Abstract
It has become increasingly difficult to treat infections caused by Enterococcus faecalis due to its high levels of intrinsic and acquired antibiotic resistance. However, few studies have explored the mechanisms that E. faecalis employs to circumvent the host innate immune response and establish infection. Capsular polysaccharides are important virulence factors that are associated with innate immune evasion. We demonstrate, using cultured macrophages (RAW 264.7), that capsule-producing E. faecalis strains of either serotype C or D are more resistant to complement-mediated opsonophagocytosis than unencapsulated strains. We show that differences in opsonophagocytosis are not due to variations in C3 deposition but are due to the ability of capsule to mask bound C3 from detection on the surface of E. faecalis. Similarly, E. faecalis capsule masks lipoteichoic acid from detection, which correlates with decreased tumor necrosis factor alpha production by cultured macrophages in the presence of encapsulated strains compared to that in the presence of unencapsulated strains. Our studies confirm the important role of the capsule as a virulence factor of E. faecalis and provide several mechanisms by which the presence of the capsule influences evasion of the innate immune response and suggest that the capsule could be a potential target for developing alternative therapies to treat E. faecalis infections.
Enterococcus faecalis is an important nosocomial pathogen associated with many types of infection, including surgical-site infections, bacteremia, urinary tract infections, and endocarditis (31). Many infections caused by E. faecalis are difficult to treat due to its increasing resistance to conventional antibiotic therapies, including vancomycin (10-12). Apart from studies of the roles of gelatinase and cytolysin (5, 22, 27), relatively little is known about the mechanisms employed by E. faecalis to circumvent host innate immune responses.
In other bacterial pathogens, the production of capsular polysaccharide is a known virulence factor, as it aids in avoidance of the host innate immune response (25, 30, 36). E. faecalis is known to produce two capsular polysaccharide serotypes (C and D) (10, 12, 14, 40) that contribute to pathogenesis and evasion of the host innate immune response (10). Hufnagel et al. reported decreased neutrophilic killing of encapsulated serotype C and D strains compared to the unencapsulated A and B strains (15). In addition, a recent comprehensive analysis of clinical E. faecalis isolates indicated that most pathogenic strains of E. faecalis belong to serotype C (19). Despite a link between capsule and virulence, little is known about the specific mechanism(s) by which capsule enhances pathogenesis.
The complement system plays a central role in the activation of the immune system and in the clearance of pathogens. Cleavage of C3 into C3b provides a highly effective opsonin in the absence of antibodies. Several reports have shown that capsule-producing species of bacteria are more resistant to opsonophagocytosis due to inhibition of the deposition and/or detection of C3b on the surface of the organism (28, 32, 42). Encapsulated bacteria employ numerous mechanisms to resist C3 opsonization and subsequent phagocytosis, including overall reduction in C3 deposition (6). The abundance of C3 deposition is known to differ between capsule-producing serotypes of Streptococcus pneumoniae (20). In Staphylococcus aureus, C3 is buried beneath the surface of the capsule, rendering C3 less accessible to complement receptors on the surfaces of macrophages and neutrophils (41).
Bacterial capsular polysaccharides are also known to aid in the avoidance of innate immune responses, including immune surveillance. Immune surveillance relies on pathogen recognition receptors (PRRs), including Toll-like receptors, to sense pathogen-associated molecular patterns (PAMPs). Two common PAMPs associated with gram-positive microorganisms are lipoteichoic acid (LTA) and peptidoglycan. Detection of these PAMPs by Toll-like receptor 2 (TLR-2), in conjunction with TLR-1 and -6, induces the production of cytokines. In other instances, capsule prevents the detection of PAMPs by PRRs, which leads to decreased or altered cytokine production (9). The altered cytokine response to encapsulated pathogens appears to contribute to pathogenicity and virulence.
Our data indicate that the E. faecalis capsular polysaccharides from serotypes C and D attenuate C3-opsonized phagocytosis and that this attenuated response is likely due to decreased recognition of bound C3 on the bacterial surface. Similarly, capsule inhibits detection of E. faecalis LTA on the surface, and the absence of recognition of this molecule and/or other surface PAMPs in the presence of capsule results in decreased tumor necrosis factor alpha (TNF-α) production by macrophages.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All relevant bacterial strains are listed in Table 1. E. faecalis strains were cultivated in Todd-Hewitt broth supplied with the appropriate antibiotics when needed (Becton Dickinson and Company, Sparks, MD).
TABLE 1.
E. faecalis strains used in this study
| Strain | Description | Reference |
|---|---|---|
| FA2-2 | Capsule+ (serotype C) | 4 |
| V583 | Capsule+ (serotype C) | 33 |
| OG1RF | Capsule− | 23 |
| 12030 | Capsule− | 13 |
| LT01 | FA2-2 ΔcpsF capsule+ (serotype D) | 40 |
| LT02 | V583 ΔcpsF capsule+ (serotype D) | 40 |
| LT05 | FA2-2 ΔcpsC capsule− | 40 |
| LT06 | V583 ΔcpsC capsule− | 40 |
| LT12 | V583 + pMV158gfp | This study |
| LT13 | LT02 + pMV158gfp | This study |
| LT14 | LT06 + pMV158gfp | This study |
Culture of macrophages.
Macrophage-like RAW 264.7 (ATCC TIB-71) cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Grand Island, NY) supplemented with 100 U penicillin per ml, 100 μg streptomycin per ml, 2 μg l-glutamine per ml, and 5% heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA).
Complement C3 deposition.
Overnight cultures of E. faecalis were diluted 1:100 in fresh medium. The cultures were allowed to reach mid-log phase (optical density at 600 nm, 0.6) and were washed three times in sterile phosphate-buffered saline (PBS), pH 7.4. Approximately 2 × 107 cells of each strain were resuspended in 10% normal CD1 mouse serum containing complement (Innovative Research, Southfield, MI) diluted in PBS. Serum for negative controls was heat inactivated prior to the addition of bacteria by incubating it at 56°C for 30 min. Bacteria were incubated in 10% serum for 30 min at 37°C with agitation. Complement deposition was stopped by the addition of EDTA to a final concentration of 10 mM, followed by incubation on ice for 5 min. The bacteria were pelleted at 4°C, washed three times with sterile PBS to remove unbound complement, and finally resuspended in 30 μl of 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer. Whole bacteria were boiled vigorously for 5 minutes, and the cell debris was removed by centrifugation. The remaining supernatants were loaded on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and electrophoresed. Proteins in the gel were transferred to nylon membranes, and detection of C3 was carried out by Western blot analysis using goat anti-mouse C3 polyclonal antibodies (Bethyl Laboratories, Montgomery, TX) and rabbit anti-goat antibody conjugated with horseradish peroxidase (HRP) as a secondary antibody (Bethyl Laboratories, Montgomery, TX), followed by development with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL).
ELISA.
The concentration of naturally occurring anti-enterococcal antibodies present in the CD1 (Innovative Research, Southfield, MI) mouse serum (used for subsequent phagocytosis assays) was analyzed by enzyme-linked immunosorbent assay (ELISA). In addition, ELISA was performed to investigate the serotype specificity conferred by the presence of CpsF among E. faecalis isolates using serotype C-specific antibodies. Briefly, log-phase E. faecalis strains were washed three times in PBS and aliquoted (50 μl) into high-binding 96-well Costar plates (Corning). The washed cells were allowed to adhere overnight at 4°C. The bound cells were then incubated with either CD1 mouse serum or rabbit anti-serotype C serum (18), followed by incubation with either goat anti-mouse immunoglobulin G (IgG)-HRP conjugate (Sigma, Saint Louis, MO) or goat anti-rabbit IgG-HRP conjugate (Jackson ImmunoResearch, West Grove, PA). ELISAs were developed using o-phenylenediamine dihydrochloride (Sigma) as the HRP substrate, and the results were read as an optical density at 490 nm on a Bio-Tek PowerWave XS 96-well plate reader.
Opsonophagocytosis assay.
E. faecalis strains V583, LT02 (V583 ΔcpsF), and LT06 (V583 ΔcpsC) were transformed by electroporation with the plasmid pMV158GFP (24), giving rise to LT12, LT13, and LT14, respectively (Table 1). Strains LT12, LT13, and LT14 constitutively express green fluorescent protein (GFP), allowing fluorescence detection during the opsonophagocytosis assay.
Log-phase bacteria were washed three times in PBS prior to being resuspended in Hanks balanced salt solution (Invitrogen) medium. Harvested RAW 264.7 cells were also resuspended in Hanks balanced salt solution medium. A bacterial concentration of 2 × 106 CFU/ml was added to 2 × 105 RAW 264.7 cells/ml, followed by the addition of complement-containing CD1 mouse serum to a concentration of 10% to give a final volume of 500 μl and a bacteria-to-macrophage ratio of 10:1. The samples were incubated at 37°C for 20 min to allow uptake of bacteria by macrophages. Trypsin was then added at a 0.25% final concentration and incubated for 10 min to remove any bacteria bound to the external surfaces of the RAW 264.7 cells. The free bacteria were removed by three PBS washes with low-speed centrifugation (750 × g) (7, 8). The washed cells were fixed to glass slides by cytocentrifugation. The samples were viewed with a 100× oil immersion lens using a Zeiss Axioplan 2 fluorescence microscope to visualize the GFP-expressing bacteria inside the RAW 264.7 cells. The intracellular bacteria of at least 100 RAW 264.7 cells were counted for each experimental replicate. The phagocytic index was calculated by dividing the number of phagocytic cells (cells that had consumed bacteria) by the total number of macrophages counted and multiplying that number by the number of bacteria per phagocytic macrophage (number of phagocytic cells/total number of cells counted × number of bacteria/number of phagocytic cells), as previously described (21, 29). The data are presented as phagocytic-index percentages, with the phagocytic index of LT14 (V583 ΔcpsC; capsule−) set to 100%. The data were compiled from three separate experiments, and the standard errors of the mean and statistical significances were calculated with Graphpad Prism software.
Slide agglutination.
Unencapsulated and encapsulated strains were tested for their reactivities to serotype A antiserum, previously reported to be specific for enterococcal LTA (39). Slide agglutination assays were performed as previously described (18, 40). Briefly, log-phase bacteria were washed three times with PBS. Following the PBS washes, 5.0 μl of LTA antiserum or preimmune serum was added to 15.0 μl of test cells on a glass slide and gently rotated for 1 minute. Agglutination was determined by visible clumping of the cells. Sterile PBS and preimmune serum were used as negative controls.
Flow cytometry.
Flow cytometry was used to determine if C3 or LTA accessibility to antibodies was altered by the presence of capsule. Log-phase bacteria were washed three times in PBS, diluted 1:2, and blocked in 5% donkey serum (Jackson ImmunoResearch). Bacteria used for analyzing C3 accessibility were incubated in 50 μl of CD1 mouse serum for 20 min at 37°C to allow C3 deposition and were washed three times in PBS prior to being blocked with donkey serum. The blocked cells were incubated for 15 min on ice with 2.0% goat anti-C3 antibodies, followed by three washes in PBS. Similarly diluted goat serum was used as an isotype control. The bacteria were then incubated with fluorescein isothiocyanate (FITC)-conjugated donkey anti-goat antibody (1:1,000; Jackson ImmunoResearch) for 15 min on ice in the dark. The bacteria were again washed three times with PBS and analyzed by flow cytometry. For detection of LTA accessibility, washed and blocked bacterial cells were incubated on ice for 15 min with 2.0% anti-LTA rabbit serum (39). Similarly diluted preimmune rabbit serum was used as an isotype control. The cells were then washed three times in PBS and incubated for 15 min on ice in the dark with FITC-conjugated donkey anti-rabbit antibody (1:100; Jackson ImmunoResearch). The bacteria were washed three times in PBS and analyzed by flow cytometry. For both the C3 and LTA experiments, flow cytometry analysis of 50,000 bacteria was performed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) at a flow rate of ∼2,000 cells per second. The data were analyzed using the WinList software program (VerityHouse, Topsham, ME).
TNF-α production.
Log-phase bacteria were washed three times in PBS and heat killed by incubation at 80°C for 30 min. RAW 264.7 cells were harvested and resuspended in fresh Dulbecco's modified Eagle's medium culture medium to a concentration of 1 × 106 cells per ml. RAW cells at a concentration of 1 × 106 cells/ml in a total volume of 2.0 ml were seeded in 24-well plates. The cells were allowed to adhere to the plate surface for 2 hours prior to induction. Bacteria were added to each well at a concentration of 1 × 107 CFU. Lipopolysaccharide from Salmonella enterica serotype Typhimurium (Sigma) was used as a positive control for TNF-α production at a concentration of 10 ng per ml. Clarified supernatants were collected from each well 4 hours after the bacterial inoculation. The amount of TNF-α present in the supernatants was determined by ELISA (eBioscience, San Diego, CA), following the manufacturer's instructions. One-way analysis of variance (ANOVA) in correlation with a Newman-Kuels post hoc test was used to evaluate statistical significance (GraphPad Prism).
RESULTS
Protective effects of capsule against opsonophagocytosis.
The capsular polysaccharides of many bacterial species confer resistance to complement-mediated opsonphagocytosis. We examined whether E. faecalis capsule conferred resistance to C3 opsonophagocytosis mediated by macrophages. We used ELISA to confirm that our complement source (CD-1 mouse serum) was free of detectable E. faecalis antibodies (data not shown).
Previous studies have shown that E. faecalis-opsonizing antibodies exist in normal human serum; however, these antibodies are directed only toward the unencapsulated serotype A and B strains of E. faecalis (2, 14). In view of these studies, we determined if E. faecalis capsule serotype C or D conferred resistance to complement-mediated opsonophagocytosis compared to an isogenic acapsular mutant. The encapsulated E. faecalis strains LT12 (serotype C) and LT13, an isogenic cpsF deletion mutant that results in the production of a serotype D capsular polysaccharide (40), and LT14, an isogenic cpsC deletion mutant that is unencapsulated (40), were compared. For this assay, we followed the method of Drevets et al. and Graveline et al. (8, 9), which calls for trypsin treatment and subsequent washes to remove externally bound bacteria as opposed to antibiotic treatment with gentamicin, which has been shown to be internalized by macrophages, leading to antibiotic killing effects independent of macrophage activity (9). Our data show a 50% reduction in the opsonophagocytosis of capsule-producing strains by macrophages in the presence of complement compared to unencapsulated strains (Fig. 1). These data also show that there is no statistical difference in opsonophagocytosis between isogenic serotype C (LT12) and serotype D (LT13) strains (Fig. 1), suggesting that the mere presence of capsule, regardless of serotype, provides protection against bacterial uptake by macrophages.
FIG. 1.
Capsule serotypes C and D are resistant to opsonophagocytosis in the presence of complement. (A) Representative micrographs depicting LT12 (V583 expressing GFP), LT13 (ΔcpsF expressing GFP), and LT14 (ΔcpsC expressing GFP) incubated with RAW 264.7 macrophage-like cells. (B) Quantification of the phagocytic index expressed as a percentage of that of the unencapsulated LT14 strain (see Materials and Methods for calculation of the phagocytic index). The light-gray bar (LT12; serotype C) and the dark-gray bar (LT13; serotype D) both show a significant reduction in the phagocytic index compared to LT14 (black bar). The error bars represent the standard errors of three replicates.
Complement C3 deposition and surface accessibility.
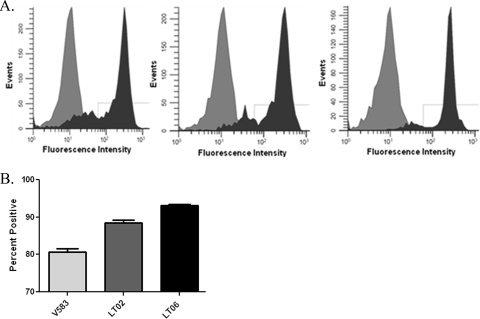
Bacterial resistance to complement-mediated opsonophagocytosis has been attributed to decreased amounts of C3 deposition on the surfaces of encapsulated strains (6). We used Western blot analysis to assess the abundance of complement C3 deposited on both encapsulated and unencapsulated E. faecalis strains. Two encapsulated strains, FA2-2 and LT01 (FA2-2ΔcpsF), and two unencapsulated strains, LT05 (FA2-2ΔcpsC) and OG1RF, were used in this experiment. Complement C3 is composed of an α and a β chain (34). The 75-kDa C3 β chain is left intact through the processing events of C3 and was used to determine differences in overall C3 deposition. Figure 2 shows the deposition of the 75-kDa β chain of C3 on different strains of E. faecalis. There was no difference in the amounts of C3 deposited on the surfaces of the unencapsulated strains OG1RF and LT05 and on the encapsulated V583 and LT01 strains (Fig. 2). The other fragments detected in the blot were known breakdown products of C3 and C3b.
FIG. 2.

Amounts of C3 deposition do not differ between strains. Western blot analysis was employed to examine the amounts of C3 deposited on the cell surfaces of serotype C (FA2-2), serotype D (LT01), and unencapsulated (LT05 and OG1RF) strains. The blot shows the 75-kDa β chain of C3 for FA2-2 (A), LT02 (B), LT05 (C), OG1RF (D), and the negative control, FA2-2 (E), incubated with heat-inactivated serum. The additional bands present on the blot are unprocessed C3, as well as C3 and C3b breakdown products recognized by the polyclonal antibodies to C3.
The amount of complement deposition does not vary between strains, but the presence of complement on the encapsulated strains could be masked from detection by complement receptors, leading to decreased phagocytosis. We used complement-opsonized strains of V583 (serotype C), LT02 (V583 ΔcpsF; serotype D), and LT06 (V583 ΔcpsC; capsule−), in conjunction with flow cytometry, to determine C3 surface accessibility to antibodies. Our data show that C3 deposited on the surface of LT06 was more detectable than C3 deposited on the surfaces of the encapsulated strains V583 and LT02 (Fig. 3). Statistical analysis using one-way ANOVA, in conjunction with a Newman-Keuls post hoc test, showed a statistically significant difference (P < 0.05) between V583 and LT06 and also between LT02 and LT06 (Fig. 3). There was also a statistically significant difference between V583 and LT02, even though they appear to be equally resistant to complement-mediated opsonophagocytosis (Fig. 1). The basis for this difference is not known, but it may be related to structural differences between the capsular polysaccharides in the two serotypes.
FIG. 3.
Complement C3 is masked from detection by capsule. Flow cytometry was used, in conjunction with anti-C3 antibodies and FITC-conjugated secondary antibodies, to evaluate the availability of C3 to detection. (A) Representative histograms depicting (from left to right) flow cytometry results for serotype C (V583), serotype D (LT02), and unencapsulated (LT06) E. faecalis strains. The isotype controls are light gray, and the C3 antibody-treated cells are dark gray. (B) Quantification of the C3-positive cells. Using one-way ANOVA, in conjunction with a Newman-Keuls posttest, statistical analysis of three replicates showed statistically significant differences (P < 0.05) in the amounts of positively labeled bacteria when V583 (light-gray bar) and LT06 (black bar) were compared and when LT02 (dark-gray bar) and LT06 were compared. Statistical analysis also revealed a significant difference in C3 detection between V583 and LT02 (P < 0.05). The error bars represent standard errors for three replicates. Approximately 50,000 bacteria were analyzed for each replicate.
LTA and capsule.
LTA and peptidoglycan are PAMPs present on E. faecalis that are known to stimulate the immune system through PRRs, including TLR-2 (35). The capsules produced by other bacteria shield PAMPs, resulting in altered cytokine production (30). We examined differences in LTA accessibility between encapsulated and unencapsulated strains by slide agglutination assays. E. faecalis serotype A antiserum is directed against enterococcal LTA (39). We tested the abilities of these antibodies to agglutinate either encapsulated or unencapsulated E. faecalis strains. The encapsulated strains V583 (serotype C) and LT02 (ΔcpsF; serotype D) were not agglutinated by the antiserum, whereas the unencapsulated strains LT06 (ΔcpsC) and 12030 (the serotype A reference strain) were both agglutinated (data not shown).
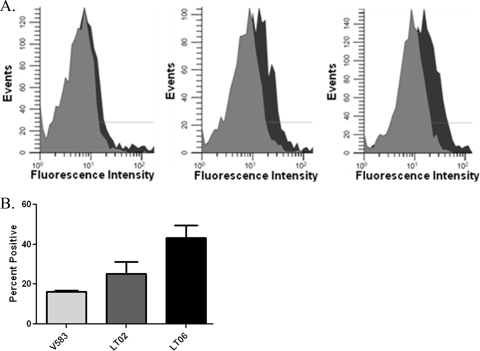
As agglutinating antibodies are generally of the IgM class, we also used flow cytometry to quantify the differences in LTA availability to the IgG class. Strains V583, LT02, and LT06 were incubated with serotype A antiserum, followed by a FITC-conjugated secondary antibody. Figure 4A and B show the percentages of the cells that were positive for FITC labeling. One-way ANOVA, followed by a Newman-Keuls post hoc test, showed significant statistical differences (P < 0.05) between V583 (serotype C) and LT06 (capsule−), and also between LT02 (serotype D) and LT06, in the amounts of LTA detected. However, there was no significant statistical difference when the encapsulated strains V583 and LT02 were compared. These data indicate that capsule produced by either serotype C or D strains masks LTA from antibody detection.
FIG. 4.
The presence of capsule masks LTA from detection by antibodies. Flow cytometry was used, in conjunction with LTA antiserum and FITC-conjugated secondary antibodies, to evaluate the levels of LTA accessibility. (A) Representative histograms depicting (from left to right) flow cytometry results for serotype C (V583), seroypte D (LT02), and unencapsulated (LT06) E. faecalis strains. The isotype controls are light gray, and the anti-LTA antibody-treated cells are dark gray. (B) Quantification of LTA detection by flow cytometry. Statistical analysis of three replicates using one-way ANOVA, in conjunction with a Newman-Keuls posttest, showed significant differences (P < 0.05) between the amounts of LTA detected in V583 (light-gray bar) and LT06 (black bar) and between LT02 (dark-gray bar) and LT06, with P values of less than 0.05. However, there was no statistical difference in LTA detection when LT02 was compared V583. The error bars represent standard errors for three replicates. Approximately 50,000 bacteria were analyzed for each replicate.
TNF-α production in response to capsule.
The presence of a capsule is known to alter the macrophage cytokine response in other microorganisms (10). To examine this possibility in E. faecalis, we used ELISA to assess the abilities of capsule-producing and nonproducing strains to induce TNF-α production by RAW 264.7 cells. We predicted that the ability of capsule to inhibit detection of LTA (Fig. 4) would translate into less TNF-α production by RAW 264.7 cells. The capsule-producing strains T-5 (serotype D), V583 (serotype C), and LT02 (serotype D), along with the unencapsulated strains (LT06, 12030, and OG1RF), were heat killed and incubated with RAW 264.7 cells. The clarified supernatants were collected at 4 h postinoculation and analyzed for TNF-α production. The amount of TNF-α produced in response to the unencapsulated strains was significantly greater than that produced in response to encapsulated strains, with P values of <0.05 using one-way ANOVA and a Newman-Keuls post hoc test analysis (Fig. 5). However, there was no statistically significant difference when the encapsulated strains were compared with each other or when the unencapsulated strains were compared with each other. Strikingly, there was no statistically significant difference in the amounts of TNF-α produced by RAW cells when the strains T-5, V583, and LT02 were compared to the uninduced RAW control cells.
FIG. 5.
E. faecalis capsule reduces TNF-α production by RAW 264.7 cells. Macrophage-like RAW 264.7 cells were incubated with serotype C (V583), serotype D (T-5 and LT02), and unencapsulated (LT06, 12030, and OG1RF) E. faecalis strains. The supernatants were collected and analyzed by ELISA for TNF-α content. The results show TNF-α production by RAW 264.7 cells in the presence of each strain. Statistical analysis of three replicates using one-way ANOVA and a Newman-Kuels post hoc test showed significant differences between the amounts of TNF-α produced in response to T-5, V583, and LT02 and those produced in response to LT06, 12030, and OG1RF. Interestingly, there was no statistically significant difference between the amounts of TNF-α produced by uninduced RAW cells and the three encapsulated strains. The error bars represent standard errors for three replicate experiments.
DISCUSSION
Capsular polysaccharides contribute to the virulence of microorganisms through multiple mechanisms, including resistance to opsonophagocytosis and masking of bacterial surface antigens from detection by the host immune system (1, 9). Several gram-positive cocci, including S. aureus (26), S. pneumoniae (1), and group B streptococci (4), produce capsular polysaccharides that are known to contribute to virulence. Previous reports have indicated that E. faecalis strains can be classified by the presence or absence of capsular polysaccharide (11, 15, 16, 40). Hancock and Gilmore (11), using a murine infection model, showed that the presence of capsule enhances persistence at infectious sites and subsequently showed that encapsulation protects the bacteria from being killed by neutrophils, whereas an unencapsulated isogenic mutant was readily killed. The killing of the unencapsulated mutant by neutrophils was dependent on the opsonic activity of complement.
Here, we demonstrate that E. faecalis capsular polysaccharide serotypes C and D provide resistance to complement-opsonized phagocytosis by macrophages. In good agreement with previously reported work on the role of the E. faecalis capsule in affecting resistance to opsonic killing by neutrophils (11, 16), we observed a 50% reduction in phagocytosis in encapsulated strains compared to the isogenic acapsular mutant. An additional cell wall polysaccharide in E. faecalis called Epa has also been shown to contribute to resistance to phagocytic killing (37) and may explain why the protective effect of the capsule is not more substantial in E. faecalis. Unlike the capsule, the Epa polymer and its genetic locus appear to be highly conserved in E. faecalis (10, 37). However, a direct comparison of the relative contributions of Cps and Epa in the same strain background has not been possible because the OG1RF strain in which Epa mutants were created lacks the capsule locus (14, 40), and we have been unable to generate Epa mutants in encapsulated strain backgrounds (L. R. Thurlow, unpublished data). A recent report by Teng et al. (38) demonstrated gross changes in the bacterial-cell shape of Epa mutants in the OG1RF background, and this may account for our inability to generate such mutants in our encapsulated strains and may partially explain the pleiotropic affects ascribed to the Epa locus in virulence studies (38, 43).
An additional benefit of the macrophage system is the use of cultured cells, which are less likely to vary from experiment to experiment than the neutrophil assay, which requires fresh isolation of neutrophils from human blood donors. Furthermore, because the strains used in this comparative study were isogenic derivatives, we could make a direct assessment of the roles of capsule and serotype differences in host immune evasion, as has been observed in other microbial pathogens (28, 32, 42, 43). Our findings show that E. faecalis capsular polysaccharides alter the detection of C3 and LTA by antibodies (Fig. 3 and 4). Paralleling these findings, we also demonstrated that the presence of capsule abrogates TNF-α production by macrophages (Fig. 5). Together, these data provide a mechanism by which the presence of capsule alters complement-mediated opsonophagocytosis by altering the accessibility of the bound C3b opsonin and by altering the production of TNF-α in response to encapsulated E. faecalis.
It is noteworthy that capsule serotype differences in an isogenic background did not result in significant changes in resistance to opsonin-mediated phagocytosis or in altered TNF-α response. McBride et al. (19) recently showed that clinical isolates of E. faecalis possessing multiple virulence factors, as well as multidrug resistance, were more likely to be identified as capsule serotype C. Our findings suggest that either of the encapsulated serotypes (C or D) benefits the bacterium in evasion of the host innate response. However, we did observe a significant difference in the amount of bound C3 detectable on the surface of isogenic serotype C compared with serotype D capsule, but this difference did not correlate with changes in the phagocytic indexes of these strains, leaving open the question of why the more pathogenic and drug-resistant clinical isolates are more frequently identified as serotype C as opposed to D. In S. aureus, comparison of the contributions of type 5 and type 8 capsule in the same strain background revealed that the presence of N-acetylation on the type 5 capsule structure conferred a fitness advantage in vivo (41). Whether a similar affect will also be observed in the comparison of E. faecalis serotype C and D strains in vivo will be the focus of future studies.
Aside from antiphagocytic properties, bacterial capsules also act as barriers that limit detection of PAMPs by PRRs (1, 9). A common PAMP shared by all strains of enterococci is LTA. The LTA of E. faecalis is known to stimulate TNF-α production via TLR-2. Although it is not fully understood, TNF-α is thought to play a key role in E. faecalis-mediated inflammatory responses (3, 26). A study involving Enterococcus faecium, which produces LTA that is serologically identical to that of E. faecalis, showed that TLR-2-mediated signaling was critical for early immune response and clearance of E. faecium (17). Based on these studies, recognition of enterococcal LTA and/or peptidoglycan by TLR-2 appears to be critical for an efficient host immune response, and the masking of these integral wall components by capsule could result in increased pathogenesis by limiting the host response to the organism. Interestingly, a study by Kau et al. (16) demonstrated that the response to E. faecalis in a urinary tract infection model is not TLR-2 dependent. The capsule phenotype of the clinical isolate used in this study is not known, and our finding that the presence of the capsule alters recognition of an important PAMP (LTA) known to be recognized by TLR-2 suggests that TLR-2 signaling might be of benefit only against E. faecalis strains that lack capsule.
Our goal was to understand the mechanism by which encapsulation enhances the resistance of E. faecalis to innate immunity. Taken together, our results show that two capsule serotypes produced by E. faecalis can subvert host innate immune responses by conferring resistance to complement-mediated phagocytosis, as well as altering the innate response to the pathogen. This study provides mechanistic evidence demonstrating that the E. faecalis capsule alters the accessibility of bound C3, supporting the observation that the most pathogenic lineages of E. faecalis are encapsulated (19, 39). By masking PAMPs on the surface of E. faecalis, the capsule also alters the host response to infection by encapsulated strains. It is our contention that the capsule produced by E. faecalis serotypes C and D is an important virulence determinant that plays multifaceted roles in evasion of host innate immune responses. Because of this, the E. faecalis capsule could serve as a target for developing future therapeutics.
Acknowledgments
We thank Sara Hoffman and Tiffany Moses (Kansas State University) for their help with cell culture. We also thank Johannes Huebner (University Medical Center Freiburg, Freiburg, Germany) for his generous donation of serotype A antiserum.
This study was supported by NIH grant RR-P20 RR017686 from the IDeA Program of the National Center for Research Resources (L.E.H. and S.D.F.), NIH grant AI061691 (S.D.F.), and a grant-in-aid from the Terry C. Johnson Cancer Center at Kansas State University (V.C.T.).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Abeyta, M., G. G. Hardy, and J. Yother. 2003. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect. Immun. 71:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arduino, R. C., B. E. Murray, and R. M. Rakita. 1994. Roles of antibodies and complement in phagocytic killing of enterococci. Infect. Immun. 62:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baik, J. E., Y. H. Ryu, J. Y. Han, J. Im, K. Y. Kum, C. H. Yun, K. Lee, and S. H. Han. 2008. Lipoteichoic acid partially contributes to the inflammatory responses to Enterococcus faecalis. J. Endod. 34:975-982. [DOI] [PubMed] [Google Scholar]
- 4.Clewell, D. B., P. K. Tomich, M. C. Gawron-Burke, A. E. Franke, Y. Yagi, and F. Y. An. 1982. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J. Bacteriol. 152:1220-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn, P. S., C. M. Pillar, B. D. Jett, W. Haas, and M. S. Gilmore. 2004. Enterococcus faecalis senses target cells and in response expresses cytolysin. Science 306:2270-2272. [DOI] [PubMed] [Google Scholar]
- 6.Cunnion, K. M., H. M. Zhang, and M. M. Frank. 2003. Availability of complement bound to Staphylococcus aureus to interact with membrane complement receptors influences efficiency of phagocytosis. Infect. Immun. 71:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drevets, D. A., and P. A. Campbell. 1991. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J. Immunol. Methods 142:31-38. [DOI] [PubMed] [Google Scholar]
- 8.Drevets, D. A., B. P. Canono, P. J. Leenen, and P. A. Campbell. 1994. Gentamicin kills intracellular Listeria monocytogenes. Infect. Immun. 62:2222-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graveline, R., M. Segura, D. Radzioch, and M. Gottschalk. 2007. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 19:375-389. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, L. E., and M. S. Gilmore. 2002. The capsular polysaccharide of Enterococcus faecalis and its relationship to other polysaccharides in the cell wall. Proc. Natl. Acad. Sci. USA 99:1574-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock, L. E., and M. S. Gilmore. 2000. Pathogenicity of enterococci, p. 251-258. In V. Fischetti, R. Novick, J. Ferretti, D. Portnoy, and J. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, DC.
- 12.Hancock, L. E., B. D. Shepard, and M. S. Gilmore. 2003. Molecular analysis of the Enterococcus faecalis serotype 2 polysaccharide determinant. J. Bacteriol. 185:4393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huebner, J., Y. Wang, W. A. Krueger, L. C. Madoff, G. Martirosian, S. Boisot, D. A. Goldmann, D. L. Kasper, A. O. Tzianabos, and G. B. Pier. 1999. Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect. Immun. 67:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hufnagel, M., L. E. Hancock, S. Koch, C. Theilacker, M. S. Gilmore, and J. Huebner. 2004. Serological and genetic diversity of capsular polysaccharides in Enterococcus faecalis. J. Clin. Microbiol. 42:2548-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hufnagel, M., A. Kropec, C. Theilacker, and J. Huebner. 2005. Naturally acquired antibodies against four Enterococcus faecalis capsular polysaccharides in healthy human sera. Clin. Diagn. Lab. Immunol. 12:930-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kau, A. L., S. M. Martin, W. Lyon, E. Hayes, M. G. Caparon, and S. J. Hultgren. 2005. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73:2461-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leendertse, M., R. J. Willems, I. A. Giebelen, P. S. van den Pangaart, W. J. Wiersinga, A. F. de Vos, S. Florquin, M. J. Bonten, and T. van der Poll. 2008. TLR2-dependent MyD88 signaling contributes to early host defense in murine Enterococcus faecium peritonitis. J. Immunol. 180:4865-4874. [DOI] [PubMed] [Google Scholar]
- 18.Maekawa, S., M. Yoshioka, and Y. Kumamoto. 1992. Proposal of a new scheme for the serological typing of Enterococcus faecalis strains. Microbiol. Immunol. 36:671-681. [DOI] [PubMed] [Google Scholar]
- 19.McBride, S. M., V. A. Fischetti, D. J. Leblanc, R. C. Moellering, Jr., and M. S. Gilmore. 2007. Genetic diversity among Enterococcus faecalis. PLoS ONE 2:e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melin, M., H. Jarva, L. Siira, S. Meri, H. Kayhty, and M. Vakevainen. 2009. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect. Immun. 77:676-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikerov, A. N., T. M. Umstead, X. Gan, W. Huang, X. Guo, G. Wang, D. S. Phelps, and J. Floros. 2008. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am. J. Physiol. Lung Cell. Mol. Physiol. 294:L121-L130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki, S., A. Ohno, I. Kobayashi, T. Uji, K. Yamaguchi, and S. Goto. 1993. Cytotoxic effect of hemolytic culture supernatant from Enterococcus faecalis on mouse polymorphonuclear neutrophils and macrophages. Microbiol. Immunol. 37:265-270. [DOI] [PubMed] [Google Scholar]
- 23.Murray, B. E., K. V. Singh, R. P. Ross, J. D. Heath, G. M. Dunny, and G. M. Weinstock. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieto, C., and M. Espinosa. 2003. Construction of the mobilizable plasmid pMV158GFP, a derivative of pMV158 that carries the gene encoding the green fluorescent protein. Plasmid 49:281-285. [DOI] [PubMed] [Google Scholar]
- 25.O'Riordan, K., and J. C. Lee. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17:218-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papasian, C. J., R. Silverstein, J. J. Gao, D. M. Bamberger, and D. C. Morrison. 2002. Anomalous role of tumor necrosis factor alpha in experimental enterococcal infection. Infect. Immun. 70:6628-6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, S. Y., K. M. Kim, J. H. Lee, S. J. Seo, and I. H. Lee. 2007. Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum. Infect. Immun. 75:1861-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson, P. K., B. J. Wilkinson, Y. Kim, D. Schmeling, and P. G. Quie. 1978. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 19:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popi, A. F., J. D. Lopes, and M. Mariano. 2004. Interleukin-10 secreted by B-1 cells modulates the phagocytic activity of murine macrophages in vitro. Immunology 113:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffatellu, M., D. Chessa, R. P. Wilson, R. Dusold, S. Rubino, and A. J. Baumler. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 73:3367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 2000. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21:510-515. [DOI] [PubMed] [Google Scholar]
- 32.Rubens, C. E., L. M. Heggen, R. F. Haft, and M. R. Wessels. 1993. Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol. Microbiol. 8:843-855. [DOI] [PubMed] [Google Scholar]
- 33.Sahm, D. F., J. Kissinger, M. S. Gilmore, P. R. Murray, R. Mulder, J. Solliday, and B. Clarke. 1989. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 33:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahu, A., and J. D. Lambris. 2001. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 180:35-48. [DOI] [PubMed] [Google Scholar]
- 35.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 36.Stollerman, G. H., and J. B. Dale. 2008. The importance of the group A Streptococcus capsule in the pathogenesis of human infections: a historical perspective. Clin. Infect. Dis. 46:1038-1045. [DOI] [PubMed] [Google Scholar]
- 37.Teng, F., K. D. Jacques-Palaz, G. M. Weinstock, and B. E. Murray. 2002. Evidence that the enterococcal polysaccharide antigen gene (epa) cluster is widespread in Enterococcus faecalis and influences resistance to phagocytic killing of E. faecalis. Infect. Immun. 70:2010-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng, F., K. V. Singh, A. Bourgogne, J. Zeng, and B. E. Murray. 2009. Further characterization of the epa gene cluster and Epa polysaccharides of Enterococcus faecalis. Infect. Immun. 77:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theilacker, C., Z. Kaczynski, A. Kropec, F. Fabretti, T. Sange, O. Holst, and J. Huebner. 2006. Opsonic antibodies to Enterococcus faecalis strain 12030 are directed against lipoteichoic acid. Infect. Immun. 74:5703-5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thurlow, L., V. C. Thomas, and L. E. Hancock. 2009. Capsular polysaccharide production in Enterococcus faecalis and the contribution of CpsF to capsule serospecificity. J. Bacteriol. 191:6203-6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watts, A., D. Ke, Q. Wang, A. Pillay, A. Nicholson-Weller, and J. C. Lee. 2005. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect. Immun. 73:3502-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wessels, M. R., A. E. Moses, J. B. Goldberg, and T. J. DiCesare. 1991. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc. Natl. Acad. Sci. USA 88:8317-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng, J., F. Teng, G. M. Weinstock, and B. E. Murray. 2004. Translocation of Enterococcus faecalis strains across a monolayer of polarized human enterocyte-like T84 cells. J. Clin. Microbiol. 42:1149-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]