Abstract
The goal of this study is to evaluate the contribution of mast cells to Helicobacter pylori immunity in a model of vaccine-induced protection. Mast cell-deficient KitlSl/KitlSl-d and control mice were immunized with H. pylori sonicate plus cholera toxin and challenged with H. pylori, and the bacterial loads, inflammatory infiltrates, and cytokine responses were evaluated and compared at 1, 2, and 4 weeks postchallenge. In vitro stimulation assays were performed using bone marrow-derived mast cells, and recall assays were performed with spleen cells of immunized mast cell-deficient and wild-type mice. Bacterial clearance was observed by 2 weeks postchallenge in mast cell-deficient mice. The bacterial load was reduced by 4.0 log CFU in wild-type mice and by 1.5 log CFU in mast cell-deficient mice. Neutrophil numbers in the gastric mucosa of immune KitlSl/KitlSl-d mice were lower than those for immune wild-type mice (P < 0.05). Levels of gastric interleukin-17 (IL-17) and tumor necrosis factor alpha (TNF-α) were also significantly lower in immune KitlSl/KitlSl-d mice than in wild-type mice (P < 0.001). Immunized mast cell-deficient and wild-type mouse spleen cells produced IFN-γ and IL-17 in response to H. pylori antigen stimulation. TNF-α and CXC chemokines were detected in mast cell supernatants after 24 h of stimulation with H. pylori antigen. The results indicate that mast cells are not essential for but do contribute to vaccine-induced immunity and that mast cells contribute to neutrophil recruitment and inflammation in response to H. pylori.
Helicobacter pylori infection of the gastric mucosa is accompanied by local inflammation consisting of neutrophils, mast cells, lymphocytes, plasma cells, and macrophages as well as H. pylori-specific immunoglobulin A (IgA) and IgG antibodies (37). The inflammation, however, fails to eradiate H. pylori, and infection generally persists for the life of the host. Mice have been used extensively as an animal model for H. pylori infection, and they can be protectively immunized against H. pylori via a number of vaccination strategies (3). Although vaccine-induced protection in mice was first observed over 15 years ago, the immunologic pathways involved in the protective immune response to H. pylori are still being elucidated. A series of studies using targeted deletions in knockout mice have demonstrated that protective immunity can be achieved in the absence of gamma interferon (IFN-γ) and interleukin-4 (IL-4) (11, 33). However, protection cannot be induced in major histocompatibility complex class II-deficient mice, demonstrating a requirement for CD4+ T cells (7, 30). Other studies have confirmed that CD4+ T cells are sufficient to transfer protective immunity to recipient mice (16). Another crucial factor in the protective immune response was demonstrated using p40 knockout mice, which fail to generate protective immunity following immunization (1, 11). Whether the importance of p40 is tied to its association with the p35 subunit of IL-12 or the p19 subunit of IL-23 remains to be determined.
The effector cells and molecules of vaccine-induced H. pylori eradication also remain ill-defined. H. pylori resides predominantly at the apical surface of the gastric epithelium, and the presence of epithelial cell tight junctions limits the immune effector mechanisms that are capable of crossing the epithelium to interact with H. pylori. Mice deficient in Ig production can be protectively immunized comparably to wild-type mice (2, 7). Several studies have begun to assess the importance of granulocytes in Helicobacter eradication. Antibody-mediated neutrophil depletion significantly compromised the ability of IL-10-deficient mice to reduce the numbers of H. felis bacteria, although bacterial clearance in that model was not vaccine induced (18). More recently, investigators determined that protective immunity in the H. felis mouse model could not be achieved with mice genetically deficient in mast cells, providing strong evidence that mast cells either kill the Helicobacter organism directly or at least are necessary to drive the mechanism responsible for bacterial clearance (36).
The involvement of mast cells in Helicobacter infection has been limited to the examination of mast cell-deficient mice that are the result of heterozygous mutations in the c-Kit gene, specifically, KitW/W-v mice. These mice have a number of phenotypic abnormalities, including being profoundly deficient in tissue mast cells in multiple anatomical sites (39). They are an extremely useful model system to probe the role of mast cells in inflammatory and other types of reactions in vivo. It is important, however, to appreciate the intricacies and limitations of the model. In particular, there have been several reports that in the settings of both cutaneous and intestinal chronic inflammation, mast cell populations develop at the inflammatory site in KitW/W-v mice, presumably due to remaining low levels of tyrosine kinase activity seen in the mutant c-kit receptor (9, 14, 38). Whereas the W mutant allele produces truncated c-Kit proteins without the transmembrane domain (29), the Wv mutant contains a point mutation in the tyrosine kinase domain, resulting in reduced, but not complete, elimination of the tyrosine kinase activity of c-Kit. No such inflammation-associated mast cells have been reported for the KitlSl/KitlSl-d mast cell deficiency model. Thus, we wished to examine mast cell involvement in Helicobacter pathogenesis using this alternative mast cell-deficient mouse model.
Additionally, although H. felis has been used extensively in the past to study Helicobacter pathogenesis and immunity, H. felis has several characteristics that distinguish it from H. pylori. H. felis lacks several important H. pylori virulence factors, such as VacA and CagA; it does not attach to the host epithelium; and it possesses pathogen-associated molecular pattern molecules that differ from those of H. pylori and consequently activate host cells via Toll-like receptor molecules much differently than H. pylori (23). In addition to these distinct mechanisms of pathogenesis, it is not possible to quantitatively culture H. felis, so that estimates of the magnitude of infection utilize less sensitive assays, such as the rapid urease test or visually counting spiral organisms in silver-stained sections. The purpose of this study, therefore, was to assess the contribution of mast cells to vaccine-induced protective immunity against challenge with H. pylori in mice, to extend this analysis to a second model of mast cell deficiency, and to begin to characterize the activity of mast cells when stimulated with H. pylori antigens. We now show that while the lack of mast cells does compromise the protective immune response, immunized mast cell-deficient mice do clear the bacteria better than unimmunized mice. We also demonstrate that the gastric mucosa of immunized mice following challenge consists of both mucosal and connective-type mast cells and that stimulation of the mucosal mast cells promotes a cytokine response that favors the recruitment of other granulocytes.
MATERIALS AND METHODS
Mice.
Wild-type C57BL/6 mice and specific-pathogen-free, mast cell-deficient mice (WCB6F1/J KitlSl/KitlSl-d; stock no. 100401) were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in autoclaved microisolator cages and provided with autoclaved water and sterilized food ad libitum. The Institutional Animal Care and Use Committee of Case Western Reserve University and the University of Maryland Medical School approved all procedures involving mice.
Bacteria and bacterial products.
H. pylori SS1 (21), H. pylori 60190 and its isogenic vacA-deficient derivative 60190-v1 (a generous gift from Richard Peek, Vanderbilt University, Nashville, TN), clinical isolates P16 and P82, and our mouse-adapted H. pylori strain HpM5 and its isogenic cagA-deficient derivative HpM5-cagA were all grown on Columbia blood agar containing 7% defibrinated horse blood (Cleveland Scientific, Bath, OH) and antibiotics under microaerophilic conditions as previously described (12). H. felis was grown on brain heart infusion medium (Difco, Sparks, MD) supplemented with horse blood and antibiotics as described previously (4). Bacterial antigen was prepared as previously described (12). Briefly, bacteria were concentrated by centrifugation and then lysed using a probe sonicator. After centrifugation, the soluble fraction was filtered (0.45 μm) and the protein concentration was determined using a bicinchoninic acid assay (Pierce, Rockford, IL). The antigen was stored at −80°C. For challenge, H. pylori SS1 was grown in static liquid cultures of Brucella broth (Difco, Detroit, MI) plus 10% fetal bovine serum (FBS) at 37°C with 10% CO2.
Characterization of H. pylori strains.
H. pylori strains were genotyped for vacA and cagA by use of purified DNA (DNeasy kit; Qiagen, Valencia, CA). PCR was performed with the respective oligonucleotide primers for vacA and cagA (Table 1), and the amplicon products were resolved on 1.5% agarose gels. The expression of CagA was determined by immunoblot analysis. H. pylori antigens were resolved on 10% polyacrylamide gels and transferred to polyvinylidene difluoride membranes. Protein was detected using a rabbit anti-CagA peptide antibody (Austral Biologicals, San Ramon, CA) (Table 2).
TABLE 1.
Oligonucleotide sequences used as primers in either standard or real-time PCR
| Gene (reference) | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| cagA | GATAACAGGCAAGCTTTTGAGG | CTGCAAAAGATTGTTTGGCAG |
| vacA | ATGGAAATACAACAAACACA | CTCCAGAACCCACACGATT |
| GAPDHa (13) | TGTAGACCATGTAGTTGAGGTCA | AGGTCGGTGTGAACGGATTTG |
| IL-17 (13) | GCTCCAGAAGGCCCTCAGA | AGCTTTCCCTCCGCATTGA |
| TNF-α (6) | CCCAAAGGGATGAGAAGT | ACAGGCTTGTCACTCGAA |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
TABLE 2.
Virulence factors identified in Helicobacter strains
| Strain | Presence (+) or absence (−) of: |
||
|---|---|---|---|
| cagA | vacA | CagA | |
| H. pylori strains | |||
| SS1 | + | + | + |
| P82 | − | − | − |
| P16 | − | + | − |
| HpM5 | + | + | + |
| HpM5-cagA | + | + | − |
| 61090 | + | + | + |
| 61090-v1 | + | − | + |
| H. felis strain | − | − | − |
Immunization and challenge.
Each experiment included an immunized-challenged (I/C) group, an unimmunized-challenged (U/C) group, and a control, naïve group. Immunizations consisted of four weekly intranasal inoculations containing 100 μg of H. pylori sonicate and 5 μg of cholera toxin in 20 μl applied directly to the nares. All animals were challenged 2 weeks after the last immunization by gavages with 0.5 ml of H. pylori liquid culture containing approximately 1.0 × 107 to 1.6 × 107 CFU/ml. Mice were sacrificed at various time points after challenge. Vaccine efficacy was established by CFU determination.
CFU determination.
A longitudinal strip of glandular stomach approximately 3 to 5 mm wide was cut aseptically from the greater curvature of the stomach for histology (see below). The remaining glandular stomach was divided longitudinally along the lesser curvature, the tissue fragments were dispensed into preweighed tubes, and one of the weighed sections was homogenized in 200 μl Brucella broth using 1.5-ml disposable polypropylene tissue grinders and pestles (Kontes, Vineland, NJ). The homogenate was diluted serially 1/10 in sterile phosphate-buffered saline (PBS) from 1/10 to 1/1,000, and 10 μl of each dilution was plated. Colonies were counted after 5 to 7 days of incubation, and representative colonies were tested for urease, oxidase, and catalase activities to confirm their identity as H. pylori.
Histologic evaluation.
Stomach strips were fixed in K-2 fixative (2% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate buffer, 0.025% CaCl2) at room temperature for 4 h on a rocker and then at 4°C overnight. The fixative was replaced with 0.1 M sodium cacodylate buffer (4°C) containing sodium azide (pH 7.35 to 7.40). Tissue was paraffin embedded and sectioned for analysis by Giemsa staining. Neutrophils and mast cells in the submucosa and gastric mucosa were enumerated per high-power field. For routine histologic evaluation, slides were stained with hematoxylin and eosin and graded for inflammation using our previously described 0-to-5 scale (12).
Real-time PCR.
Total RNA was extracted from half stomachs by using Trizol (Invitrogen). RNA (1 μg) was reverse transcribed into cDNA by using a reverse transcription kit (Qiagen, Valencia, CA). PCR amplification was performed with an Eppendorf Realplex instrument (Eppendorf AG, Hamburg, Germany), using 96-well microtiter plates. For each sample, the PCR was performed in duplicate with SYBR green supermix (Fermentas, Glen Burnie, MD). Samples were heated at 95°C for 10 min and then subjected to 40 cycles consisting of 95°C for 15 s and 55°C for 10 s. The primers (2 μM) used for each reaction are listed in Table 2. Gastric tissue RNA from a naïve mouse from each group was chosen as a calibrator by use of relative-analysis real-time PCR. Differences in expression of genes in the tissue were calculated as 2−ΔΔCT (where CT is the threshold cycle).
Mast cell cultures and in vitro stimulation assays.
Mouse bone marrow cells were recovered from the femoral and tibia bones of 6- to 8-week-old C57BL/6J mice. Recovered cells were cultured in RPMI medium supplemented with 20% (vol/vol) conditioned WEHI-3 cell medium (32), 10% heat-inactivated FBS, 4 mM l-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, 0.25 μg/ml amphotericin B, 25 mM HEPES, and 0.05 mM 2-mercaptoethanol (complete RPMI). Cells were fed fresh medium biweekly by replacement of half of the volume. After 2 weeks, the cell concentration was adjusted to 1.0 × 106 cells/ml. After 4 weeks, the purity of the mast cells was determined by flow cytometry using fluorescein isothiocyanate-labeled anti-CD117 and phycoerythrin-labeled anti-FcɛRIα antibodies. Greater than 97% of the cells were double positive. Bone marrow-derived mast cells (BMMC) were plated at 2 × 105 cells/well in 96-well, flat-bottomed plates in complete RPMI and were stimulated with the indicated concentration of H. pylori SS1 sonicate (see the figure legends) for 24 h at 37°C. The levels of tumor necrosis factor alpha (TNF-α), IFN-γ, CXCL1/keratinocyte-derived chemokine (KC), and IL-10 in the culture supernatants were determined by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Escherichia coli O111:84 serotype lipopolysaccharide was used as a control.
Recall assay.
Mast cell-deficient KitlSl/KitlSl-d mice, wild-type littermate control mice, and C57BL/6 mice were immunized with H. pylori sonicate plus cholera toxin as described above, and spleens were harvested 2 weeks after the final immunization. Single-cell suspensions were prepared after lysis of red blood cells, and cells were plated at 1.0 × 106 cells per well in 96-well, flat-bottomed plates in RPMI 1640-10% FBS medium. Cells were stimulated with H. pylori SS1 sonicate at the indicated concentration, in triplicate. Concanavalin A (Sigma Chemical Co.) stimulation at 10 ng/ml was used as a positive control. Supernatants were collected after 48 h of incubation, and the concentrations of IFN-γ and IL-17 were determined using a quantitative sandwich ELISA according to the manufacturer's instructions (R&D Systems).
Statistics analysis.
Within each experiment, all treatment groups of knockout and wild-type mice were compared by analysis of variance and Fisher's protected least-significant-difference post hoc testing, using SigmaState 3.5 (Systat Software, San Jose, CA).
RESULTS
The absence of mast cells reduces but does not eliminate the protective immune response against H. pylori.
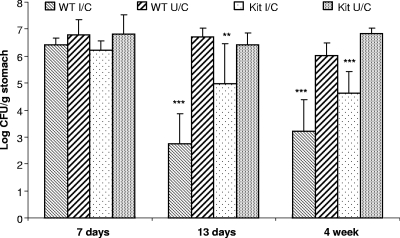
The ability of mast cell-deficient mice to be protected from H. pylori by immunization was evaluated by direct comparison of mast cell-deficient mice and wild-type C57BL/6 mice at multiple time points. We observed high bacterial numbers in all mice 1 week after challenge, including I/C C57BL/6 mice, as previously shown (12) (Fig. 1). By 2 weeks postchallenge, however, immunized C57BL/6 mice displayed a 4.0-log reduction in H. pylori CFU compared to CFU for nonimmunized mice (P < 0.001), while immunized KitlSl/KitlSl-d mice had a lesser but marked reduction in bacterial load of 1.5 log compared to the bacterial load for nonimmunized mice (P < 0.01). By 4 weeks postchallenge, however, there was protective immunity in mast cell-deficient mice, as measured by a reduction in bacterial load of greater than 2 log (2.2-log reduction in CFU for I/C deficient mice versus CFU for U/C deficient mice). Immunization of wild-type mice resulted in a greater reduction in bacterial load than that for unimmunized control mice (2.8 log CFU; P < 0.001). By 11 weeks postchallenge, although the bacterial load in I/C deficient mice remained consistent with the levels observed for I/C deficient mice at 4 weeks postchallenge, the bacterial loads in the U/C deficient mice varied widely, and therefore no significant difference was recorded at this time point. Therefore, the kinetics of bacterial clearance for both wild-type and mast cell-deficient mice are similar to that previously observed for wild-type mice but with reduced levels of clearance in mast cell-deficient mice. A histologic evaluation of the gastric tissue demonstrated that mast cell-deficient KitlSl/KitlSl-d mice were indeed completely devoid of mast cells. Despite the lack of mast cells, these mice responded to challenge with a robust postimmunization gastritis that was similar in overall magnitude to that for wild-type mice (data not shown). These results suggest that mast cells make a partial but not absolute contribution to protective immunity.
FIG. 1.
Mast cell-deficient mice exhibit compromised immunity to vaccine-induced H. pylori-specific immunity. Immunized and unimmunized wild-type (WT) and mast cell-deficient (Kit) mice were challenged with H. pylori, and subgroups of mice were assessed for bacterial load at 1, 2, and 4 weeks by CFU determination. Each group contained 6 to 11 mice. Error bars indicate standard deviations from the means. **, P < 0.01; ***, P < 0.001 (compared to U/C control mice).
Neutrophil recruitment to the gastric mucosa is reduced in mast cell-deficient mice.
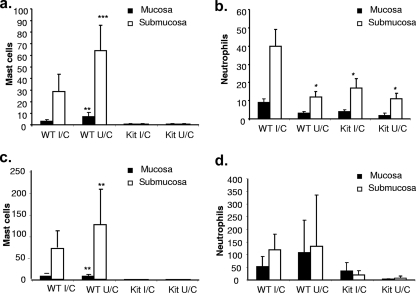
A histologic evaluation of longitudinal gastric biopsy specimens taken from mice sacrificed 2 weeks postchallenge confirmed a complete absence of mast cells in KitlSl/KitlSl-d mice (Fig. 2a and c). In wild-type mice, there were comparable but low numbers of mast cells in the mucosa of I/C and U/C mice, but when the submucosa was assessed we observed an increase in the number of mast cells in unimmunized mice compared to that for immunized mice. Coincident with H. pylori clearance, however, we observed significantly increased numbers of neutrophils in the submucosa of wild-type I/C mice compared to those for U/C mice (P < 0.05) (Fig. 2b). There was no difference in the numbers of neutrophils in mast cell-deficient KitlSl/KitlSl-d I/C mice and KitlSl/KitlSl-d U/C mice. The numbers of neutrophils in U/C mice were the same for both strains. By 4 weeks postchallenge, there was an increase in the number of granulocytes in wild-type mice (Fig. 2d). U/C mast cell-deficient mice showed no increase in neutrophils, but there were increased numbers in the mucosa for I/C mast cell-deficient mice.
FIG. 2.
Immunized mast cell-deficient mice have reduced neutrophil counts in the gastric mucosa postchallenge compared to the counts for immunized wild-type mice. Immunized and unimmunized wild-type (WT) and mast cell-deficient (Kit) mice were challenged with H. pylori, and the gastric mucosa was assessed at 2 (a and b) and 4 (c and d) weeks postchallenge for granulocyte recruitment. Tissue sections were Giemsa stained, and the numbers of mast cells (a and c) and neutrophils (b and d) were quantified in a blinded fashion. Each group contained 6 to 11 mice. Error bars indicate standard deviations from the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to the wild-type I/C group).
Mast cells produce neutrophil chemotactic cytokines in response to H. pylori antigens.
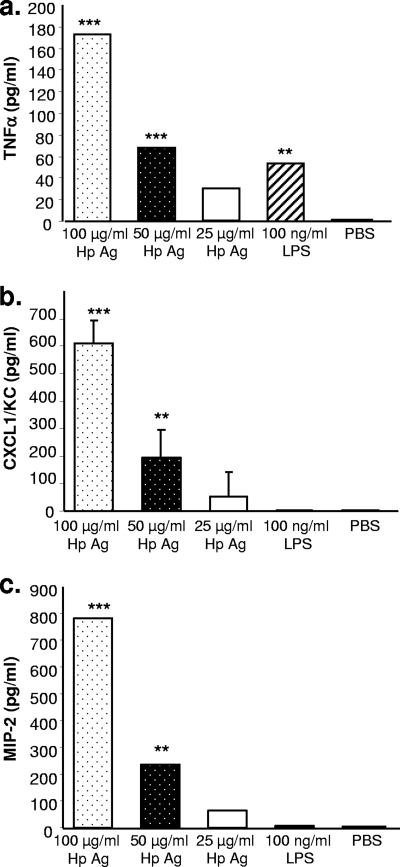
Since neutrophil numbers were reduced in mast cell-deficient mice, we assessed the potential of mast cells to influence neutrophil recruitment. BMMC were stimulated with increasing concentrations of H. pylori SS1 sonicate antigen, and supernatants were evaluated at 3, 6, 24, and 48 h for the production of TNF-α, macrophage inflammatory protein 2 (MIP-2), and CXCL1/KC. H. pylori sonicate antigen was chosen to stimulate mast cells in vitro because the tissue mast cells reside in the lamina propria and are not naturally exposed to whole bacteria during infection with H. pylori. This antigen preparation contains most of the major H. pylori protein antigens, as demonstrated by gel electrophoresis (data not shown); stimulates T cells and macrophages in vitro; and is a potent immunogen when used to vaccinate mice against H. pylori (11, 12, 16). All three cytokines were detectable by 24 h, and their production occurred in a dose-dependent manner, maximizing at 100 μg/ml of antigen, with production of 173 pg/ml, 608 pg/ml, and 783 pg/ml of TNF-α, CXCL1, and MIP-2, respectively (Fig. 3) (P < 0.001). No further increase was observed at 48 h, and no IFN-γ or IL-10 could be detected.
FIG. 3.
BMMC produce neutrophil recruitment and activate cytokines in response to stimulation with H. pylori antigens. Mast cells were grown from mouse bone marrow cells for 4 weeks and then stimulated with decreasing concentrations of H. pylori lysate antigens (Hp Ag) or E. coli lipopolysaccharide (LPS) in vitro for 24 h. TNF-α (a), CXCL1/KC (b), and MIP-2 (c) concentrations were determined by quantitative ELISA. The data are representative of two independent experiments. Assays were performed in quadruplicate. Error bars indicate standard deviations from the means. **, P < 0.01; ***, P < 0.001 (compared to unstimulated cells [PBS]).
Mast cell cytokine production is independent of H. pylori genotype.
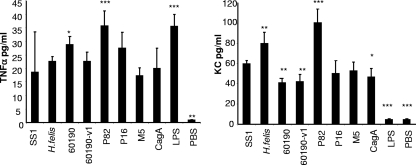
The response of mast cells to Helicobacter antigens was further characterized by stimulation of BMMC with sonicate antigens prepared from multiple H. pylori strains of various virulence factor genotypes and phenotypes, as well as the related animal model pathogen H. felis. Strains were chosen based on their ability to produce CagA or VacA and included strains that are naturally deficient in as well as isogenic mutants for specific genes (Table 2). When BMMC were stimulated with antigen, all bacterial strains induced significant amounts of TNF-α and KC compared to the levels for PBS controls (Fig. 4). Although there were variations in the degrees of stimulation between strains, no pattern emerged in which the presence or absence of VacA or CagA was determinative of TNF-α or KC production by mast cells compared to that for the H. pylori SS1 challenge strain used for infection of mice.
FIG. 4.
Helicobacter-induced cytokine production by BMMC is not strain dependent. Mast cells were grown from mouse bone marrow cells for 4 weeks, and then 2 × 105 cells were stimulated with 100 μg/ml of bacterial lysate prepared from one of seven H. pylori strains of various genotypes and phenotypes or from H. felis. Cell culture supernatants were assessed for TNF-α and KC at 24 h by ELISA. Assays were performed in quadruplicate. Error bars indicate standard deviations from the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to stimulation with H. pylori SS1). LPS, lipopolysaccharide.
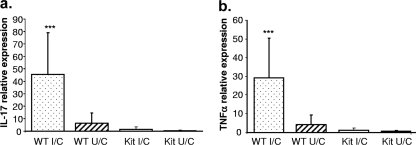
Levels of TNF-α and IL-17 expression are elevated in the gastric mucosa of immunized mice.
We observed elevated neutrophil numbers in wild-type mice compared to those for mast cell-deficient mice at 2 weeks postchallenge (Fig. 2b). Since mast cells produced neutrophil recruitment of cytokines TNF-α, MIP-2, and CXCL1/KC in vitro (Fig. 3), we tested the stomachs of wild-type and mast cell-deficient mice for the presence of mRNA for TNF-α as well as IL-17, cytokines that help recruit neutrophils. Real-time PCR was performed with mRNA isolated from the stomachs of mice sacrificed 2 weeks postchallenge, and the increase in the amount of message compared to that for naïve control mice was determined (Fig. 5). There was an ∼45-fold increase in IL-17 expression in wild-type immunized mice, compared to a 5-fold increase for unimmunized mice (Fig. 5a) (P < 0.001). The increase for mast cell-deficient mice was less than twofold regardless of immunization status. A similar pattern was observed for TNF-α, where immunized wild-type mice had almost a 30-fold increase in expression, compared to less than 5-fold for unimmunized mice and very little expression for mast cell-deficient mice (Fig. 5b) (P < 0.001).
FIG. 5.
Immunized mast cell-deficient mice have reduced levels of IL-17 and TNF-α in the gastric mucosa 2 weeks after challenge. Immunized and unimmunized wild-type (WT) and mast cell-deficient (Kit) mice were challenged with H. pylori. In one experiment, real-time PCR analysis was performed with the mice that were evaluated at 2 weeks postchallenge for the presence of IL-17 (a) and TNF-α (b) expression in the gastric mucosa. Values obtained for challenged mice are expressed as relative units indicating the increase (n-fold) over the level for naïve control samples. Each group contained 10 mice. Error bars indicate standard deviations from the means. ***, P < 0.001 (compared to all other groups).
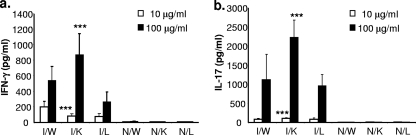
Mast cell deficiency does not impact the inductive phase of H. pylori-specific immunity.
The above-described results indicate that after challenge of immunized mice, mast cell-deficient animals are not protected to the same degree as wild-type mice. In addition, we observed lower levels of CD4 expression and fewer neutrophils in the stomachs of I/C mast cell-deficient mice than in the stomachs of wild-type animals. These results do not indicate, however, whether reduced H. pylori clearance in mast cell-deficient animals results from a failure to induce anti-H. pylori adaptive T- and B-cell immunity or from a failure of adaptive immune effector mechanisms in immunized mast cell-deficient mice. To begin to address this issue, we investigated whether mast cell deficiency blunts the induction of adaptive anti-H. pylori immune responses. Mast cell-deficient KitlSl/KitlSl-d mice, wild-type littermate control mice, and C57BL/6 mice were vaccinated using our established protocol but were not challenged. Two weeks after the final immunization, isolated spleen cells were used to perform a recall assay to assess the memory response to H. pylori antigens, as measured by the production of IFN-γ and IL-17 (Fig. 6). Mast cell-deficient mice responded with significantly greater IL-17 production than either immunized wild-type mice or littermate controls when stimulated with either 10 or 100 μg/ml bacterial antigen (P < 0.001). Similar results were obtained for IFN-γ production when mice were stimulated with 100 μg/ml antigen. Although immunized wild-type mice produced more IFN-γ than did mast cell-deficient mice when stimulated with 10 μg/ml antigen, the response of the littermate controls was equivalent to that of the mast cell-deficient mice.
FIG. 6.
The T-cell cytokine recall response of immunized mast cell-deficient mice is not reduced compared to that for immunized wild-type mice. Wild-type C57BL/6 mice (W), KitlSl/KitlSl-d mast cell-deficient mice (K), and KitlSl/KitlSl-d wild-type littermates (L) were immunized, and the spleen cells were removed 2 weeks after the final booster. Spleen cells (1 × 106 cells per well) were stimulated with either 10 or 100 μg/ml H. pylori lysate for 48 h. Supernatants were assessed by ELISA for IFN-γ (a) and IL-17 (b). Assays were performed in triplicate. I, immunized (two mice in each group); N, naïve (two mice in each group). Error bars indicate standard deviations from the means. ***, P < 0.001 (compared to I/W mice).
DISCUSSION
In the present study, we extend previous observations about the importance of mast cells in vaccine-induced immunity against H. felis in mice (36) to an H. pylori mouse model. We also demonstrate that the impact of the mast cell deficiency on the protective immune response occurs in the effector phase and has little bearing on the development of a strong T-cell recall response. Although we show that H. pylori antigen induces mast cells to produce neutrophil activation factors and that KitlSl/KitlSl-d mast cell-deficient mice also have reduced levels of neutrophils in the gastric mucosa, the mechanisms by which mast cells participate in the clearance of H. pylori remain unclear.
We demonstrate that immunized KitlSl/KitlSl-d mast cell-deficient mice can reduce the bacterial load in the gastric mucosa more than 2 log CFU compared to that for unimmunized mice by 4 weeks postchallenge. However, this reduction was significantly less than the reduction which was observed for immunized wild-type mice at both 2 weeks and 4 weeks postchallenge. Velin et al. were unable to achieve any reductions in bacterial load when using mast cell-deficient mice to immunize against H. felis, thereby demonstrating an absolute requirement for mast cells in protective immunity versus H. felis (36). Our results with the H. pylori model are not as striking but nevertheless demonstrate a blunting of vaccine efficacy in the absence of mast cells. These different results may be attributable to some of the differences between H. felis and H. pylori as well as the differences in the inflammatory responses induced by these two species.
H. felis lacks many of the H. pylori virulence factors, such as VacA, CagA, and the type IV secretion system. It also fails to bind to the gastric epithelium and form adhesion pedestals. Although the impact of these phenotypic distinctions is difficult to assess, the VacA toxin can directly activate mast cells for migration and production of proinflammatory cytokines in vitro (34). Despite lacking these virulence factors, H. felis is known to induce much more inflammation in the gastric mucosa of mice than H. pylori. It is possible that the H. felis-mediated inflammation is more dependent upon mast cells than the H. pylori-mediated inflammation, even when immunized mice are challenged. There are of course also differences between the mouse strains employed in the two studies and in the mechanisms of bacterial quantification. However, while the degrees of impact may vary between studies, we also demonstrate that mast cells play a role in the protective immunity induced against Helicobacter by prophylactic immunization.
Mast cells are derived from CD34+/c-kit+ multipotent hematopoietic progenitor bone marrow cells that migrate to virtually all tissues throughout the body, where they undergo final maturation in response to local factors (17). Classically, mast cell activation occurs primarily through cross-linking of FcɛR1 via IgE antibodies and results in degranulation and release of histamine and other bioactive mediators that impact the microenvironment, including heparin and serotonin. Activated mast cells can also produce lipid mediators and a variety of cytokines and chemokines. More recently, the role of mast cells in the innate immune response to infectious organisms has been expanded to include a direct response to microbial antigens via Toll-like receptors and CD48 independently of IgE (24, 31). Under these circumstances, mast cells have been demonstrated to rapidly and selectively produce appropriate mediators to enhance effector-cell recruitment that complement other effector components of the immune system (25). In the case of the host response to H. pylori, mast cells have been reported to be present at higher densities in the gastric mucosa in biopsy specimens of H. pylori-infected subjects than in those of H. pylori-negative subjects (19, 28). Since H. pylori is rarely spontaneously eradicated in the absence of immunization in mice, increased mast cell numbers and activity may be a result of effector T-cell signaling following challenge of immunized mice.
Our data show that immunized KitlSl/KitlSl-d mast cell-deficient mice have reduced neutrophil recruitment in both the mucosa and the submucosa compared to the levels for immunized wild-type mice, even though the mast cell-deficient mice displayed greater than a 2-log reduction in H. pylori CFU compared to the CFU for unimmunized knockout mice. The primary function of mast cells in vaccine-induced H. pylori immunity, therefore, may be the recruitment or activation of neutrophils.
The in vitro analysis we performed using BMMC shows that stimulation with H. pylori antigens induces the production of TNF-α, CXCL1/KC, and MIP-2 chemokines in a dose-dependent manner. These cytokines are potent inducers of neutrophil activation. Mast cell-derived TNF-α plays a crucial role in the influx of neutrophils into bacterium-infected sites. Mast cell-derived MIP-2 has also been reported to be produced at infected sites and influences the recruitment of neutrophils (26). In the present study, mast cell-deficient mice had reduced inflammation and reduced levels of TNF-α compared to the levels for wild-type mice in the immunization model. Our previous data have shown that recruitment of neutrophils to the gastric mucosa may be required for protection in the H. pylori mouse model (5).
TNF-α has been described as an immunoprotective factor produced by mast cells to efficiently recruit neutrophils to bacterium-infected tissues (15, 20, 22, 27, 35). Previous studies with other bacterial infection models showed that no cells expressing TNF-α mRNA were identified in mast cell-deficient KitW/W-v mice even though there were numerous cells expressing TNF-α transcripts within the mucosa, submucosa, and muscularis of normal mice. Models in which mice are reconstituted with mast cells showed that TNF-α is an important mediator of mast cell-dependent leukocyte recruitment in response to IgE and antigen or immune complexes (8, 10, 40). The proinflammatory cytokine IL-17 can also directly induce neutrophil recruitment. In this H. pylori vaccinated animal model, the reduction of IL-17 mRNA levels in the gastric mucosa of immunized KitlSl/KitlSl-d mast cell-deficient mice might offer another explanation for the reduced recruitment of neutrophils in the lamina propria. Nakae et al. reported that mast cell and mast cell-derived TNF-α can significantly enhance the Th17-dependent inflammatory response associated with significant neutrophil recruitment in the infected airway by FcRγ-independent mechanisms (27).
The influence of mast cells on expression of the T-cell-associated immune response has recently been studied (36). Mast cells secrete cytokines in response to both T-cell and B-cell development and function, including IL-3, -4, -5, -6, -10, -13, and -16 and TNF-α. Vaccination-induced immunity against H. pylori infection requires CD4+ T cells, as has been demonstrated previously (7, 16, 30). We now present evidence to show that the absence of mast cells in mice does not impact the inductive phase of the anti-H. pylori immune response following immunization. We postulate that T cells and mast cells interact either directly or indirectly at the gastric mucosa following bacterial challenge to promote effector mechanisms capable of eradicating H. pylori infection. Mast cell activation has been reported to induce T-cell migration either directly by the release of chemokines or indirectly by the induction of adhesion molecule expression on endothelial cells (25). It is also likely that activated T cells influence the recruitment and activation of mast cells in the gastric mucosa of I/C mice.
In conclusion, we have shown that mast cells, while not essential for vaccine-induced protective immunity against H. pylori, do contribute to protection in immunized mice. We also demonstrate that the T-cell recall response to vaccination is not diminished in the absence of mast cells, indicating that the reduction in immunity to H. pylori challenge is not due to a compromise in the inductive phase of the immune response. The ability of BMMC to produce proinflammatory cytokines in response to H. pylori antigens suggests that mast cells may participate in further promoting protective inflammation. The downstream event associated with activated mast cells in the gastric mucosa of I/C mice will be the focus of future studies.
Acknowledgments
This research was supported by NIH grants AI055710 (T.G.B.) and DK46461 (S.J.C.).
Editor: S. R. Blanke
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell. Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard, T. G., J. C. Eisenberg, and Y. Matsumoto. 2004. Clearance of Helicobacter pylori infection through immunization: the site of T cell activation contributes to vaccine efficacy. Vaccine 22:888-897. [DOI] [PubMed] [Google Scholar]
- 4.Czinn, S. J., A. Cai, and J. G. Nedrud. 1993. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine 11:637-642. [DOI] [PubMed] [Google Scholar]
- 5.DeLyria, E. S., R. W. Redline, and T. G. Blanchard. 2009. Vaccination of mice against H. pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology 136:247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton, K. A., L. H. Benson, J. Haeger, and B. M. Gray. 2006. Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice. Infect. Immun. 74:4673-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuta, G. T., A. Schmidt-Choudhury, M. Y. Wang, Z. S. Wang, L. Lu, R. I. Furlano, and B. K. Wershil. 1997. Mast cell-dependent tumor necrosis factor alpha production participates in allergic gastric inflammation in mice. Gastroenterology 113:1560-1569. [DOI] [PubMed] [Google Scholar]
- 9.Galli, S. J., N. Arizono, T. Murakami, A. M. Dvorak, and J. G. Fox. 1987. Development of large numbers of mast cells at sites of idiopathic chronic dermatitis in genetically mast cell-deficient WBB6F1-W/Wv mice. Blood 69:1661-1666. [PubMed] [Google Scholar]
- 10.Galli, S. J., and B. K. Wershil. 1996. The two faces of the mast cell. Nature 381:21-22. [DOI] [PubMed] [Google Scholar]
- 11.Garhart, C. A., J. G. Nedrud, F. P. Heinzel, N. E. Sigmund, and S. J. Czinn. 2003. Vaccine-induced protection against Helicobacter pylori in mice lacking both antibodies and interleukin-4. Infect. Immun. 71:3628-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garhart, C. A., R. W. Redline, J. G. Nedrud, and S. J. Czinn. 2002. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect. Immun. 70:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godinez, I., T. Haneda, M. Raffatellu, M. D. George, T. A. Paixao, H. G. Rolan, R. L. Santos, S. Dandekar, R. M. Tsolis, and A. J. Bäumler. 2008. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect. Immun. 76:2008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon, J. R., and S. J. Galli. 1990. Phorbol 12-myristate 13-acetate-induced development of functionally active mast cells in W/Wv but not Sl/Sld genetically mast cell-deficient mice. Blood 75:1637-1645. [PubMed] [Google Scholar]
- 15.Gordon, J. R., and S. J. Galli. 1991. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J. Exp. Med. 174:103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottwein, J. M., T. G. Blanchard, O. S. Targoni, J. C. Eisenberg, B. M. Zagorski, R. W. Redline, J. G. Nedrud, M. Tary-Lehmann, P. V. Lehmann, and S. J. Czinn. 2001. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J. Infect. Dis. 184:308-314. [DOI] [PubMed] [Google Scholar]
- 17.Hu, Z. Q., W. H. Zhao, and T. Shimamura. 2007. Regulation of mast cell development by inflammatory factors. Curr. Med. Chem. 14:3044-3050. [DOI] [PubMed] [Google Scholar]
- 18.Ismail, H. F., P. Fick, J. Zhang, R. G. Lynch, and D. J. Berg. 2003. Depletion of neutrophils in IL-10(-/-) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J. Immunol. 170:3782-3789. [DOI] [PubMed] [Google Scholar]
- 19.Kayaselcuk, F., E. Serin, Y. Gumurdulu, S. Bircan, and I. Tuncer. 2002. Relationship between gastritis severity, Helicobacter pylori intensity and mast cell density in the antrum and corpus. Turk. J. Gastroenterol. 13:154-158. [PubMed] [Google Scholar]
- 20.Kempuraj, D., M. Tagen, B. P. Iliopoulou, A. Clemons, M. Vasiadi, W. Boucher, M. House, A. Wolfberg, and T. C. Theoharides. 2008. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell-dependent stimulation of Jurkat T cells. Br. J. Pharmacol. 155:1076-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardised mouse model of Helicobacter pylori infection. Introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 22.Malaviya, R., T. Ikeda, E. Ross, and S. N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381:77-80. [DOI] [PubMed] [Google Scholar]
- 23.Mandell, L., A. P. Moran, A. Cocchiarella, J. Houghton, N. Taylor, J. G. Fox, T. C. Wang, and E. A. Kurt-Jones. 2004. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via Toll-like receptor 2 but not Toll-like receptor 4. Infect. Immun. 72:6446-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall, J. S. 2004. Mast-cell responses to pathogens. Nat. Rev. Immunol. 4:787-799. [DOI] [PubMed] [Google Scholar]
- 25.Mekori, Y. A., and D. D. Metcalfe. 1999. Mast cell-T cell interactions. J. Allergy Clin. Immunol. 104:517-523. [DOI] [PubMed] [Google Scholar]
- 26.Mercer-Jones, M. A., M. S. Shrotri, M. Heinzelmann, J. C. Peyton, and W. G. Cheadle. 1999. Regulation of early peritoneal neutrophil migration by macrophage inflammatory protein-2 and mast cells in experimental peritonitis. J. Leukoc. Biol. 65:249-255. [DOI] [PubMed] [Google Scholar]
- 27.Nakae, S., H. Suto, G. J. Berry, and S. J. Galli. 2007. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood 109:3640-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima, S., N. Bamba, and T. Hattori. 2004. Histological aspects and role of mast cells in Helicobacter pylori-infected gastritis. Aliment. Pharmacol. Ther. 20(Suppl. 1):165-170. [DOI] [PubMed] [Google Scholar]
- 29.Nocka, K., J. C. Tan, E. Chiu, T. Y. Chu, P. Ray, P. Traktman, and P. Besmer. 1990. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 9:1805-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocha-de-Souza, C. M., B. Berent-Maoz, D. Mankuta, A. E. Moses, and F. Levi-Schaffer. 2008. Human mast cell activation by Staphylococcus aureus: interleukin-8 and tumor necrosis factor alpha release and the role of Toll-like receptor 2 and CD48 molecules. Infect. Immun. 76:4489-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rottem, M., S. Barbieri, J. P. Kinet, and D. D. Metcalfe. 1992. Kinetics of the appearance of Fc epsilon RI-bearing cells in interleukin-3-dependent mouse bone marrow cultures: correlation with histamine content and mast cell maturation. Blood 79:972-980. [PubMed] [Google Scholar]
- 33.Sawai, N., M. Kita, T. Kodama, T. Tanahashi, Y. Yamaoka, Y. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect. Immun. 67:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Supajatura, V., H. Ushio, A. Wada, K. Yahiro, K. Okumura, H. Ogawa, T. Hirayama, and C. Ra. 2002. Cutting edge: VacA, a vacuolating cytotoxin of Helicobacter pylori, directly activates mast cells for migration and production of proinflammatory cytokines. J. Immunol. 168:2603-2607. [DOI] [PubMed] [Google Scholar]
- 35.Tartaglia, L. A., D. V. Goeddel, C. Reynolds, I. S. Figari, R. F. Weber, B. M. Fendly, and M. A. Palladino, Jr. 1993. Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J. Immunol. 151:4637-4641. [PubMed] [Google Scholar]
- 36.Velin, D., D. Bachmann, H. Bouzourene, and P. Michetti. 2005. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology 129:142-155. [DOI] [PubMed] [Google Scholar]
- 37.Warren, J. R. 2000. Gastric pathology associated with Helicobacter pylori. Gastroenterol. Clin. N. Am. 29:705-751. [DOI] [PubMed] [Google Scholar]
- 38.Waskow, C., S. Bartels, S. M. Schlenner, C. Costa, and H. R. Rodewald. 2007. Kit is essential for PMA-inflammation-induced mast-cell accumulation in the skin. Blood 109:5363-5370. [DOI] [PubMed] [Google Scholar]
- 39.Wershil, B. K. 2000. IX. Mast cell-deficient mice and intestinal biology. Am. J. Physiol. Gastrointest. Liver Physiol. 278:G343-G348. [DOI] [PubMed] [Google Scholar]
- 40.Wershil, B. K., T. Murakami, and S. J. Galli. 1988. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J. Immunol. 140:2356-2360. [PubMed] [Google Scholar]