Abstract
Pseudomonas aeruginosa is a leading cause of pneumonia, and many components of the innate immune system have been proposed to exert important effects in preventing lung infection. However, a vigorous experimental system to identify an overriding, key effector mediating innate immunity to lung infection has not been utilized. As many of the important components of innate immunity are involved in recruitment and activation of polymorphonuclear neutrophils (PMNs) to infected tissues, we hypothesized that the cells and factors needed for their proper recruitment to the lung comprised the major mediators of innate immunity. In neutropenic mice, intranasal (i.n.) doses of P. aeruginosa as low as 10 to 100 CFU/mouse produced a fatal lung infection, compared with 107 to >108 CFU for nonneutropenic mice. There was only a very modest increased mortality in mice lacking mature lymphocytes and no increased mortality in mice depleted of alveolar macrophages when administered i.n. P. aeruginosa. Recombinant mouse granulocyte colony-stimulating factor increased survival of neutropenic mice after i.n. P. aeruginosa inoculation. MyD88−/− mice, which cannot recruit PMNs to the lungs, were highly susceptible to fatal P. aeruginosa lung infection, with bacterial doses of <120 CFU being lethal. Activation of a MyD88-independent pathway for PMN recruitment to the lungs in MyD88−/− mice resulted in enhanced protection against P. aeruginosa lung infection. Overall, in the absence of PMNs, mice cannot resist P. aeruginosa lung infection from extremely small bacterial doses. There is an inescapable requirement for local PMN recruitment and activation to mediate innate immunity to P. aeruginosa lung infection.
Pseudomonas aeruginosa is a cause of significant morbidity and mortality in hospitalized patients, particularly those with compromised immune systems. P. aeruginosa is a leading cause of ventilator-associated pneumonia and of chronic pulmonary infections in cystic fibrosis patients (7, 20). Given that the lung represents the largest sterile epithelial surface of the body in contact with the external environment, it is not surprising that an elaborate defense system is in place to protect this organ from infectious pathogens. The pulmonary innate host defense consists of structural (integumentary) barriers provided by respiratory tract epithelial cells, mucociliary clearance, antimicrobial molecules (i.e., defensins, lysozyme, lactoferrin, and collectins) produced in the airways, and phagocytic defenses such as resident alveolar macrophages (AM) and recruited polymorphonuclear neutrophils (PMNs).
With regard to both resident and recruited immune cells (specifically, AM, PMNs, and lymphocytes), it is unclear what the relative contribution of each of these cellular immune components is in preventing acute P. aeruginosa pulmonary infection. Alveolar macrophages are thought to provide the first line of defense against microorganisms that reach the lower airways, and they have well-defined roles in both the innate and adaptive immune responses (48). However, in studies using methods to selectively deplete AM to investigate the importance of macrophage phagocytosis in lung host defense against acute P. aeruginosa lung infection, the results have been somewhat contradictory (9), and no major increase in infectious pathology or increased mortality following low challenge doses of P. aeruginosa has been found. Most of the evidence indicates a role for AM in regard to cytokine secretion and nonessential PMN recruitment but no direct role in mediating bacterial killing to protect the lung. The role of lymphocytes in innate defense against acute P. aeruginosa lung infection has not been well characterized, although there is some suggestion that a T-cell immunomodulatory effect may be important (15). Evidence for enhanced susceptibility of patients with AIDS to nosocomial P. aeruginosa infection has also been reported (18), although this has been mostly judged to be secondary to defects in PMN numbers or function. More recently, a murine study suggested that CD1d-restricted T cells play a role in host defense in acute P. aeruginosa pulmonary infections (33). In contrast, it is generally well-accepted that neutrophils comprise a major component of host resistance to P. aeruginosa infection, particularly in the acute settings of bacteremia and sepsis (32). Neutrophil depletion (49, 55) or inhibition of PMN recruitment to the lungs by blocking CXC chemokine receptor 2 (58) resulted in increased mortality after P. aeruginosa infection, but these studies used high doses of single strains of P. aeruginosa that were chosen due to their known high virulence in the setting of murine pneumonia and did not encompass a systematic study of the relationship of PMN depletion to resultant infectious doses of multiple isolates of P. aeruginosa that could cause pneumonia, sepsis, and death. Nor did these studies rule out additional effects from their interventions on disruption of local mediators of immunity, such as AM function or mucociliary clearance, contributing to some of the enhanced susceptibility to P. aeruginosa pneumonia.
Having observed that neutropenia alone was sufficient to allow P. aeruginosa systemic dissemination from the gastrointestinal tract of colonized mice (24), we set out to determine how essential neutrophils were for the host innate defense system against acute P. aeruginosa pulmonary infection, or if, in their absence, other cellular factors such as resident AM, mucociliary clearance, or lymphocyte recruitment and activation could remove very low bacterial inocula efficiently. To evaluate these factors, we utilized selective neutrophil depletion, selective AM depletion, and mice lacking mature lymphocytes (recombinase activating gene-deficient mice [rag−/−]) or unable to recruit PMNs to infected tissues (myeloid differentiation factor 88-deficient mice [MyD88−/−]) in a model of murine acute pneumonia initiated by intranasal (i.n.) inoculation of bacteria onto the nares of anesthetized mice. In neutropenic mice, or MyD88−/− mice, doses of P. aeruginosa in the range of 10 to 100 CFU/animal produced a lethal pneumonia in >88% of infected animals. Restoration of PMN recruitment by injection of recombinant murine granulocyte colony-stimulating factor (r-mGCSF) or by inducing MyD88-independent recruitment of PMNs to the lungs of Myd88-deficient mice significantly increased resistance to P. aeruginosa pneumonia. These findings collectively indicate that in the absence of neutrophil recruitment to the lung, other factors of the innate immune system of mice cannot adequately control infections initiated by very low infectious doses of most strains of P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains of P. aeruginosa used are listed in Table 1. The variety of strains, a mixture of laboratory strains and clinical isolates, was chosen to illustrate that the results obtained were not strain specific but were applicable to diverse strains of P. aeruginosa. For infecting mice, P. aeruginosa cultures were made from frozen stocks, streaked onto cetrimide agar plates, grown overnight at 37°C, and resuspended in sterile phosphate-buffered saline (PBS) with 1% fetal calf serum. P. aeruginosa concentrations were estimated using a spectrophotometer, and actual CFU counts (averages of four replicates) were verified by enumeration on LB and cetrimide agars. Escherichia coli strain HB101 was grown in LB medium. For experiments using killed E. coli cells, 4% paraformaldehyde was added to the culture and incubated overnight at 4°C; bacterial cells were then harvested and washed five times with PBS, and then the cells were resuspended in PBS. The concentration was determined by counting dilutions of the cell suspension in a hemocytometer. Cell death was confirmed by inoculating 0.1 ml of the paraformaldehyde-treated cells onto tryptic soy agar, on which no growth was observed after 48 h at 37°C.
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Source, reference, or description |
|---|---|---|
| PAO1 | Wild-type serogroup O2/O5 st noncytotoxic, chloramphenicol sensitive, pilC+ | M. Vasil |
| IT-4 | Clinical isolate from bacteremia, serogroup O1 | Clinical laboratory |
| 170003 | Wild-type serogroup O2/O5 strain, LPS smooth, noncytotoxic | 23 |
| PA14 | Wild-type serogroup O10 strain, cytotoxic (ExoU+) | F. Ausubel |
| N13 | Wild-type serogroup O6 strain, early isolate from CF lung | 11 |
| 6077 | Wild-type serogroup O11 strain, cytotoxic (ExoU+) | 17 |
| N8 | Wild-type serogroup O11 strain, cytotoxic (ExoU+), early isolate from cystic fibrosis patient lung | 11 |
Animals.
Six- to eight-week-old female C3H/HeN mice were obtained from Harlan Sprague Dawley, Inc. Six- to eight-week-old female RAG1-deficient mice (C57BL/6 background) were obtained from The Jackson Laboratory. MyD88−/− mice were kindly provided by Douglas Golenbock, University of Massachusetts Medical School, and were backcrossed to C57BL/6 mice for >10 generations and then intercrossed to generate MyD88−/− mice in the B6 background. Six- to eight-week-old male and female MyD88−/− mice (sex matched for experiments) were subsequently bred at the animal facility at Harvard Medical School. Age- and sex-matched C57BL/6 mice (The Jackson Laboratory) were used as controls. Mice were housed in groups of four in sterilized cages equipped with filter hoods and supplied with sterile bedding, sterile water, and sterile mouse chow and were maintained under specific-pathogen-free conditions in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee guidelines.
Murine model of acute pneumonia.
Mice were treated with different immunosuppressive regimens (Table 2) 24 h prior to infection. To initiate lung infection, mice were injected intraperitoneally (i.p.) with ketamine (90 mg/kg of body weight/dose) and xylazine (10 mg/kg/dose), and after anesthesia was induced, the P. aeruginosa inoculum was administered i.n. (10 μl per nare). Neutropenic mice received 0.100 mg of gentamicin/ml (Research Product International, Mt. Prospect, IL) added to the drinking water to prevent translocation of normal enteric bacteria from the gastrointestinal tract to the oropharynx. Mice were monitored twice daily for 7 days, by which time PMN counts had returned to normal (16). Moribund mice (i.e., with labored or rapid breathing, decreased motility, ruffled or abnormal-looking fur, or other obvious signs of distress) were euthanized and counted as dead for purposes of these experiments, and along with animals that died between the observation periods, bodies were frozen at −20°C. Spleens and lungs were resected, homogenized in 1% proteose peptone, serially diluted in proteose peptone, and plated on MacConkey and cetrimide agars to confirm that lethality was associated with dissemination of P. aeruginosa, and not other organisms, to these organs. All deaths reported were from moribund/euthanized mice, and dead mice were confirmed to have P. aeruginosa disseminated to the spleens and lungs.
TABLE 2.
Immunosuppressive regimens
To evaluate the effect of MyD88-independent neutrophil recruitment into the lungs of MyD88−/− mice, the acute pneumonia model was modified in the following manner: MyD88−/− and wild-type (WT) C57BL/6 mice were randomized to receive either live E. coli strain HB101 (1 × 108 CFU, i.n.) or paraformaldehyde-killed E. coli strain HB101 (1 × 108 CFU, i.n.) 24 h prior to challenge with P. aeruginosa strains. To confirm PMNs were the key components elicited by this treatment, additional Myd88−/− mice treated with E. coli were also made neutropenic as described below.
Differential staining of BAL.
Mice were euthanized by carbon dioxide inhalation. Bronchoalveolar lavage (BAL) fluid was obtained by exposing the trachea and injecting, then removing, 0.8 ml of PBS with 0.5 M EDTA three times from the lungs. BAL samples were centrifuged to pellet cells; cells were washed and resuspended in PBS. Cells were centrifuged onto microscope slides (cytospins), slides were air dried, and cells were stained using a modified Wright-Giemsa stain (Diff-Quick; Baxter Scientific, Miami, FL). For each sample, a total of 100 cells were counted from randomly chosen high-power fields.
Cyclophosphamide-induced neutropenia, lymphopenia, and monocytopenia.
A cyclophosphamide (Cy; Sigma-Aldrich, St. Louis, MO) dose of 150 mg/kg was administered i.p. every other day for three doses to produce neutropenia (absolute neutrophil count, <500/mm3) lasting for 5 days (24).
Production of RB6-8C5 monoclonal antibody and induction of selective neutropenia.
The RB6-8C5 rat anti-mouse monoclonal antibody (MAb) specific for the Ly-6 antigen highly expressed by PMNs was produced by growing hybridoma cells in culture (Dulbecco's modified Eagle's medium with 10% fetal calf serum), followed by purification of the antibody by affinity chromatography, as previously described (24). A single dose of 0.2 mg of RB6-8C5 was administered i.p. to mice to produce a severe neutropenia (absolute neutrophil count, <100/mm3) lasting for 5 days (24).
Histological analysis of lungs.
C3H/HeN mice were given either 100 CFU of P. aeruginosa strain PAO1 i.n. after anesthesia with ketamine and xylazine, 0.2 mg of RB6-8C5 i.p., or 0.2 mg of RB6-8C5 i.p., followed 24 h later by 100 CFU of PAO1 i.n. Animals were sacrificed 24 h after administration of MAb RB6-8C5 or challenge with P. aeruginosa, the trachea was exposed with a midline neck incision, and 1 ml of PBS containing 1% paraformaldehyde was instilled into the lungs by means of a tracheal catheter. The lungs were removed, fixed in PBS with 1% paraformaldehyde for 1 h at room temperature, and then placed in 70% ethanol in water at 4°C overnight prior to paraffin embedding. Sections were stained with hematoxylin and eosin and reviewed by a veterinary pathologist.
Alveolar macrophage depletion.
CL2MBP (clodronate) was a gift from Roche Diagnostics (Mannheim, Germany). Liposomes containing CL2MBP (250 mg/ml) were prepared as described previously (59). For assessment of AM depletion, three uninfected mice per group were anesthetized as described above and then inoculated i.n. with 100 μl of CL2MBP liposomes or PBS-containing liposomes (28). Twenty-four hours later, BAL samples from the three mice were obtained and analyzed as above.
Neutrophil reconstitution.
Recombinant murine granulocyte colony-stimulating factor was a gift from Amgen (Thousand Oaks, CA). To determine whether r-mGCSF reconstituted neutrophils after RB6-8C5 neutrophil depletion, two groups of four C3H/HeN mice each received MAb RB6-8C5 i.p. on day zero. The groups were then randomized to receive either r-mGCSF (0.150 mg/kg/dose, i.p.) (51) or mouse albumin (0.150 mg/kg/dose, i.p.) daily on days 1 to 5. Each group of mice was bled on days 1 to 6, and blood samples were sent for complete blood count and differential analysis to the hematology laboratory at Children's Hospital Boston, Boston, MA.
To evaluate the effect of neutrophil reconstitution, the acute pneumonia model was modified in the following manner: (i) MAb RB6-8C5 was administered on day zero; (ii) mice were randomized to receive either r-mGCSF at 0.150/mg/kg/dose, i.p., or mouse albumin at 0.150 mg/kg/dose, i.p., daily for 5 days (days 1 to 5), starting 24 h after RB6-8C5 administration; and (iii) mice were challenged with P. aeruginosa strains on day 3 after administration of MAb RB6-8C5.
Neutrophil recruitment.
To evaluate MyD88-independent PMN recruitment, anesthetized MyD88−/− mice (n = 3 per group) were given i.n. one of the following inocula: P. aeruginosa strain PAO1 (50 CFU), 10 μg lipopolysaccharide (LPS) from E. coli strain 0111:B4 (List Biological Labs, Campbell, CA), 1 × 108 CFU of paraformaldehyde-killed E. coli strain HB101, 10 μg of poly I:C (Sigma Aldrich, St. Louis, MO), or 1 × 108 CFU of live E. coli strain HB101. Twenty-four hours later the mice were euthanized and BAL was performed. BAL samples were then analyzed for neutrophil levels by using cytospin slides and Wright-Giemsa stain.
Immunofluorescent staining of lung tissue.
Lungs from mice infected for 24 h were dissected after euthanasia by CO2 overdose and fixed in Bouin's solution, and paraffin-embedded sections were cut and mounted on slides. Five-micrometer sections of lung tissue were deparaffinized in xylene and rehydrated in a series of reverse ethanol washes (100%, 95%, and 80% ethanol, sequentially). The samples were then blocked by incubation in histology blocking buffer (PBS containing 1% bovine serum albumin and 2% normal rat serum [Sigma-Aldrich, St. Louis, MO]) for 15 min at 37°C. Samples were then incubated with MAb RB6-8C5 (0.01 mg/ml) for 90 min at 37°C, then washed with PBS and incubated with goat anti-rat immunoglobulin G conjugated to the Alexa 488 fluorophore (0.005 mg/ml) for 90 min at 37°C. To detect background fluorescence, an appropriate isotype control MAb (rat immunoglobulin G2bκ) was used. The samples were then washed and sections visualized with the 10× alpha-plan lens on a Zeiss Axioplan 2 microscope fitted with filter sets for green fluorescent protein and a cooled charge-coupled-device Hamamatsu Orca camera. Images were acquired and processed using MetaMorph (Molecular Devices Corporation, Sunnyvale, CA) and Adobe Photoshop software (30).
Statistical analyses.
Survival data were analyzed with Fisher's exact test, and the survival curves were analyzed by the Kaplan-Meier log rank test using the GraphPad Prism software (San Diego, CA).
RESULTS
Murine model of acute P. aeruginosa pneumonia.
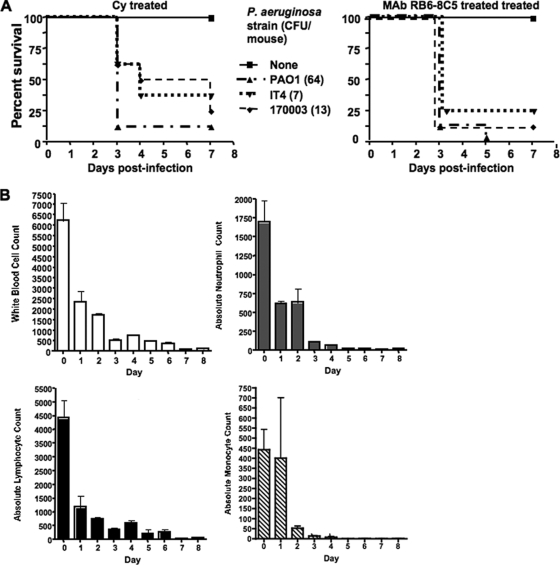
Mice that were made neutropenic with Cy or MAb RB6-8C5 treatment were inoculated with P. aeruginosa strains PAO1 (64 ± 5.2 CFU, average ± standard deviation [SD]), IT4 (7 ± 2.0 CFU), or 170003 (13 ± 4.8 CFU). Mortality ranged from 62.5% to 100%, and median survival of mice treated with Cy or MAb RB6-8C5 and subsequent i.n. P. aeruginosa challenge was significantly lower than that of mice treated with either Cy or RB6-8C5 alone (Fig. 1). All deceased mice given either Cy or RB6-8C5 and then inoculated with P. aeruginosa strains were confirmed to have P. aeruginosa in the lungs that also disseminated to the spleen and liver.
FIG. 1.
(A) Survival curves of female 6- to 8-week-old C3H/HeN mice treated with Cy (150 mg/kg i.p. once daily every other day for 3 days) or MAb RB6-8C5 (200 μg i.p.) and subsequently challenged i.n. with P. aeruginosa strain PAO1, IT4, or 17003. Median survival of mice given Cy or MAb RB6-8C5 and P. aeruginosa was significantly lower than that of mice treated only with Cy or MAb RB6-8C5 (P < 0.01, log rank test). Each group contained eight mice. Values in parentheses indicate the average P. aeruginosa CFU inoculum. (B) Effect of cyclophosphamide (150 mg/kg i.p. once daily every other day for 3 days) on murine white blood cell, absolute neutrophil, absolute lymphocyte, and absolute monocyte blood counts. Data are means ± standard errors of the means (in cells/mm3) for four mice per group.
Effect of lack of functional lymphocytes.
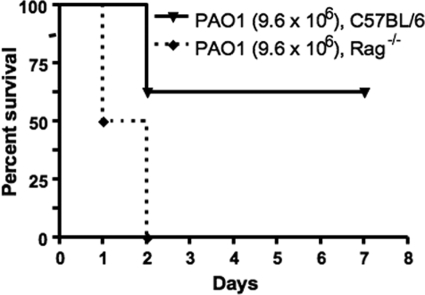
RAG−/− mice and their C57BL/6 wild-type counterparts (n = 8 in each group) all survived after being inoculated i.n. with an average of 6.2 × 106 CFU (±1.5 × 106 CFU) of P. aeruginosa strain PAO1. When the i.n. inoculum was increased slightly more than twofold, to an average 1.4 × 107 CFU (SD, 2.1 × 106 CFU), all mice in both groups died and had high levels of P. aeruginosa in both lungs and spleens. Interestingly, when an intermediate inoculum of 9.2 × 106 CFU (SD, 1.1 × 106 CFU) was administered i.n., none of the RAG−/− mice survived, whereas 62.5% of the wild-type mice survived (Fig. 2) (P = 0.0028 by log rank test). Overall, there was an increased susceptibility of C57BL/6 RAG−/− mice lacking functional lymphocytes to acute P. aeruginosa pneumonia compared to their WT counterparts, but this was only observed at a single challenge dose just 1.5 times the dose that was nonlethal for any RAG−/− or WT mice and 50% lower than a dose lethal to all of the challenged RAG−/− and WT mice.
FIG. 2.
Survival curves of Rag−/− mice and wild-type counterparts (C57BL/6) after i.n. administration of P. aeruginosa strain PAO1. Median survival of wild-type mice was higher than that of Rag−/− mice (P = 0.0028, log rank test). Each group contained eight mice. Values in parentheses indicate the average P. aeruginosa CFU inoculum.
Effect of macrophage depletion.
Treatment with liposomal clodronate reduced AM levels by an average of 83%, comparable to previously reported results (28). Analysis of AM in the control group gave a mean value of 55.3% macrophages in BAL fluid (SD, 5.2%), whereas the liposomal clodronate group had an average of 9.7% macrophages (SD, 1.8%) in BAL fluid. When C3H/HeN mice were given either liposomal clodronate (100 μl) or empty liposomes in PBS (100 μl) 24 h prior to i.n. challenge with three different doses of P. aeruginosa strain PAO1 (6.4 × 105 ± 2.8 × 104 CFU, 2.6 × 106 ± 5.7 × 105 CFU, or 3.8 × 107 ± 2.8 × 106) we saw no difference in survival between controls and AM-depleted mice (Table 3). P. aeruginosa was present in the lungs and spleens of all deceased mice.
TABLE 3.
Survival of C3H/HeN mice after i.n. administration of liposomal clodronate followed by i.n. challenge with P. aeruginosa strain PAO1
| Agent | No. of survivors/no. challenged with the indicated inoculum (CFU/mouse) |
||
|---|---|---|---|
| 6.4 × 105 | 2.6 × 106 | 3.8 × 107 | |
| Liposomal clodronate | 8/8 | 2/8 | 0/8 |
| Liposomes in PBS | 8/8 | 3/8 | 0/8 |
Histological analysis of murine lungs after RB6-8C5 treatment.
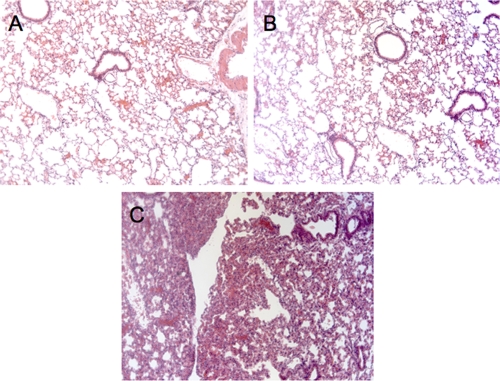
There was no histologic evidence of any lung injury in the lungs of mice given PAO1 or MAb RB6-8C5 alone (Fig. 3) as determined in a review of the dissected tissues by a veterinary pathologist. In contrast, the lungs of mice given MAb RB6-8C5 followed by P. aeruginosa strain PAO1 given i.n. 24 h later and then sacrificed 24 h subsequent to challenge showed significant consolidation, atelectasis, and alveolar wall thickening (Fig. 3).
FIG. 3.
Histological analysis of murine lungs after MAb RB6-8C5 administration. Histological sections of lungs from C3H/HeN mice that were given PAO1 (100 CFU) only (A), MAb RB6-8C5 (0.200 mg i.p. once only) (B), or MAb RB6-8C5 followed by P. aeruginosa strain PAO1 (100 CFU i.n. 24 h after RB6-8C5 administration) (C). Mice were sacrificed 24 h after either MAb RB6-8C5 or PAO1 challenge. Magnification, ×40. Images in panels A and B show normal histologic appearance, whereas the section in panel C shows consolidation and histologic changes consistent with an acute pneumonia.
Neutrophil reconstitution with r-mGCSF.
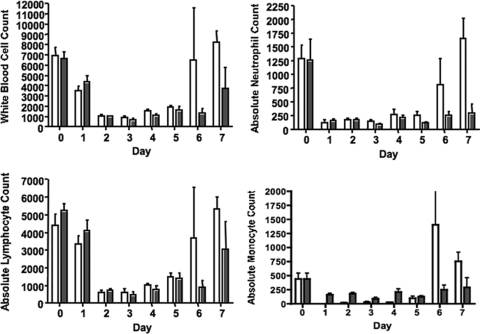
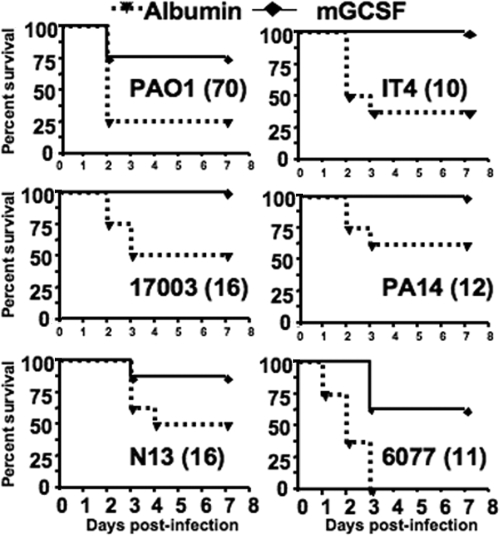
Mice given r-mGCSF after neutrophil depletion with MAb RB6-8C5 showed an increase in the absolute neutrophil counts by days 5 and 6 compared to the control group (Fig. 4). Injection of r-mGCSF after neutrophil depletion with MAb RB6-8C5 and subsequent i.n. challenge with P. aeruginosa strains PAO1, IT4, 170003, PA14, N13, or 6077 increased median survival compared to the control group treated with albumin (Fig. 5), and in five of the six groups there was also significantly increased survival (P ≤ 0.06). P. aeruginosa was present in the lungs and spleens of all deceased or moribund/euthanized mice. Mice treated with MAb RB6-8C5 and subsequently given either albumin or r-mGCSF but not infected with P. aeruginosa all survived (n = 8 mice per group).
FIG. 4.
Effects of r-mGCSF on murine white blood cell, absolute neutrophil, absolute lymphocyte, and absolute monocyte blood counts. C3H/HeN mice were administered MAb RB6-8C5 (0.200 mg, i.p. once on day −1). Mice were then randomized to receive either 0.150 mg/kg r-mGCSF i.p. or 0.150 mg/kg albumin i.p. daily for five doses, starting on day zero. Data are means ± standard errors of the means of cells/mm3 for four mice per group. White bars, r-mGCSF; dark bars, albumin (control).
FIG. 5.
Survival curves of neutropenic C3H/HeN mice treated i.p. with either albumin (control) or r-mGCSF and challenged i.n. with the P. aeruginosa strain indicated in each survival curve graph and the dose (CFU/mouse) indicated in parentheses. P values by log-tank test: PAO1, P = 0.05; IT4, P = 0.008; 170003, P = 0.02; PA14, P = 0.06; N13, P = 0.12; 6077, P = 0.002. Each group contained eight mice.
Acute P. aeruginosa infection in MyD88−/− mice.
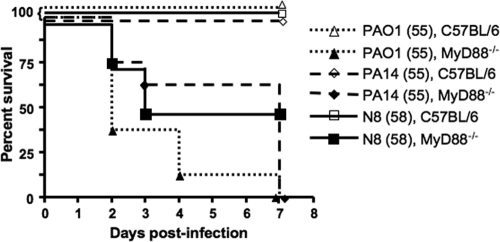
Age- and sex-matched MyD88−/− mice and wild-type C57BL/6 mice were inoculated with P. aeruginosa strains PAO1 (55 ± 3.2 CFU), PA14 (55 ± 7.0 CFU), or N8 (58 ± 1.2 CFU). Mortality ranged from 62.5% to 100% for the MyD88−/− mice and was 0% for all of the wild-type mice. The median survival of MyD88−/− mice given P. aeruginosa i.n. was significantly lower than that of wild-type C57BL/6 mice: PAO1 group, P < 0.0001; PA14 group, P = 0.0001; N8 group, P = 0.025 (all by log rank test). (Fig. 6). P. aeruginosa was present in the lungs and spleens of all deceased or moribund/euthanized mice.
FIG. 6.
Survival curves of WT C57BL/6 or MyD88−/− mice after i.n. challenge with P. aeruginosa strain PAO1, PA14, or N8. Median survival of MyD88−/− mice infected i.n. with P. aeruginosa was significantly lower (P ≤ 0.025, log-rank test) than that of WT C57BL/6 mice infected i.n. with P. aeruginosa (all showed 100% survival). Each group contained eight mice. Values in parentheses indicate the average P. aeruginosa CFU inoculum.
Effect of r-mGCSF treatment in MyD88−/− mice and i.n. challenge with P. aeruginosa.
We investigated whether r-mGCSF treatment could prevent fatal lung infections in MyD88−/− mice. MyD88−/− mice (n = 7) were given 150 μg r-mGCSF/kg/dose i.p. daily on days 1 to 5. On day 3, P. aeruginosa strain PA14 (55 ± 3.2 CFU) was given i.n. to all mice. Only one of seven of the r-mGCSF-treated MyD88−/− mice survived. P. aeruginosa was present in the lungs and spleens of all deceased or euthanized mice. Thus, r-mGCSF could not restore PMN recruitment to the lungs of MyD88−/− mice.
Effects of MyD88-independent PMN recruitment on P. aeruginosa lung infection.
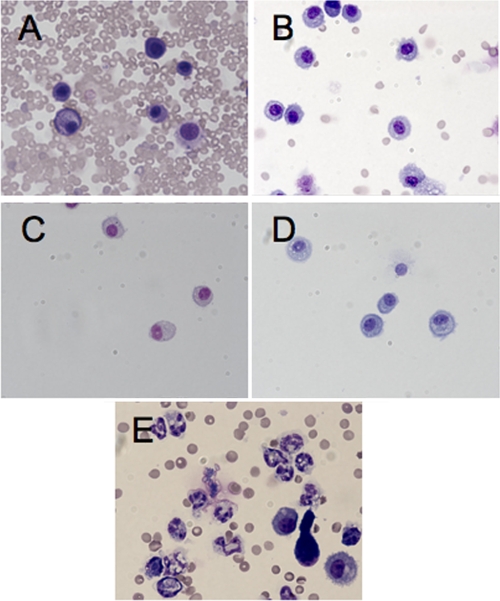
To determine if PMNs could be recruited to the lungs of MyD88−/− mice in a MyD88-independent fashion, we administered various stimuli by the i.n. route and compared the PMNs in BAL fluid 24 h after inoculation with that observed following low-dose P. aeruginosa strain PAO1 infection. No neutrophils per 100 cells counted were detected in the BAL fluid of MyD88−/− mice given only 50 CFU of P. aeruginosa i.n., nor were any PMNs detected in BAL fluid of mice given 10 μg of LPS from E. coli strain O111:B4, 1 × 108 CFU of paraformaldehyde-killed E. coli strain HB101, or 10 μg of poly(I:C) (Fig. 7). Only mice given 1 × 108 CFU of live E. coli HB101 i.n. had neutrophils in the BAL fluid (62 ± 3.1 neutrophils/100 cells counted [mean ± standard error]) (Fig. 7E). Of note, there was a marked number of red blood cells in the BAL fluid of mice that were administered live bacteria (Fig. 7A and E).
FIG. 7.
Murine BAL fluid analysis for the presence of neutrophils in lung sections from MyD88−/− mice administered various bacterial preparations. BAL fluid from 6- to 8-week-old female MyD88−/− mice was obtained 24 h after i.n. administration of the following: 50 CFU of P. aeruginosa strain PAO1 (A); 10 μg of E. coli strain 0111:B4 LPS (B); 108 CFU of paraformaldehyde-killed E. coli strain HB101 (C); 10 μg of poly(I:C) (D); 108 CFU of live E. coli strain HB101 (E). Differential cell counts were determined from Wright-Giemsa-stained cytospins (100 cells per slide were counted). Objective lens, 10×. Only panel E shows the presence of polymorphonuclear lymphocytes in the BAL fluid.
Lung tissues of MyD88−/− mice inoculated i.n. with P. aeruginosa strain PAO1 (100 CFU), live E. coli HB101 (1 × 108 CFU), or live E. coli HB101 (1 × 108 CFU) and then challenged 24 h later with either P. aeruginosa strain PAO1 (100 CFU) or no bacteria were next analyzed for the presence of neutrophils in the lung tissues by using immunofluorescence. Twenty-four hours after the challenge, mice were euthanized and lungs were resected, preserved in Bouin's solution, and stained for the Ly-6 antigen using the RB6-8C5 MAb. Cells presumed to be neutrophils (i.e., eosinophils and monocytes also express Ly-6G and Ly-6C) were detected only in the lungs of mice that were intranasally inoculated with live E. coli strain HB101 (data not shown).
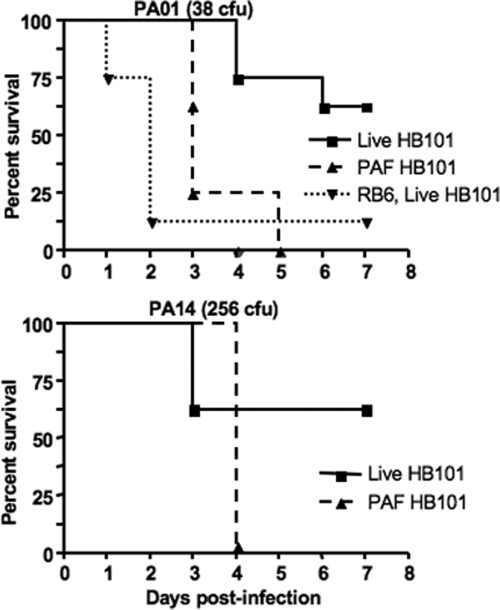
We next evaluated whether the MyD88−/−-independent recruitment of neutrophils to the lungs following inoculation of live E. coli HB101 could affect the survival of MyD88−/− mice from a subsequent P. aeruginosa pneumonia. Mice were initially randomized to receive either 1 × 108 CFU live E. coli strain HB101 i.n. or 1 × 108 CFU paraformaldehyde-killed E. coli strain HB101 i.n. Twenty-four hours later MyD88 −/− mice were challenged with P. aeruginosa strains PAO1 (average 38 CFU) or PA14 (average 256 CFU). MyD88−/− mice treated with the live E. coli HB101 and challenged with strain PAO1 had a decreased time to death (P = 0.001, log rank test) and overall increased survival (five of eight versus zero of eight; P = 0.03, Fisher's exact test), whereas mice challenged with strain PA14 did not have a significant difference in time to death (P = 0.19, log rank test) but did have an overall significantly increased survival (five of eight versus zero of eight; P = 0.03, Fisher's exact test). When the infectious inoculum of P. aeruginosa strain PAO1 was increased to 775 CFU, no MyD88−/− mice survived after pretreatment with either the live E. coli HB101 or paraformaldehyde-treated E. coli HB101 groups (data not shown). Wild-type C57BL/6 mice randomized to receive either live E. coli HB101 or paraformaldehyde-treated E. coli HB101 and then given the same P. aeruginosa strains and inocula as their MyD88−/− counterparts had no mortality (data not shown). In order to discern whether the effect of live E. coli HB101 on augmenting P. aeruginosa clearance was truly due to PMN recruitment, versus a non-PMN-dependent mechanism, a group of MyD88−/− mice were depleted of neutrophils first by administration of MAb RB6-8C5 and then given live HB101 followed by infection with P. aeruginosa strain PAO1; this group showed 88.5% mortality (P = 0.0059 compared to the group given live HB101 and challenged with strain PAO1 group but not pretreated with RB6-8C5; log rank test) (Fig. 8.) P. aeruginosa was present in the lungs and spleens of all deceased or euthanized mice.
FIG. 8.
Survival curves of MyD88 −/− mice after i.n. administration of live or paraformaldehyde (PAF)-killed E. coli strain HB101 followed by challenge with 38 CFU of P. aeruginosa strain PAO1 or 256 CFU of strain PA14 i.n. The RB6, live HB101 group was given MAb RB6-8C5, 0.200 mg i.p., once on day zero, E. coli strain HB101 i.n. on day 1 (24 h); or 38 CFU of PAO1 i.n. on day 2 (48 h). Median survival of mice given live HB101 and subsequent i.n. P. aeruginosa was higher than that of mice treated with PAF-HB101 (P = 0.0012 for PAO1 group, log rank test). Each group contained eight mice. Values in parentheses indicate the average P. aeruginosa inoculum CFU.
DISCUSSION
Although it is clear from human (3, 42) and animal (34, 49) studies that PMNs are essential cellular components of resistance to P. aeruginosa lung infection, it is also true that many other innate immune or tissue clearance factors in the lung, such as mucociliary clearance, antimicrobial peptides, lysozyme, collectins such as surfactants and sugar-binding lectins, epithelial cell responses, lymphocytes and lymphocyte components, and alveolar macrophages, have also been implicated as providing a means to significantly resist infection (61). From these observations a reasonable hypothesis is that neutropenia induced by a specific depletion of PMNs or a genetic inability to recruit PMNs to the lungs would enhance susceptibility to P. aeruginosa pneumonia, but these other host factors would nonetheless provide a substantial measure of resistance to lung infection. However, previously published studies have not systemically tested this hypothesis with a range of P. aeruginosa isolates in a setting of neutropenia induced by specific PMN depletion or in animals unable to recruit PMN to the lungs. We therefore utilized a model of acute P. aeruginosa pneumonia in mice under these defined parameters to study the relative contributions of host factors needed to resist development of lung infection, systemic spread of bacteria, and death. By either chemically administered or genetically induced immunosuppression—via systemic chemotherapy that resulted in neutropenia, lymphopenia, and epithelial cell mucosal damage (cyclophosphamide), specific neutrophil depletion (RB6-8C5 MAb), which also induced a modest lymphopenia, lack of mature lymphocytes (Rag1−/− mice), alveolar macrophage depletion (via liposomal clodronate), or an inability to recruit PMNs to the lungs (MyD88−/− mice)—followed by i.n. P. aeruginosa infection with very low bacterial doses, we found PMNs to be unassailably essential for host resistance to lung infection and death from P. aeruginosa. Infectious doses as low as 8 CFU/mouse for some P. aeruginosa strains resulted in high levels of lung infection and death, with doses of <120 CFU/mouse of all P. aeruginosa strains evaluated being nearly 100% lethal in the absence of effective neutrophil numbers or recruitment to the lungs. Importantly, we found this nearly absolute requirement for PMNs was manifest in C3H mice, which do not show the T helper cell biases of other strains, such as C57BL/6 (43) and BALB/c (43), although we did use the former strain to also show that Myd88 deficiency, which abrogates PMN recruitment to an infected lung, was also a major factor in resistance of this mouse strain to P. aeruginosa lung infection. Although no single manipulation of the murine immune system provided an absolute setting wherein only neutropenia was present without other immune system factors affected, the only common factor among the five experimental settings we tested that resulted in profound susceptibility to low doses of P. aeruginosa was neutropenia. These results imply that among the host factors commonly invoked to explain innate resistance to P. aeruginosa pneumonia, or the expectation that protective effects from factors such as mucociliary clearance, antimicrobial peptides, surfactants, and the like could augment resistance to P. aeruginosa lung infection in mice, we found only lung-recruited neutrophils to have an essential and critical role in clearing very low challenge doses of P. aeruginosa from the mouse lung.
The lack of resistance of neutropenic mice to P. aeruginosa is striking when compared to mice with intact neutrophil numbers and function. The 50% lethal dose (LD50) for P. aeruginosa strain PAO1 in this murine model of acute P. aeruginosa pneumonia in normal 6- to 8-week-old female C3H/HeN mice is 3 × 107 CFU, for strain IT4 it is 6 × 108 CFU, and for strain 170003 it is 8 × 107 CFU (40). Remarkably, when giving either Cy or MAb RB6-8C5 followed by a very-low-dose i.n. inoculum of one of these P. aeruginosa strains, 62.5% to 100% mortality was achieved. In many cases there was a 6- to nearly 8-log decrease in inoculum needed to achieve lethality in 50% or more of the animals. Perhaps the most notable example is strain IT4, which is relatively avirulent in intact mice (LD50, 6 × 108 CFU/mouse), but 8 to 10 CFU applied to the nose was lethal to 62.5% to 75% of neutropenic mice. Likewise, based on work previously done in this laboratory utilizing the same murine model of acute pneumonia, the LD50 for PA14 is 3 × 106 CFU (1, 39, 40), and the LD100 for N13 is 5 × 107 CFU (36). Thus, this acute susceptibility of neutropenic mice to low-inoculum P. aeruginosa acute pneumonia does not appear to be relegated to one or two strains but is generalizable to all seven strains used in this study.
Recently, Jayaseelan and colleagues (21) demonstrated that neutrophils are crucial in controlling P. aeruginosa lung infection by showing enhanced susceptibility to infection and death of both MyD88-deficient mice and WT mice treated with MAb RB6-8C5; the study, however, only utilized one strain of P. aeruginosa (PAO1) and administered a much higher inoculum (1 × 106 CFU) than what we used. Similarly, others have previously established that neutropenia induced by either Cy or MAb RB6-8C5 lowers the inoculum of P. aeruginosa needed to produce pneumonia and sepsis (49, 55), but no systematic study of the relative contributions of neutrophils versus other mediators of innate immunity were analyzed. Again, only one strain of P. aeruginosa for each treatment was used in these other studies, whereas we used a range of strains, including numerous clinical isolates, and the inoculum used in one of these studies was also substantially higher (1 × 106 CFU) (55).
We initially evaluated susceptibility of mice to P. aeruginosa lung infection following induction of neutropenia by Cy, as this agent has been used to induce neutropenia in prior models of P. aeruginosa infection (49). However, as Cy produces neutropenia (24), lymphopenia (24), and mucosal damage (6), its use often makes it unclear what components of the innate immune system are truly critical for preventing acute P. aeruginosa pneumonia. As evidenced in Fig. 1B, Cy resulted in profound neutropenia, lymphopenia, and monocytopenia in the mice that we tested. We thus compared effects of Cy treatment to the more specific PMN-depleting agent MAb RB6-8C5. Neutrophil depletion by MAb RB6-8C5 has been used in a number of animal models of infection (4, 8, 14, 56) and reacts with a common epitope on Ly-6G and Ly-6C. In the peripheral blood, MAb RB6-8C5 recognizes granulocytes (neutrophils and eosinophils) and monocytes (12, 26, 57). Although the absolute lymphocyte count also decreased after administration of RB6-8C5 MAb, counts were generally over 1,000 cells/mm3 (Fig. 4) and the degree of lymphopenia was not as profound (<100 cells/mm3) as that seen with Cy administration (Fig. 1B). RB6-8C5 MAb has also been reported to almost completely eliminate a subset of Ly-6C+ memory-type CD8+ T cells (31). Therefore, to determine if the decreased lymphocyte count could contribute to the increased susceptibility to P. aeruginosa pneumonia, we used Rag1−/− mice and found that a lack of mature lymphocytes made only a very modest contribution to the increased susceptibility to acute P. aeruginosa pneumonia. This was evidenced by the finding that an i.n. inoculum of 9.2 × 106 CFU of P. aeruginosa strain PAO1 resulted in 100% mortality for Rag1−/− mice and only 37.5% mortality in WT mice. Interestingly, a 70% reduction in inoculum resulted in 100% survival for both Rag1−/− and WT mice, and yet a 0.5-log increase in inoculum resulted in 0% survival for both groups. This steep dose-response curve has been observed with other strains of P. aeruginosa in this model (unpublished observation). Although lack of mature lymphocytes modestly increased susceptibility to acute P. aeruginosa infection, this was not nearly as dramatic as the 6- to 7-log-increased susceptibility to P. aeruginosa infection found in the setting of neutropenia or an inability to recruit PMNs to the lungs.
Alveolar macrophages have long been considered one of the first lines of defense against microorganisms that reach the lower respiratory tract. Several receptors (i.e., Fc receptors, complement receptors 1 and 3, and mannose-binding lectin receptor) have been identified and associated with phagocytosis of P. aeruginosa (46, 54), and multiple mechanistic steps involved with AM phagocytosis of P. aeruginosa have been elucidated (27, 53). However, a direct role for AM in surviving acute P. aeruginosa infection seems to be minor (9, 25). One study (25) showed continued administration of liposomal clodronate resulted in persistent neutrophilic alveolitis and decreased bacterial clearance, leading those authors to conclude that macrophages were critical for coordinating the innate immune response (in particular, recruitment of neutrophils) in acute P. aeruginosa infection, but there was no overall difference in mortality between mice with sufficient or depleted AM. Consistent with these findings, we saw no effect on mortality following liposomal clodronate depletion of AM in mice given >6 × 106 CFU of P. aeruginosa i.n.
To implicate PMNs further as the essential mediators of resistance to P. aeruginosa pneumonia, we administered r-mGCSF to neutropenic mice to see if we could augment resistance to an otherwise-fatal dose of P. aeruginosa by stimulating the proliferation, differentiation, and release of granulocytes from the bone marrow. We were able to markedly increase circulating neutrophils in mice treated with r-mGCSF after depletion of PMNs with RB6-8C5 MAb and reduce the mortality from i.n. P. aeruginosa administration. These results are similar to those reported by others who have used G-CSF in neutropenic mice infected with P. aeruginosa (2), but higher bacterial challenge doses or coadministration of antibiotics or defensins were also studied in these other experimental protocols. Human G-CSF has been used to treat neutropenic patients with a wide range of clinical conditions (35) and has shown benefit in resisting P. aeruginosa infection, and G-CSF is often induced during infections in both mice (41) and humans (22). It should be noted, however, that G-CSF not only stimulates the bone marrow to produce neutrophils but also stem cells. This may explain why both murine lymphocyte and monocyte counts also increased after administration of r-mGCSF (Fig. 4).
Given that the absolute number of circulating neutrophils appears to be critical in the defense against acute P. aeruginosa lung infection, the next logical step was to investigate the importance of neutrophil recruitment. We previously showed that Myd88 is needed for the rapid nuclear translocation of NF-κΒ following P. aeruginosa lung infection (45), which leads to production of interleukin-6 and interleukin-8 and increases in intercellular adhesion molecule 1, all factors needed for PMN recruitment (44). Here we found that MyD88-deficient mice exhibited a nearly complete deficit in neutrophil recruitment to the lung following P. aeruginosa infection and were nearly as susceptible to lethal pneumonia and sepsis as were neutropenic mice. We confirmed that the MyD88−/− mice had normal numbers of PMNs in the systemic circulation compared to wild-type C57BL/6 mice (A. Y. Koh and G. B. Pier, unpublished observation). However, when PMNs were recruited to the lungs via a MyD88-independent pathway by prior administration of live E. coli HB101, we could augment resistance of MyD88−/− mice to low-dose P. aeruginosa infection. This did not occur in either mice pretreated with paraformaldehyde-killed E. coli HB101, which failed to induce PMN recruitment in MyD88−/− mice or in MyD88-deficient mice given live, i.n. E. coli HB101 to augment PMN entry into the lungs but also given MAb RB6-8C5 to deplete PMNs. This finding indicates that PMN recruitment into the lungs following infection with P. aeruginosa is nearly completely dependent on MyD88-mediated signaling.
Prior studies of P. aeruginosa infection in MyD88−/− mice (38, 52) showed blunted early cytokine production and inflammatory responses and development of fatal pneumonia with P. aeruginosa (52), but these studies did not look at low infectious inocula and their ability to cause infection and dissemination. The study of Skerrett et al. (52) utilized only a single bacterial strain and a high aerosol inoculum to indicate an important role for MyD88 in resistance to P. aeruginosa lung infection but did not analyze the degree to which the MyD88−/− mice were more susceptible to P. aeruginosa in terms of the effect of infectious inocula. Another study showed reduced early clearance of a single P. aeruginosa strain in MyD88−/− mice (38), but this factor was dispensable for delayed clearance (37), wherein recruitment of neutrophils into the lung operated via an MyD88-independent mechanism (e.g., TLR-TRAM/TRIF pathway). However, this latter study used a mucoid, LPS rough clinical isolate of P. aeruginosa that does not readily cause, and is not representative of the strains that are isolated from cases of, ventilator-associated acute pneumonia and sepsis, and it is also a strain that can be cleared from the lungs and blood due to its sensitivity to the bactericidal effects of complement. Thus, this finding in MyD88−/− mice is not completely applicable to the setting of acute P. aeruginosa pneumonia with LPS smooth, nonmucoid, serum-resistant strains. Notably, a recent report documented nine children with autosomal recessive MyD88 deficiency that experienced severe, often life-threatening pyogenic bacterial infections with pathogens, including P. aeruginosa, Streptococcus pneumoniae, and Staphylococcus aureus (60), indicating that MyD88 is also important in human resistance to P. aeruginosa infection. Finally, there are several studies that support the notion that most of the initial colonizing strains in patients with cystic fibrosis tend to be nonmucoid and LPS smooth (5, 16, 29).
Our findings and conclusions clearly challenge some well-established dogmas in regard to host resistance to P. aeruginosa lung infection, wherein factors other than PMNs have been implicated as making a significant contribution to clearing this pathogen from the lung. These include structural (integumentary) barriers provided by respiratory tract epithelial cells, mucociliary clearance (61), and antimicrobial molecules (i.e., defensins [50], lysozyme [10], lactoferrin [47], and collectins [19]). It is possible that extending our findings and conclusions to humans requires appropriate caution, particularly with regard to mucociliary clearance. For example, the absolute size of a P. aeruginosa cell is the same whether infecting a mouse or human, but the smaller amount of tissue providing mucociliary clearance in a mouse versus the much larger tissue area in a human may mean that 10 CFU of P. aeruginosa given i.n. to a neutropenic mouse is able to overcome the mucociliary defense system of this species, whereas mucociliary clearance in a neutropenic human may be more effective in resisting low-dose infections. Similarly, antimicrobial molecules may have a more prominent role in human defense against acute P. aeruginosa pulmonary infection that we could not demonstrate in our mouse model. However, as almost all experimental studies of mammalian resistance to P. aeruginosa infection use mice, and essentially all studies to date in transgenic animals use mice, the utility of this species for such studies is considered to provide a strong basis for insights into human resistance and susceptibility to this pathogen.
In conclusion, by utilizing a murine model of acute P. aeruginosa pulmonary infection and manipulating distinct arms of the cellular innate immune system (i.e., neutrophils, AM, and mature lymphocytes) under five different experimental conditions, we found that the lack of effective PMN activity in the lungs was the one factor common to these conditions that allowed for the extreme susceptibility to a lethal P. aeruginosa lung infection. Thus, despite the fact that the pulmonary innate defense system is a complicated amalgam of structural barriers, mucociliary clearance, antimicrobial molecules produced in the airways, and cellular defenses, ultimately PMNs, and in particular recruitment of PMNs to the lung, are the component of the innate host defense needed for resisting very low doses of P. aeruginosa in an acute model of pulmonary infection. In the absence of circulating PMNs, or PMN recruitment into the lung, fatal infections can be induced by aspiration of very small inocula of P. aeruginosa.
Acknowledgments
We thank Amgen for provision of r-mGCSF.
This work was supported by NIH grants RO1 AI22535 (G.B.P.), AI48917 (G.B.P.), AI62983 (A.Y.K.), and AI 50036 (G.P.P.).
We have no financial conflicts of interest.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babalola, C. P., C. H. Nightingale, and D. P. Nicolau. 2004. Adjunctive efficacy of granulocyte colony-stimulating factor on treatment of Pseudomonas aeruginosa pneumonia in neutropenic and non-neutropenic hosts. J. Antimicrob. Chemother. 53:1098-1100. [DOI] [PubMed] [Google Scholar]
- 3.Bodey, G. P. 2001. Pseudomonas aeruginosa infections in cancer patients: have they gone away? Curr. Opin. Infect. Dis. 14:403-407. [DOI] [PubMed] [Google Scholar]
- 4.Buendia, A. J., L. Del Rio, N. Ortega, J. Sanchez, M. C. Gallego, M. R. Caro, J. A. Navarro, F. Cuello, and J. Salinas. 2002. B-cell-deficient mice show an exacerbated inflammatory response in a model of Chlamydophila abortus infection. Infect. Immun. 70:6911-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campodonico, V. L., M. Gadjeva, C. Paradis-Bleau, A. Uluer, and G. B. Pier. 2008. Airway epithelial control of Pseudomonas aeruginosa infection in cystic fibrosis. Trends Mol. Med. 14:120-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavicchioli, L., G. De Zan, V. Zappulli, R. Cadrobbi, A. Dedja, S. Hutabba, L. Ravarotto, E. Cozzi, E. Ancona, and M. Castagnaro. 2007. Histopathological findings in the gastrointestinal tract of primate recipients of porcine renal xenografts following different immunosuppressive regimens. Xenotransplantation 14:145-156. [DOI] [PubMed] [Google Scholar]
- 7.Chastre, J., and J. Y. Fagon. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165:867-903. [DOI] [PubMed] [Google Scholar]
- 8.Cheminay, C., D. Chakravortty, and M. Hensel. 2004. Role of neutrophils in murine salmonellosis. Infect. Immun. 72:468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung, D. O., K. Halsey, and D. P. Speert. 2000. Role of pulmonary alveolar macrophages in defense of the lung against Pseudomonas aeruginosa. Infect. Immun. 68:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole, A. M., D. R. Thapa, V. Gabayan, H. I. Liao, L. Liu, and T. Ganz. 2005. Decreased clearance of Pseudomonas aeruginosa from airways of mice deficient in lysozyme M. J. Leukoc. Biol. 78:1081-1085. [DOI] [PubMed] [Google Scholar]
- 11.Coleman, F. T., S. Mueschenborn, G. Meluleni, C. Ray, V. J. Carey, S. O. Vargas, C. L. Cannon, F. M. Ausubel, and G. B. Pier. 2003. Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection. Proc. Natl. Acad. Sci. USA 100:1949-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cryz, S. J., Jr., E. Furer, and R. Germanier. 1983. Passive protection against Pseudomonas aeruginosa infection in an experimental leukopenic mouse model. Infect. Immun. 40:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czuprynski, C. J., J. F. Brown, R. D. Wagner, and H. Steinberg. 1994. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect. Immun. 62:5161-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunkley, M. L., R. L. Clancy, and A. W. Cripps. 1994. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology 83:362-369. [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell, P. M., Z. Li, M. R. Kosorok, A. Laxova, C. G. Green, J. Collins, H. C. Lai, L. M. Makholm, M. J. Rock, and M. L. Splaingard. 2003. Longitudinal evaluation of bronchopulmonary disease in children with cystic fibrosis. Pediatr. Pulmonol. 36:230-240. [DOI] [PubMed] [Google Scholar]
- 17.Fleiszig, S. M. J., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franzetti, F., A. Grassini, M. Piazza, M. Degl'innocenti, A. Bandera, L. Gazzola, G. Marchetti, and A. Gori. 2006. Nosocomial bacterial pneumonia in HIV-infected patients: risk factors for adverse outcome and implications for rational empiric antibiotic therapy. Infection 34:9-16. [DOI] [PubMed] [Google Scholar]
- 19.Giannoni, E., T. Sawa, L. Allen, J. Wiener-Kronish, and S. Hawgood. 2006. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 34:704-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918-951. [DOI] [PubMed] [Google Scholar]
- 21.Jeyaseelan, S., S. K. Young, M. Yamamoto, P. G. Arndt, S. Akira, J. K. Kolls, and G. S. Worthen. 2006. Toll/IL-1R domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J. Immunol. 177:538-547. [DOI] [PubMed] [Google Scholar]
- 22.Kawakami, M., H. Tsutsumi, T. Kumakawa, H. Abe, M. Hirai, S. Kurosawa, M. Mori, and M. Fukushima. 1990. Levels of serum granulocyte colony-stimulating factor in patients with infections. Blood 76:1962-1964. [PubMed] [Google Scholar]
- 23.Knirel, Y. A., E. V. Vinogradov, N. A. Kocharova, N. A. Paramonov, N. K. Kochetkov, B. A. Dmitriev, E. S. Stanislavsky, and B. Lányi. 1988. The structure of O-specific polysaccharides and serological classification of Pseudomonas aeruginosa. Acta Microbiol. Hung. 35:3-24. [PubMed] [Google Scholar]
- 24.Koh, A. Y., G. P. Priebe, and G. B. Pier. 2005. Virulence of Pseudomonas aeruginosa in a murine model of gastrointestinal colonization and dissemination in neutropenia. Infect. Immun. 73:2262-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooguchi, K., S. Hashimoto, A. Kobayashi, Y. Kitamura, I. Kudoh, J. Wiener-Kronish, and T. Sawa. 1998. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect. Immun. 66:3164-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagasse, E., and I. L. Weissman. 1996. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods 197:139-150. [DOI] [PubMed] [Google Scholar]
- 27.Lee, D. J., D. Cox, J. Li, and S. Greenberg. 2000. Rac1 and Cdc42 are required for phagocytosis, but not NF-κB-dependent gene expression, in macrophages challenged with Pseudomonas aeruginosa. J. Biol. Chem. 275:141-146. [DOI] [PubMed] [Google Scholar]
- 28.Leemans, J. C., N. P. Juffermans, S. Florquin, N. van Rooijen, M. J. Vervoordeldonk, A. Verbon, S. J. van Deventer, and T. van der Poll. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 166:4604-4611. [DOI] [PubMed] [Google Scholar]
- 29.Li, Z., M. R. Kosorok, P. M. Farrell, A. Laxova, S. E. West, C. G. Green, J. Collins, M. J. Rock, and M. L. Splaingard. 2005. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581-588. [DOI] [PubMed] [Google Scholar]
- 30.Lyczak, J. B., and G. B. Pier. 2002. Salmonella enterica serovar Typhi modulates cell surface expression of its receptor, the cystic fibrosis transmembrane conductance regulator, on the intestinal epithelium. Infect. Immun. 70:6416-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzaki, J., T. Tsuji, K. Chamoto, T. Takeshima, F. Sendo, and T. Nishimura. 2003. Successful elimination of memory-type CD8+ T cell subsets by the administration of anti-Gr-1 monoclonal antibody in vivo. Cell Immunol. 224:98-105. [DOI] [PubMed] [Google Scholar]
- 32.Mizgerd, J. P. 2002. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin. Immunol. 14:123-132. [DOI] [PubMed] [Google Scholar]
- 33.Nieuwenhuis, E. E., T. Matsumoto, M. Exley, R. A. Schleipman, J. Glickman, D. T. Bailey, N. Corazza, S. P. Colgan, A. B. Onderdonk, and R. S. Blumberg. 2002. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat. Med. 8:588-593. [DOI] [PubMed] [Google Scholar]
- 34.Oishi, K., F. Sonoda, A. Iwagaki, P. Ponglertnapagorn, K. Watanabe, T. Nagatake, A. Siadak, M. Pollack, and K. Matsumoto. 1993. Therapeutic effects of a human antiflagella monoclonal antibody in a neutropenic murine model of Pseudomonas aeruginosa pneumonia. Antimicrob. Agents Chemother. 37:164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozer, H., J. O. Armitage, C. L. Bennett, J. Crawford, G. D. Demetri, P. A. Pizzo, C. A. Schiffer, T. J. Smith, G. Somlo, J. C. Wade, J. L. Wade III, R. J. Winn, A. J. Wozniak, M. R. Somerfield, et al. 2000. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J. Clin. Oncol. 18:3558-3585. [DOI] [PubMed] [Google Scholar]
- 36.Pier, G. B., D. Boyer, M. Preston, F. T. Coleman, N. Llosa, S. Mueschenborn-Koglin, C. Theilacker, H. Goldenberg, J. Uchin, G. P. Priebe, M. Grout, M. Posner, and L. Cavacini. 2004. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J. Immunol. 173:5671-5678. [DOI] [PubMed] [Google Scholar]
- 37.Power, M. R., J. S. Marshall, M. Yamamoto, S. Akira, and T. J. Lin. 2006. The myeloid differentiation factor 88 is dispensable for the development of a delayed host response to Pseudomonas aeruginosa lung infection in mice. Clin. Exp. Immunol. 146:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Power, M. R., Y. Peng, E. Maydanski, J. S. Marshall, and T. J. Lin. 2004. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J. Biol. Chem. 279:49315-49322. [DOI] [PubMed] [Google Scholar]
- 39.Priebe, G. P., C. R. Dean, T. Zaidi, G. J. Meluleni, F. T. Coleman, Y. S. Coutinho, M. J. Noto, T. A. Urban, G. B. Pier, and J. B. Goldberg. 2004. The galU gene of Pseudomonas aeruginosa is required for corneal infection and efficient systemic spread following pneumonia but not for infection confined to the lung. Infect. Immun. 72:4224-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Priebe, G. P., G. J. Meluleni, F. T. Coleman, J. B. Goldberg, and G. B. Pier. 2003. Protection against fatal Pseudomonas aeruginosa pneumonia in mice after nasal immunization with a live, attenuated aroA deletion mutant. Infect. Immun. 71:1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinton, L. J., S. Nelson, D. M. Boe, P. Zhang, Q. Zhong, J. K. Kolls, and G. J. Bagby. 2002. The granulocyte colony-stimulating factor response after intrapulmonary and systemic bacterial challenges. J. Infect. Dis. 185:1476-1482. [DOI] [PubMed] [Google Scholar]
- 42.Ramphal, R. 2004. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin. Infect. Dis. 39(Suppl. 1):S25-S31. [DOI] [PubMed] [Google Scholar]
- 43.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 44.Reiniger, N., J. K. Ichikawa, and G. B. Pier. 2005. Influence of cystic fibrosis transmembrane conductance regulator on gene expression in response to Pseudomonas aeruginosa infection of human bronchial epithelial cells. Infect. Immun. 73:6822-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiniger, N., M. M. Lee, F. T. Coleman, C. Ray, D. E. Golan, and G. B. Pier. 2007. Resistance to Pseudomonas aeruginosa chronic lung infection requires cystic fibrosis transmembrane conductance regulator-modulated interleukin-1 (IL-1) release and signaling through the IL-1 receptor. Infect. Immun. 75:1598-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhein, L. M., M. Perkins, N. P. Gerard, and C. Gerard. 2008. FcγRIII is protective against Pseudomonas aeruginosa pneumonia. Am. J. Respir. Cell Mol. Biol. 38:401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogan, M. P., C. C. Taggart, C. M. Greene, P. G. Murphy, S. J. O'Neill, and N. G. McElvaney. 2004. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J. Infect. Dis. 190:1245-1253. [DOI] [PubMed] [Google Scholar]
- 48.Sadikot, R. T., H. Zeng, M. Joo, M. B. Everhart, T. P. Sherrill, B. Li, D.-S. Cheng, F. E. Yull, J. W. Christman, and T. S. Blackwell. 2006. Targeted immunomodulation of the NF-κB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J. Immunol. 176:4923-4930. [DOI] [PubMed] [Google Scholar]
- 49.Scarff, J. M., and J. B. Goldberg. 2008. Vaccination against Pseudomonas aeruginosa pneumonia in immunocompromised mice. Clin. Vaccine Immunol. 15:367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shu, Q., Z. Shi, Z. Zhao, Z. Chen, H. Yao, Q. Chen, A. Hoeft, F. Stuber, and X. Fang. 2006. Protection against Pseudomonas aeruginosa pneumonia and sepsis-induced lung injury by overexpression of beta-defensin-2 in rats. Shock 26:365-371. [DOI] [PubMed] [Google Scholar]
- 51.Sionov, E., and E. Segal. 2003. Polyene and cytokine treatment of experimental aspergillosis. FEMS Immunol. Med. Microbiol. 39:221-227. [DOI] [PubMed] [Google Scholar]
- 52.Skerrett, S. J., H. D. Liggitt, A. M. Hajjar, and C. B. Wilson. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 172:3377-3381. [DOI] [PubMed] [Google Scholar]
- 53.Speert, D. P., and S. Gordon. 1992. Phagocytosis of unopsonized Pseudomonas aeruginosa by murine macrophages is a two-step process requiring glucose. J. Clin. Investig. 90:1085-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speert, D. P., S. D. Wright, S. C. Silverstein, and B. Mah. 1988. Functional characterization of macrophage receptors for in vitro phagocytosis of unopsonized Pseudomonas aeruginosa. J. Clin. Investig. 82:872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun, L., R. F. Guo, M. W. Newstead, T. J. Standiford, D. R. Macariola, and T. P. Shanley. 2008. Effect of IL-10 on neutrophil recruitment and survival after Pseudomonas aeruginosa challenge. Am. J. Respir. Cell Mol. Biol. 41:76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 57.Tepper, R. I., R. L. Coffman, and P. Leder. 1992. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257:548-551. [DOI] [PubMed] [Google Scholar]
- 58.Tsai, W. C., R. M. Strieter, B. Mehrad, M. W. Newstead, X. Zeng, and T. J. Standiford. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 68:4289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174:83-93. [DOI] [PubMed] [Google Scholar]
- 60.von Bernuth, H., C. Picard, Z. Jin, R. Pankla, H. Xiao, C. L. Ku, M. Chrabieh, I. B. Mustapha, P. Ghandil, Y. Camcioglu, J. Vasconcelos, N. Sirvent, M. Guedes, A. B. Vitor, M. J. Herrero-Mata, J. I. Arostegui, C. Rodrigo, L. Alsina, E. Ruiz-Ortiz, M. Juan, C. Fortuny, J. Yague, J. Anton, M. Pascal, H. H. Chang, L. Janniere, Y. Rose, B. Z. Garty, H. Chapel, A. Issekutz, L. Marodi, C. Rodriguez-Gallego, J. Banchereau, L. Abel, X. Li, D. Chaussabel, A. Puel, and J. L. Casanova. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321:691-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, P., W. R. Summer, G. J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39-51. [DOI] [PubMed] [Google Scholar]