Abstract
Parasitic helminth infection has been shown to modulate pathological inflammatory responses in allergy and autoimmune disease. The aim of this study was to examine the effects of infection with a helminth parasite, Heligmosomoides polygyrus, on type 1 diabetes (T1D) in nonobese diabetic (NOD) mice and to elucidate the mechanisms involved in this protection. H. polygyrus inoculation at 5 weeks of age protected NOD mice from T1D until 40 weeks of age and also inhibited the more aggressive cyclophosphamide-induced T1D. Moreover, H. polygyrus inoculation as late as 12 weeks of age reduced the onset of T1D in NOD mice. Following H. polygyrus inoculation of NOD mice, pancreatic insulitis was markedly inhibited. Interleukin-4 (IL-4), IL-10, and IL-13 expression and the frequency of CD4+ CD25+ FoxP3+ regulatory T cells were elevated in mesenteric and pancreatic lymph nodes. Depletion of CD4+ CD25+ T cells in vivo did not abrogate H. polygyrus-induced T1D protection, nor did anti-IL-10 receptor blocking antibody. These findings suggest that infection with H. polygyrus significantly inhibits T1D in NOD mice through CD25- and IL-10-independent mechanisms and also reduces the severity of T1D when administered late after the onset of insulitis.
Helminth parasites infect about 1.5 billion people worldwide, especially in developing countries, and cause chronic infection that leads to malnutrition, anemia, impaired growth, and significant mortality. Intestinal nematode parasites can produce strong polarized Th2-type responses in mice. This immune response is characterized by eosinophilia, mucosal mast cell hyperplasia, elevated immunoglobulin E (IgE) secretion, and increased production of Th2 cytokines, such as interleukin-4 (IL-4) and IL-13. Recent studies have suggested that helminth infection can regulate infectious, allergic, or autoimmune inflammatory diseases. Helminth infection enhances susceptibility to certain infectious diseases, like tuberculosis (11, 35) and viral hepatitis (10, 17). Conversely, helminth infection is protective in murine models of asthma (19), multiple sclerosis (40), and inflammatory bowel disease (42).
Type 1 diabetes (T1D) is a life-threatening disease that affects approximately 1 out of 400 children in westernized societies (18). Over the past 3 decades, the rate of T1D has increased by approximately 4% per year in both Europe and the United States (8, 12, 39). This increase in disease incidence may result in part from a dysregulated immune system due to lack of exposure to certain environmental pathogens, such as helminth parasites (5, 7, 32). Studies with nonobese diabetic (NOD) mice showed that inoculation with Trichinella spiralis, Heligmosomoides polygyrus, or Schistosoma mansoni markedly reduced the rate of T1D and suppressed lymphoid infiltration in the islets (9, 37). T1D was also prevented in NOD mice by injection of whole eggs or soluble antigens from the schistosome egg antigen or the schistosome worm antigen, but only if treatment was started at 4 weeks of age (49). Moreover, the addition of oral insulin B chain to schistosome egg antigen-treated mice augmented the induction of regulatory T cells (Tregs) that secreted IL-4, IL-10, and transforming growth factor beta (TGF-β) (27).
We were interested in further examining potential mechanisms contributing to the control of T1D during infection with the intestinal nematode parasite H. polygyrus. This strictly enteric parasite triggers a potent Th2-type response without eliciting an associated Th1-type response (4). We found that H. polygyrus infection exerted significant protection against T1D in NOD mice when administered at 5 and 7 weeks of age and even when given as late as 12 weeks of age (30% protection). This was associated with reduced lymphoid infiltration in the islets and an increased frequency of CD25+ Tregs with augmented Th2-type responses, including induction of alternatively activated macrophages (AAMΦs) and IL-10 mRNA in pancreatic lymph nodes (PLN). When H. polygyrus-inoculated NOD mice were treated with cyclophosphamide (Cyp), an agent known to accelerate T1D, T1D prevention was sustained. Similarly, when H. polygyrus-inoculated NOD mice were treated with anti-CD25 monoclonal antibody (MAb) in vivo, we observed no change in insulitis between this group and those receiving a control monoclonal Ig. Furthermore, in Cyp-treated NOD mice, administration of an anti-IL-10 receptor (IL-10R) blocking MAb did not abrogate H. polygyrus-induced protection from T1D. These findings suggest that H. polygyrus inoculation suppressed T1D even after the development of insulitis and that suppression of T1D in H. polygyrus-treated NOD mice is accomplished through CD25- and IL-10-independent mechanisms.
MATERIALS AND METHODS
Mice.
Four-week-old female NOD mice were purchased from Jackson Laboratories, Bar Harbor, ME. All of the mice were maintained in a specific-pathogen-free facility during the experiments. The studies reported here conformed to the principles for laboratory animal research outlined by the Animal Welfare Act and the Department of Health, Education, and Welfare (National Institutes of Health) guidelines for the experimental use of animals and were approved by the IACUC at New Jersey Medical School.
Parasite infection and blocking-antibody (Ab) treatments.
Five-, 7-, and 12-week-old female NOD mice were inoculated orally with 200 infective third-stage H. polygyrus larvae using a rounded gavage tube as previously described (45). In certain experiments, groups of mice were also intraperitoneally administered 500 μg of anti-CD25 Ab (PC61) or anti-IL-10R MAb (1B1.3A) every 5 days (BioXCell, West Lebanon, NH) at doses previously shown to be effective at depleting CD25+ T cells (25) or IL-10 function (28) in vivo. Control IgG1 Ab (horseradish peroxidase-conjugated IgG1 Ab)-treated groups were included in all experiments.
Glucose monitoring.
The mice were screened for blood glucose levels using a BD glucose monitor and were considered diabetic when the glucose levels reached >200 mg/dl. In some experiments, the mice were sacrificed after two successive positive readings. The animals were also evaluated daily for clinical signs of diabetes (e.g., weight loss), morbidity, and mortality.
Acceleration of diabetes by Cyp.
For induction of diabetes with Cyp, female NOD mice received intraperitoneal injections at 7 and 9 weeks of age at a dose of 250 mg/kg body weight. Glucose levels were monitored weekly, and diabetes was defined as a blood glucose level of >200 mg/dl.
Flow cytometry.
Mesenteric lymph nodes (MLN) and PLN were collected from treated and control mice at the times indicated, and single-cell suspensions were prepared from the material. Cells (1 × 106) were blocked with Fc Block (BD Pharmingen, San Diego, CA) and stained with different combinations of Abs as follows: anti-CD11c-phycoerythrin (PE), anti-CD8-fluorescein isothiocyanate (FITC), anti-CD11b-allophycocyanin (APC), anti-CD4-peridinin chlorophyll protein, anti-CD62L-PE, and anti-CD44-APC (all from BD Pharmingen). The Treg populations were stained with anti-CD4-peridinin chlorophyll protein (BD Pharmingen) and anti-CD25-PE (Invitrogen), and then intracellular staining was done with anti-FoxP3-APC (eBioscience, San Diego, CA). After washes, the cells were analyzed by flow cytometry with a FACS Calibur (Becton Dickinson). The results were analyzed with Winlist software (Verity Software House, Topsham, ME).
ELISPOT assay.
The frequency of IL-4-producing cells was determined by an enzyme-linked immunospot (ELISPOT) assay as previously described (23). Briefly, single-cell lymph node suspensions were prepared in RPMI 1640 containing 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (all from Gibco BRL, NY). Cells (0.5 × 106) were seeded into each well of an anti-IL-4 (clone BVD4-1D11.2; a gift from Fred D. Finkelman)-coated Immulon IV 96-well microtiter plate (Microtiter, Chantilly, VA). After overnight culture, the plate was washed several times with phosphate-buffered saline (PBS), followed by washes with PBS-Tween 20. Secondary biotinylated anti-IL-4 Ab was diluted in PBS-0.05% Tween-5% fetal calf serum, added at 100 μl/well, and incubated overnight at 4°C. The plates were then washed, and a 1/2,000 dilution of streptavidin-AKP (Jackson Immunoresearch, West Grove, PA) was added. The plates were developed, and the results were counted as described previously (23).
Quantification of serum Igs.
Total serum IgE levels were measured using an ELISA, as previously described (24).
Histology.
Pancreata were removed and fixed in 10% neutral buffered formalin. The pancreata were embedded in paraffin and step sectioned at ∼0.6-mm increments, yielding five or six sections per pancreas. The sections were stained with hematoxylin and eosin (H&E). All islets larger than 20 cells were evaluated for the extent of lymphocytic infiltration. Islets with infiltrates occupying 10% or more of their areas were identified as positive for insulitis. Islets were scored using the following scale: no infiltrates, 0% infiltration; peri-insulitis, 1 to 10% infiltration; moderate insulitis, 11 to 50% infiltration; severe insulitis, >50% infiltration. We typically counted ∼50 to 100 islets per experiment from six to eight mice, depending on the number of islets that were present in the sections.
Cytokine gene expression by RT-PCR.
PLN and MLN were collected from treated and control mice at the times indicated. For reverse transcription (RT)-PCR, total RNA was extracted from tissue and then reverse transcribed as previously described (23). Real-time PCR kits (PE Applied Biosystems, Foster City, CA) specific for individual cytokines or rRNA were used to quantify differences in gene expression, and all data were normalized to constitutive rRNA values. The Applied Biosystems 7700 sequence detector (PE Applied Biosystems, Foster City, CA) was used for amplification of target mRNA, and differences between treatment groups were calculated and quantified according to the manufacturer's instructions.
Immunohistological staining.
Pancreata and PLN harvested from individual mice were frozen in liquid nitrogen, and 6-μm tissue sections were obtained using a HM505E cryostat (Richard-Allan Scientific, Kalamazoo, MI) and stored at −80°C. The tissue sections were allowed to dry at room temperature for 30 min, fixed in cold acetone for 10 min, and then stained with polyclonal guinea pig anti-swine insulin (Dako, Carpinteria, CA), followed by Alexa 488-goat anti-guinea pig IgG (Molecular Probes, Eugene, OR) or Alexa 488-anti-mouse CD206 and PE-anti-mouse CD8, Gr-1, F4/80, or CD11c, as well as Alexa 647-anti-mouse CD4, B220, F4/80, CD11b, or CD11c. Coverslips were applied to the slides using Fluoromount G (Southern Biotechnology Associates, Inc., Birmingham, AL). Pancreatic islets were examined and individually photographed at ×400 magnification using a SPOT2 cooled charge-coupled-device camera (Diagnostic Instruments, Inc., Sterling Heights, MI) mounted on a Leica DMRXA (Leica Microsystems, Inc., Bannockburn, IL) computerized fluorescence microscope, and analyzed using Image Pro software (Caffeine Software, Santa Clara, CA). Each fluorescence channel was photographed separately, and the three sets of ×400 images were merged using Image Pro software (Caffeine Software, Santa Clara, CA) to create the final image of the islet.
Ex vivo cytokine measurement.
Lymphocytes were harvested from spleens of different groups and cultured in 96-well round-bottom plates (2 × 105 cells/well) precoated with 1.5 μg/ml anti-mouse CD3 MAb (2C11; BD Pharmingen) plus 2 μg/ml anti-CD28 MAb (Invitrogen). After 48 h, the supernatants were collected and stored at −70°C. The levels of IL-4 and gamma interferon (IFN-γ) in supernatants were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Statistical analysis.
Statistical analyses were performed using GraphPad Prism 4 for Windows version 4.03 (GraphPad Software, Inc., La Jolla, CA). For life table analysis, we used the log rank test to compare survival curves. For comparisons of three or more variables, we used a one-way analysis of variance with posttest Tukey analysis if P was <0.05. For comparison of two variables, we used the unpaired Student's t test with Welch's correction for unequal variances.
RESULTS
Infection with H. polygyrus prevents the development of T1D in NOD mice.
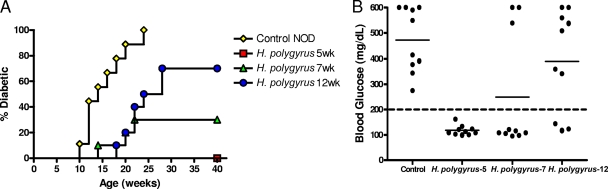
Female NOD mice were orally inoculated with 200 third-stage H. polygyrus larvae at 5, 7, or 12 weeks of age. Age-matched untreated female NOD mice were used as controls. The incidence of diabetes was monitored by blood glucose measurements every second week for a total of 40 weeks. We defined diabetes as a blood glucose level of >200 mg/dl on two consecutive occasions. As shown in Fig. 1A, by 25 weeks of age, 100% of the untreated NOD mice became diabetic. In contrast, a single inoculation of H. polygyrus given at 5 weeks of age completely protected NOD mice from developing diabetes, with 0/10 diabetic mice at 40 weeks of age. When given at 7 weeks of age, H. polygyrus significantly protected NOD mice from diabetes (3/10 diabetic mice at 40 weeks), but to a lesser extent than when the helminth infection was established at 5 weeks of age. When H. polygyrus was given as late as 12 weeks of age, a time when insulitis is fully established, the incidence of diabetes was decreased from 100% to 70% by 40 weeks of age. As seen in Fig. 1B, at 32 weeks of age, untreated NOD mice had extremely high blood glucose, with all levels over 200 mg/ml and some values reaching 600 mg/ml or higher. H. polygyrus infection at 5 weeks of age sustained normal glucose levels at this time point. H. polygyrus inoculation of NOD mice at 7 and 12 weeks of age was not as effective in blocking the development of diabetes; however, significant reduction in the incidence and severity of disease was obtained even when infection was introduced at these later time points after disease progression.
FIG. 1.
(A) Infection with H. polygyrus blocks the development of T1D in NOD mice. H. polygyrus was administered orally as a single dose to female NOD mice at 5, 7, and 12 weeks of age. Blood glucose was monitored in alternate weeks. A blood glucose value of >200 mg/dl on two consecutive occasions was considered to indicate diabetes. The mouse cohorts were followed for 40 weeks (P < 0.0001, control versus H. polygyrus at 5 weeks; P = 0.0003, control versus H. polygyrus at 7 weeks; P = 0.0005, control versus H. polygyrus at 12 weeks). (B) Blood glucose values were plotted at 32 weeks of age. Similar results were obtained in two experiments. The solid horizontal bar indicates the mean blood glucose for each group.
H. polygyrus infection prevents Cyp-induced diabetes in NOD mice.
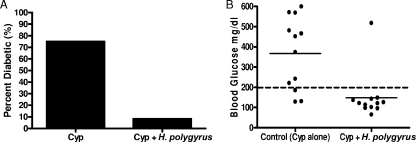
Cyp, a cytotoxic chemotherapeutic agent, has been used to accelerate diabetes in NOD mice (6). Compared to spontaneous diabetes in NOD mice, Cyp treatment induces synchronized, accelerated T1D in 2 to 4 weeks (1). Recently, Treg apoptosis has been shown to be induced in Cyp-treated NOD mice, at least partly explaining their increased susceptibility to T1D (6). To determine whether H. polygyrus infection also prevented Cyp-induced T1D, we treated female NOD mice at 5 weeks of age with H. polygyrus and then administered Cyp via intraperitoneal injections at 7 and 9 weeks of age. We monitored blood glucose levels for development of T1D. As shown in Fig. 2A, by 13 weeks of age, H. polygyrus treatment dramatically blocked the development of diabetes (1/12; 8.3%) compared to the untreated NOD group (9/12; 75%). In Fig. 2B, the majority of the mice in the uninfected group had high glucose levels. In contrast, the glucose levels in 11 out of 12 H. polygyrus-infected mice were all below 200 mg/dl. These studies demonstrate that H. polygyrus-induced control of T1D occurs even in this highly aggressive and rapidly developing experimental model.
FIG. 2.
H. polygyrus infection blocks the development of Cyp-induced diabetes in NOD mice. NOD mice were orally inoculated with H. polygyrus at 5 weeks, and Cyp was given at 7 and 9 weeks of age. (A) The incidence of diabetes at 10 weeks of age. (B) Blood glucose was monitored every week; a blood glucose value of >200 mg/dl (dashed line) on two consecutive occasions was considered to indicate diabetes. Blood glucose levels at 10 weeks of age are shown. Similar results were obtained in two experiments. The solid horizontal bar indicates the mean blood glucose for each group.
H. polygyrus infection protects the pancreatic islets from lymphoid infiltration.
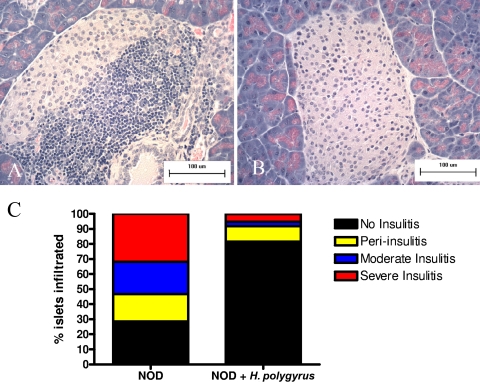
To directly examine whether H. polygyrus infection influenced immune cell infiltration of the islets, histological analysis was performed on pancreatic tissues from H. polygyrus-inoculated and untreated NOD mice. NOD mice were inoculated with H. polygyrus at 5 weeks of age. At 13 weeks of age, pancreata were harvested from mice for H&E staining. Figure 3A and B demonstrates a characteristic H&E-stained pancreatic section from untreated (A) and H. polygyrus-inoculated (B) NOD mice. Control NOD mice had typical invasive insulitis, while H. polygyrus-treated mice had minimal islet infiltrate. We scored the frequency of normal islets, islets with peri-infiltration, islets with moderate infiltration, and islets with invasive infiltration by randomly counting 100 islets from serial sections of paraffin-embedded, H&E-stained pancreas in each group of mice (n = 8). We quantified the differences in insulitis seen between these two groups of mice (Fig. 3C). A dramatic reduction in all types of lymphocyte infiltration was noted in H. polygyrus-treated NOD mice compared to untreated NOD mice. In untreated NOD mice, 32% of islets developed severe, invasive insulitis; 21% of islets had moderate insulitis; 18% had peri-insulitis; and 29% of islets remained normal. In contrast, H. polygyrus infection markedly reduced all type of insulitis (severe, invasive insulitis, 5%; moderate insulitis, 3%; and peri-insulitis, 10%), while 82% of the islets remained normal. Thus, these results indicated that H. polygyrus infection markedly reduced lymphoid infiltration, resulting in marked increases in normal islets.
FIG. 3.
Infection with H. polygyrus prevents lymphoid infiltration and insulitis in the islets of NOD mice. (A and B) H&E-stained section of representative pancreata from control female (n = 9) and H. polygyrus-treated (n = 9) NOD mice. Control mice displayed characteristic invasive insulitis (A), while H. polygyrus-treated mice typically had no inflammatory infiltrates (B). Similar results were obtained in two experiments. (C) NOD mice were infected with H. polygyrus at 5 weeks of age and sacrificed at 13 weeks. Pancreata were removed and fixed in 10% neutral buffered formalin. Islets were scored using the following scale: no infiltrates, 0% infiltration; peri-insulitis, 1 to 10% infiltration; moderate insulitis, 11 to 50% infiltration; severe insulitis, >50% infiltration. We typically counted 50 to 100 islets per experiment from six mice depending on the number of islets that were present in the sections.
Effects of H. polygyrus infection on specific immune cell populations infiltrating the pancreatic islets.
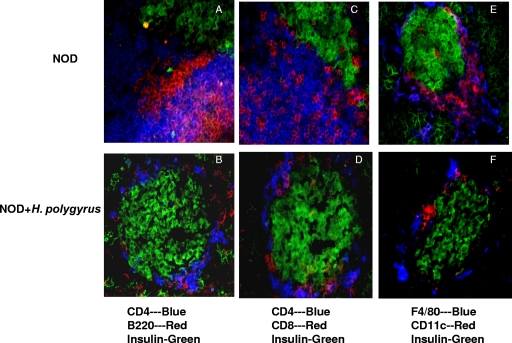
H. polygyrus infection significantly suppressed cellular infiltration into the pancreatic islets. To determine whether H. polygyrus infection affected the composition of immune cells infiltrating the islets, we performed three-color immunofluorescent staining of the pancreas. At 5 weeks of age, female NOD mice were either inoculated with H. polygyrus or left untreated. At 13 weeks of age, the mice were sacrificed and pancreata were collected, snap-frozen, and stored at −80°C. Serial sections of acetone-fixed pancreas were costained for insulin, CD4+ T cells, CD8+ T cells, B cells, dendritic cells, and macrophages by using different combinations of fluorescence-conjugated Abs. As shown in Fig. 4A, we found significant numbers of CD4+ T cells and B220+ B cells infiltrated into the β-cells in untreated NOD mice. In contrast, H. polygyrus-treated NOD mice demonstrated reduced numbers of CD4+ T cells and B220+ B cells invading or surrounding the islets (Fig. 4B). Significant numbers of CD8+ T cells were also detected around the islets of untreated NOD mice (Fig. 4C), whereas H. polygyrus-infected NOD mice had relatively few CD8+ T cells (Fig. 4D). Moreover, pronounced numbers of F4/80+ macrophages and CD11c+ dendritic cells were found in the islets of untreated NOD mice (Fig. 4E), whereas few of these cells were seen around the islets in the H. polygyrus-infected NOD mice (Fig. 4F). Thus, our data suggested that H. polygyrus infection markedly reduced the infiltration of all the major cell populations we evaluated, but the actual compositions and proportions of different cell populations in the infiltrate remained similar.
FIG. 4.
Analysis of cellular infiltration in the pancreata of H. polygyrus-treated and untreated NOD mice. NOD mice inoculated with H. polygyrus at 5 weeks were sacrificed at week 13, and the pancreata were frozen in liquid nitrogen; sectioned; stained for anti-mouse CD4, CD8, B220, CD11c, F4/80, and insulin; and analyzed using an immunofluorescence microscope. The sections in panels A, C, and E were obtained from untreated NOD mice (n = 9), while the sections in panels B, D, and F were from H. polygyrus-treated NOD mice (n = 9). Shown is one representative islet from each group of mice. Untreated NOD mice demonstrated characteristic islet infiltration with CD4+, CD8+, B220+, F4/80+ (macrophage), and CD11c+ cell populations. H. polygyrus-treated mice demonstrated all of the same cell populations, but to a lesser extent.
H. polygyrus infection alters T-cell composition and results in increased AAMΦs in the PLN.
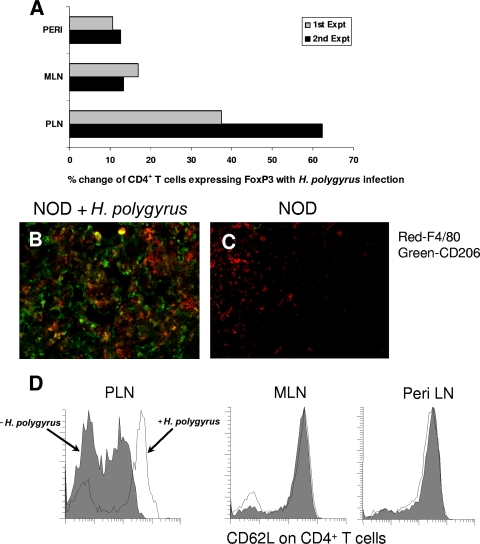
Our data showed that H. polygyrus infection blocked the infiltration of innate cells and lymphocytes into the islets, suggesting that there might be alterations in immune cell phenotypes in the draining PLN. To address whether H. polygyrus infection changed the regulatory-cell composition in the draining PLN relative to MLN and peripheral lymph nodes, we harvested these lymph nodes from H. polygyrus-infected and uninfected NOD mice at 11 weeks of age. Cell suspensions were prepared and stained for CD4, CD25, and FoxP3, and the data were collected by fluorescence-activated cell sorter (FACS). We analyzed the data as the percentage of CD4+ T cells expressing FoxP3. As shown in Fig. 5A, H. polygyrus-inoculated NOD mice showed three to fivefold more FoxP3+ cells in the CD4+ T-cell subset in the PLN than in either the peripheral lymph nodes or MLN. The increases in FoxP3+ cells in the PLN after H. polygyrus infection were 38% in experiment 1 and 62% in experiment 2 compared to 10 to 15% increases in MLN and peripheral lymph nodes.
FIG. 5.
H. polygyrus infection induces AAMΦs, increases the frequency of CD4+ FoxP3+ Tregs, and decreases CD4+ T-cell activation in PLN. Five-week-old female NOD mice were inoculated with H. polygyrus or left uninoculated and sacrificed at weeks 11 to 13. (A) Cell suspensions obtained from PLN, MLN, and peripheral lymph nodes (PERI) were stained with anti-CD4-FITC and anti-FoxP3-PE Abs and analyzed by flow cytometry. H. polygyrus-treated NOD mice showed a marked increase in CD4+ T cells expressing FoxP3 in the total CD4+ T-cell populations. Expt, experiment. (B and C) PLN (nine mice per treatment group) were sectioned, fixed, and stained with anti-F4/80-PE and anti-CD206-FITC. H. polygyrus-treated mice had numerous cells that stained for both markers (yellow-orange) in the PLN, indicative of AAMΦs. (D) Cell suspensions prepared from PLN, MLN, and peripheral lymph nodes (Peri LN) were individually stained with CD4 and CD62L and analyzed by flow cytometry. H. polygyrus-treated mice had an increase in CD62L+ CD4+ T cells, indicative of naive T cells in the PLN.
Previous studies had shown that AAMΦs can be induced by IL-4 produced by Th2 cells during helminth parasite infection (26). To address whether the AAMΦs were increased in the PLN after H. polygyrus infection, we sectioned the PLN and performed immunofluorescent staining with anti-CD206-Alexa 488 and anti-F4/80-PE, with dually stained cells being AAMΦs. As shown in Fig. 5B, there were considerably more CD206+ F4/80+ double-positive cells in the PLN from H. polygyrus-infected mice than in those from untreated NOD mice (Fig. 5C). Thus, our data demonstrated that H. polygyrus infection in NOD mice increased AAMΦs in the lymph nodes draining the pancreas.
H. polygyrus infection increases Tregs and modulates T-cell activation in draining PLN.
Increased numbers of Tregs have been associated with several helminth infections (37, 47). In addition, a recent study showed that H. polygyrus infection decreased the percentage of CD62Lhi CD25− cells but increased the frequency of CD62Llow CD25+ cells in the spleens of NOD mice (37). To study whether H. polygyrus infection in NOD mice elicited changes in CD62L, we infected female NOD mice at 5 weeks of age and sacrificed the mice at 11 weeks of age. Untreated female NOD mice were used as controls. The PLN, MLN, and peripheral lymph nodes (such as inguinal and axillary lymph nodes) were collected, and cell suspensions were obtained. The cells were then stained for surface CD4 and CD62L expression as described in Materials and Methods using flow cytometry. We found that H. polygyrus treatment of NOD mice increased CD62L expression on CD4+ T cells in PLN compared to PLN from untreated NOD mice but did not alter CD62L expression on CD4+ T cells from MLN and peripheral lymph nodes (Fig. 5D). These results suggested that the pronounced T-cell activation associated with the loss of cell surface CD62L and aggressive β-cell infiltration of NOD mice was preferentially inhibited in the draining PLN following H. polygyrus inoculation.
H. polygyrus infection drives a Th2-type cytokine response in PLN and spleens.
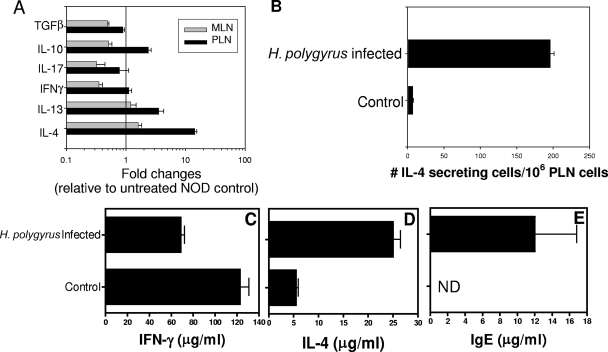
Helminth parasites can induce strong Th2-type immune responses in the host. Since it had been shown that a Th1-type response predominates in the development of T1D, we were interested in determining whether H. polygyrus infection in NOD mice was capable of inducing an alternative Th2-type immune response, particularly in the lymph nodes draining the pancreas, where the Th1-type autoimmune response develops. Female NOD mice were treated with H. polygyrus at 5 weeks of age, and draining MLN and PLN were harvested at 13 weeks of age. Real-time fluorogenic quantitative PCR was used to assess changes in cytokine gene expression. As shown in Fig. 6A, H. polygyrus-treated NOD mice demonstrated a >10-fold increase in IL-4 mRNA in PLN compared to untreated mice (P < 0.001). A lesser, but significant, two- to threefold increase in IL-13 (P = 0.007) and IL-10 (P = 0.002) was also noted. In contrast, IFN-γ, IL-17, and TGF-β mRNA levels were similar to those in untreated NOD mice. We also used an ELISPOT assay to detect IL-4-secreting cells from PLN cells. As shown in Fig. 6B, pronounced increases in the number of IL-4-secreting cells were found in PLN from H. polygyrus-infected NOD mice compared to untreated NOD mice. Single-cell suspensions of splenocytes were also stimulated in vitro with anti-CD3 (1.5 μg/ml) and anti-CD28 (2 μg/ml) Abs for 48 h at 37°C. IL-4 and IFN-γ protein levels in the supernatants were quantified by ELISA. As shown in Fig. 6C and D, H. polygyrus inoculation at 5 weeks of age induced increased IL-4 production and decreased IFN-γ production. We also determined that H. polygyrus infection at 5 weeks of age significantly increased total serum IgE levels (Fig. 6E). Thus, our results suggest that H. polygyrus inoculation stimulated a potent Th2-type response in NOD mice and suppressed the Th1-type response associated with T1D.
FIG. 6.
H. polygyrus infection drives a Th2 immune response in PLN and spleen. Five-week-old female NOD mice were orally inoculated with H. polygyrus and then sacrificed at week 13. (A) Cytokine gene expression in PLN and MLN was detected by quantitative real-time RT-PCR. (B) The number of IL-4-producing cells in PLN was determined by ELISPOT assay. (C and D) Splenocytes were restimulated with anti-CD3 and anti-CD28 for 48 h. The splenocyte supernatants were used to detect IFN-γ (C) and IL-4 (D) by ELISA. (E) The serum IgE level was determined by ELISA. The means and standard errors for nine mice per treatment group are shown. Similar results were obtained in two experiments.
H. polygyrus infection prevents diabetes and insulitis through a CD25-independent mechanism.
Tregs bearing the cell surface marker CD25 are thought to play an important role in mediating protection against T1D in NOD mice (46). In our studies described above, we showed that H. polygyrus infection increased the frequencies of CD4+ CD25+ FoxP3+ Tregs in both PLN and MLN in NOD mice. To directly investigate the role of CD25+ Tregs in our helminth-induced diabetes protection model, NOD mice were treated with depleting anti-CD25 Ab (PC61).
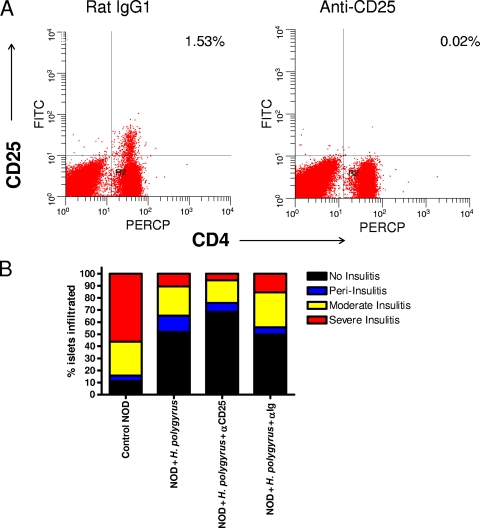
NOD mice were infected with H. polygyrus at 5 weeks of age, and anti-CD25 MAb was given every 5 days starting from the day before infection. Rat IgG1 Ab was used as an isotype control. At 13 weeks of age, the mice were sacrificed, PLN were collected for FACS analysis, and pancreata were removed for histological examination. We chose to use severe insulitis as our surrogate readout for diabetes development because we previously demonstrated that H. polygyrus infection dramatically inhibited severe insulitis in NOD mice. The cost of treating NOD mice with anti-CD25 Ab for a full 40 weeks was prohibitive. To test whether anti-CD25 MAb was effective at depleting CD25+ cell populations in vivo, we stained the cells from PLN with anti-CD4-FITC and anti-CD25-APC and performed FACS analysis. As shown in Fig. 7A, we found that, compared to the mice given control Abs, the anti-CD25 MAb treatment specifically eliminated expression of CD4+ CD25+ cells (>90% reduction). When we examined the effect of H. polygyrus-induced Tregs in regulating the development of insulitis in the pancreas, our H&E staining results (Fig. 7B) showed that H. polygyrus infection dramatically inhibited immune cell infiltration into the pancreatic islets and the development of severe insulitis. Administration of either anti-CD25 MAb or the control MAb did not increase the percentage of the islets with immune cell infiltration. Taken together with our findings that H. polygyrus effectively blocked accelerated Cyp-induced T1D, our studies indicate that CD25+ Tregs are not required for H. polygyrus control of T1D.
FIG. 7.
H. polygyrus-induced prevention of insulitis is mediated through a CD25-independent mechanism. Five-week old female NOD mice (n = 8) were orally inoculated with H. polygyrus. Anti-CD25 and control Abs were given intraperitoneally every 5 days starting from the day before H. polygyrus infection. At 13 weeks of age, the mice were sacrificed. (A) The cells from PLN were stained with anti-CD4-FITC and anti-CD25-APC. The percentages of CD4+ CD25+ cells are shown. (B) Pancreata were fixed in 10% neutral buffered formalin and stained with H&E. The frequencies of normal islets and islets with peri-insulitis, moderate insulitis, or severe insulitis were determined in each group of mice. PERCP, peridinin chlorophyll protein.
H. polygyrus infection-mediated prevention of diabetes is independent of IL-10.
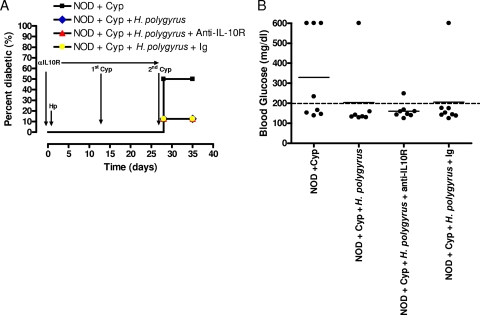
Our data demonstrated that H. polygyrus infection increased IL-10 mRNA in PLN, suggesting that IL-10 might have contributed to H. polygyrus-induced inhibition of T1D. To address this, female NOD mice were infected with H. polygyrus at 5 weeks of age. Commercially obtained anti-IL-10R MAb or control MAb was given intraperitoneally every 5 days starting from the day before infection. The dosage and frequency of administration were similar to those used previously with this clone to block IL-10 interactions (28). At 7 and 9 weeks of age, the mice were treated with Cyp to induce accelerated T1D. The more rapid and synchronized development of Cyp-induced T1D made in vivo anti-IL-10R MAb treatment more practical. Blood glucose levels were monitored every week. In this experiment, we chose to use blood glucose as our readout instead of the insulitis score because Cyp induces rapid-onset hyperglycemia in NOD mice. At 10 weeks of age, the mice were sacrificed. As shown in Fig. 8A, by 10 weeks of age, 50% of the Cyp-treated NOD mice became diabetic. However, only 12% of Cyp-treated mice in the H. polygyrus-infected group had T1D. Administration of either anti-IL-10R or control Abs did not increase the incidence of T1D after H. polygyrus infection. Similarly, without H. polygyrus infection, by 10 weeks of age, four out of eight mice had high blood glucose readings (Fig. 8B). In contrast, H. polygyrus infection prevented Cyp-induced diabetes in the majority of mice (six of seven). Anti-IL-10R MAb or control Ab treatment did not reverse the diabetes protection in H. polygyrus-infected mice. Thus, these results suggest that the H. polygyrus-mediated protection of Cyp-induced T1D occurs through an IL-10-independent mechanism.
FIG. 8.
H. polygyrus-induced diabetes protection is mediated through an IL-10-independent mechanism. Five-week-old female NOD mice (n = 7 to 8) were orally inoculated with H. polygyrus. At 5 and 7 weeks of age, the mice were intraperitoneally administered Cyp. Anti-IL-10R MAb or control Ab was given intraperitoneally every 5 days starting from the day before H. polygyrus infection. Blood glucose levels were monitored throughout the experiment, and at 10 weeks of age, the mice were sacrificed. (A) Incidence of T1D. (B) Blood glucose levels (mg/dl) for individual mice at 10 weeks of age. The solid horizontal bar indicates the mean blood glucose for each group.
DISCUSSION
Our studies demonstrate that H. polygyrus infection protects NOD mice from developing diabetes. The potent inhibitory effect of H. polygyrus infection on autoimmune inflammation was also observed in the aggressive Cyp-induced T1D model. Oral H. polygyrus inoculation of NOD mice inhibited lymphoid infiltration into the islets and, in the draining PLN, induced changes in immune cell composition consistent with preferential development of regulatory-cell populations. In vivo depletion of CD4+ CD25+ Tregs by anti-CD25 MAb or in vivo treatment with blocking anti-IL-10R MAb did not reverse H. polygyrus-induced prevention of T1D. Thus, our data suggest that H. polygyrus infection protects NOD mice from the onset of T1D through CD25- and IL-10-independent mechanisms.
Previously, it had been shown that infection with helminth parasites, such as T. spiralis, H. polygyrus, or S. mansoni, significantly inhibited the incidence of T1D (9, 37). In this study, we confirmed these findings by showing that infection with H. polygyrus at 5 weeks of age completely suppresses the incidence of T1D in NOD mice. We further extended these findings by demonstrating that the diabetes-inhibitory function of H. polygyrus became less effective when H. polygyrus was given at 7 and 12 weeks of age. However, unlike S. mansoni eggs, which inhibited T1D only when given before 8 weeks of age (49), H. polygyrus inoculation still effectively reduced the severity of T1D when given as late as 12 weeks of age, a time point after invasive insulitis had already developed.
T1D is an autoimmune disease resulting in the destruction of insulin-producing β-cells by a localized Th1-type inflammatory response (20). During its pathogenesis, lymphocyte infiltration into the islets is not only an initial characteristic of pathology, but also a fundamental step in disease development (3). Understanding how H. polygyrus affected this localized inflammatory response could provide important insights into how H. polygyrus prevented T1D in NOD mice. Two checkpoints have been suggested in the destruction of β-cells: T effector cell infiltration from PLN into the islets to form “benign insulitis” and progression of “benign insulitis” to “invasive insulitis,” which destroys the islets (3). Previously, administration of S. mansoni eggs, S. mansoni egg antigen, or S. mansoni worm antigen to NOD mice decreased the number of islets with infiltration (49). Our H&E staining showed similar results and further suggested that H. polygyrus preferentially inhibited the development of invasive insulitis, while changes in peri-insulitis were less pronounced.
It has been shown that CD4+ T cells, CD8+ T cells, and macrophages all contribute to the pathogenesis of T1D (16, 34). It is possible that H. polygyrus infection prevents T1D by blocking the infiltration of specific cells, such as pathogenic CD4+ T cells, CD8+ T cells, or macrophages, into the islets or by induced infiltration of regulatory immune cell populations that inhibit islet destruction. Our studies examined for the first time the effects of helminth infection on specific immune cell infiltrates in and around the islet cells and pancreas. Using immunofluorescent in situ staining, we demonstrated that although H. polygyrus infection markedly attenuated islet infiltration by immune cells, it did not change the overall immune cell composition of the infiltrate associated with the islets, as CD4+ T cells, CD8+ T cells, macrophages, and dendritic cells were all present surrounding the islets, albeit in greatly reduced numbers. These findings are consistent with previous flow cytometry results showing that H. polygyrus infection significantly reduced the percentages of CD4+ T cells, CD8+ T cells, B220+ B cells, and CD11c+ dendritic cells in total pancreata of NOD mice by 13 to 14 weeks (37). Our further observation of increased CD62L expression on CD4+ effector T cells suggests a reduced frequency of activated T helper cells. These findings are consistent with previous results showing that S. mansoni egg treatment of NOD mice induced reduced proliferative splenocyte responses when the splenocytes were stimulated with concanavalin A (49). Moreover, H. polygyrus infection did not increase CD62L expression on CD4+ T cells from MLN or peripheral lymph nodes, further indicating that H. polygyrus infection induced a localized alteration of the immune-regulatory environment in the draining PLN. Analysis of potential antigen-presenting cell populations in the PLN of H. polygyrus-inoculated mice indicated increased frequencies of CD11c+ cells, CD11b+ cells, and CD11c+ CD11b+ double-positive cells. CD11c expression is characteristic of dendritic cells (38), and CD11b+ CD11c+ cells include primarily myeloid dendritic cells, which have recently been shown to play a primary role in supporting the development of T1D in NOD mice (38). In future studies, it will be interesting to examine whether myeloid dendritic cells differ functionally in H. polygyrus-infected and uninfected NOD mice.
H. polygyrus infection in NOD mice also resulted in the accumulation of AAMΦs (F4/80+ CD206+) in the PLN. These AAMΦs are distinguished from other activated macrophages by their expression of CD206 and by metabolism of arginine to ornithine by arginase instead of conversion to nitric oxide by inducible nitric oxide synthase (33, 36). AAMΦs may have several important functions during parasite infection, including (i) downregulating pathological Th1-type inflammation (15), (ii) contributing to collagen production for wound recovery (44), (iii) mediating parasite expulsion (22), and (iv) upregulation of arginase, thereby reducing arginine availability for inducible nitric oxide synthase-mediated generation of NO. As classically activated macrophages have been implicated in β-cell destruction during T1D development in NOD mice (2, 16, 21), it is possible that their alternative activation reduces macrophage-mediated pathogenesis.
Helminth parasites have potent immunoregulatory functions, as they induce IL-10, TGF-β, and Tregs in the host and are able to manipulate the ability of the host immune system to respond to unrelated antigens (26, 48). Our finding of increased IL-10 mRNA expression in PLN of H. polygyrus-inoculated NOD mice was consistent with previous findings that, following infection with H. polygyrus or S. mansoni eggs, the splenocytes produced more IL-10 after restimulation with either concanavalin A or parasite antigens (37, 49). In contrast, levels of TGF-β mRNA remained low. Recent studies also showed that administration or gene transfer of IL-10 prevented the onset of T1D (13, 31). It is thus possible that H. polygyrus-induced IL-10 is important in suppressing the development of T1D. To further study the role of IL-10 in the H. polygyrus-induced regulation of diabetes, we used anti-IL-10R MAb to block IL-10 function in vivo in the context of H. polygyrus infection. Our data showed that in vivo IL-10 blockade did not change the T1D-suppressive function of H. polygyrus in a Cyp-induced T1D model, suggesting that increased IL-10 production was not a critical element for the H. polygyrus-mediated diabetes prevention. This is consistent with other studies suggesting that Th1-type inflammatory responses can be inhibited during a helminth-induced response by IL-10-independent mechanisms (15), although it should be noted that helminth control of allergic responses can be IL-10 dependent (43). Further support for our findings comes from a study that used a murine gammaherpesvirus 68 infection in NOD mice (41), where anti-IL-10R Ab administration did not alter CD4+ T-cell trafficking, activation, or proliferation after adoptive transfer of autoreactive T cells. An important caveat, however, is that the blocking-Ab treatment might have incompletely inhibited IL-10 function. Future studies using genetically deficient NOD/IL-10 knockout mice should be more definitive in determining the role of IL-10 in helminth-induced regulation of T1D.
In addition to IL-10, Tregs have been shown to exert a protective effect in the development of T1D (14, 30). We also evaluated regulatory-cell populations in lymphoid organs using flow cytometry. Our findings indicated a preferential accumulation of FoxP3+ Tregs in lymph nodes draining the site of infection or pancreatic inflammation (i.e., MLN and PLN). Previous studies have suggested that Cyp-induced T1D results in more aggressive β-islet destruction and T1D development because of preferential ablation of Tregs (6). Our findings that H. polygyrus infection effectively blocked the development of Cyp-induced T1D suggested that H. polygyrus might be capable of controlling T1D through a CD25+ Treg-independent mechanism. To directly examine the role of CD25+ Tregs under conditions where mice spontaneously develop T1D, we depleted CD25+ cells in vivo using an anti-CD25 MAb. Our findings that anti-CD25 Ab treatment had no effect on the inhibition of insulitis by H. polygyrus inoculation indicated that H. polygyrus likely controls β-islet cell infiltration through CD25+ Treg-independent mechanisms. Of interest, we also treated noninfected 5-week-old female NOD mice with anti-CD25 Ab and evaluated insulitis at 12 weeks. In comparison, female NOD mice that did not receive anti-CD25 had severe insulitis in 39.7% of islets (n = 6 mice) while Ab-treated mice had severe insulitis in 70% of islets (n = 6 mice; data not shown). These results are consistent with previous studies (30) suggesting that CD25+ Tregs are important in attenuating the severity of T1D in uninfected mice and show the effectiveness of our Ab preparation in depleting Tregs. An important consideration in such studies is the possibility that the anti-CD25 Ab might affect other CD25+ cell populations. Future studies with NOD mice deficient in Tregs should help to resolve this concern.
One possible unexplored explanation for the fact that our anti-CD25 and anti-IL-10R treatments did not reverse diabetes protection is that these two mechanisms are redundant. To examine this possibility, it will be necessary to treat NOD mice with both Abs or to use a combination of genetically altered mice plus Ab treatment.
Helminth parasites are strong inducers of Th2-type responses. Infection of NOD mice with S. mansoni eggs markedly enhanced the production of IL-4, IL-5, and IL-13 (49). Infection of NOD mice with H. polygyrus also elevated IL-4, but not IFN-γ, production by splenocytes and increased the percentage of IL-4-producing CD4+ T cells in the cells isolated from the pancreas (37). These results are consistent with our findings that IL-4 and IL-13 mRNA expression was upregulated in PLN and that IL-4, but not IFN-γ, production by splenocytes was boosted after H. polygyrus inoculation. Furthermore, serum IgE levels were markedly elevated in H. polygyrus-infected NOD mice compared to uninfected controls, consistent with a Th2 response. Several studies have shown that IL-4 has a protective function in T1D, as overexpression of IL-4 inhibited the onset of T1D and downregulated β-cell infiltration (29). H. polygyrus-induced IL-4 thus not only indicated that a Th2 response was sustained in NOD mice, but raised the possibility that the cytokine might have modulated T1D. As the overall Th2-type response to H. polygyrus is IL-4 dependent (4), it should be noted that blocking IL-4 would be expected to abrogate any possible regulatory effects of nematode parasite infection.
In conclusion, we have demonstrated that H. polygyrus potently reduced the spontaneous onset of diabetes in NOD mice when given as late as 12 weeks of age and even prevented the much more aggressive Cyp-induced T1D in NOD mice. Associated with an H. polygyrus-induced Th2-type response, we observed marked increases in IL-10, FoxP3+ Tregs, and AAMΦs. However, our studies indicate that neither IL-10 nor CD25 Tregs are essential for H. polygyrus-induced T1D protection. In future studies, it will be interesting to elucidate the other important H. polygyrus-induced regulatory mechanism(s) affecting diabetes prevention.
Acknowledgments
This work was supported by the Foundation for Diabetes Research of New Jersey.
The opinions or assertions contained in the article are the private views of the authors and should not be construed as official or necessarily reflecting the views of the University of Medicine and Dentistry of New Jersey.
We report no financial conflicts of interest.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 14 September 2009.
REFERENCES
- 1.Ablamunits, V., D. Elias, and I. R. Cohen. 1999. The pathogenicity of islet-infiltrating lymphocytes in the non-obese diabetic (NOD) mouse. Clin. Exp. Immunol. 115:260-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alleva, D. G., R. P. Pavlovich, C. Grant, S. B. Kaser, and D. I. Beller. 2000. Aberrant macrophage cytokine production is a conserved feature among autoimmune-prone mouse strains: elevated interleukin (IL)-12 and an imbalance in tumor necrosis factor-alpha and IL-10 define a unique cytokine profile in macrophages from young nonobese diabetic mice. Diabetes 49:1106-1115. [DOI] [PubMed] [Google Scholar]
- 3.Andre, I., A. Gonzalez, B. Wang, J. Katz, C. Benoist, and D. Mathis. 1996. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc. Natl. Acad. Sci. USA 93:2260-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony, R. M., L. I. Rutitzky, J. F. Urban, Jr., M. J. Stadecker, and W. C. Gause. 2007. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7:975-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bach, J. F. 2005. Infections and autoimmune diseases. J. Autoimmun. 25(Suppl.):74-80. [DOI] [PubMed] [Google Scholar]
- 6.Brode, S., T. Raine, P. Zaccone, and A. Cooke. 2006. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J. Immunol. 177:6603-6612. [DOI] [PubMed] [Google Scholar]
- 7.Christen, U., and M. G. von Herrath. 2005. Infections and autoimmunity—good or bad? J. Immunol. 174:7481-7486. [DOI] [PubMed] [Google Scholar]
- 8.Cinek, O., V. Lanska, S. Kolouskova, Z. Sumnik, M. Snajderova, K. S. Ronningen, J. Vavrinec, et al. 2000. Type 1 diabetes mellitus in Czech children diagnosed in 1990-1997: a significant increase in incidence and male predominance in the age group 0-4 years. Diabet. Med. 17:64-69. [DOI] [PubMed] [Google Scholar]
- 9.Cooke, A., P. Tonks, F. M. Jones, H. O'Shea, P. Hutchings, A. J. Fulford, and D. W. Dunne. 1999. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 21:169-176. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, M. J., O. Buchatska, M. Ashton, M. Montoya, Q. D. Bickle, and P. Borrow. 2005. Reciprocal immunomodulation in a schistosome and hepatotropic virus coinfection model. J. Immunol. 175:6275-6285. [DOI] [PubMed] [Google Scholar]
- 11.Elias, D., H. Akuffo, C. Thors, A. Pawlowski, and S. Britton. 2005. Low dose chronic Schistosoma mansoni infection increases susceptibility to Mycobacterium bovis BCG infection in mice. Clin. Exp. Immunol. 139:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner, S. G., P. J. Bingley, P. A. Sawtell, S. Weeks, E. A. Gale, et al. 1997. Rising incidence of insulin dependent diabetes in children aged under 5 years in the Oxford region: time trend analysis. BMJ 315:713-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goudy, K., S. Song, C. Wasserfall, Y. C. Zhang, M. Kapturczak, A. Muir, M. Powers, M. Scott-Jorgensen, M. Campbell-Thompson, J. M. Crawford, T. M. Ellis, T. R. Flotte, and M. A. Atkinson. 2001. Adeno-associated virus vector-mediated IL-10 gene delivery prevents type 1 diabetes in NOD mice. Proc. Natl. Acad. Sci. USA 98:13913-13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregori, S., N. Giarratana, S. Smiroldo, and L. Adorini. 2003. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J. Immunol. 171:4040-4047. [DOI] [PubMed] [Google Scholar]
- 15.Herbert, D. R., C. Holscher, M. Mohrs, B. Arendse, A. Schwegmann, M. Radwanska, M. Leeto, R. Kirsch, P. Hall, H. Mossmann, B. Claussen, I. Forster, and F. Brombacher. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20:623-635. [DOI] [PubMed] [Google Scholar]
- 16.Jun, H. S., C. S. Yoon, L. Zbytnuik, N. van Rooijen, and J. W. Yoon. 1999. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Exp. Med. 18:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamal, S. M., J. W. Rasenack, L. Bianchi, A. Al Tawil, K. El Sayed Khalifa, T. Peter, H. Mansour, W. Ezzat, and M. Koziel. 2001. Acute hepatitis C without and with schistosomiasis: correlation with hepatitis C-specific CD4+ T-cell and cytokine response. Gastroenterology 121:646-656. [DOI] [PubMed] [Google Scholar]
- 18.Karvonen, M., M. Viik-Kajander, E. Moltchanova, I. Libman, R. LaPorte, J. Tuomilehto, et al. 2000. Incidence of childhood type 1 diabetes worldwide. Diabetes Care 23:1516-1526. [DOI] [PubMed] [Google Scholar]
- 19.Kitagaki, K., T. R. Businga, D. Racila, D. E. Elliott, J. V. Weinstock, and J. N. Kline. 2006. Intestinal helminths protect in a murine model of asthma. J. Immunol. 177:1628-1635. [DOI] [PubMed] [Google Scholar]
- 20.Koarada, S., Y. Wu, G. Olshansky, and W. M. Ridgway. 2002. Increased nonobese diabetic Th1:Th2 (IFN-gamma:IL-4) ratio is CD4+ T cell intrinsic and independent of APC genetic background. J. Immunol. 169:6580-6587. [DOI] [PubMed] [Google Scholar]
- 21.Kolb, H., V. Burkart, B. Appels, H. Hanenberg, G. Kantwerk-Funke, U. Kiesel, J. Funda, U. Schraermeyer, and V. Kolb-Bachofen. 1990. Essential contribution of macrophages to islet cell destruction in vivo and in vitro. J. Autoimmun. 3:117-120. [DOI] [PubMed] [Google Scholar]
- 22.Kreider, T., R. M. Anthony, J. F. Urban, Jr., and W. C. Gause. 2007. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 19:448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, Z., Q. Liu, H. Hamed, R. M. Anthony, A. Foster, F. D. Finkelman, J. F. Urban, Jr., and W. C. Gause. 2005. IL-2 and autocrine IL-4 drive the in vivo development of antigen-specific Th2 T cells elicited by nematode parasites. J. Immunol. 174:2242-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, Z., Q. Liu, J. Pesce, J. Whitmire, M. J. Ekkens, A. Foster, J. VanNoy, A. H. Sharpe, J. F. Urban, Jr., and W. C. Gause. 2002. Nippostrongylus brasiliensis can induce B7-independent antigen-specific development of IL-4-producing T cells from naive CD4 T cells in vivo. J. Immunol. 169:6959-6968. [DOI] [PubMed] [Google Scholar]
- 25.Lohr, J., B. Knoechel, S. Jiang, A. H. Sharpe, and A. K. Abbas. 2003. The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat. Immunol. 4:664-669. [DOI] [PubMed] [Google Scholar]
- 26.Maizels, R. M., A. Balic, N. Gomez-Escobar, M. Nair, M. D. Taylor, and J. E. Allen. 2004. Helminth parasites—masters of regulation. Immunol. Rev. 201:89-116. [DOI] [PubMed] [Google Scholar]
- 27.Maron, R., V. Palanivel, H. L. Weiner, and D. A. Harn. 1998. Oral administration of schistosome egg antigens and insulin B-chain generates and enhances Th2-type responses in NOD mice. Clin. Immunol. Immunopathol. 87:85-92. [DOI] [PubMed] [Google Scholar]
- 28.Mekala, D. J., R. S. Alli, and T. L. Geiger. 2005. IL-10-dependent infectious tolerance after the treatment of experimental allergic encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Proc. Natl. Acad. Sci. USA 102:11817-11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller, R., L. M. Bradley, T. Krahl, and N. Sarvetnick. 1997. Mechanism underlying counterregulation of autoimmune diabetes by IL-4. Immunity 7:411-418. [DOI] [PubMed] [Google Scholar]
- 30.Ott, P. A., M. R. Anderson, M. Tary-Lehmann, and P. V. Lehmann. 2005. CD4+CD25+ regulatory T cells control the progression from periinsulitis to destructive insulitis in murine autoimmune diabetes. Cell Immunol. 235:1-11. [DOI] [PubMed] [Google Scholar]
- 31.Pennline, K. J., E. Roque-Gaffney, and M. Monahan. 1994. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin. Immunol. Immunopathol. 71:169-175. [DOI] [PubMed] [Google Scholar]
- 32.Prioult, G., and C. Nagler-Anderson. 2005. Mucosal immunity and allergic responses: lack of regulation and/or lack of microbial stimulation? Immunol. Rev. 206:204-218. [DOI] [PubMed] [Google Scholar]
- 33.Raes, G., L. Brys, B. K. Dahal, J. Brandt, J. Grooten, F. Brombacher, G. Vanham, W. Noel, P. Bogaert, T. Boonefaes, A. Kindt, R. Van den Bergh, P. J. Leenen, P. De Baetselier, and G. H. Ghassabeh. 2005. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J. Leukoc. Biol. 77:321-327. [DOI] [PubMed] [Google Scholar]
- 34.Rajagopalan, G., A. K. Mangalam, M. M. Sen, Y. C. Kudva, and C. S. David. 2007. Distinct local immunogenic stimuli dictate differential requirements for CD4+ and CD8+ T cell subsets in the pathogenesis of spontaneous autoimmune diabetes. Autoimmunity 40:489-496. [DOI] [PubMed] [Google Scholar]
- 35.Resende Co, T., C. S. Hirsch, Z. Toossi, R. Dietze, and R. Ribeiro-Rodrigues. 2007. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin. Exp. Immunol. 147:45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Sosa, M., A. R. Satoskar, R. Calderon, L. Gomez-Garcia, R. Saavedra, R. Bojalil, and L. I. Terrazas. 2002. Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2-biasing ability. Infect. Immun. 70:3656-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunders, K. A., T. Raine, A. Cooke, and C. E. Lawrence. 2007. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 75:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saxena, V., J. K. Ondr, A. F. Magnusen, D. H. Munn, and J. D. Katz. 2007. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J. Immunol. 179:5041-5053. [DOI] [PubMed] [Google Scholar]
- 39.Schoenle, E. J., M. Lang-Muritano, S. Gschwend, J. Laimbacher, P. E. Mullis, T. Torresani, A. Biason-Lauber, and L. Molinari. 2001. Epidemiology of type I diabetes mellitus in Switzerland: steep rise in incidence in under 5 year old children in the past decade. Diabetologia 44:286-289. [DOI] [PubMed] [Google Scholar]
- 40.Sewell, D., Z. Qing, E. Reinke, D. Elliot, J. Weinstock, M. Sandor, and Z. Fabry. 2003. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int. Immunol. 15:59-69. [DOI] [PubMed] [Google Scholar]
- 41.Smith, K. A., S. Efstathiou, and A. Cooke. 2007. Murine gammaherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J. Immunol. 179:7325-7333. [DOI] [PubMed] [Google Scholar]
- 42.Smith, P., N. E. Mangan, C. M. Walsh, R. E. Fallon, A. N. McKenzie, N. van Rooijen, and P. G. Fallon. 2007. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. 178:4557-4566. [DOI] [PubMed] [Google Scholar]
- 43.Smits, H. H., H. Hammad, M. van Nimwegen, T. Soullie, M. A. Willart, E. Lievers, J. Kadouch, M. Kool, J. Kos-van Oosterhoud, A. M. Deelder, B. N. Lambrecht, and M. Yazdanbakhsh. 2007. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J. Allergy Clin. Immunol. 120:932-940. [DOI] [PubMed] [Google Scholar]
- 44.Song, E., N. Ouyang, M. Horbelt, B. Antus, M. Wang, and M. S. Exton. 2000. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 204:19-28. [DOI] [PubMed] [Google Scholar]
- 45.Urban, J. F., Jr., I. M. Katona, and F. D. Finkelman. 1991. Heligmosomoides polygyrus: CD4+ but not CD8+ T cells regulate the IgE response and protective immunity in mice. Exp. Parasitol. 73:500-511. [DOI] [PubMed] [Google Scholar]
- 46.Waid, D. M., G. M. Vaitaitis, N. D. Pennock, and D. H. Wagner, Jr. 2008. Disruption of the homeostatic balance between autoaggressive (CD4+CD40+) and regulatory (CD4+CD25+FoxP3+) T cells promotes diabetes. J. Leukoc. Biol. 84:431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson, M. S., and R. M. Maizels. 2006. Regulatory T cells induced by parasites and the modulation of allergic responses. Chem. Immunol. Allergy 90:176-195. [DOI] [PubMed] [Google Scholar]
- 48.Wilson, M. S., M. D. Taylor, A. Balic, C. A. Finney, J. R. Lamb, and R. M. Maizels. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202:1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaccone, P., Z. Fehervari, F. M. Jones, S. Sidobre, M. Kronenberg, D. W. Dunne, and A. Cooke. 2003. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol. 33:1439-1449. [DOI] [PubMed] [Google Scholar]