness with or without pneumonia and/or hepatitis (30, 31) or (ii) a chronic disease manifesting most frequently as endocarditis and/or hepatitis (40, 41).
C. burnetii isolates have been obtained from natural Q fever infections in humans and other animals. Several theories have been proposed to explain the dichotomy in development of acute and chronic Q fever. Unique sequence differences between genomic groups are correlated with the clinical expression of Q fever (44). Biochemical markers have grouped C. burnetii isolates from chronic-disease patients separately from acute-disease/arthropod/domestic animal isolates, but whether these groupings predict virulence potential and acute/chronic-disease outcomes has not yet been fully resolved (20). Samuel et al. were the first to separate these isolates and their resulting diseases based on plasmid patterns (44). Hackstadt used variations in lipopolysaccharide (LPS) banding patterns to divide isolates of C. burnetii into three groups, and group distinction was noted in correlation with acute or chronic disease (16). Hendrix et al. separated C. burnetii isolates into six genomic groups (20). Group I to III isolates have a QpH1 plasmid and have been isolated from ticks, acute human Q fever cases, cow's milk, and livestock abortions. Groups IV and V have a QpRS plasmid or no plasmid (with plasmid-related sequences integrated into the chromosome), respectively, and have been associated with livestock abortions and human chronic endocarditis or hepatitis. Group VI isolates were collected from wild rodents in Dugway, UT, and were infectious but avirulent in rodent models of disease (47, 48). Jager et al. used restriction fragment length polymorphism (RFLP) to differentiate 80 C. burnetii isolates and reproduced distinguishable patterns for reference isolates in groups I, IV, V, and VI (23). More recently, multiple-locus variable nucleotide tandem repeat analyses (49) have validated these groupings. Infrequent-restriction-site PCR of 14 livestock and tick isolates resulted in six groups; subsequent multiple-locus variable-number tandem repeat analysis typing of 42 isolates revealed 36 genotypes (2). Glazunova et al. used multispacer sequence typing to analyze 173 isolates, a majority of which were acquired from chronic-disease patients, and identified 30 genotypes in three monophyletic groups; an association between the plasmid type, some genotypes, and the nature of disease was observed (15). These monophyletic groups supported the early RFLP groups and placed groups I, II, and III in one monophyletic group; group IV in the second monophyletic group; and group V in the third monophyletic group. A comprehensive microarray-based whole-genome comparison by Beare et al. confirmed the relatedness of RFLP-grouped isolates and added two more genomic groups, VII and VIII (4). Differences in novel gene contents and pseudogenes may be factors in the variations in virulence seen among group I, IV, V, and VI isolates (5). It has been shown in an intraperitoneal (i.p.)-challenge guinea pig model that 101 organisms of the acute-disease-associated group I isolate Nine Mile RSA493 (NM) caused fever, but 106 chronic-disease-associated group IV isolate MSU Goat Q177 (Priscilla) organisms were required to induce fever (36).
In opposition to the theory of genotype/pathotype correlation, Stein and Raoult evaluated 28 human isolates and found that isolates bearing the QpH1 plasmid were present in both acute and chronic Q fever patients in France and that isolates without the QpH1 plasmid were able to cause acute disease (46). QpH1 plasmid-containing isolates have also been isolated from chronic-endocarditis patients (50). Several groups have speculated that host factors are primarily responsible for the outcome of infection with C. burnetii. Individual differences in immune function lead to varying sensitivity to infection and disease development. In this model, acute and chronic disease could be caused by organisms from the same isolate group, and chronic disease could develop because of compromised resistance of the host rather than as a consequence of a specific property of the pathogen. For example, human immunodeficiency virus infection is a risk factor for the development of chronic Q fever endocarditis (9, 29). Deficiencies in the host-specific cell-mediated immune response in Q fever patients have been associated with the suppression of monocyte and macrophage activities (25), and monocytes from chronic-Q fever patients have been shown to be defective in phagosome maturation and to have impaired C. burnetii-killing potential, regulated in part by elevated interleukin-10 (IL-10) expression (14). There is strong clinical evidence to support the role of increased host production of IL-10 in the development of both Q fever endocarditis and chronic fatigue syndrome (11, 12, 21, 39). A recent study suggested that chronic Q fever endocarditis may be associated with atypical M2 polarization and stimulation of bacterial replication (7), but the pathogenic process that mediates this polarization was undefined.
The route of infection may also be an important determining factor in the manifestation of acute and chronic Q fever. La Scola et al. and Marrie et al. demonstrated that the route of infection and the size of the inoculum affected clinical illness and pathology associated with infection in mouse and guinea pig models (26, 33). Differences in the geographic distributions of the diseases have also been noted (32); in Nova Scotia, for example, the primary manifestation of acute Q fever is pneumonia (34), but in France it is hepatitis, possibly due to ingestion of raw milk and unpasteurized cheeses (51).
The pathogenicity of C. burnetii has been evaluated using guinea pigs, mice, and chicken embryos. Febrile response, splenomegaly, and mortality in guinea pigs; splenomegaly and mortality in mice; and mortality in chicken embryos are indicators of virulence for C. burnetii. The establishment of an aerosol model of C. burnetii infection in guinea pigs (43) provides a relevant model in which to test isolate virulence. Additionally, severe combined immunodeficient (SCID) mice are highly sensitive to the C. burnetii prototype (NM isolate) (1), and the 50% lethal dose (LD50) of NM in SCID mice was at least 108 times less than in wild type mice. We speculated that with these highly sensitive rodent models it may be possible to observe intra- and intergroup pathogenicity differences of C. burnetii isolates. To confirm whether SCID mice could be used to model isolate-specific virulence, we gave multiple infectious doses of a group IV Q fever isolate to immune-competent CB-17 and SCID mice (on the same background) to compare them with previously reported group I isolate (NM) infections (1). Eight isolates from four genomic groups (Table 1) were then evaluated for the ability to cause acute disease in SCID mouse i.p.-challenge and guinea pig aerosol challenge models. We hypothesized that isolates within the same genotypic group would cause similar diseases and that there would be a distinct difference in disease manifestations between isolate groups. Finally, we evaluated the potential of a vaccine composed of one C. burnetii isolate to protect guinea pigs against infection with an isolate from another group, since cross-protection between disparate isolate groups is a further indication of antigenic relatedness.
TABLE 1.
Isolates evaluated for virulence
| Genomic group | Isolate | Notation in this study | Original source |
|||
|---|---|---|---|---|---|---|
| Sample | Yr | Location | Disease | |||
| I | Nine Mile RSA493 | NM | Tick | 1935 | Montana, US | NAa (acute; flu-like in humans) |
| African RSA334 | African | Human blood | 1949 | Central Africa | Acute; Congolese Red Fever | |
| Ohio 314 RSA270 | Ohio | Cow's milk | 1956 | Ohio, US | Persistent | |
| IV | MSU Goat Q177 | Priscilla | Goat Cotyledon | 1980 | Montana, US | Abortion |
| P Q173 | P | Human heart valve | 1979 | California, US | Endocarditis | |
| V | G Q212 | G | Human heart valve | 1981 | Nova Scotia, Canada | Endocarditis |
| S Q217 | S | Human liver biopsy specimen | 1981 | Montana, US | Hepatitis | |
| VI | Dugway 5J108-111 | Dugway | Rodents | 1958 | Utah, US | NA |
NA, not applicable.
MATERIALS AND METHODS
Animals.
The female 6- to 7-week-old CB-17/Icr-scid/scid (SCID) and wild-type CB-17/Icr+/+ (CB-17) mice used in Japan were purchased from Japan CLEA (Tokyo, Japan); A/J mice were purchased from Japan SLC (Shizuoka, Japan). A/J mice were used because they are considered more susceptible to C. burnetii than other inbred mouse strains (45). The female 6- to 8-week-old SCID and wild-type CB-17 mice used in the United States were purchased from Taconic (Hudson, NY). Female Hartley guinea pigs weighing approximately 350 to 450 g were purchased from Charles River Laboratories (Wilmington, MA).
All infected animals were housed in approved animal biosafety level 3 facilities, and immunodeficient mice were housed under sterile conditions. All animals used in this study were acclimated to the facility and assessment procedures during the week prior to infection to decrease stress-related abnormalities. Animal health was assessed daily by a veterinarian.
Mouse experiments performed in Japan adhered to the guidelines for animal experiments at Gifu University. The Texas A&M University Laboratory Animal Care Committee reviewed and approved the mouse and guinea pig research at Texas A&M University, and experiments were carried out in AAALAC-approved facilities in accordance with university and federal regulations.
C. burnetii.
Eight C. burnetii isolates from four genomic groups (Table 1) were used. For the initial dose-effect experiment in Japan, C. burnetii MSU Goat Q177 (Priscilla), obtained from J. Kazar, Institute of Virology, Brastislava, Slovakia, was maintained in mice by passage in spleen homogenates at Gifu University. The spleen homogenates were stored at −80°C until they were used. The absence of contamination with other pathogens was confirmed by direct staining (Giménez and Gram staining), detection of Mycoplasma DNA using a PCR Mycoplasma detection set (Takara, Shiga, Japan), and inoculation of the spleen homogenate into cell culture and SCID mice (independent experimental infection from the study described here). The bacterial dose was evaluated as the 50% tissue culture infectious dose (TCID50) in BGM cells (buffalo green monkey fibroblasts), the 50% infectious dose (ID50) in CB-17 mice, and the LD50 in SCID mice. The TCID50 was determined by detecting the bacteria 6 days after infection using immunofluorescence staining with anti-C. burnetii rabbit antiserum. The ID50 was determined by detecting seroconversion (immunoglobulin G [IgG], >1:16) using indirect microimmunofluorescence. The LD50 was determined as reported previously (1).
For all subsequent experiments, all of the C. burnetii isolates were maintained at the Texas A&M Health Science Center. The C. burnetii isolates were cultivated in embryonated chicken eggs, purified by gradient centrifugation as previously reported (19, 44, 53), and stored at −80°C until they were used. The absence of contamination by other pathogens was confirmed as described above. C. burnetii was quantified by optical density (OD) (53), direct viable-particle count using the Live/Dead BacLight Bacterial Viability Kit (Molecular Probes, Eugene, OR), and quantitative real-time PCR (qPCR) using primers amplifying the com1 gene (8) (see Table S1 in the supplemental material). The bacterial dose used for mouse infections was determined by qPCR; guinea pig doses were calculated using the OD.
Experimental infection in mice. (i) Dose/effect experiment with the Priscilla isolate.
Six mice per group were used for the dose/effect experiment. SCID, CB-17, and A/J mice were inoculated i.p. with serial 10-fold dilutions of Priscilla (102 to 10−7 TCID50 per animal) or sterile phosphate-buffered saline (PBS) (sham infection). SCID mice were observed for 112 days (16 weeks), and CB-17 and A/J mice were observed for 30 days.
(ii) Genomic group comparison.
Four mice per group were used for the genomic group comparison. Each of eight C. burnetii isolates described in Table 1 (105 genome copies/animal) or PBS was administered i.p. to SCID and CB-17 mice. Two independent infections were performed, and the mice were observed for 28 days (for all of the C. burnetii isolates in SCID and CB-17 mice) or until death (for four representative C. burnetii isolates in SCID mice).
Clinical signs were evaluated every 2 days by visual observation (ruffled fur, hunched-back appearance, and lethargy) and body weight measurement. Body weight changes were evaluated using a body weight index (BWI) derived as follows: BWI = relative body weight/mean relative body weight of the control group; relative body weight = body weight on day “x” of infection/body weight on the day of infection. Cachexia was diagnosed when a mouse was lethargic and had a BWI of less than 0.85. At necropsy, the spleen weight was measured as an indicator of C. burnetii infection (54), and tissues were collected. To quantify the growth of C. burnetii, DNA was extracted from spleen tissue and C. burnetii com1 gene copies were detected by qPCR as previously described (8). The heart, lung, liver, spleen, kidney, and femur were formalin fixed, embedded in paraffin, sliced, and then prepared by hematoxylin-eosin staining and immunocytochemistry, as described previously (1, 8), to evaluate histopathologic changes and bacterial distribution in tissues. The degree of inflammation present in each tissue sample was scored numerically by the following system: 0, none; 1, mild; 2, moderate; 3, marked; 4, severe. IgG titers for phase I and II C. burnetii in the sera of CB-17 mice were measured by microimmunofluorescence as described elsewhere (1). For cytokine assays, blood was collected from the lateral saphenous vein at 3, 7, 10, 14, and 21 days postinfection (p.i.) and via cardiac puncture at 28 days p.i. after euthanasia, and the group pooled sera were stored at −80°C until they were used. Sixteen cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-12p40, IL-12p70, IL-10, granulocyte-macrophage colony-stimulating factor, gamma interferon [IFN-γ], KC, macrophage inflammatory protein 1α [MIP-1α], RANTES, and tumor necrosis factor alpha [TNF-α]) were measured using the Bio-Plex cytokine assay system (Bio-Rad, Hercules, CA) following the manufacturer's protocol. The cytokine quantification assay was performed in duplicate for each sample. The cytokine levels of infected sera were evaluated as the induction values compared to the values of uninfected sera.
Experimental infection in guinea pigs.
A chamber specially designed to deliver droplet nuclei directly to the alveolar spaces (College of Engineering Shops, University of Wisconsin, Madison), allowing the infection of multiple guinea pigs simultaneously and ensuring uniform infection within each challenge group (35, 43, 52), was used for all guinea pig infection studies. (i) Three guinea pigs per group were infected with low (102), mid-level (104), or high (106) doses of one of the phase I C. burnetii isolates described in Table 1. Four negative control animals were sham infected with sterile PBS. Body weight, rectal temperature, and behavioral attitude were recorded, along with any abnormalities noted on thoracic auscultation and abdominal palpation. A rectal temperature of ≥39.5°C was defined as fever. The guinea pigs were observed for 28 days p.i. The spleens and livers were weighed at necropsy. Tissues were collected and formalin fixed for histopathologic evaluation. Serum was obtained from each animal for serologic testing. (ii) In a separate experiment, three guinea pigs per group were exposed to PBS or 2 × 106 particle equivalents of NM, P, G, or Dugway. Daily assessment of these animals was performed as described above, and the organs were weighed at necropsy 14 days p.i. to detect splenomegaly and/or hepatomegaly. (iii) In the heterologous-protection study, guinea pigs were vaccinated twice with 40 μg of formalin-inactivated group I (NM) or group V (S) C. burnetii in Freund's incomplete adjuvant or with adjuvant alone, with 2-week intervals between the vaccinations and infection. The animals were then infected with high doses of either NM or S. Three animals per group were separated into the following six groups: (a) nonvaccinated, NM infected; (b) nonvaccinated, S infected; (c) NM vaccinated, NM infected; (d) S vaccinated, S infected; (e) NM vaccinated, S infected; and (f) S vaccinated, NM infected. The guinea pigs were monitored for 14 days p.i. for development of fever and other clinical signs of illness.
Histopathologic samples were prepared by hematoxylin and eosin staining or by immunohistochemistry using a Vectastain ABC kit and a Vector NovaRed substrate kit (Vector Laboratories, Burlingame, CA) and in-house-generated rabbit anti-C. burnetii NM (3) and by counterstaining them with hematoxylin. All slides were evaluated in a blinded fashion. Serum samples collected at necropsy were tested by enzyme-linked immunosorbent assay for IgG titers against phase I C. burnetii NM antigen as previously described (43). Sera from uninfected guinea pigs were used as negative controls.
Statistical analyses.
The results were expressed as means for each group and were compared using one- and two-way analysis of variance or Student's t test, as appropriate. Differences were considered significant at a P value of <0.05.
RESULTS
C. burnetii Priscilla is infective and exhibits delayed virulence in SCID mice.
A detailed analysis of dose-effect in an immunocompromised-mouse model supported the previous study by Moos and Hackstadt that evaluated the ability of the Priscilla isolate to cause fever in i.p.-challenged guinea pigs (36). The infectious titer of the Priscilla isolate in the splenic homogenate used for the multiple-dose infection was 2 × 104 TCID50/ml in BGM cells, 2 × 109.3 ID50/ml in CB-17 mice, and 2 × 1010 LD50/ml in SCID mice (1 TCID50 corresponded to 105.3 ID50 in CB-17 mice and to 106 LD50 in SCID mice). The LD50 in CB-17 mice could not be determined because no CB-17 mice died from any infectious dose used in this study, and the ID50 in SCID mice could not be determined due to lack of antibody production. The ID50 in CB-17 mice and the LD50 in SCID mice were similar, suggesting that SCID mice could be lethally infected with very few viable organisms.
Multiple-dose infection of SCID mice with the Priscilla isolate resulted in slow, progressive, and long-term-persistent disease. Clinical signs included ruffled fur, extremely distended abdomens, and death. Body weight loss, inactivity, and cachexia were not observed until a few days prior to death. Survival time ranged from 55 to 109 days p.i. Progression of clinical signs and survival times were dose dependent, with shorter times corresponding to higher infectious doses (see Table S2 in the supplemental material). Similar lesions were found in all of the SCID mice that died, most notably severe hepatosplenomegaly, and all organs had cellular infiltration, primarily macrophages containing bacteria. The severity of the lesions in infected SCID mice was not dependent on the C. burnetii challenge dose.
On the other hand, CB-17 and A/J mice displayed transitory clinical signs only after infection with the highest dose of Priscilla. Both mouse strains showed ruffled fur from 4 to 13 days p.i., but only A/J mice demonstrated transient body weight loss (data not shown). No other clinical signs were observed. At 28 days p.i., CB-17 and A/J mice had mild splenomegaly and seroconversion as evidence of infection (data not shown). Small granulomas were present in the spleen and liver, but bacterial antigen was not detectable by immunohistochemistry.
Genomic-group-specific virulence in mice.
It was important to establish whether the results of infection seen with the Priscilla isolate and those previously noted with the NM isolate were genomic group specific (24). To determine this, the pathogenicities of multiple isolates were compared by delivering a single dose of eight C. burnetii isolates from four genomic groups (Table 1) to mice by i.p. injection. The infections were initially compared in SCID and CB-17 mice sacrificed at 28 days p.i.
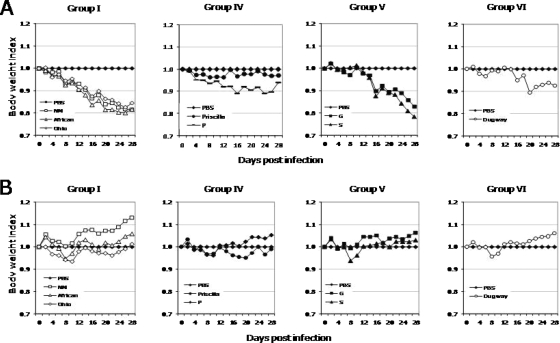
All C. burnetii isolates caused disease in SCID mice, with various clinical courses. There was no mortality during the 28-day infection period. Clinical signs, including significant body weight loss (P < 0.05) and cachexia, summarized in Fig. 1A and in Fig. S1A in the supplemental material, were most apparent in mice infected with group I isolates, followed by those given group V, IV, and VI isolates. In CB-17 mice, only mild transient disease was noted, with minimal loss of body weight, in response to all isolates and noticeably ruffled fur with group I isolate infection (Fig. 1B).
FIG. 1.
Average body weight changes in SCID mice (A) and CB-17 mice (B) infected with C. burnetii isolates during 28 days of infection. Body weights were significantly lower in SCID mice throughout the infection period and transiently in CB-17 mice infected with all isolates except Priscilla compared to PBS-injected controls (P < 0.05).
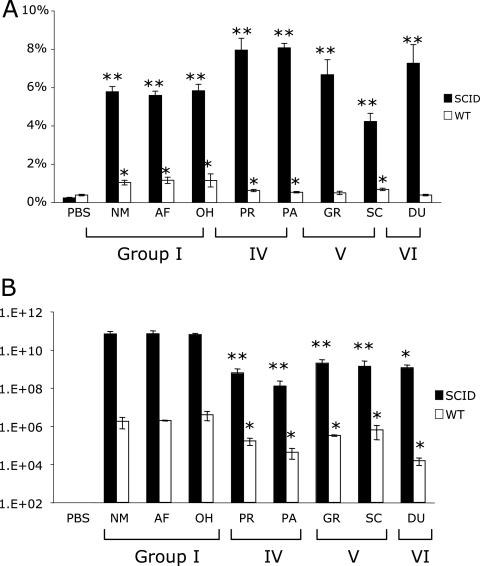
Splenomegaly in response to infection was more severe in SCID than in CB-17 mice (Fig. 2A). The number of bacteria in the spleens was determined by qPCR (Fig. 2B), and consistently higher numbers of com1 genes were detected in SCID than in CB-17 mice. SCID mice showed phylogenetic-group-characteristic spleen size and growth of bacteria. Splenomegaly was greatest in SCID mice with mild clinical disease infected with bacteria from groups IV and VI. However, the number of organisms in the spleen was greater in mice with severe clinical disease following infection with phylogenetic groups I and V. In CB-17 mice, splenic enlargement and numbers of bacteria increased with the severity of clinical disease. CB-17 mice displayed differences between infection with the C. burnetii isolates that caused acute disease (phylogenetic group I) and infection with the C. burnetii isolates that caused chronic disease (phylogenetic groups IV and V), but there was no difference between groups infected with isolates that caused chronic disease. All infected mice developed significant splenomegaly, but mice infected with group IV, V, and VI isolates had significantly fewer splenic bacteria than mice infected with group I isolates (P < 0.05).
FIG. 2.
Splenomegaly (A) and splenic bacterial loads (B) in mice at 28 days p.i. (A) All infected animals developed significant splenomegaly compared to controls, and infected SCID mice had significantly larger spleens than CB-17 mice (P < 0.05). (B) Mice infected with group IV, V, and VI isolates had significantly fewer bacteria than those infected with group I isolates (P < 0.05). *, P < 0.05. The error bars indicate standard deviations.
Evaluation of histopathology at 28 days p.i. revealed more lesions in SCID mice than in CB-17 mice (see Table S3 in the supplemental material). SCID mice showed histopathologic changes in all organs investigated. Group I isolates caused the most inflammation, followed by groups V, IV, and VI. The inflammatory-cell populations were similar in all groups and consisted of few neutrophils and numerous macrophages containing abundant intracytoplasmic bacteria. C. burnetii antigen was diffusely distributed in all organs examined. CB-17 mice had mild histopathologic changes in some organs, but even in the tissues with an inflammatory response, C. burnetii antigen was rarely detected.
Circulating cytokines are altered in C. burnetii-infected CB-17 mice.
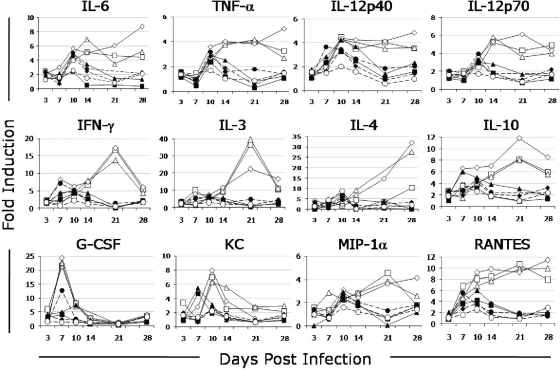
The variations in pathology and inflammation associated with these isolate group infections suggest differences in the immune responses. To expand on this observation, the serum levels of 16 cytokines and chemokines were measured. In CB-17 mice, serum cytokine levels differed between mice infected with group I isolates and those given isolates from other groups. Group I isolates induced persistently high cytokine secretion throughout the 28-day experiment; group IV and V isolates caused moderate cytokine secretion at the peak of clinical disease (7 to 14 days p.i.) (Fig. 3). After 14 days p.i., group I isolates induced higher secretion of IL-3, IL-4, IL-6, IL-10, IL-12p40, IL-12p70, IFN-γ, TNF-α, MIP-1α, and RANTES than other groups. The KC and granulocyte-macrophage colony-stimulating factor levels of mice infected with group I isolates were higher than those in mice infected with other groups prior to 14 days p.i. Serum IL-1α, IL-1β, IL-2, and IL-5 levels and eotaxin secretion were not increased during the infection period (data not shown).
FIG. 3.
Mean circulating cytokine levels in response to infection in CB-17 mice with different C. burnetii isolates. Isolates from genomic group I induced persistently high cytokine secretion with increased levels of IL-6, TNF-α, IL-12p40, IL-12p70, IFN-γ, IL-3, IL-4, IL-10, MIP-1α, and RANTES compared with other genogroups (P < 0.05). ⧫, PBS; □, NM; ▵, African; ⋄, Ohio; •, Priscilla; ⧫, P; ▪, G; ▴, S; ○, Dugway.
Lethal potentials of all genomic groups in SCID mice.
The lethal potentials of representative isolates from each phylogenetic group were investigated in SCID mice, and it was determined that all of the isolates evaluated could eventually lead to clinical illness and death in the immunodeficient model (see Fig. S1B in the supplemental material). Isolates that caused a long period of cachexia led to severe body weight loss in infected mice (see Fig. S2 in the supplemental material). A group I isolate (NM) induced the earliest and longest period of cachexia and, correspondingly, the most severe body weight loss. Mice infected with isolates from groups V (G) and VI (Dugway) had similar survival times, but those given group V isolates had longer periods of cachexia and more severe body weight loss than group VI-infected mice. Infection with group IV isolates (Priscilla and P) resulted in the shortest period of cachexia, and body weight loss was not observed until the terminal stage of infection. The survival time was shortest in mice challenged with group I isolates (32.0 ± 0.8 days), followed by those infected with groups V (36.0 ± 0.0 days), VI (35.5 ± 1.0 days), and IV (47.5 ± 0.6 days for P and 77.3 ± 2.8 days for Priscilla). The probable cause of death was multiple-organ failure due to massive systemic infection.
The pathological changes in SCID mice at mortality were more advanced than those observed at 28 days p.i. (data not shown). The severity of inflammatory changes in the liver and spleen was similar in all groups of infected mice, but animals given group I isolates exhibited a greater degree of inflammation in the heart and lungs than those given group IV, V, and VI isolates. The extent of splenomegaly changed with survival time; however, the numbers of bacteria in the spleen were similar in all groups, suggesting that the number of bacteria (1010 genome copies/spleen) detected is the saturation point in SCID mice. C. burnetii antigen was diffusely distributed in all tissue sections.
Genomic-group-specific outcome of acute Q fever pneumonia in the guinea pig aerosol model.
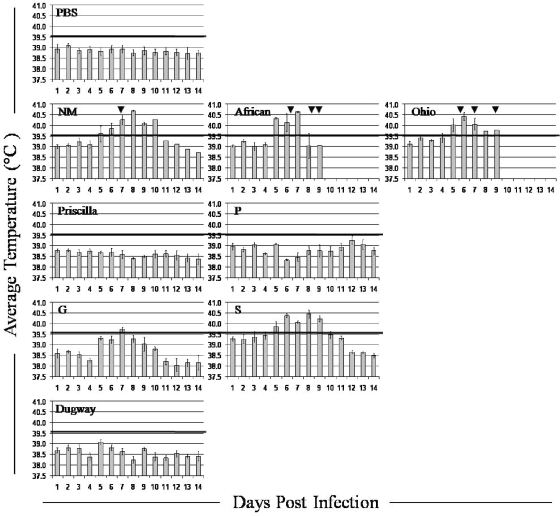
Aerosol challenge in the guinea pig provides a physiologically relevant model that simulates both the natural route of infection and common clinical presentations associated with human acute Q fever, making this a choice model for evaluating the comparative levels of virulence of different C. burnetii isolates, and thus, it was used in the logical progression of experiments after different levels of virulence were observed in mouse models of infection. Guinea pigs challenged with group I and V isolates developed significant fever in response to infection (P < 0.01), whereas those given isolates from groups IV and VI were afebrile even at the highest challenge dose (Fig. 4).
FIG. 4.
Fever responses of guinea pigs to infection with high doses of C. burnetii isolates. The mean daily temperatures ± standard errors of the mean (n = 3) of animals infected with 2 × 106 bacteria of each C. burnetii isolate. Temperatures of ≥39.5°C (black lines) were considered fever. The arrows indicate days on which death occurred in NM-, African-, and Ohio-infected groups.
Fever response, weight loss, and other clinical signs displayed a dose-dependent relationship in guinea pigs infected with the group I C. burnetii isolates African and Ohio, as has been described for the reference isolate in this group, NM (43). All animals that received African or Ohio organisms at a high dose died within 7 to 9 days p.i., as did two of three that received NM; lower infectious doses were not lethal. Gross lung consolidation and overall lack of normal body fat were noted on necropsy at 7 to 9 days p.i. in guinea pigs infected with the highest dose of organisms. Histologically, these animals had severe panleukocytic bronchointerstitial pneumonia with bronchial and alveolar exudates. Lung tissues from the surviving NM-infected guinea pig and those given the mid-level dose of group I organisms were evaluated at 28 days p.i. for comparison to animals infected with other isolates evaluated at this time, and they exhibited moderate multifocal lymphohistiocytic pneumonia with granuloma formation.
No significant fever or other overt clinical signs were noted in guinea pigs infected with group IV isolates. Mild lymphohistiocytic pneumonia was seen histologically at 28 days p.i. in animals given the highest dose of organisms.
Group V isolate-infected guinea pigs all developed fever when given the highest challenge dose, and dose-dependent temperature increases and other clinical signs were again noted, with no fever development, in those animals receiving the lowest dose of organism. Though auscultation confirmed respiratory compromise, none of the infections were lethal. At 28 days p.i., the lungs had mild to moderate lymphohistiocytic interstitial pneumonia and a few small granulomas.
No major clinical or pathological changes were noted in guinea pigs infected with the group VI isolate or in negative control animals. Table S4 in the supplemental material compares the severity of histopathologic changes in guinea pigs infected with high doses of C. burnetii isolates from each group at 28 days p.i. Immunohistochemistry confirmed the presence of C. burnetii organisms, primarily in macrophages, in the lungs, livers, and spleens of infected animals.
Experimental guinea pigs in all dose groups for each isolate seroconverted by the time of euthanasia, with the exception of animals infected with high doses of NM, African, and Ohio necropsied at 1 week p.i. and low-dose Dugway-infected guinea pigs. The degree of seroconversion was dose dependent and varied among isolates (data not shown). No PBS-injected control animals seroconverted.
Genomic-group-specific severity of hepatitis and splenomegaly in guinea pigs.
The doughnut granulomas common in human acute Q fever hepatitis (31) had not been previously described in animals experimentally infected with C. burnetii and were also not seen in the guinea pigs in this study. Mild hepatitis and severe hepatic lipidosis were noted at death 7 days p.i. in guinea pigs challenged with high doses of group I isolates, as had been previously reported for NM aerosol-infected guinea pigs (43). Tissue sections from the remaining NM-infected guinea pig and those infected with mid-level doses of the group I organisms were evaluated for comparison with animals infected with other isolates at 28 days p.i. and revealed vacuolization and degeneration of centrilobular hepatocytes, lymphocyte infiltration in periportal regions, and multiple small granulomas.
Group IV-infected guinea pigs also had periportal lymphocytic infiltration, as well as multiple granulomas of various sizes. The granulomas in Priscilla- and P-infected guinea pigs were more defined, with more histiocytic involvement than was seen in guinea pigs infected with group I isolates. Subjectively, of all animals necropsied from each isolate group, hepatic granulomas from those infected with P were the greatest in size and number.
The livers of guinea pigs infected with the group V isolates G and S contained a few small granulomas and mild to moderate infiltration of lymphocytes along portal tracts. The hepatic changes observed in guinea pigs infected with group V isolates suggested that isolates from this group are less hepatovirulent than group IV isolates but more so than group I isolates.
No hepatic granulomas or other significant pathological changes were noted in guinea pigs infected with the group VI isolate Dugway. Liver weights did not vary significantly within or between genomic groups.
There were no significant differences in spleen weights at 28 days p.i. within or between genomic or dose groups. Animals infected with all isolates examined at 14 days p.i. (NM, P, G, and Dugway) had significantly larger spleens than PBS-injected control animals, and spleens from NM- and G-infected guinea pigs were significantly larger (P < 0.01 and P < 0.05, respectively) than those of P- and Dugway-infected animals (see Fig. S3 in the supplemental material). Pathological findings included multiple small granulomas in the spleens of group I-infected guinea pigs; fewer small granulomas were occasionally noted in animals infected with group IV and V isolates.
Heterologous protection of cross-vaccination and challenge in guinea pigs.
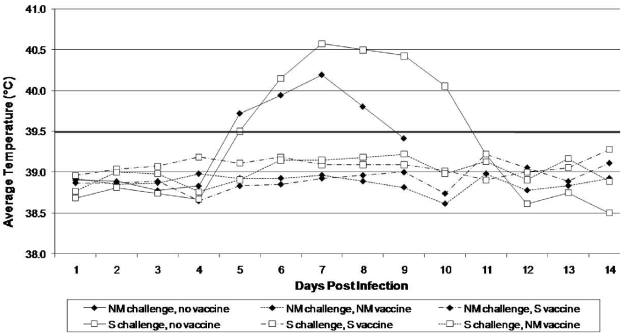
The infection studies described here illustrate that there is pathotype diversity between C. burnetii isolates from different genogroups, and they are consistent with phylogenetic studies cataloging distinct gene contents (4). We therefore strove to determine whether this diversity was great enough to affect the ability of vaccines to protect against infection. Guinea pigs were given group I (NM) or group IV (S) vaccine and cross-challenged to evaluate potential heterologous protection against high-dose infection. Nonvaccinated guinea pigs developed a noticeable fever response by day 5 p.i., and infection was lethal in three of three NM- and one of three S-challenged animals. Guinea pigs vaccinated with either formalin-killed NM or S were completely protected against fever development and death when challenged with either NM or S (Fig. 5).
FIG. 5.
Heterologous vaccination and challenge in guinea pigs. Shown are average daily temperatures of animals vaccinated with NM (dashed and dotted line), S (dashed line), or adjuvant alone (solid line) and challenged with high doses of NM (⧫) or S (□). Temperatures of ≥39.5°C were considered fever.
DISCUSSION
The potential for genomic-group-specific pathogenicity of C. burnetii was evaluated using immunocompetent mice and guinea pigs and immunodeficient mice. The hypotheses that isolates belonging to the same genomic group would cause similar disease and that there would be distinctions in disease manifestations between isolate groups were supported by the findings presented here.
A detailed analysis of the Priscilla isolate dose-effect in SCID mice revealed differences in virulence of C. burnetii isolates. Disease development after Priscilla infection was progressive but slower than the development of the disease caused by NM previously reported in SCID mice (1); the survival time of SCID mice infected with Priscilla was longer with the same LD50. This result supports the previous study by Moos and Hackstadt that evaluated the lesser ability of the Priscilla isolate to cause fever in i.p.-challenged guinea pigs (36). Interestingly, the mice infected with Priscilla did not exhibit cachexia until the terminal stages of infection, when they had extremely severe hepatosplenomegaly. Although the disease caused by Priscilla was milder than that associated with NM, all mice that developed clinical illness died. This result confirms the high infectivity and lethal potential of C. burnetii, which is not restricted to isolates that cause acute disease, and suggests that the SCID mouse model can be useful for evaluation of C. burnetii virulence.
The virulence of C. burnetii isolates tested in SCID mice was determined to be genomic group specific. Acute-Q fever-associated group I isolates caused the most rapidly progressing disease and the most severe pathological changes. Groups IV and V, isolates associated with chronic Q fever, caused a slower progression of disease. Overall, pathological changes in mice infected with group IV and V isolates were milder than those of group I-infected mice. The number of bacteria in the spleen at 28 days p.i. was greater in mice with severe disease from infection with group I isolates; however, the bacterial loads at the time of death were similar in all infected mice. This suggests that the rate of proliferation of C. burnetii in vivo may be virulence related. An in vitro comparison of infection in L929 cells using NM, Priscilla, and S isolates showed that all of the isolates could persistently infect, but Priscilla required a greater period of time to establish an infection (42), and it has been shown that inclusion-forming units produced by NM and Priscilla isolates were similar in Vero cells (36). However, because of developmental differences in clinical signs and pathological changes, the replication rate does not seem to be the only virulence factor involved, since clinical signs would then be similar with differences only in disease progression. At both time points, 28 days p.i. and the time of death due to infection, heart and lung lesions caused by group IV, V, and VI isolates were milder than those produced by infection with group I isolates. This observation seems to conflict with the hypothesis that isolates from chronic disease cause chronic Q fever, including heart disease. However, our observation is consistent with the report that isolates from heart lesions of chronic-Q fever patients have genetic characteristics similar to those of isolates from acute disease (46). The hypothesis that isolates from acute disease do not cause endocarditis has been supported by two other research groups (17, 24). The correlation between virulence and phylogeny has been controversial because of a lack of comprehensive studies. One study detected genes specific to isolates from acute disease in isolates from chronic Q fever patients and concluded that the isolates were not disease specific (46). The isolates used in the study were isolated by cell culture, and although the cell culture system is highly effective for isolation, isolates from acute disease are known to infect cultured cells more efficiently than isolates from chronic disease, so there remains a potential that the study collected only cell culture-adapted isolates. Several in vivo studies have reported isolate-specific virulence using guinea pig and mouse models (17, 24, 36); however, the number of isolates used in these studies was limited, making it difficult to conclude that there was genomic-group-specific virulence. The present study using eight isolates from four phylogenetic groups strongly supports the variation in virulence among C. burnetii isolate groups.
In the absence of functional T and B cells, cytokine profiles showed no group-specific differences. In immunocompetent mice, group I isolates caused a stronger immune response with high levels of multiple cytokines over a longer time than other groups. Interestingly, Dugway (group VI) induced the least change in CB-17 mice. The inflammatory-cytokine changes in immunocompetent mice in this study were similar to those in humans with acute Q fever (10): TNF-α and IL-6 were upregulated, but IL-1β was not. IFN-γ increased in CB-17 mice infected with group I isolates, and it is associated with the control of bacterial growth, stimulates phagosome-lysosome fusion, and may enable monocytes/macrophages to kill C. burnetii (13, 14). A difference in vacuole formation between isolates has also been shown, with NM and S developing within single large vacuoles while Priscilla occupied several smaller vacuoles per cell (18). This in vitro study suggested a difference in isolate ecology within host cells, which may be correlated with their virulence in vivo.
The ability to cause fever and respiratory illness was isolate and dose dependent in the guinea pig aerosol challenge model, with isolates from groups I and V causing disease consistent with human acute Q fever. Isolates within the same genomic group produced similar clinical illnesses, strongly supporting the mouse experiments demonstrating that genomic differences in the bacterial isolates do play a role in virulence. It was shown here that isolates associated with chronic disease, G and S, have the ability to cause acute disease in the guinea pig model. Our study confirmed and expanded the observations of Kazar et al. that the virulence of NM and S isolates was greater than that of Priscilla.
Lesny et al. compared the cross-immunity of whole-cell and soluble Q fever vaccines made from phase I NM, S, Priscilla, and Luga isolates. They found that vaccines from NM and Priscilla afforded a higher degree of protection than S and Luga vaccines and that whole-cell vaccines were more effective than soluble vaccines (28). In the guinea pig challenge study presented here, killed whole-cell vaccines made from isolates differing in LPS banding pattern (16), plasmid type (44), and genomic group (20), specifically isolates from groups I and V, conferred heterologous protection against virulent high-dose challenge in accordance with previous studies (28). This suggests that although the manifestations of disease and genomic contents differ among various isolate groups, the antigenic properties of whole-cell vaccines are shared enough that cross-protection is possible. Such information is valuable for the design of new vaccines and could be of the utmost importance in offering reliable protection in the event of an outbreak.
The differences in perceived infectious doses noted when ODs, particle counts, and genome copy enumerations were compared underline the importance of using multiple quantitation methods to compare studies with earlier observations. Some of the differences in disease manifestations seen in guinea pigs in this study could be due to slight differences in the infectious doses delivered. For instance, Priscilla and P both induced hepatic changes, although guinea pigs infected with P appeared to develop more severe lesions than those infected with Priscilla, which had a lower infectious dose by OD and qPCR. The difference in infectious dose as determined by the genome copy number could account for this variation. However, G and S both caused fever, and although guinea pigs infected with G did not attain the same degree of febrile response as S-infected animals, quantitation by particle count and real-time PCR showed infectious doses of S to be over a log unit lower than those of G. It could be argued that Priscilla-infected guinea pigs did not develop fever because fewer bacteria were present in the aerosol challenge; however, the group IV isolates did not induce fever at any of the challenge doses while group I isolates induced fever even at the lowest dose. We believe that, despite the variation in the infectious dose depending on the enumeration technique, the significant differences noted among genotypic groups are valid.
Phase variation is the only well-characterized phenotypic difference that is related to virulence in C. burnetii (50). Although LPS may be a major virulence determinant, and isolate LPS banding patterns have been correlated with acute or chronic disease (16), other components alone or in association with LPS may be responsible for differences in mortality in SCID mice and fever development in aerosol-challenged guinea pigs. It has been hypothesized that differences in the lipid A component are responsible for the variations in virulence, but lipid structural information indicates they are similar. The combination of a variety of factors expressed by phase I bacteria likely governs the ability of C. burnetii to infect cells and to maintain continuous growth within the phagolysosome. Indeed, the combination of pathotype variation of disease in infected guinea pigs and cross-protection of different isolates suggests conserved predominant antigenic components with virulence determinant specificity.
A recent report compared all open reading frames of NM phase I to those of African, Ohio, P, G, S, and Dugway, among others (4), and a majority of the open reading frames deleted from NM in the other isolates were either hypothetical or nonfunctional; however, a few were associated with assorted cellular functions. Beare et al. compared the complete genome sequences of NM, K, G, and Dugway and found distinct collections of pseudogenes and unique gene contents that may contribute to pathotype-specific virulence, including type II and type IV secreted effector molecules (5). Integrating our in vivo data with these molecular details, as well as with other in vitro studies, may reveal the critical virulence determinants of C. burnetii and ultimately identify targets for vaccine and therapeutic intervention.
Isolates of phase I C. burnetii have the potential to cause a range of clinical signs, including fever, pneumonia, hepatitis, and splenomegaly. Isolates from one human chronic-disease group induced mild to moderate acute disease in the physiologically relevant guinea pig aerosol challenge model, while a separate isolate group representing several chronic-disease isolates caused no acute disease. All isolates examined were capable of producing disease in the immunocompromised SCID mouse model, and genogroup-consistent trends were noted in cytokine production in response to infection in the immunocompetent-mouse model. In these studies, isolates within the same genomic group caused similar pathological responses, with a distinction in strain virulence between established genogroups, sustaining the theory that genetic differences in the bacterial isolates affect their virulence.
Supplementary Material
Acknowledgments
This work was supported by funding from NIH NIAID grants KO8 AI055664, U54 AI057156, and RO1 AI057768 and Science Research Grant number 13460142 from the Ministry of Education, Science, Sports and Culture of Japan.
We are grateful to Laura R. Hendrix for critical review of the manuscript.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 28 September 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Andoh, M., T. Naganawa, A. Hotta, T. Yamaguchi, H. Fukushi, T. Masegi, and K. Hirai. 2003. SCID mouse model for lethal Q fever. Infect. Immun. 71:4717-4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arricau-Bouvery, N., Y. Hauck, A. Bejaoui, D. Frangoulidis, C. C. Bodier, A. Souriau, H. Meyer, H. Neubauer, A. Rodolakis, and G. Vergnaud. 2006. Molecular characterization of Coxiella burnetii isolates by infrequent restriction site-PCR and MLVA typing. BMC Microbiol. 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner, W., H. Dettinger, N. Schmeer, and E. Hoffmeister. 1988. Evaluation of different fixatives and treatments for immunohistochemical demonstration of Coxiella burnetti in paraffin-embedded tissues. J. Clin. Microbiol. 26:2044-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beare, P. A., J. E. Samuel, D. Howe, K. Virtaneva, S. F. Porcella, and R. A. Heinzen. 2006. Genetic diversity of the Q fever agent, Coxiella burnetii, assessed by microarray-based whole-genome comparisons. J. Bacteriol. 188:2309-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beare, P. A., N. Unsworth, M. Andoh, D. E. Voth, A. Omsland, S. D. Gilk, K. P. Williams, B. W. Sobral, J. J. Kupko III, S. F. Porcella, J. E. Samuel, and R. A. Heinzen. 2008. Comparative genomics reveal extensive transposon-mediated genomic plasticity and diversity among potential effector proteins within the genus Coxiella. Infect. Immun. 77:642-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benenson, A. S., and W. D. Tigertt. 1956. Studies on Q fever in man. Trans. Assoc. Am. Physicians 69:98-104. [PubMed] [Google Scholar]
- 7.Benoit, M., E. Ghigo, C. Capo, D. Raoult, and J. L. Mege. 2008. The uptake of apoptotic cells drives Coxiella burnetii replication and macrophage polarization: a model for Q fever endocarditis. PLoS Pathog. 4:e1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan, R. E., and J. E. Samuel. 2003. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay. J. Clin. Microbiol. 41:1869-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouqui, P. 1993. Chronic Q fever. Arch. Inern. Med. 153:642-648. [DOI] [PubMed] [Google Scholar]
- 10.Capo, C., N. Amirayan, E. Ghigo, D. Raoult, and J. Mege. 1999. Circulating cytokine balance and activation markers of leucocytes in Q fever. Clin. Exp. Immunol. 115:120-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capo, C., Y. Zaffran, F. Zugan, P. Houpikian, D. Raoult, and J. L. Mege. 1996. Production of interleukin-10 and transforming growth factor β by peripheral blood mononuclear cells in Q fever endocarditis. Infect. Immun. 64:4143-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghigo, E., C. Capo, D. Raoult, and J. L. Mege. 2001. Interleukin-10 stimulates Coxiella burnetii replication in human monocytes through tumor necrosis factor down-modulation: role in microbicidal defect of Q fever. Infect. Immun. 69:2345-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghigo, E., C. Capo, C. H. Tung, D. Raoult, J. P. Gorvel, and J. L. Mege. 2002. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-gamma mediates its restoration and bacterial killing. J. Immunol. 169:4488-4495. [DOI] [PubMed] [Google Scholar]
- 14.Ghigo, E., A. Honstettre, C. Capo, J. P. Gorvel, D. Raoult, and J. L. Mege. 2004. Link between impaired maturation of phagosomes and defective Coxiella burnetii killing in patients with chronic Q fever. J. Infect. Dis. 190:1767-1772. [DOI] [PubMed] [Google Scholar]
- 15.Glazunova, O., V. Roux, O. Freylikman, Z. Sekeyova, G. Fournous, J. Tyczka, N. Tokarevich, E. Kovacava, T. J. Marrie, and D. Raoult. 2005. Coxiella burnetii genotyping. Emerg. Infect. Dis. 11:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackstadt, T. 1986. Antigenic variation in the phase I lipopolysaccaride of Coxiella burnetii isolates. Infect. Immun. 52:337-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackstadt, T. 1990. The role of lipopolysaccharides in the virulence of Coxiella burnetii. Ann. N. Y. Acad. Sci. 590:27-32. [DOI] [PubMed] [Google Scholar]
- 18.Hechemy, K. E., M. McKee, M. Marko, W. A. Samsonoff, M. Roman, and O. Baca. 1993. Three-dimensional reconstruction of Coxiella burnetii-infected L929 cells by high-voltage electron microscopy. Infect. Immun. 61:4485-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrix, L., and L. P. Mallavia. 1984. Active transport of proline by Coxiella burnetii. J. Gen. Microbiol. 130:2857-2863. [DOI] [PubMed] [Google Scholar]
- 20.Hendrix, L. R., J. E. Samuel, and L. P. Mallavia. 1991. Differentiation of Coxiella burnetii isolates by analysis of restriction-endonuclease-digested DNA separated by SDS-PAGE. J. Gen. Microbiol. 137:269-276. [DOI] [PubMed] [Google Scholar]
- 21.Honstettre, A., G. Imbert, E. Ghigo, F. Gouriet, C. Capo, D. Raoult, and J. L. Mege. 2003. Dysregulation of cytokines in acute Q fever: role of interleukin-10 and tumor necrosis factor in chronic evolution of Q fever. J. Infect. Dis. 187:956-962. [DOI] [PubMed] [Google Scholar]
- 22.Huebner, R. J., W. L. Jellison, and M. D. Beck. 1949. Q fever studies in southern California. III. Effects of pasteurization on survival of Coxiella burnetii in naturally infected milk. Public Health Rep. 64:499-511. [PMC free article] [PubMed] [Google Scholar]
- 23.Jager, C., H. Willems, D. Thiele, and G. Baljer. 1998. Molecular characterization of Coxiella burnetii isolates. Epidemiol. Infect. 120:157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazar, J., M. Lesny, P. Propper, D. Valkova, and R. Brezina. 1993. Comparison of virulence for guinea pigs and mice of different Coxiella burnetii phase I strains. Acta Virol. 37:437-448. [PubMed] [Google Scholar]
- 25.Koster, F. T., J. C. Williams, and J. S. Goodwin. 1985. Cellular immunity in Q fever: modulation of responsiveness by a suppressor T cell-monocyte circuit. J. Immunol. 135:1067-1072. [PubMed] [Google Scholar]
- 26.La Scola, B., H. Lepidi, and D. Raoult. 1997. Pathologic changes during acute Q fever: influence of the route of infection and inoculum size in infected guinea pigs. Infect. Immun. 65:2443-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lennette, E. H., W. H. Clark, M. M. Abinanti, O. Brunetti, and J. M. Covert. 1952. Q fever studies. XIII. The effect of pasteurization on Coxiella burnetii in naturally infected milk. Am. J. Hyg. 55:246-253. [PubMed] [Google Scholar]
- 28.Lesny, M., J. Kazar, P. Propper, and M. Lukacova. 1991. Virulence and cross-immunity study on guinea pigs infected with different phase I Coxiella burnetii strains, p. 666-673. In J. Kazar and D. Raoult (ed.), Rickettsiae and rickettsial diseases. Publishing House of the Slovak Academy of Sciences, Bratislava, Slovakia.
- 29.Madariaga, M. G., J. Pulvirenti, M. Sekosan, C. D. Paddock, and S. R. Zaki. 2004. Q fever endocarditis in HIV-infected patients. Emerg. Infect. Dis. 10:501-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrie, T. J. 1990. Acute Q fever, p. 125-160. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, FL. [Google Scholar]
- 31.Marrie, T. J. 1990. Q fever hepatitis, p. 171-178. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, FL. [Google Scholar]
- 32.Marrie, T. J. 2004. Q fever pneumonia. Curr. Opin. Infect. Dis. 17:137-142. [DOI] [PubMed] [Google Scholar]
- 33.Marrie, T. J., A. Stein, D. Janigan, and D. Raoult. 1996. Route of infection determines the clinical manifestations of acute Q fever. J. Infect. Dis. 173:484-487. [DOI] [PubMed] [Google Scholar]
- 34.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMurray, D. N. 1994. Guinea pig model of tuberculosis, p. 135-147. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, DC.
- 36.Moos, A., and T. Hackstadt. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect. Immun. 55:1144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ormsbee, R. A. 1965. Q fever rickettsia, p. 1144-1160. In F. L. Horsfall and I. Tamm (ed.), Viral and rickettsial diseases of man. J. B. Lippencott, Philadelphia, PA.
- 38.Reference deleted.
- 39.Penttila, I. A., R. J. Harris, P. Storm, D. Haynes, D. A. Worswick, and B. P. Marmion. 1998. Cytokine dysregulation in the post-Q-fever fatigue syndrome. QJM 91:549-560. [DOI] [PubMed] [Google Scholar]
- 40.Raoult, D., and T. Marrie. 1995. Q Fever. Clin. Infect. Dis. 20:489-496. [DOI] [PubMed] [Google Scholar]
- 41.Raoult, D., A. Raza, and T. J. Marrie. 1990. Q fever endocarditis and other forms of chronic Q fever, p. 179-120. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, FL. [Google Scholar]
- 42.Roman, M. J., H. A. Crissman, W. A. Samsonoff, K. E. Hechemy, and O. G. Baca. 1991. Analysis of Coxiella burnetii isolates in cell culture and the expression of parasite-specific antigens on the host membrane surface. Acta Virol. 35:503-510. [PubMed] [Google Scholar]
- 43.Russell-Lodrigue, K. E., G. Q. Zhang, D. N. McMurray, and J. E. Samuel. 2006. Clinical and pathologic changes in a guinea pig aerosol challenge model of acute Q fever. Infect. Immun. 74:6085-6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuel, J. E., M. E. Frazier, and L. P. Mallavia. 1985. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect. Immun. 49:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott, G. H., J. C. Williams, and E. H. Stephenson. 1987. Animal models in Q fever: pathological responses of inbred mice to phase I Coxiella burnetii. J. Gen. Microbiol. 133:691-700. [DOI] [PubMed] [Google Scholar]
- 46.Stein, A., and D. Raoult. 1993. Lack of pathotype specific gene in human Coxiella burnetii isolates. Microb. Pathog. 15:177-185. [DOI] [PubMed] [Google Scholar]
- 47.Stoenner, H. G., R. Holdenried, D. Lackman, and J. S. Orsborn. 1959. The occurrence of Coxiella burnetii, Brucella, and other pathogens among fauna of the Great Salt Lake Desert in Utah. Am. J. Trop. Med. Hyg. 8:590-595. [DOI] [PubMed] [Google Scholar]
- 48.Stoenner, H. G., and D. B. Lackman. 1960. The biologic properties of Coxiella burnetii isolated from rodents collected in Utah. Am. J. Hyg. 71:45-51. [DOI] [PubMed] [Google Scholar]
- 49.Svraka, S., R. Toman, L. Skultety, K. Slaba, and W. L. Homan. 2006. Establishment of a genotyping scheme for Coxiella burnetii. FEMS Microbiol. Lett. 254:268-274. [DOI] [PubMed] [Google Scholar]
- 50.Thiele, D., and H. Willems. 1994. Is plasmid based differentiation of Coxiella burnetii in ‘acute’ and ‘chronic’ isolates still valid? Eur. J. Epidemiol. 10:427-434. [DOI] [PubMed] [Google Scholar]
- 51.Tissot Dupont, H., D. Raoult, P. Brouqui, F. Janbon, D. Peyramond, P. J. Weiller, C. Chicheportiche, M. Nezri, and R. Poirier. 1992. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am. J. Med. 93:427-434. [DOI] [PubMed] [Google Scholar]
- 52.Wiegeshaus, E. H., D. N. McMurray, A. A. Grover, G. E. Harding, and D. W. Smith. 1970. Host-parasite relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am. Rev. Respir. Dis. 102:422-429. [DOI] [PubMed] [Google Scholar]
- 53.Williams, J. C., M. G. Peacock, and T. F. McCaul. 1981. Immunological and biological characterization of Coxiella burnetii, phase I and phase II, separated from host components. Infect. Immun. 32:840-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, G. Q., and J. E. Samuel. 2003. Identification and cloning potentially protective antigens of Coxiella burnetii using sera from mice experimentally infected with Nine Mile phase I. Ann. N. Y. Acad. Sci. 990:510-520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.