Abstract
Human monocytotropic ehrlichiosis (HME), an emerging and often life-threatening tick-transmitted disease, is caused by the obligately intracellular bacterium Ehrlichia chaffeensis. HME is modeled in C57BL/6 mice using Ehrlichia muris, which causes persistent infection, and Ixodes ovatus Ehrlichia (IOE), which is either acutely lethal or sublethal depending on the dose and route of inoculation. A persistent primary E. muris infection, but not a sublethal IOE infection, protects mice against an ordinarily lethal secondary IOE challenge. In the present study, we determined the role of persistent infection in maintenance of protective memory immune responses. E. muris-infected mice were treated with doxycycline or left untreated and then challenged with an ordinarily lethal dose of IOE. Compared to E. muris-primed mice treated with doxycycline, untreated mice persistently infected with E. muris had significantly greater numbers of antigen-specific gamma interferon-producing splenic memory T cells, significant expansion of CD4+ CD25+ T regulatory cells, and production of transforming growth factor β1 in the spleen. Importantly, E. muris-primed mice treated with doxycycline showed significantly greater susceptibility to challenge infection with IOE compared to untreated mice persistently infected with E. muris. The study indicated that persistent ehrlichial infection contributes to heterologous protection by stimulating the maintenance of memory T-cell responses.
Human monocytotropic ehrlilchiosis (HME), an emerging tick-borne disease in the United States, is caused by the obligately intracellular bacterium Ehrlichia chaffeensis, which resides in the host mononuclear phagocytic cells (24, 34). HME manifests initially as a mild flulike illness with nonspecific symptoms (12, 22), and the disease can progress to a severe life-threatening condition resulting from liver dysfunction, multiorgan failure, meningoencephalitis, and adult respiratory distress syndrome (11, 25, 26, 28). Immune-mediated pathology is attributed in severe cases of HME to the severity of inflammation observed in the absence of high numbers of ehrlichiae in the tissues (28). It is unknown whether E. chaffeensis establishes persistent infection in humans, although a few studies reported prolonged infection with E. chaffeensis (9, 27). It is also unknown whether immunocompetent patients recovered from HME are immune to reinfection with a genetically identical or distinct E. chaffeensis strain. However, a case of reinfection with a distinct strain of E. chaffeensis after a 2-year interval in a liver transplant patient who was receiving immunosuppressive therapy has been reported (21).
In a previous study, using murine models of persistent ehrlichiosis and fatal ehrlichiosis, we have established that ehrlichial species that differ in their ability to cause acute or chronic persistent infection also differ in stimulation of a long-term protective memory immune response (33). Ehrlichia muris, a mildly virulent Ehrlichia which causes persistent infection in C57BL/6 mice, but not sublethal infection with highly virulent Ixodes ovatus Ehrlichia (IOE), which causes acute infection only, induces strong memory immune responses and confers protection against an ordinarily lethal IOE challenge. The cross-protection induced by persistent E. muris infection is associated with the generation and maintenance of strong Ehrlichia-specific IFN-γ-producing CD4+ and CD8+ memory T-cell responses in the spleen and production of Ehrlichia-specific serum immunoglobulin G (IgG) responses (33). The correlation between persistent primary E. muris infection and heterologous protection against secondary lethal IOE challenge suggested that persistent infection or antigen persistence could play a role in the maintenance of protective immunity. Antigen persistence or continuous exposure to low levels of antigen is thought to be required for maintenance of protective memory immune responses (3, 14, 37). In the present study we addressed the role of persistent ehrlichial infection in conferring protection against lethal heterologous ehrlichiosis. The results suggested that persistence of E. muris infection in C57BL/6 mice contributes to protective heterologous immunity against IOE infection by stimulating maintenance of memory T-cell and Ehrlichia-specific antibody responses.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old female C57BL/6 mice were used in all experiments. Mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed and cared for in the Animal Research Center at the University of Texas Medical Branch in accordance with the Institutional Animal Care and Use Committee guidelines under whose review and approval the experiments were conducted.
Bacteria, infection of mice, and doxycycline treatment.
Two ehrlichial species, namely, E. muris (provided by Y. Rikihisa, Ohio State University, Columbus) and IOE isolated from I. ovatus ticks in Japan (kindly provided by M. Kawahara, Nagoya City Public Health Research Institute, Nagoya, Japan), were used in the study. Ehrlichial stocks were prepared (17). Mice were inoculated with the splenic stock containing E. muris (∼105 bacterial genomes) i.p., and the IOE challenge infection consisted of either ∼2 × 103 or ∼1 × 104 bacterial genomes given by the intraperitoneal (i.p.) route. Antibiotic treatment of E. muris-infected mice consisted of doxycycline injections given i.p. for 7 consecutive days (10 mg/kg [body weight], one injection per day) starting on day 16 after primary infection.
Determination of ehrlichial copy numbers in E. muris and IOE stocks and quantification of ehrlichial load in tissue samples.
Ehrlichial copy numbers in stocks and tissue samples were determined by a quantitative real-time PCR method (32). Briefly, DNA was extracted from infected tissues, and a portion of the E. muris or IOE dsb gene, which encodes a disulfide bond formation protein (GenBank accession no. AY236484 and AY236485), and the host housekeeping gene, murine gapdh, were amplified and detected using the primers and the probes described previously (32). The absolute number of copies of the dsb gene and the gapdh gene and the number of host cells present in stocks were used to calculate the number of ehrlichiae present per milliliter of the stock. The bacterial load in each organ was expressed as the number of copies of E. muris or IOE dsb gene present per microgram of total DNA. The limit of detection of the plasmid carrying the dsb gene by the PCR method used in the study was found to be 100 copies.
Preparation of E. muris antigen.
E. muris antigen was prepared from Percoll density gradient purified E. muris cultured in the DH82 canine macrophage cell line as described previously (17). Total protein concentration in the antigen preparations was determined by using a bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL).
Splenocyte cultures and in vitro assay of immune responses.
Single-cell suspensions were prepared from spleens harvested from mice. Splenocytes were cultured in vitro in complete medium (RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 10 mM HEPES buffer, 50 μM 2-mercaptoethanol, and antibiotics [penicillin [100 U/ml] and streptomycin [100 μg/ml]) in a 12-well plate (Costar, Corning, NY). The cells were cultured at a concentration of 5 × 106 cells per well, along with irradiated naive syngeneic spleen cells (5 × 106 per well) as antigen-presenting cells. The cells were stimulated in vitro with E. muris antigen (5 μg/ml) for 18 h, followed by 4 h of incubation with brefeldin A (BD Biosciences, San Diego, CA) for intracellular cytokine staining and analysis by flow cytometry. Positive and negative control wells included cells stimulated with 5 μg of concanavalin A/ml or medium, respectively. Splenocyte culture supernatants were collected at 48 h for measurement of interleukin-10 (IL-10) and transforming growth factor β1 (TGF-β1) concentrations by enzyme-linked immunosorbent assay by using Quantikine immunoassay kits (R&D Systems, Minneapolis, MN). The detection limits of the Quantikine enzyme-linked immunosorbent assay for IL-10 and TGF-β1 are 4.0 and 4.61 pg/ml, respectively.
For flow cytometric analysis, cells were incubated with anti-Fc II/III receptor monoclonal antibodies (MAbs; BD Biosciences) in fluorescence-activated cell sorting buffer to block Fc receptors. Cells were then labeled with fluorochrome-conjugated MAbs (BD Biosciences) specific for mouse CD3 (clones 17A2 or 145-2C11), CD4 (RM4-5), CD8 (clone 53-6.7), CD62L (clone MEL-14), CD44 (clone IM7), or CD25 (clone PC61) molecules. The expression of gamma interferon (IFN-γ) by T cells was assessed by intracellular cytokine staining (clone XMG1.2). Staining for Foxp3 expression was carried using the phycoerythrin-labeled anti-mouse Foxp3 (clone FJK-16S) and Foxp3 staining buffer set (eBioscience, San Diego, CA). Flow cytometric data were collected by using FACSCanto (BD Immunocytometry Systems, San Jose, CA). Lymphocytes were gated based on forward scatter and side scatter, and data were analyzed by using FCS Express (version 3; De Novo Software, Los Angeles, CA).
Histology.
Tissue samples of liver and lung were fixed in 10% neutral buffered formaldehyde, embedded in paraffin, cut at 4-μm thickness, and stained with hematoxylin and eosin.
IFA.
An indirect immunofluorescence assay (IFA) was used to determine the titer of Ehrlichia-specific IgG antibodies in serum samples (23). Antigen slides prepared from E. muris grown in DH82 canine macrophage cell line were incubated with twofold dilutions of serum samples, followed by incubation with fluorescein-labeled horse anti-mouse IgG (gamma-chain-specific) secondary antibodies (Vector Laboratories, Burlingame, CA) for 30 min each at 37°C. Antibody titer was expressed as the reciprocal of the highest dilution of serum at which specific fluorescence was observed.
Statistical analysis.
Analysis of the experimental data was carried out by using the GraphPad Prism software version 5.01 for windows (GraphPad Software, San Diego, CA). The data were transformed by taking the square root of individual values, and analyzed using one-way analysis of variance with Tukey's post test for comparison of multiple groups. The log-rank test was used to compare survival curves. Statistical significance was determined at 95% (P < 0.05), and asterisks indicate levels of statistical significance (*, P = 0.01 to 0.05; **, P = 0.001 to 0.01; ***, P < 0.001).
RESULTS
Persistent E. muris infection stimulates maintenance of splenic memory CD4+ and CD8+ T-cell populations.
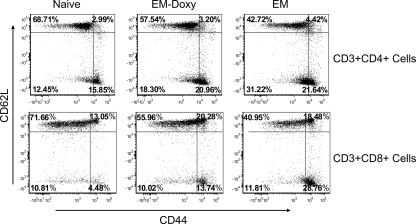
Our previous study had demonstrated that cross-protection of E. muris-primed mice and lack of homologous protection against lethal IOE challenge are associated with the presence and absence of persistent infection, respectively, and distinct memory immune responses (33). In the present study, we addressed the role of persistent infection in maintenance of protective memory immune responses during ehrlichial infections by antibiotic treatment of persistent E. muris infection prior to challenge with IOE. C57BL/6 mice were infected with E. muris by the i.p. route and treated with i.p. doxycycline on days 16 to 22 postinfection. The doxycycline treatment resulted in undetectable levels of ehrlichial DNA in different organs as measured by real-time PCR on day 36 after E. muris infection (e.g., in the lungs, there were less than 100 copies of the ehrlichial dsp gene per μg of total DNA/μg in the treated group versus 241 ± 48 copies of the ehrlichial dsp gene per μg of total DNA/μg in untreated persistently infected mice). Flow cytometric analysis of splenic memory T-cell populations revealed the presence of approximately three- and fourfold greater total numbers of memory CD4+ and CD8+ T cells, respectively, in the spleens of untreated mice persistently infected with E. muris compared to E. muris-primed mice treated with doxycycline on day 36 after E. muris infection. We detected significantly greater percentages of effector memory (CD44high CD62Llow) CD4+ and CD8+ T cells in the spleens of untreated mice persistently infected with E. muris and E. muris-primed mice treated with doxycycline on day 36 after primary infection compared to uninfected naive mice (Fig. 1). However, due to substantial expansion of the CD4+ and CD8+ T-cell populations, the absolute numbers of both effector memory and central memory (CD44high CD62Lhigh) CD4+ and CD8+ T cells were significantly higher in the spleens of mice persistently infected with E. muris than in the spleens of E. muris-primed mice treated with doxycycline (data not shown). The results of the present study suggest that persistence of E. muris stimulates maintenance of memory T cells, particularly effector memory CD4+ and CD8+ T cells, which are known to migrate to sites of infection and exhibit immediate effector functions.
FIG. 1.
Greater numbers of memory CD4+ and CD8+ T-cell populations are maintained in the spleen in the presence of persistent E. muris. Significantly greater frequencies (P < 0.05) of effector/effector memory CD4+ and CD8+ T cells were present in the spleens of E. muris-primed mice treated with doxycycline and in mice persistently infected with E. muris on day 36 after primary infection compared to naive mice. The data are representative of three independent experiments with three mice per group.
Persistent E. muris infection does not lead to T-cell effector dysfunction or exhaustion.
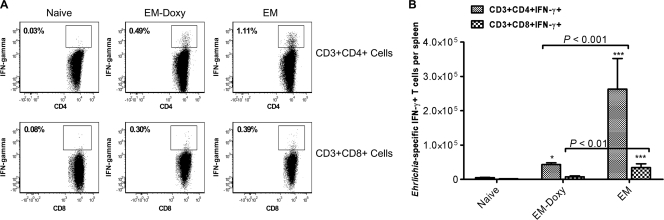
We next examined the proliferation and functional status of T cells during persistence of E. muris infection. CD4+ and CD8+ T cells from day 36 E. muris-primed mice responded to in vitro antigenic stimulation by producing IFN-γ (Fig. 2), and CD4+ T cells proliferated in vitro in response to antigenic stimulation as measured by the incorporation of bromodeoxyuridine (data not shown). These results suggested that T cells do not appear to enter a state of dysfunction or exhaustion during persistent E. muris infection, which correlates with low antigen load during the persistent phase of E. muris infection.
FIG. 2.
Persistent E. muris infection does not lead to T-cell dysfunction or exhaustion. (A) Significantly higher percentages of Ehrlichia-specific IFN-γ-secreting CD4+ T cells were present in the spleens of untreated mice persistently infected with E. muris than in E. muris-primed mice treated with doxycycline on day 36 after primary infection. (B) Compared to E. muris-primed mice treated with doxycycline, untreated mice persistently infected with E. muris had significantly greater absolute numbers of Ehrlichia-specific IFN-γ-producing CD4+ and CD8+ T cells in the spleen. The data are representative of two experiments. Three mice per group were included for analysis. Asterisks indicate significant differences from the naive group. *, P < 0.01; ***, P < 0.001.
We next examined whether the differences in the number of memory T cells maintained in the presence or absence of persistent E. muris infection are associated with differences in the magnitude of antigen-specific T-cell responses. Compared to uninfected mice, significantly greater percentages of Ehrlichia-specific IFN-γ-producing CD4+ and CD8+ type 1 cells were present in the spleens of untreated mice persistently infected with E. muris and E. muris-primed mice treated with doxycycline on day 36 after infection (Fig. 2). However, the absolute numbers of Ehrlichia-specific IFN-γ-producing CD4+ T cells and CD8+ T cells in the spleens of untreated mice persistently infected with E. muris were approximately six- and fourfold higher than in E. muris-primed mice treated with doxycycline, respectively (Fig. 2B). IL-4-producing CD4+ and CD8+ type 2 cells were not detected in both groups of mice (data not shown). These results suggest that absence of persistent infection significantly compromises the magnitude of antigen-specific type 1 memory cells (8).
Persistent E. muris infection induces higher titers of Ehrlichia-specific serum IgG antibodies than that detected in the absence of persistent infection.
Studies from our laboratory and others have established the importance of antibodies in protection against ehrlichial infection (10, 17, 19). Examination of Ehrlichia-specific IgG antibody responses indicated that sera from E. muris-primed mice treated with doxycycline obtained on day 36 after primary infection had approximately twofold lower Ehrlichia-specific IgG antibody titers than untreated mice persistently infected with E. muris (Table 1) . Similar differences were observed in Ehrlichia-specific IgG antibody titers on day 7 after IOE challenge, suggesting that persistent E. muris infection promotes substantial IgG antibody responses.
TABLE 1.
Serum anti-Ehrlichia IgG titers in E. muris-primed mice, untreated or treated with doxycycline, before and after challenge with a lethal dose of IOEa
| Mouse group | IFA titer on day 36 after primary E. muris infection |
|---|---|
| Naive uninfected mice | NA |
| E. muris-doxycycline | 1:256 to 1:512 |
| E. muris | 1:1,024 |
| Mouse group | IFA titer on day 7 after IOE challenge |
|---|---|
| IOE | ND |
| E. muris-doxycycline/IOE | 1:512 to 1:1,024 |
| E. muris/IOE | 1:2,048 to 1:4,096 |
Serum anti-Ehrlichia IgG antibody titers were determined by using an IFA. NA, not applicable; ND, not detected.
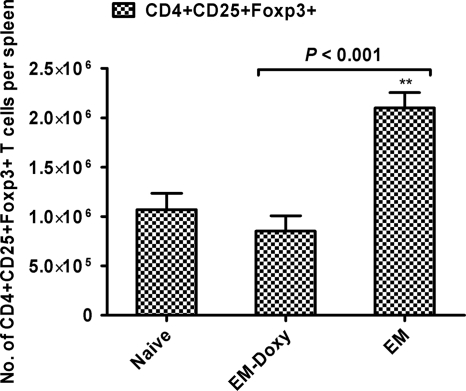
Persistent E. muris infection induces expansion of CD4+ CD25+ Foxp3+ T regulatory cells.
We examined the potential role of CD4+ CD25+ Foxp3+ T regulatory cells in the maintenance of persistent ehrlichial infection. Compared to E. muris-primed mice treated with doxycycline, untreated mice persistently infected with E. muris had similar percentages but significantly greater absolute numbers of CD4+ CD25+ Foxp3+ T regulatory cells in the spleen on day 36 after primary infection (Fig. 3). These data suggest that the persistence of ehrlichiae is required for expansion of T regulatory cells in the spleen.
FIG. 3.
Persistent E. muris infection induces expansion of CD4+ CD25+ Foxp3+ T regulatory cells in the spleen. Mice persistently infected with E. muris had similar percentages (data not shown) but significantly greater absolute numbers of CD4+ CD25+ Foxp3+ T regulatory cells in the spleen compared to E. muris-primed mice treated with doxycycline. Three mice per group are included for analysis. The data are representative of three independent experiments. Asterisks indicate significant differences from the naive group. **, P < 0.01.
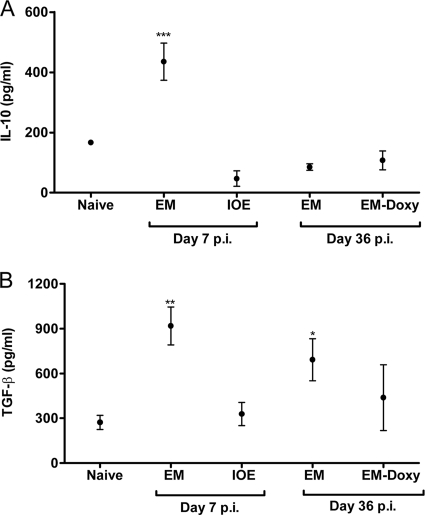
E. muris infection induces immunoregulatory cytokines IL-10 and TGF-β1 during the acute stage and TGF-β1 during the chronic phase of the infection.
Further, we examined the possible roles of immunoregulatory cytokines IL-10 and TGF-β1 in persistence of E. muris infection. Splenocytes from E. muris-infected mice, but not IOE-infected mice, produced significantly greater quantities of IL-10 and TGF-β1 on day 7 after acute infection (Fig. 4). Furthermore, splenocytes from untreated mice persistently infected with E. muris, but not E. muris-primed mice treated with doxycycline, produced significantly greater quantities of TGF-β1 during the chronic stage of infection (day 36 postinfection) compared to uninfected naive mice (Fig. 4B).
FIG. 4.
E. muris infection, but not IOE infection, induces immunoregulatory cytokine IL-10 in the spleen during the acute stage and TGF-β1 during the acute and chronic phases of the infection. (A and B) Splenocytes from mice infected with E. muris produced significantly greater quantities of IL-10 and TGF-β1 on day 7 after primary infection compared to IOE-infected mice and naive uninfected mice. Significantly greater quantities of TGF-β1 were produced by splenocytes from mice persistently infected with E. muris on day 36 after E. muris infection compared to splenocytes harvested from naive mice (B). The data are presented as means ± the standard deviations, and three mice per group are included for analysis. Asterisks indicate significant differences from the naive group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Persistent E. muris infection is critical for protection against virulent IOE challenge.
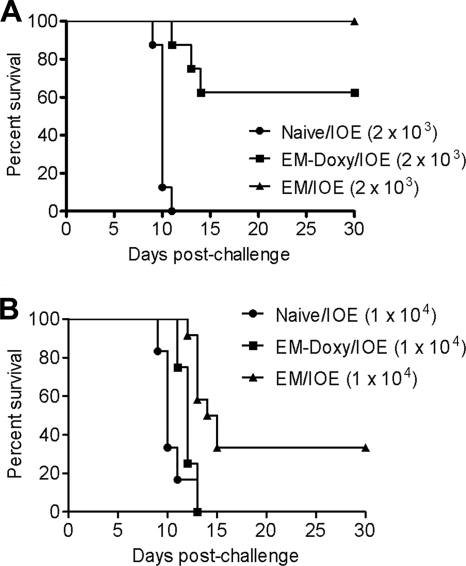
We next investigated whether persistent infection is essential for protection against fatal IOE challenge. Mice previously infected via the i.p. route with E. muris and treated with doxycycline or left untreated were challenged on day 36 after E. muris infection with either a low or a high (∼2 × 103 or ∼1 × 104 bacterial genomes) dose of virulent IOE. Naive mice were also challenged with the same doses of IOE, and survival of all groups was assessed up to 30 days after IOE challenge. All unprimed mice challenged with IOE succumbed to infection between days 9 and 13 after the challenge (Fig. 5). Doxycycline-treated mice also exhibited significant susceptibility, with ∼38 and 100% of treated mice succumbing to challenge with the low dose and the high dose of IOE compared to 0 and ∼66%, respectively, of untreated mice persistently infected with E. muris (Fig. 5).
FIG. 5.
Persistent E. muris infection contributes to heterologous protection against IOE infection. C57BL/7 mice persistently infected with E. muris and E. muris-primed mice treated with doxycycline were challenged on day 36 postinfection with either an ordinarily lethal lower (A) or a higher (B) dose of IOE (∼2 × 103 or ∼1 × 104 bacterial genomes, respectively). The survival rates after the lethal low-dose IOE challenge were statistically significant in untreated mice persistently infected with E. muris (P < 0.0001) and in E. muris-primed mice treated with doxycycline (P = 0.0001) compared to naive mice. Compared to E. muris-primed mice treated with doxycycline, the higher survival rates after the lethal low-dose and the high-dose IOE challenges were marginally (P = 0.062) and highly (P = 0.0029) significant, respectively, in untreated mice persistently infected with E. muris (A and B). The log-rank test was used for the analysis of survival curves. The data are representative of two independent experiments with eight mice/group per experiment (n = 8).
Persistent E. muris infection prevents the development of IOE-induced immune-mediated pathology.
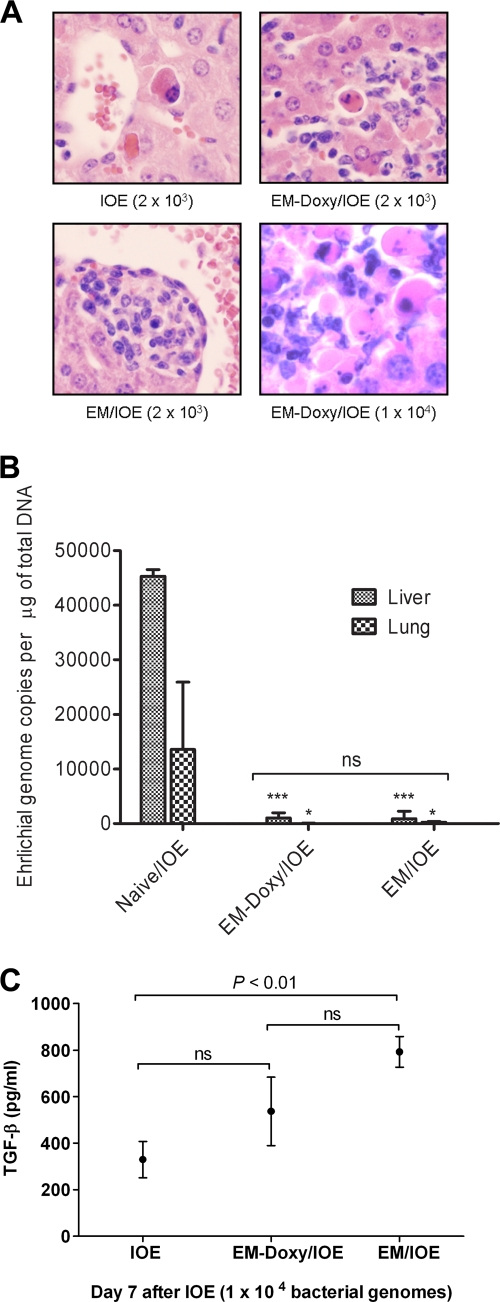
We next evaluated the mechanism of increased susceptibility of E. muris-primed mice treated with doxycycline to challenge IOE infection. Similar to our previous studies (17, 33), naive mice challenged with either the lethal low dose or the high dose of IOE had extensive apoptotic cells in the liver parenchyma with few inflammatory cells on day 7 after IOE challenge (Fig. 6A). After IOE challenge, untreated E. muris-primed mice had fewer apoptotic cells in the liver associated with inflammatory foci and well-formed granulomas. However, a greater number of apoptotic cells, which were concentrated mainly in the inflammatory foci, were observed on day 7 after IOE challenge in the livers of E. muris-primed mice treated with doxycycline than in untreated mice persistently infected with E. muris and challenged with IOE (Fig. 6A). Interestingly, the higher rate of mortality of doxycycline-treated E. muris-primed mice challenged with the high dose IOE (∼104 bacterial genomes) was associated with substantially higher degree of apoptosis and necrosis in the liver with inflammatory cells compared to untreated mice persistently infected with E. muris and challenged with either the low or the high dose of IOE or doxycycline-treated E. muris-primed mice challenged with the low dose of IOE (Fig. 6A). These data suggest that fatal disease in E. muris-primed mice treated with doxycycline and challenged with IOE is most likely due to immune-mediated host cell injury similar to that observed in naive mice infected with the same lethal doses of IOE.
FIG. 6.
Persistent E. muris infection confers protection against IOE-mediated immunopathology. (A) Doxycycline-treated mice infected with E. muris have a greater number of apoptotic cells concentrated in the inflammatory foci after challenge with a lethal low dose of IOE (EM-Doxy/IOE) than was observed in untreated E. muris-primed mice, but the levels were comparable to those detected in naive mice challenged with the same dose of IOE. A higher degree of apoptosis in the liver was more pronounced in E. muris-primed mice treated with doxycycline after challenge with a lethal high-, than low-, dose IOE challenge. Original magnifications, ×400. The data are representative of sections from one mouse in each group with similar results in three independent experiments with three mice/group. (B) Comparable, but significantly lower numbers of IOE bacteria were present in the livers (P < 0.001) and lungs (P < 0.05) of E. muris-primed mice treated with doxycycline and of untreated mice persistently infected with E. muris than in naive mice infected with the same dose of IOE on day 7 after a lethal low-dose IOE challenge. Similar results were observed after a lethal high-dose IOE challenge (data not shown). Asterisks indicate significant differences from the IOE group: *, P < 0.05; ***, P < 0.001; NS, not significant (P > 0.05). (C) Splenocytes from untreated mice persistently infected with E. muris, but not E. muris-primed mice treated with doxycycline, produced significantlygreater quantities of TGF-β1 on day 7 postinfection compared to naive mice challenged with the high-dose IOE. The data are presented as means plus the standard deviations with three mice per group. The data are representative of two independent experiments.
We next analyzed bacterial burdens after IOE challenge in the different groups of mice. Bacterial burdens in the livers and lungs of E. muris-primed mice treated with doxycycline were similar to those in untreated mice persistently infected with E. muris on day 7 after challenge with either the low dose (Fig. 6B) or the high dose (data not shown) of IOE. This result suggests that the failure of cross-protection against IOE-associated pathology in doxycycline-treated, E. muris-primed mice is not due to ineffective clearance of IOE.
Heterologous protection against IOE induced by persistent E. muris infection is associated with increased TGF-β responses.
Our previous studies showed that primary fatal ehrlichial infection is due to an overactivation of proinflammatory responses and the generation of pathogenic T-cell responses that lead to severe tissue injury. Therefore, we examined whether the lack of immunoregulatory cytokines such as IL-10 and TGF-β1 in doxycycline-treated mice may account for the development of immunopathology after IOE infection. IL-10 production was very low and not significantly different among untreated mice persistently infected with E. muris, E. muris-primed mice treated with doxycycline, and naive mice after IOE challenge (data not shown). In contrast, splenocytes from untreated mice persistently infected with E. muris, but not E. muris-primed mice treated with doxycycline, produced significantly greater quantities of TGF-β1 after IOE challenge than naive mice challenged with IOE (Fig. 6C). Thus, the lower levels of protection observed in E. muris-primed mice treated with doxycycline against lethal IOE challenge may be attributable to decreased TGF-β1 production, which in turn leads to a less efficient downregulation of inflammatory and pathogenic T-cell responses after IOE challenge.
DISCUSSION
The generation and maintenance of memory immune responses after infection with pathogens that establish persistent infection are not well understood (7, 31). In the present study, we addressed the role of the persistence of ehrlichial infection in maintenance of heterologous protective immunity. The results suggested that persistent infection contributes to heterologous protective immunity by promoting the maintenance of type 1 memory T-cell responses and preventing the development of IOE-induced immune-mediated pathology. Our data showed that E. muris-infected/doxycycline-treated mice challenged with the lethal high dose of IOE had marked susceptibility to fatal disease, whereas those challenged with the ordinarily lethal lower dose of IOE had some degree (∼60%) of protection. Our data suggest that the elimination of persistent infection by doxycycline treatment generates a single distinct “intermediate” or “suboptimal” cell-mediated and humoral immune phenotype compared to naive or untreated mice persistently infected with E. muris. This suboptimal phenotype can either lead to partial or no protection against fatal disease depending on the secondary-challenge dose of IOE (Fig. 5). In agreement with this conclusion, we have reported previously that a higher frequency of CD4+ Th1 cells and strong cell-mediated responses are associated with complete protection against primary infection with a lethal dose of IOE (16, 18).
The cross-protection induced by E. muris against IOE infection is unlikely to be nonspecific immunity mediated by inflammatory responses or activation of nonspecific T cells. Bitsaktsis et al. (5) have demonstrated that nonspecific inflammatory responses and/or nonspecific T cells do not provide protection against IOE infection. That study also demonstrated that the protection induced was systemic and not due to persistent E. muris infection in the peritoneal cavity since E. muris-primed mice were protected against IOE challenge by the intravenous route as well (5). In addition, studies from our laboratory have demonstrated the importance of specific T- and B-cell responses in protection against monocytotropic ehrlichiosis. Adoptive transfer of Ehrlichia-specific effector/memory CD4+ and CD8+ type 1 cells and Ehrlichia-specific IgG antibodies from E. muris/IOE-infected mice, which survived ordinarily fatal IOE infection, protected naive mice from ordinarily lethal IOE challenge (17).
Chronic viral infections with persisting high antigen loads such as lymphocytic choriomeningitis virus result in T-cell exhaustion or T-cell effector dysfunction and interfere with development of memory T cells (2, 30, 35). Similarly, infection with Anaplasma marginale, a ruminant bacterial pathogen that causes high-level bacteremia during persistent infection, is associated with the loss of antigen-specific T cells and immunologic memory (15). In contrast, viral infections that establish latency with a low antigen load such as gammaherpesvirus infections do not appear to interfere with T-cell functions and the generation of memory T cells (7). However, antigen-specific T cells during persistent ehrlichiosis do not appear to enter a state of exhaustion or depletion since both CD4+ and CD8+ T cells from mice persistently infected with E. muris responded to in vitro antigenic stimulation by secreting IFN-γ and CD4+ T cells proliferated in response to in vitro antigenic stimulation. However, further phenotypic and functional characterization of T cells in C57BL/6 mice with or without persistent E. muris infection is required to examine the occurrence of any partial T-cell exhaustion or altered memory T-cell functions (29, 30).
The present study demonstrates that the magnitude of T cells maintained in the presence or absence of persistent E. muris infection is greatly different. The persistence of antigen is required for the generation and maintenance of a high frequency of multifunctional (i.e., capable of simultaneously secreting IFN-γ, tumor necrosis factor alpha, and IL-2) effector memory CD4+ Th1 cells and protection against L. major infection (8). The presence of low levels of antigen during persistent infection is thought to condition effector memory T cells for optimal function (8). Our data here clearly demonstrate that substantially greater numbers of antigen-specific IFN-γ-producing type 1 memory cells were maintained in mice persistently infected with E. muris than in mice primed with E. muris and treated with doxycycline. Further studies are required to establish whether the substantial quantity of effector memory T cells maintained during persistent E. muris infection is multifunctional.
Higher levels of serum Ehrlichia-specific IgG antibodies were maintained in untreated mice persistently infected with E. muris than in E. muris-primed mice treated with doxycycline. Studies suggest that antigen persistence is not required for long-term maintenance of humoral memory responses since bone marrow-residing long-lived plasma cells contribute to the long-term antibody response in the absence of persisting antigen and independent of memory B cells (1, 13, 36). However, elevated levels of antigen during persistent E. muris infection could have influenced the maintenance of the germinal center reaction, early B-cell proliferation, and the development of memory B cells, which would produce a high titer of IgG antibodies in the presence of antigen-dependent memory T-cell help during chronic infection (13). In conclusion, our data suggest that the lack of protection against fatal IOE challenge, in the absence of persistent E. muris infection, is most likely due to the compromised or suboptimal cell-mediated and humoral immune responses.
The findings of the present study appear to contradict earlier observations made by Bitsaktsis et al. (5). These authors indicated that protection induced against IOE infection in mice previously primed with E. muris is mostly dependent on T-cell-independent humoral immunity. The discrepancy between our results and those in the study by Bitsaktsis et al. could be due to (i) differences in the challenge doses of IOE used in these studies or (ii) the presence of compensatory mechanisms in the knockout mice used in the Bitsaktsis study (5). Of note, Bitsaktsis et al. observed (i) significantly lower titers of antibodies to Ehrlichia outer membrane protein (OMP-19) in CD4+ T-cell-deficient mice compared to wild-type mice, (ii) the generation of T-cell-dependent immunoglobulin subclasses in CD4−/− knockout mice, and (iii) partial protection against IOE challenge in mice that received anti-E. muris serum from major histocompatibility complex class II−/− mice compared to those that received immune serum from E. muris-infected wild-type mice (20% versus 60%). As suggested by these authors, their data indicate that other T cells, such as NKT cells and γ/δ T cells, could provide B-cell help, which would result in immunoglobulin class switch and low-titer antibodies in the absence of CD4+ T cells. Nevertheless, these researchers did not rule out the role of CD4+ T cells in providing B-cell help.
Although comparable bacterial burdens in the organs were found after IOE challenge, E. muris-primed mice treated with doxycycline had greater tissue damage than untreated mice persistently infected with E. muris. The enhanced tissue damage in the absence of higher bacterial burdens after IOE challenge in E. muris-primed mice treated with doxycycline is similar to immune-mediated pathology associated with a complete lack of homologous protection against secondary IOE challenge in mice primed with sublethal doses of IOE (33). The latter does not cause persistent infection (4, 33). These results suggest that immune-mediated pathology plays a role in IOE-induced disease and that protective immunity against IOE reinfection in E. muris-primed mice involves at least two components: (i) the protective mechanisms that prevent bacterial replication and eliminate IOE and (ii) the regulatory mechanisms that prevent IOE-induced immune-mediated pathology. Overproduction of tumor necrosis factor alpha by antigen-specific CD8+ T cells and effector/memory CD8+ T cells is implicated in IOE-induced immune-mediated pathology during primary and secondary IOE infections, respectively (6, 17).
Higher numbers of CD4+ CD25+ Foxp3+ T cells and a higher level of TGF-β1 in the spleen were observed in the presence of persistent E. muris infection compared to E. muris-primed mice treated with doxycycline. The correlation between the generation and maintenance of higher frequencies of memory T cells in persistently infected mice, minimal tissue injury, and higher levels of TGF-β1 compared to the treated mice (Fig. 2 and 6A and C) suggests that TGF-β1 may exert multiple functions, including (i) the maintenance of memory T cells and (ii) the inhibition of inflammatory tissue injury and the downregulation of pathogenic T-cell inflammatory responses after IOE challenge. This conclusion is consistent with the study showing that TGF-β signaling positively regulates T-cell activation, differentiation, development, and homeostasis, as well as the maintenance of peripheral Foxp3-expressing regulatory T cells and the survival of CD4+ T cells (20). Further studies are required to decipher the roles of CD4+ CD25+ Foxp3+ T regulatory cells and/or TGF-β in ehrlichial persistence and immune regulation after lethal infection.
In conclusion, we demonstrate here that persistent E. muris infection contributes to heterologous protective immunity against IOE infection in mice by stimulating the maintenance of type 1 memory T-cell responses. Our findings also suggest that T cells maintained during persistent E. muris infection do not appear to enter a state of exhaustion or dysfunction.
Acknowledgments
We thank Doris Baker for excellent secretarial assistance.
This study was supported by the National Institute of Allergy and Infectious Diseases grant AI31431 to D.H.W., and N.R.T. was supported by a fellowship from the McLaughlin Endowment for Infection and Immunity, UTMB Institute for Human Infections and Immunity.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Ahuja, A., S. M. Anderson, A. Khalil, and M. J. Shlomchik. 2008. Maintenance of the plasma cell pool is independent of memory B cells. Proc. Natl. Acad. Sci. USA 105:4802-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 3.Bell, E. B., and J. Westermann. 2008. CD4 memory T cells on trial: immunological memory without a memory T cell. Trends Immunol. 29:405-411. [DOI] [PubMed] [Google Scholar]
- 4.Bitsaktsis, C., J. Huntington, and G. Winslow. 2004. Production of IFN-γ by CD4 T cells is essential for resolving Ehrlichia infection. J. Immunol. 172:6894-6901. [DOI] [PubMed] [Google Scholar]
- 5.Bitsaktsis, C., B. Nandi, R. Racine, K. C. Macnamara, and G. Winslow. 2007. T-cell-independent humoral immunity is sufficient for protection against fatal intracellular Ehrlichia infection. Infect. Immun. 75:4933-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitsaktsis, C., and G. Winslow. 2006. Fatal recall responses mediated by CD8 T cells during intracellular bacterial challenge infection. J. Immunol. 177:4644-4651. [DOI] [PubMed] [Google Scholar]
- 7.Cush, S. S., K. M. Anderson, D. H. Ravneberg, J. L. Weslow-Schmidt, and E. Flano. 2007. Memory generation and maintenance of CD8+ T-cell function during viral persistence. J. Immunol. 179:141-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darrah, P. A., D. T. Patel, P. M. De Luca, R. W. Lindsay, D. F. Davey, B. J. Flynn, S. T. Hoff, P. Andersen, S. G. Reed, S. L. Morris, M. Roederer, and R. A. Seder. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843-850. [DOI] [PubMed] [Google Scholar]
- 9.Dumler, J. S., J. E. Dawson, and D. H. Walker. 1993. Human ehrlichiosis: hematopathology and immunohistologic detection of Ehrlichia chaffeensis. Hum. Pathol. 24:391-396. [DOI] [PubMed] [Google Scholar]
- 10.Feng, H. M., and D. H. Walker. 2004. Mechanisms of immunity to Ehrlichia muris: a model of monocytotropic ehrlichiosis. Infect. Immun. 72:966-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fichtenbaum, C. J., L. R. Peterson, and G. J. Weil. 1993. Ehrlichiosis presenting as a life-threatening illness with features of the toxic shock syndrome. Am. J. Med. 95:351-357. [DOI] [PubMed] [Google Scholar]
- 12.Fishbein, D. B., J. E. Dawson, and L. E. Robinson. 1994. Human ehrlichiosis in the United States, 1985 to 1990. Ann. Intern. Med. 120:736-743. [DOI] [PubMed] [Google Scholar]
- 13.Gatto, D., S. W. Martin, J. Bessa, E. Pellicioli, P. Saudan, H. J. Hinton, and M. F. Bachmann. 2007. Regulation of memory antibody levels: the role of persisting antigen versus plasma cell life span. J. Immunol. 178:67-76. [DOI] [PubMed] [Google Scholar]
- 14.Gray, D. 2002. A role for antigen in the maintenance of immunological memory. Nat. Rev. Immunol. 2:60-65. [DOI] [PubMed] [Google Scholar]
- 15.Han, S., J. Norimine, G. H. Palmer, W. Mwangi, K. K. Lahmers, and W. C. Brown. 2008. Rapid deletion of antigen-specific CD4+ T cells following infection represents a strategy of immune evasion and persistence for Anaplasma marginale. J. Immunol. 181:7759-7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismail, N., E. C. Crossley, H. L. Stevenson, and D. H. Walker. 2007. Relative importance of T-cell subsets in monocytotropic ehrlichiosis: a novel effector mechanism involved in Ehrlichia-induced immunopathology in murine ehrlichiosis. Infect. Immun. 75:4608-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail, N., L. Soong, J. W. McBride, G. Valbuena, J. P. Olano, H. M. Feng, and D. H. Walker. 2004. Overproduction of TNF-α by CD8+ type 1 cells and downregulation of IFN-γ production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 172:1786-1800. [DOI] [PubMed] [Google Scholar]
- 18.Ismail, N., H. L. Stevenson, and D. H. Walker. 2006. Role of tumor necrosis factor alpha (TNF-α) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection. Infect. Immun. 74:1846-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, A. A. Reilly, F. K. Chu, and G. M. Winslow. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855-1862. [DOI] [PubMed] [Google Scholar]
- 20.Li, M. O., S. Sanjabi, and R. A. Flavell. 2006. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25:455-471. [DOI] [PubMed] [Google Scholar]
- 21.Liddell, A. M., J. W. Sumner, C. D. Paddock, Y. Rikihisa, A. Unver, R. S. Buller, and G. A. Storch. 2002. Reinfection with Ehrlichia chaffeensis in a liver transplant recipient. Clin. Infect. Dis. 34:1644-1647. [DOI] [PubMed] [Google Scholar]
- 22.Olano, J. P., E. Masters, W. Hogrefe, and D. H. Walker. 2003. Human monocytotropic ehrlichiosis, Missouri. Emerg. Infect. Dis. 9:1579-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olano, J. P., G. Wen, H. M. Feng, J. W. McBride, and D. H. Walker. 2004. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am. J. Pathol. 165:997-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel, R. G., and M. A. Byrd. 1999. Near fatal acute respiratory distress syndrome in a patient with human ehrlichiosis. South. Med. J. 92:333-335. [DOI] [PubMed] [Google Scholar]
- 26.Ratnasamy, N., E. D. Everett, W. E. Roland, G. McDonald, and C. W. Caldwell. 1996. Central nervous system manifestations of human ehrlichiosis. Clin. Infect. Dis. 23:314-319. [DOI] [PubMed] [Google Scholar]
- 27.Roland, W. E., G. McDonald, C. W. Caldwell, and E. D. Everett. 1995. Ehrlichiosis: a cause of prolonged fever. Clin. Infect. Dis. 20:821-825. [DOI] [PubMed] [Google Scholar]
- 28.Sehdev, A. E., and J. S. Dumler. 2003. Hepatic pathology in human monocytic ehrlichiosis: Ehrlichia chaffeensis infection. Am. J. Clin. Pathol. 119:859-865. [DOI] [PubMed] [Google Scholar]
- 29.Shin, H., S. D. Blackburn, J. N. Blattman, and E. J. Wherry. 2007. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204:941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin, H., and E. J. Wherry. 2007. CD8 T-cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 19:408-415. [DOI] [PubMed] [Google Scholar]
- 31.Snyder, C. M., K. S. Cho, E. L. Bonnett, G. R. Shellam, and A. B. Hill. 2008. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29:650-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson, H. L., J. M. Jordan, Z. Peerwani, H. Q. Wang, D. H. Walker, and N. Ismail. 2006. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect. Immun. 74:4856-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thirumalapura, N. R., H. L. Stevenson, D. H. Walker, and N. Ismail. 2008. Protective heterologous immunity against fatal ehrlichiosis and lack of protection following homologous challenge. Infect. Immun. 76:1920-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker, D. H., N. Ismail, J. P. Olano, J. W. McBride, X. J. Yu, and H. M. Feng. 2004. Ehrlichia chaffeensis: a prevalent, life-threatening, emerging pathogen. Trans. Am. Clin. Climatol. Assoc. 115:375-384. [PMC free article] [PubMed] [Google Scholar]
- 35.Wherry, E. J., D. L. Barber, S. M. Kaech, J. N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 101:16004-16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrammert, J., and R. Ahmed. 2008. Maintenance of serological memory. Biol. Chem. 389:537-539. [DOI] [PubMed] [Google Scholar]
- 37.Zinkernagel, R. M., and H. Hengartner. 2006. Protective ‘immunity’ by preexistent neutralizing antibody titers and preactivated T cells but not by so-called ‘immunological memory’. Immunol. Rev. 211:310-319. [DOI] [PubMed] [Google Scholar]