Abstract
Recent findings have implicated interleukin-1β (IL-1β) as an important mediator of the inflammatory response in the female genital tract during chlamydial infection. But how IL-1β is produced and its specific role in infection and pathology are unclear. Therefore, our goal was to determine the functional consequences and cellular sources of IL-1β expression during a chlamydial genital infection. In the present study, IL-1β−/− mice exhibited delayed chlamydial clearance and decreased frequency of hydrosalpinx compared to wild-type (WT) mice, implying an important role for IL-1β both in the clearance of infection and in the mediation of oviduct pathology. At the peak of IL-1β secretion in WT mice, the major producers of IL-1β in vivo are F4/80+ macrophages and GR-1+ neutrophils, but not CD45− epithelial cells. Although elicited mouse macrophages infected with Chlamydia muridarum in vitro secrete minimal IL-1β, in vitro prestimulation of macrophages by Toll-like receptor (TLR) ligands such as lipopolysaccharide (LPS) purified from Escherichia coli or C. trachomatis L2 prior to infection greatly enhanced secretion of IL-1β from these cells. By using LPS-primed macrophages as a model system, it was determined that IL-1β secretion was dependent on caspase-1, potassium efflux, and the activity of serine proteases. Significantly, chlamydia-induced IL-1β secretion in macrophages required bacterial viability but not growth. Our findings demonstrate that IL-1β secreted by macrophages and neutrophils has important effects in vivo during chlamydial infection. Additionally, prestimulation of macrophages by chlamydial TLR ligands may account for the elevated levels of pro-IL-1β mRNA observed in vivo in this cell type.
The obligate intracellular pathogen Chlamydia trachomatis is the most common cause of sexually transmitted infection worldwide. Infection can lead to oviduct pathology and infertility in females. Cells respond to infection with C. trachomatis by upregulating a wide assortment of genes, including those for proinflammatory cytokines such as tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-6 (34, 54, 55, 68). The cellular paradigm of chlamydial pathogenesis states that tissue damage resulting from chlamydial infection is caused by excessive production of these proinflammatory cytokines (61). In support of this theory, caspase-1 knockout (KO) and Toll-like receptor 2 (TLR2) KO mice exhibit less upper genital tract pathology than wild-type (WT) mice, despite equivalent courses of infection (12, 15). This finding is noteworthy because caspase-1 KO mice lack the protease required to activate IL-1β (32) and TLR2 KO fibroblasts express less mRNA for IL-1β and lower levels of other inflammatory cytokines than WT fibroblasts during in vitro chlamydial infections (15). Additionally, administration of an IL-1β antagonist prevents C. trachomatis-induced cytotoxicity in a human fallopian tube organ culture model (30). Overall, these observations suggest that IL-1β may be a potentially important factor in the development of oviduct pathology, underscoring the need to define the role of IL-1β during in vivo chlamydial infections and to mechanistically determine how the IL-1β-converting enzyme caspase-1 is activated upon contact with Chlamydia spp. to produce mature IL-1β.
Caspase-1 is the central component of the host “inflammasome” (reviewed in reference 38) and exists at rest as a 45-kDa zymogen. When activated by proteolysis, it forms a tetramer consisting of two 10- to 20-kDa heterodimers (65), with the 20-kDa portion containing the active-site cysteine necessary to cleave the cytokine precursors pro-IL-1β and pro-IL-18 and also the TH2 cytokine precursor pro-IL-33 (10, 24, 59, 63). Caspase-1 has many other substrates in the cell (60), but its two main targets relevant to inflammation are IL-1β (10) and IL-18 (24). Both IL-1β and IL-18 require this processing for biologic activity. IL-18 plays a role in stimulating gamma interferon production from T cells (18, 56) and natural killer cells (11), suggesting a protective role for IL-18 during genital chlamydial infection. This leads to the theory that the detrimental role of caspase-1 for oviduct pathology during chlamydial infection is mediated mainly via overproduction of the proinflammatory cytokine IL-1β.
Several studies have shown that caspase-1 activation during intracellular bacterial infection involves active contribution from the bacteria. For instance, activation occurs following bacterial type III secretion (T3S)-dependent introduction into the cytosol of Yersinia pestis YopJ (35), Pseudomonas aeruginosa flagellin (42), or Salmonella enterica flagellin (20). Early work examining the role of caspase-1 during chlamydial infection by using HeLa cells demonstrated that the activation of caspase-1 late in the infection cycle (36 to 48 h postinfection) is dependent on both chlamydial viability and protein synthesis (36). Additionally, the T3S antagonist INP0007 prevents activation of caspase-1 in C. trachomatis-infected HeLa cells (66). Low-level production of IL-1β was abolished in caspase-1 KO mouse peritoneal macrophages infected in vitro with mouse chlamydia C. muridarum (12), which can replicate effectively in mouse macrophages (46). However, it is not known if the infected epithelial cells or the inflammatory cells recruited to the site of infection are the main producers of IL-1β during an in vivo genital infection. Furthermore, the specific role for IL-1β during in vivo genital infection has not been addressed. In this study, we show that IL-1β is required for optimal chlamydial clearance but also contributes to the development of genital tract pathology. Additionally, macrophages and neutrophils and not epithelial cells account for the high expression levels of IL-1β in vivo. We further describe mechanistic details of IL-1β production by using in vitro infections of lipopolysaccharide (LPS)-primed macrophages. Overall, these macrophages produced IL-1β by both caspase-1- and serine protease-dependent mechanisms, but surprisingly, IL-β secretion by these cells occurred in a bacterial protein synthesis-independent manner, although chlamydial viability was required.
MATERIALS AND METHODS
Chlamydial stocks and cell lines.
The C. muridarum Nigg strain was propagated in Mycoplasma sp.-free McCoy cells grown in Dulbecco's modified Eagle medium supplemented with 100 μM nonessential amino acids (Invitrogen), 2 mM l-glutamine (Invitrogen), 10% fetal bovine serum, 50 mg/ml gentamicin sulfate, and 0.5 mg/ml cycloheximide. Infectious elementary bodies (EBs) were isolated from McCoy cells by sonication, washed in phosphate-buffered saline (PBS), resuspended in SPG buffer (250 mM sucrose, 10 mM sodium phosphate, and 5 mM l-glutamic acid, pH 7.2), and stored at −80°C.
Chemicals and reagents.
The TLR ligands Pam3CSK4 (1 mg/ml), Escherichia coli LPS (1 μg/ml), and poly(I:C) (2.5 mg/ml) were purchased from Invivogen and reconstituted with endotoxin-free water. Chlamydial LPS was purified from C. trachomatis serovar L2 as described previously (58) and reconstituted at 500 μg/ml. Potassium chloride (1 M), glycine (1 M; Fisher Chemicals), and Nα-tosyl-phenylalanine chloromethyl ketone (TPCK [10 mM; Calbiochem]) were dissolved in Millipore water and stored at 4°C. Stock rifampin (rifampicin [30 mg/ml; Fisher Scientific]), INP0007 (10 mM; Chembridge), caspase-1 inhibitor I (Ac-YVAD-CHO [10 mM; Calbiochem]), MG-132 (10 mM; Calbiochem), and cytochalasin D (3 mg/ml; Sigma) were reconstituted in dimethyl sulfoxide (DMSO) and stored at −20°C. The enzyme apyrase and ATP (Sigma) were dissolved in endotoxin-free water at 0.5 U/μl and 100 mM, respectively, and stored at −20°C.
Mice and intravaginal infections.
IL-1β KO mice backcrossed in the B10.RIII background (35, 29) were provided as a gift from Wee Yong (University of Calgary, Canada). For experiments using IL-1β KOs, B10.RIII mice (Jackson Laboratories, Bar Harbor, ME) were used as controls. The remainder of experiments used C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME). Seven days prior to infection, each mouse received 2.5 mg of Depo-Provera subcutaneously in 100 μl of PBS. A week later, mice were anesthetized with pentobarbital sodium (Nembutal) and infected by administration of either 107 inclusion-forming units (IFU; for infection course/pathology experiments) or 3 × 106 IFU (for fluorescence-activated cell sorting experiments) of C. muridarum in 10 μl SPG buffer directly into the vaginal vault. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences.
IFU enumeration and specimen collection.
Chlamydial shedding was evaluated by swabbing the cervices and vaginal vaults of infected mice with a calcium alginate swab (Spectrum Medical Instruments, Los Angeles, CA) at various times postinfection. Numbers of IFU were calculated by reinfecting a fresh McCoy cell monolayer in 96-well black plates. Inclusions were visualized with Alexa Fluor 488-conjugated anti-mouse immunoglobulin G (Southern Biotechnology) and a mouse monoclonal antibody (EVI H1) recognizing chlamydial LPS (14). Chlamydial inclusions were then counted using an Olympus fluorescence microscope to calculate the number of IFU per milliliter in each sample. Additionally, genital secretions were collected 1 day prior to infection (day 0) and also throughout the duration of infection and processed for cytokine analysis as described previously (15). Briefly, mice were anesthetized with pentobarbital sodium, and absorbent sponges were placed into the vaginas for 30 min. Sponges were removed and stored at −70°C until further processing. Cytokines were eluted from the sponges with a solution containing PBS, 0.5% bovine serum albumin, and 0.05% Tween 20.
Histopathology.
At the conclusion of the study, mice were sacrificed at day 44 postinfection and the genital tracts were excised and fixed in 10% buffered formalin. The tissues were then embedded in paraffin. Longitudinal sections (4 μm) were stained with hematoxylin and eosin and evaluated using a previously elaborated scoring system (15) by a pathologist blinded to the experimental design.
Isolation of genital tract cells, flow cytometry analysis, and sorting.
On day 4 postinfection, infected mice were sacrificed and the lower genital tract in each animal, from the cervix to the beginning of the horns, was excised. The tissues were minced and incubated individually in collagenase I (5 mg/ml; Sigma) for 30 min at 37°C and then passed through a 70-μm nylon mesh filter. Cells from all the mice were then pooled, resuspended in RPMI medium, and treated with 900 μg/ml RNase A-200 μg/ml DNase I (Sigma) for 30 min at room temperature before being passed through a second nylon filter. Red cells were lysed by resuspension of the cell pellet in a red blood cell lysis solution (17 mM Tris, pH 7.4, and 140 mM NH4Cl) for 5 min at 37°C, followed by neutralization with RPMI medium. Single-cell suspensions (107 cells) in PBS supplemented with 0.5% bovine serum albumin and 2 mM EDTA were incubated with an Fc blocking agent (clone 2.4G2 [5 μg/ml; BD Pharmingen]) for 10 min at 4°C. The incubation was followed by staining for 20 min on ice with Ly-6G-fluorescein isothiocyanate antibody (clone RB6-8C5; BD Pharmingen), allophycocyanin-conjugated F4/80 antibody (clone BM8; Biolegend), and peridinin chlorophyll protein-Cy5.5-conjugated CD45 antibody (clone 30-F11; BD Pharmingen), each at a titer of 0.25 μg/106 cells. As the Ly-6G (clone RB6-8C5) antibody stains Gr-1 antigen expressed on both neutrophils and macrophages, macrophages were identified as CD45+ F4/80+ Gr-1+ cells and neutrophils were identified as CD45+ F4/80− GR-1+ cells. Cells were analyzed, and gated populations were sorted into RPMI medium supplemented with 10% fetal bovine serum by using a BD-FACSAria cell sorter. The sorted subsets were reanalyzed on the flow cytometer to determine the purity of each population. Analysis of flow cytometry data was carried out using FlowJo software (Tree Star, Inc.). For subsequent analysis of IL-1β expression in the sorted cells, each cell population was enumerated and divided equally for RNA and protein analyses. For IL-1β protein analysis, the cells were treated with lysis buffer (10 mM Tris, pH 8.0, 150 mM NaCl, and 1% NP-40) and the resulting cell lysates were evaluated by an enzyme-linked immunosorbent assay (ELISA).
Prestimulation of macrophages and in vitro infections.
Peritoneal macrophages were isolated from 8- to 12-week-old female C57BL/6J mice (Jackson Laboratory) 3 days after intraperitoneal injection with 1 ml of 3% thioglycolate, and the cells were cultured as described previously (46, 52). For studies using prestimulation, a TLR ligand [100 ng/ml E. coli LPS, 25 μg/ml poly(I:C), or 2 μg/ml Pam3CSK4] was added to the culture 6 h prior to infection. For all studies utilizing pharmacologic compounds such as Ac-YVAD-CHO (50 μM), TPCK (50 μM), rifampin (150 μg/ml), INP0007 (50 μM), cytochalasin D (0.3 to 3.0 μg/ml), and MG-132 (10 μM), the macrophages were pretreated for 30 min prior to exposure to C. muridarum. After C. muridarum was added at a multiplicity of infection of 1, cells were centrifuged at 1,690 × g and 37°C for 1 h. Next, the medium was aspirated and replaced with fresh complete medium. Importantly, pharmacologic agents were added back into the culture medium while prestimulating ligands such as LPS and poly(I:C) were not. For experiments examining the role of cellular potassium efflux, 130 mM KCl was added to the medium to eliminate the concentration gradient across the plasma membrane for this ion. For all infections utilizing prestimulated macrophages, supernatants were collected 18 h postinfection and stored at −80°C until further analysis. To confirm that the cells were infected, macrophages in wells containing glass coverslips were fixed with methanol at 24 h postinfection for 30 min at room temperature and either stained with the fluorescein isothiocyanate-conjugated Pathfinder antichlamydial monoclonal antibody (Bio-Rad) or processed for IFU enumeration on a fresh McCoy monolayer (7). For ATP-induced caspase-1 activation, cells pretreated for 6 h with LPS were pulsed for 20 min with 5 mM ATP. Supernatants were collected 2 h after the withdrawal of ATP and the addition of fresh culture medium.
Preparation of lysates from HeLa cells infected with C. muridarum and macrophage priming.
Confluent HeLa cell monolayers were infected with C. muridarum at a multiplicity of infection of 5. At 16 h postinfection, cells were trypsinized and resuspended in SPG buffer at a concentration of 2.5 × 106 cells/ml. In parallel, suspensions of uninfected HeLa cells at the same density were also collected. Both sets of cell suspensions were sonicated and then aliquoted and stored at −70°C. Prior to macrophage priming, these lysates were thawed and sonicated again. The aliquot of infected lysates was then heat treated for 30 min at 60°C to kill any infectious C. muridarum. For priming, 106 macrophages were incubated for 6 h with lysates derived from 2.5 × 104 or 2.5 × 105 HeLa cells. After 6 h, the cell supernatants were removed and one set of cells was processed for RNA analysis to measure pro-IL-1β mRNA levels. Another set was infected with C. muridarum in fresh medium as described previously. Chloramphenicol (100 μg/ml) was present for the duration of priming but not during the subsequent chlamydial infection.
RNA extraction and real-time PCR analysis.
RNA was isolated from the sorted cells or macrophage monolayer by using the RNeasy kit (Qiagen). RNA samples (1 μg) were treated with 1.0 U of RNase-free DNase I (Promega) for 30 min at 37°C and then subjected to inactivation for 10 min at 70°C. Subsequently, 200 ng of RNA was reverse transcribed with SuperScript III enzyme according to the instructions of the manufacturer (Invitrogen) by using random hexamers and oligo(dT) for priming. Quantitative PCR analysis of samples was performed using an iQ SYBR green mix (Bio-Rad) and an iQ5 iCycler instrument (Bio-Rad). The amount of cDNA present for each gene was determined with standard curves, and the result was normalized to the value for the β-actin housekeeping gene before analysis. All primers were designed using Beacon Design software (Bio-Rad). The following primers were used: β-actin gene, sense, GGCTATGCTCTCCCTCACG, and antisense, CGCTCGGTCAGGATCTTCAT; IL-1β gene, sense, ACAGCAGCACATCAACAAGAG, and antisense, CCAGCAGGTTATCATCATCATCC; and chlamydial rs16 gene, sense, AGACAACAAGGACGCAAGAACC, and antisense, GGATCATACCACCCTAACAACTCG.
Cytokine analysis.
The levels of IL-1β protein in genital secretions, culture supernatants, and cellular lysates were determined using an ELISA kit (MLB00B; R&D Systems). For analysis of IL-1β in genital secretions, eluates were diluted 1:10 prior to the ELISA. For analysis of IL-1β in cell lysates, values were normalized by dividing the total IL-1β protein levels by the number of cells used to make the lysates. Optical densities at 450 nm for quantification were measured using a BioTek plate reader.
Statistical analysis.
Statistical comparisons between the two mouse strains (IL-1β KO and WT) for IFU quantification over the course of infection were made by a two-way analysis of variance (ANOVA) with the post hoc Tukey test as a multiple-comparison procedure. The Fisher exact test was used for the determination of significant differences in the frequencies of hydrosalpinx observed in the two groups. For analysis of pathology scores, the Friedman repeated-measures (RM) ANOVA by ranks, followed by the Student-Newman-Keuls multiple-comparison procedure, was performed. For analysis of results from in vitro studies with multiple independent experiments, statistical significance tests were performed using SigmaStat (Systat Software Inc.). For experiments with only two groups, unpaired t tests were used. For experiments including more than two treatment groups, a one-way ANOVA with pairwise multiple comparisons (Holm-Sidak method) was performed to determine statistically significant differences (P, <0.05 or <0.01).
RESULTS
IL-1β is required for optimal chlamydial clearance and contributes to oviduct pathology in C. muridarum-infected mice.
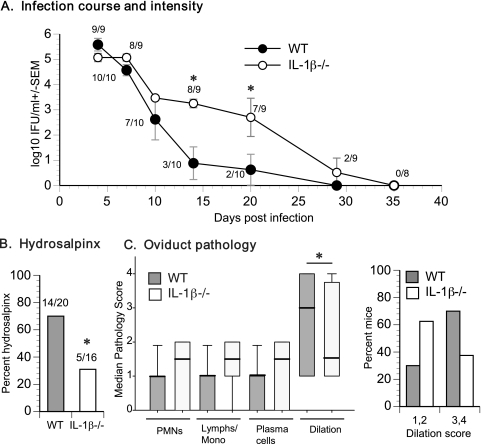
To determine whether IL-1β production plays an important role in vivo, the course of C. muridarum genital infection in WT and IL-1β KO mice was monitored. The absence of IL-1β led to delayed clearance of C. muridarum during infection (Fig. 1A). Further, the infectious burden in IL-1β KO mice was significantly greater than that in WT mice from days 7 to 21 postinfection (Fig. 1A). Despite their prolonged infection and increased bacterial burden, IL-1β KO mice exhibited decreased incidence of gross hydrosalpinx at day 44 postinfection (Fig. 1B). Histopathological examination of the genital tracts showed no significant differences in levels of acute (neutrophil), chronic (lymphocyte), or plasma cell infiltrations, but the median score for dilatation in the oviducts of IL-1β KO mice was decreased (Fig. 1C), consistent with the observation of reduced gross pathology. These results indicate that IL-1β is necessary for optimal chlamydial clearance and is a contributing factor in oviduct pathology during infection.
FIG. 1.
IL-1β deficiency delays chlamydial clearance and reduces oviduct pathology. (A) C. muridarum infection course in WT (n = 10) and IL-1β KO (n = 9) mice. Log10 numbers of IFU per milliliter were calculated as described in Materials and Methods and graphed as means ± standard errors of the means (SEM). Significance (P = 0.002) was determined by two-way RM ANOVA and is denoted by asterisks. Numbers next to datum points indicate the fraction of animals infected at the particular time point. (B) Percent hydrosalpinx among WT (n = 20) and IL-1β KO (n = 16) oviducts (2 oviducts/mouse) harvested 42 days postinfection. The asterisk indicates a significant difference (P < 0.05) in the proportion of oviducts with hydrosalpinx as determined by the Fisher exact test. (C) The median group pathology score for each category of cells from oviducts harvested from WT and IL-1β KO mice 42 days postinfection is graphed. The asterisk denotes a statistically significant difference (P < 0.05) in oviduct dilation between the two groups as determined using Friedman RM ANOVA by ranks, followed by Student-Newman-Keuls multiple-comparison procedure. The distribution of individual dilation scores is also shown. PMNs, polymorphonuclear leukocytes; lymphs/mono, lymphocytes/monocytes.
Macrophages and neutrophils are the source of IL-1β during genital chlamydial infection in vivo.
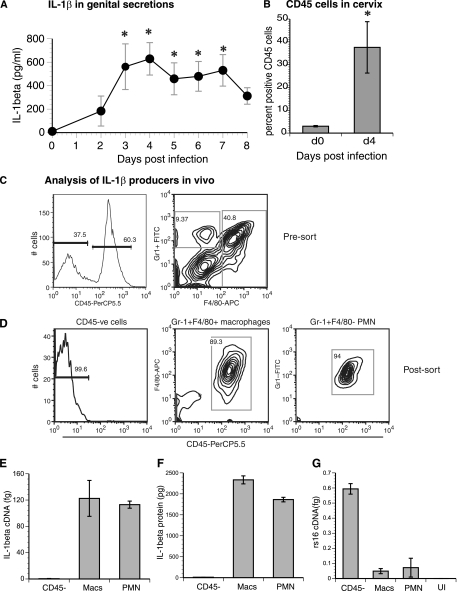
Consistent with previously published results (16), IL-1β levels in genital secretions were significantly elevated (P < 0.05) in infected mice between days 3 and 7 postinfection, peaking at day 4 (Fig. 2A). There are minimal CD45+ cells in the mouse cervix before infection (49), and basal IL-1β levels preinfection (day 0) were <10 pg/ml. Gr-1+ cells accounted for less than 1% of the population at the onset of C. muridarum infection, but the proportion of these cells steadily increased thereafter (data not shown). At the peak of IL-1β secretion (day 4 postinfection), there was a 10- to 15-fold increase in the percentage of CD45 cells entering the cervix (Fig. 2B). Also at this time point, influx of a high proportion of CD45+ F4/80+ macrophages and, to a lesser extent, CD45+ Gr-1+ neutrophils was observed (Fig. 2C). To determine if one or both of these populations or resident CD45− cells were responsible for IL-1β production, the cells were enriched by fluorescence-activated sorting into CD45+ Gr-1+ F4/80+ macrophages, CD45+ Gr-1+ F4/80− neutrophils, and CD45− cells (Fig. 2D). Overall, macrophages and neutrophils expressed approximately 200- to 250-fold more pro-IL-1β mRNA than CD45− cells (Fig. 2E). To take advantage of the fact that IL-1β can be detected intracellularly in cells infected in vitro with C. muridarum (12), lysates from each of the sorted cell populations were made. Consistent with the mRNA induction data, F4/80+ and Gr-1+ F4/80− cell lysates but not CD45− cell lysates possessed high levels of intracellular IL-1β (Fig. 2F). To determine if this expression correlates with the degree of chlamydial growth inside the cell, levels of C. muridarum rRNA gene rs16 expression were also analyzed. Among the three populations, CD45− cells showed the highest level of rs16 expression (Fig. 2G), implying that infected epithelial cells are likely included in the CD45− population. Additionally, the data also illustrated that higher IL-1β expression in macrophages and neutrophils than in epithelial cells was not due to an increased chlamydial burden in the former cells. Macrophages and neutrophils seem to produce nearly equivalent amounts of IL-1β mRNA and protein, when amounts are represented on a per-cell basis (Fig. 2E and F). However, the approximately fourfold greater number of the former cell type than of the latter in the genital tract at day 4 postinfection in this experiment indicates that macrophages are larger contributors to IL-1β expression in vivo than neutrophils at this time point.
FIG. 2.
Macrophages and neutrophils are the principal source of IL-1β in vivo. (A) Genital tract secretions from C57BL6/J mice (n = 5) infected with C. muridarum (3 × 105 IFU/mouse) were isolated and analyzed for IL-1β protein. Data are means ± standard errors of results representative of those from three independent experiments. Asterisks denote significant differences (P < 0.05 by one-way RM ANOVA) from day 0 baseline levels. (B) Percent CD45+ cells in the cervices of mice (n = 3) at day 0 (d0; preinfection) and day 4 (d4) postinfection with 3 × 105 IFU of C. muridarum/mouse. The asterisk denotes a P value of <0.05 by the t test. (C and D) Populations of CD45− epithelial cells, CD45+ Gr-1+ F4/80+ macrophages, and CD45+ Gr-1+ F4/80− neutrophils before (C) and after (D) sorting are depicted. Values represent percentages of cells within the indicated gate. The infection dose was 3 × 106 IFU/mouse. CD45-PerCP5.5, peridinin chlorophyll protein-Cy5.5 CD45 antibody; Gr1-FITC, fluorescein isothiocyanate Gr-1 antibody; F4/80-APC, allophycocyanin F4/80 antibody; CD45-ve, CD45-positive; PMN, polymorphonuclear leukocytes. (E and F) RNAs and cellular lysates from CD45−, macrophage (Macs), and neutrophil (PMN) cell populations were analyzed for IL-1β mRNA (E) and intracellular IL-1β protein (F) expression. IL-1β mRNA levels were normalized with respect to actin gene expression, while IL-1β protein levels in cell lysates were normalized with respect to the cell number (results are expressed in picograms per 105 cells). (G) The same RNA samples and RNA from an uninfected (UI) mouse cervix were assayed for chlamydial rs16 RNA. Data in panels E, F, and G are means ± standard deviations of results for samples assayed in duplicate. Results from one of two similar experiments are shown.
Prestimulation with TLR ligands, including chlamydial LPS, amplifies IL-1β secretion from C. muridarum-infected macrophages.
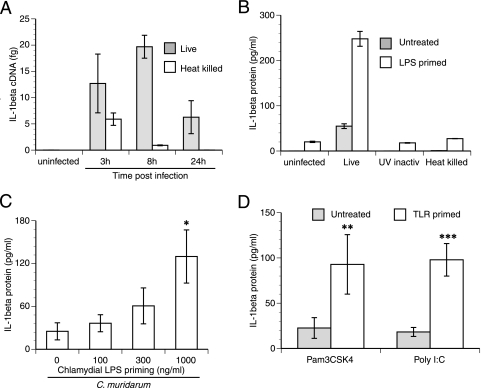
In order to address the mechanism of chlamydia-induced IL-1β secretion, infections of elicited macrophages were performed in vitro. Macrophages infected with C. muridarum in vitro induced IL-1β mRNA rapidly, within 3 h postinfection, with sustained expression until 24 h (Fig. 3A). But surprisingly, these cells secreted minimal amounts of cytokine into the supernatant (Fig. 3B). Heat-killed (Fig. 3A) and UV-inactivated (data not shown) C. muridarum cells were able to induce IL-1β mRNA until 8 h postinfection, albeit at lower levels than viable cells, but without resulting in the secretion of any IL-1β (Fig. 3B). We hypothesized that the difference in levels of IL-1β during in vitro versus in vivo infection could be attributed to prestimulation of macrophages by chlamydial TLR ligands in vivo. Prestimulating macrophages with small amounts of E. coli LPS, a TLR4 ligand, prior to infection can trigger expression of pro-IL-1β (28), providing more substrate for caspase-1 to cleave when an infection triggers its activation. This model has been effectively used to address the mechanism of caspase-1 activation during infections with Salmonella spp., Yersinia spp., and Pseudomonas spp. or following exposure to bacterial DNA or flagellin (20, 21, 35, 37, 41, 43, 51, 62). As expected, priming of macrophages with E. coli LPS prior to infection with C. muridarum amplified the secretion of IL-1β approximately 5- to 10-fold (Fig. 3B). Importantly, heat-killed and UV-inactivated C. muridarum cells were still unable to induce secretion of IL-1β by macrophages, demonstrating that viable chlamydiae are required for caspase-1 activation and IL-1β secretion. LPS pretreatment alone led to minimal IL-1β secretion (Fig. 3B). Like E. coli LPS, chlamydial LPS can signal via TLR4 (53), so it would be expected to have a similar effect on macrophage priming. Prestimulation with purified LPS isolated from C. trachomatis serovar L2 increased the amount of IL-1β secreted by infected macrophages in a dose-dependent manner (Fig. 3C), but the magnitude of the overall response was lower than that of the response to E. coli LPS. Similar to E. coli or chlamydial LPS, both the TLR3 ligand poly(I:C) and the TLR2 ligand Pam3CSK4 can induce IL-1β mRNA (data not shown). Prestimulation with both of these TLR ligands also amplified the secretion of IL-1β following infection with C. muridarum (Fig. 3D), indicating that prestimulation with any TLR ligand should be sufficient to prime the macrophage. These data suggest that during in vivo infection, prestimulation of macrophages by chlamydial TLR ligands may account for the elevated levels of pro-IL-1β mRNA observed in vivo.
FIG. 3.
TLR prestimulation of macrophages enhances chlamydia-induced IL-1β secretion. (A) Peritoneal macrophages were infected in vitro with live or heat-killed C. muridarum, and the upregulation of IL-1β mRNA at 3, 8, and 24 h postinfection was monitored. Data are mean levels of IL-1β cDNA ± standard deviations for samples assayed in duplicate in a representative experiment. (B) Macrophages were pretreated with either 100 ng/ml E. coli LPS or medium alone for 6 h before infection with viable, UV-inactivated (UV inactiv), or heat-killed C. muridarum. Data are means ± standard deviations of results for samples assayed in duplicate in a representative experiment. (C) Macrophages were prestimulated with increasing doses of C. trachomatis L2 LPS and then infected with C. muridarum as described in the legend to panel B. The asterisk denotes a significant difference (P < 0.05 by one-way ANOVA) from levels in infected, unprimed cells. (D) Peritoneal macrophages were prestimulated with either 25 μg/ml of the TLR3 ligand poly(I:C) or 2 μg/ml of the TLR2 ligand Pam3CSK4 and infected with C. muridarum. For analyses in panels B, C, and D, supernatants were harvested at 18 h postinfection and assayed for IL-1β. Double and triple asterisks denote significant differences (**, P = 0.025, and ***, P = 0.002 by an unpaired t test) between primed and unprimed cells. Data in panels C and D are means ± standard deviations of values obtained from three independent experiments.
Chlamydia-induced IL-1β secretion in macrophages is dependent on potassium chloride efflux-induced caspase-1 activation and the activity of serine proteases.
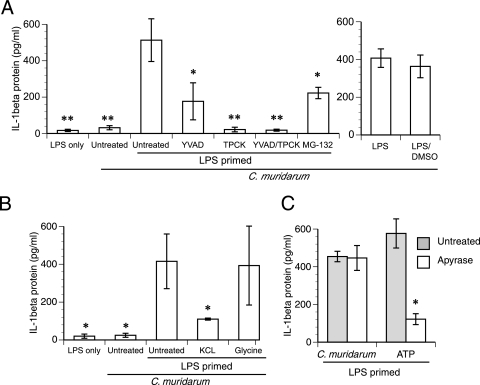
To address the mechanism of IL-1β secretion in macrophages, E. coli LPS-primed macrophages were used as a model system. Caspase-1 is the main protease responsible for IL-1β maturation following infection of unstimulated macrophages with C. muridarum (12). However, treatment with a cell-permeable caspase-1 inhibitor, Ac-YVAD-CHO, led only to approximately 70% inhibition of IL-1β secretion from LPS-primed macrophages (P < 0.05) (Fig. 4A). The fact that Ac-YVAD-CHO did not completely abolish the secretion of IL-1β suggested that there might be caspase-1-independent secretion of IL-1β from LPS-primed macrophages. Along those lines, serine proteases have been recently linked to the maturation of IL-1β in neutrophils (25). Treatment with serine protease inhibitor TPCK led to almost complete inhibition of IL-1β secretion (P < 0.01) (Fig. 4A). Since TPCK also inhibits the activity of the proteasome (2, 13), it is possible that the effect of TPCK on IL-1β maturation may be mediated at least partially through proteasome inhibition. However, treatment with specific proteasome inhibitor MG-132 did not inhibit IL-1β secretion to the same degree as TPCK, although >50% inhibition of IL-1β secretion was consistently observed (P < 0.05). These data suggest that the effect of TPCK on IL-1β secretion is likely to occur through other mechanisms in addition to proteasome inhibition (Fig. 4A). Dual treatment with YVAD and TPCK led to the greatest inhibition (P < 0.01) (Fig. 4A). LPS-primed and infected cells treated with vehicle control (DMSO) alone showed no significant difference from untreated cells in the levels of IL-1β secretion (Fig. 4A). Based on these results, IL-1β secretion appears to be dependent on the activities of serine proteases and caspase-1 in prestimulated macrophages. Further, the data suggest that a certain serine protease(s) likely functions upstream of caspase-1 activation.
FIG. 4.
IL-1β secretion from primed macrophages requires caspase-1, potassium efflux, and the activity of serine proteases. (A) E. coli LPS-prestimulated (primed) macrophages were treated with 50 μM Ac-YVAD-CHO and 50 μM TPCK individually or in tandem, 10 μM MG-132, or vehicle (DMSO) alone and then infected with C. muridarum as described in Materials and Methods. (B) E. coli LPS-prestimulated macrophages were treated with 130 mM KCl or 5 mM glycine prior to infection. Asterisks in panels A and B denote significant differences (*, P < 0.05, and **, P < 0.01 by one-way ANOVA) from results for LPS-primed macrophages infected with C. muridarum. (C) E. coli LPS-primed macrophages were treated with 5 mM ATP or infected with C. muridarum in the presence or absence of 2.5 U/ml of apyrase. The asterisk denotes a significant difference (P = 0.016 by unpaired t test) between ATP-induced IL-1β secretion levels in the presence and absence of apyrase. For all experiments, supernatants were harvested at 18 h postinfection and assayed for IL-1β. Data in all panels are means ± standard deviations of values obtained from three independent experiments.
As a significant portion of IL-1β secretion from prestimulated cells infected with C. muridarum was caspase-1 dependent, the role of caspase-1 was explored further. Potassium efflux can trigger the activation of caspase-1 via cryopyrin, a central component of the inflammasome, following activation of the pannexin channel (31, 37). Blocking this efflux by maintaining an isomolar concentration of potassium chloride in the culture medium inhibited the secretion of IL-1β to an extent similar to that achieved by the caspase-1 inhibitor (P < 0.05) (Fig. 4B), confirming the role for caspase-1. Conversely, the cytoprotectant glycine (22) had no effect on this response, indicating that cell lysis is not contributing to the release of IL-1β into the supernatant (Fig. 4B). Potassium efflux can also be triggered by the binding of ATP to the P2X7 receptor (P2X7R), leading to caspase-1 activation (37). However, the addition of an exogenous ATPase (apyrase) to the culture medium had no effect on IL-1β secretion following infection with C. muridarum (Fig. 4C), although it efficiently inhibited secretion of IL-1β from prestimulated macrophages pulsed with ATP (P = 0.016). These data indicate that during chlamydial infection, potassium efflux-dependent caspase-1 activation occurs independently of ATP binding to the P2X7R.
Chlamydia-induced IL-1β secretion in macrophages occurs independently of bacterial protein synthesis and growth.
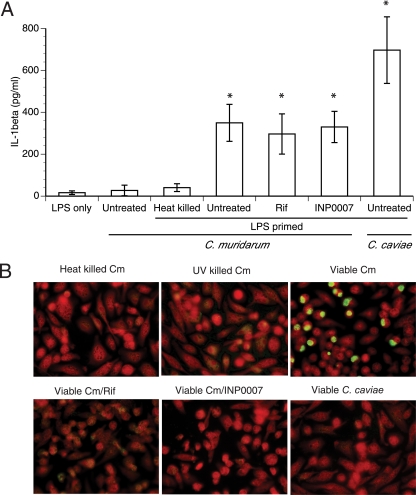
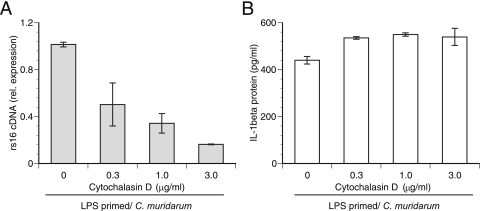
For C. trachomatis and C. muridarum, it was shown previously that caspase-1 activation correlates with the replicative phase of chlamydiae (12, 36), indicating that activation was likely a growth-dependent event. However, in prestimulated macrophages, IL-1β protein levels increased by four- to fivefold from basal levels as early as 2 h postinfection, and approximately 65% of total IL-1β secretion from these macrophages occurred in the first 6 h (data not shown); both the 2- and 6-h time points occur prior to chlamydial replication. This finding implies that under conditions in which the substrate pro-IL-1β is not limiting, infection, not growth, is the main trigger for caspase-1 activation and IL-1β secretion. Consequently, pretreatment of LPS-primed macrophages with the bacterial protein synthesis inhibitor rifampin did not decrease the secretion of IL-1β (Fig. 5A), implying that caspase-1 activation can occur independently of growth. The T3S antagonist INP0007 also prevents chlamydial growth (44, 52, 66), and similar to rifampin, it had no effect on IL-1β secretion (Fig. 5A). Growth in mouse macrophages is not universal among different chlamydial strains, so to test the hypothesis that IL-1β secretion can proceed independently of growth, LPS-primed macrophages were infected with C. caviae, which did not develop mature inclusions in mouse macrophages (Fig. 5B). As predicted, C. caviae was able to induce large amounts of IL-1β from macrophages despite this deficiency (Fig. 5A). Additionally, we have observed that inside macrophages at 2 h postinfection, C. muridarum EBs reside in endosomes while C. caviae EBs are found in phagocytic vacuoles (unpublished observation). Likewise, UV- and heat-killed bacteria are expected to end up in phagocytic vacuoles (6, 67). Since C. muridarum and C. caviae elicit similar IL-1β responses in prestimulated macrophages, cytochalasin D, an inhibitor of actin polymerization (9) and consequently the internalization of C. trachomatis (8), was added to the LPS-primed infected cells to determine the role of bacterial phagocytosis in IL-1β secretion. The addition of cytochalasin D decreased the number of chlamydial inclusions in a dose-dependent manner as visualized by using immunofluorescence (data not shown), leading to decreased chlamydial rs16 expression (Fig. 6A). However, cytochalasin D did not cause a similar decrease in IL-1β secretion following infection (Fig. 6B), suggesting that phagocytosis of chlamydiae does not play a role in chlamydia-induced IL-1β secretion. Together, these data demonstrate that although chlamydial growth is dispensable for caspase-1 activation in primed macrophages, interaction of macrophages with viable chlamydiae is necessary for IL-1β secretion, suggesting a role for growth-independent early chlamydial effector proteins in caspase-1 activation/IL-1β secretion.
FIG. 5.
IL-1β secretion from primed macrophages requires chlamydial viability but not growth. (A) E. coli LPS-primed macrophages were infected with either heat-killed C. muridarum (Cm) or viable C. muridarum or C. caviae. Additionally, certain macrophages receiving live C. muridarum were also treated with either 150 μg/ml rifampin (Rif) or 50 μM INP0007. Supernatants were harvested at 18 h postinfection and assayed for IL-1β. Data are means ± standard deviations of values obtained from three independent experiments. Asterisks denote significant differences (P < 0.05 by one-way ANOVA) from the results for LPS-only treatment. (B) Staining for chlamydial inclusions in all the treated groups described in the legend to panel A.
FIG. 6.
Inhibition of phagocytic uptake of C. muridarum does not impair IL-1β secretion. E. coli LPS-primed macrophages were infected following pretreatment with increasing doses of cytochalasin D. Chlamydial rs16 expression from cellular RNA (A) and IL-1β levels in culture supernatants (B) were assayed. Error bars in panel A represent means ± standard deviations of results for samples assayed in duplicate in a single experiment, while error bars in panel B represent means ± standard deviations of values obtained from two independent experiments.
In vitro priming of macrophages by heat-killed chlamydial RBs released from infected cells enhances IL-1β secretion.
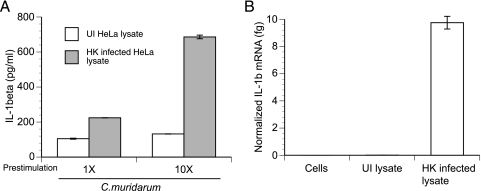
We have shown that macrophages have high levels of expression of IL-1β in vivo and that primed macrophages are potent IL-1β-secreting cells in vitro. We hypothesize that the high concentration of IL-1β detected in genital secretions (Fig. 2A) may be caused by priming of macrophages in vivo by chlamydial LPS, killed chlamydiae, or other chlamydial TLR ligands released from dying epithelial cells. In an attempt to model this phenomenon in vitro, macrophages were prestimulated with lysates from HeLa cells infected with heat-killed C. muridarum. As a control, macrophages were prestimulated under identical conditions with lysates from uninfected HeLa cells. Prestimulation with these cell lysates enriched with heat-killed reticulate-bodies (RBs) resulted in a >5-fold-higher level of IL-1β secretion than that from controls following infection with C. muridarum EBs (Fig. 7A). The amount of IL-1β secreted correlates with the amount of pro-IL-1β mRNA induced during priming (Fig. 7B). These results suggest that killed chlamydial RBs released during infection may cause prestimulation of macrophages, likely through TLR signaling.
FIG. 7.
Cellular lysates containing heat-killed chlamydial RBs are sufficient to prestimulate macrophages. Macrophages were prestimulated for 6 h with lysates from C. muridarum-infected or uninfected (UI) HeLa cells. 1X and 10X correspond to lysates derived from 2.5 × 104 and 2.5 × 105 cells, respectively. Where indicated, the lysates were subjected to heat treatment for heat killing (HK) of any viable RBs/EBs. (A) After prestimulation, macrophages were infected with C. muridarum and IL-1β protein levels were assayed at 18 h postinfection. (B) In parallel, IL-1β mRNA in macrophages was also quantitated after the initial priming step and immediately prior to infection. Error bars in panels A and B represent means ± standard deviations of results for samples assayed in duplicate.
DISCUSSION
Caspase-1 has been linked to exacerbation of upper genital tract pathology during infection with C. muridarum (12), suggesting that the caspase-1-dependent cytokine IL-1β may be a major player in disease progression. However, little is known about how much IL-1β actually contributes to the phenotype of the caspase-1 KO mice, what the cellular source of this cytokine is in vivo, or how it is made. In the present study, we have determined that IL-1β contributes to both the resolution of infection and the development of pathology following chlamydial genital infection. The major sources of this cytokine in vivo are macrophages and neutrophils. Additionally, using a model of in vitro LPS priming of mouse macrophages, we have shown that secretion of IL-1β is dependent on caspase-1, serine protease(s), and chlamydial viability but independent of chlamydial protein synthesis. We speculate that during an in vivo infection, macrophages and possibly neutrophils are primed by chlamydial TLR ligands to produce pro-IL-1β but require interaction with viable chlamydiae for IL-1β secretion.
In contrast to caspase-1 KO mice (12), IL-1β KO mice exhibit delayed clearance of C. muridarum. This discrepancy suggests that caspase-1-independent pathways may be capable of producing sufficient bioactive IL-1β in caspase-1 KO mice to control infection. Overall, the infection course in IL-1β KO mice is reminiscent of that in tumor necrosis factor receptor KO mice, which also exhibit delayed chlamydial clearance (50). Together, these results suggest that rapid clearance is facilitated by the activity of proinflammatory cytokines in recruiting neutrophils to the local site. However, this inflammation can be viewed as a double-edged sword. IL-1β KO mice actually displayed reduced severity of oviduct dilatation and decreased incidence of hydrosalpinx formation following infection compared to WT mice, demonstrating that excessive production of IL-1β by a combination of caspase-1-dependent and -independent pathways is a contributing factor in the development of pathology. These results are in agreement with recent findings that increased IL-1β levels in humans and mice result in increased Th17-dominant immunopathology (40).
Superficial epithelial cells in the cervix are the primary targets of chlamydial infection. However, we observed that the majority of IL-1β expression was limited to macrophages and neutrophils, which account for a large proportion of the CD45+ cells in the cervix at this time point. Other leukocytes tested at this time point included NK cells, but these cells were few in number and expressed minimal IL-1β (data not shown). The contribution from dendritic cells is unknown. Importantly, CD45− cells constituting the epithelial cell population showed minimal expression of IL-1β at the mRNA and protein levels. In support of this observation, no IL-1β was detected in supernatants of mouse embryonic fibroblasts or oviduct epithelial cells (BM1.1) that were infected with C. muridarum for 24 h in vitro (data not shown). Moreover, neutrophils have already been observed to enter the infection site between 12 and 24 h postinfection (R. G. Rank and U. M. Nagarajan, unpublished data) and likely account for the major output of this cytokine. A significant corollary to this observation is that polymorphonuclear leukocytes, in particular, and perhaps also macrophages play a significant role in amplifying the inflammatory response at the local site of infection. Further, our data strongly suggest that it is the TLRs on the polymorphonuclear leukocytes and macrophages, rather than infected epithelial cells, which are critical for this amplification via the production of IL-1β. However, this study cannot rule out the possibility that epithelial cells may be producing small amounts of IL-1β in response to C. muridarum during the first 24 to 48 h of infection. A recent study has shown that cervical epithelial HeLa cells can activate caspase-1 in a process requiring chlamydial replication, the generation of reactive oxygen species, and the host protein NALP3 (1), although the authors of this study did not measure IL-1β secretion.
Although the bulk of the in vitro mechanistic work involving the activation of caspase-1 during C. trachomatis infection has been performed with HeLa cells (1, 36, 66), our findings provide a strong rationale for looking more rigorously at how this cytokine is produced by macrophages. In resting macrophages infected in vitro with C. muridarum, there is minimal secretion of IL-1β, despite the upregulation of IL-1β mRNA in the first 8 h of infection. Overall, this conundrum illustrates a disconnect between the large amounts of IL-1β secreted in vivo and the miniscule amounts detected with in vitro models. One possible explanation for this discrepancy may be the absence in the in vitro models of “danger signals” such as the release of ATP and that of uric acid crystals into the microenvironment by dying epithelial cells. Both of these danger signals are sufficient to trigger caspase-1 activation in macrophages (37, 39). ATP requires binding to the host P2X7R for this activation (37). However, the finding that P2X7R KO mice show only a partial decrease in IL-1β secretion in vivo following infection with C. muridarum and only on certain days postinfection (16) suggests that danger signals may be only an ancillary factor. An alternative possibility to explain the dissonance in IL-1β secretion levels is that the cytokine milieu and the microenvironment in vivo following infection may be such that infiltrating neutrophils and macrophages are primed to induce pro-IL-1β prior to their exposure to viable C. muridarum. In support of this theory, the levels of pro-IL-1β mRNA were found to be over sixfold higher in macrophages isolated from the genital tracts of infected animals than in macrophages infected in vitro (Fig. 2E and 3A). In order to model this phenomenon, peritoneal macrophages were prestimulated with small amounts of E. coli LPS prior to infection to induce pro-IL-1β mRNA. As expected, this priming step greatly amplified the amount of IL-1β secreted by the cell. Overall, any TLR ligand used in the priming phase to induce pro-IL-1β mRNA, including the low-potency endotoxin chlamydial LPS (27), and cellular lysates containing killed chlamydial RBs were sufficient to enhance IL-1β secretion. It is likely that TLR priming of macrophages involves other events in addition to the upregulation of pro-IL-1β expression, such as rapid redistribution of the key inflammasome component ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) to the cytoplasm, where it can facilitate caspase-1 activation (4). It can be speculated that during an in vivo infection, macrophages and neutrophils encounter and are prestimulated by chlamydial TLR4 ligands LPS (53) and Hsp60 (5), chlamydial TLR2 ligands (3, 17, 47), or killed chlamydial RBs/EBs released from infected cells, leading to enhanced secretion of IL-1β upon subsequent exposure to viable chlamydiae.
In our study, inhibition of caspase-1 led to decreased IL-1β secretion in infected LPS-primed macrophages, consistent with the findings of a previous study using unprimed macrophages (12). However, this block was incomplete, consistent with the effect of the caspase-1 inhibitor YVAD on IL-1β secretion following C. caviae infection of the monocytic cell line THP-1 (48). Caspase-1 activation has been shown to be linked with potassium efflux following the treatment of cells with potassium ionophores (64), during bacterial infection (51), or after exposure to certain bacterial toxins (26, 64). We also found that blocking this efflux diminished chlamydia-induced IL-1β. Surprisingly, the serine protease inhibitor TPCK caused almost complete inhibition instead of the expected partial block. Recently, serine proteases were linked with IL-1β maturation in neutrophils (25). It has also been shown that TPCK can block the proteasome (2, 13), whose activity may be involved in caspase-1 activation (19). These data suggest that a certain serine protease(s) may be involved in IL-1β maturation by directly cleaving pro-IL-1β in addition to functioning upstream of caspase-1 activation.
Overall, the most surprising finding in this study was that chlamydial growth was unnecessary for IL-1β secretion by primed macrophages. This result conflicts with previous reports that activation of caspase-1 or production of the caspase-1 substrate IL-18 by HeLa cells was absent when growth was impaired by chloramphenicol or INP0007 (36, 66). Similarly, production of IL-1β by human dendritic cells infected with C. trachomatis serovar L2 requires bacterial protein synthesis (23). But what was also unique in our study was the rapidity of IL-1β secretion, suggesting possible cell type-specific activation of caspase-1. Macrophages may possess an alternative rapid caspase-1 activation pathway that the other cell types do not. The fact that C. caviae infection of LPS-primed macrophages causes IL-1β secretion supports the idea that growth is not essential for caspase-1 activation. Overall, this idea has important ramifications for the human biovars that do not grow as well in macrophages as C. muridarum (33, 57, 69), implying that macrophages may be a prime source of IL-1β during human genital tract infections. In general, the only steadfast bacterial requirement for IL-1β secretion by LPS-primed macrophages was viability, as UV- or heat-killed EBs did not elicit this response.
Bacterial T3S has been widely implicated in the activation of caspase-1. Additionally, the T3S effector protein YopJ is necessary to activate the inflammasome during infection with Y. pestis strain KIM (35), suggesting that presynthesized chlamydial T3S effector proteins secreted via T3S may be activating caspase-1. UV inactivation or heat treatment would block this effect by preventing translocation. In contrast, rifampin would not block the effect because it would block only de novo T3S effector synthesis. However, our data did not demonstrate a block in IL-1β secretion in LPS-primed cells treated with the T3S antagonist INP0007. Although this finding appears to dismiss a role for T3S effectors, it should be noted that INP compounds are unable to block the initial translocation of the chlamydial T3S effector Tarp (66) or other potential effectors required for cellular invasion (45), supporting the hypothesis that early EB-derived T3S effectors secreted despite the presence of INP0007 are sufficient to trigger caspase-1 activation and IL-1β secretion in primed macrophages. Looking forward, it will be important to identify these chlamydial effectors and the host inflammasome pathway(s) they trigger.
Acknowledgments
The project described herein was supported by grant number AI067678 (from NIAID) to U.M.N., in part by grant number AI054624 (from NIAID) to T.D., by the Arkansas Children's Hospital Research Institute, by the Arkansas Biosciences Institute, and by resources from the UAMS Graduate Student Research Fund (to D.P.).
We thank Wee V. Yong for providing IL-1β KO mice. We thank David Ojcius, Richard Morrison, Vladimir Lupashin, and Shanmugam Nagarajan for critical reading of the manuscript and Horacio Gomez for statistical consultation.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Abdul-Sater, A. A., E. Koo, G. Hacker, and D. M. Ojcius. 2009. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. J. Biol. Chem. 284:26789-26796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backhed, F., S. Normark, and A. Richter-Dahlfors. 2002. TLR4-dependent lipopolysaccharide signalling in epithelial cells is independent of extracellular protease activity. Cell. Microbiol. 4:297-303. [DOI] [PubMed] [Google Scholar]
- 3.Bas, S., L. Neff, M. Vuillet, U. Spenato, T. Seya, M. Matsumoto, and C. Gabay. 2008. The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. J. Immunol. 180:1158-1168. [DOI] [PubMed] [Google Scholar]
- 4.Bryan, N. B., A. Dorfleutner, Y. Rojanasakul, and C. Stehlik. 2009. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol. 182:3173-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulut, Y., E. Faure, L. Thomas, H. Karahashi, K. S. Michelsen, O. Equils, S. G. Morrison, R. P. Morrison, and M. Arditi. 2002. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J. Immunol. 168:1435-1440. [DOI] [PubMed] [Google Scholar]
- 6.Byrne, G. I., and J. W. Moulder. 1978. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect. Immun. 19:598-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carabeo, R. A., S. S. Grieshaber, E. Fischer, and T. Hackstadt. 2002. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect. Immun. 70:3793-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casella, J. F., M. D. Flanagan, and S. Lin. 1981. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature 293:302-305. [DOI] [PubMed] [Google Scholar]
- 10.Cerretti, D. P., L. T. Hollingsworth, C. J. Kozlosky, M. B. Valentine, D. N. Shapiro, S. W. Morris, and N. Nelson. 1994. Molecular characterization of the gene for human interleukin-1 beta converting enzyme (IL1BC). Genomics 20:468-473. [DOI] [PubMed] [Google Scholar]
- 11.Chaix, J., M. S. Tessmer, K. Hoebe, N. Fuseri, B. Ryffel, M. Dalod, L. Alexopoulou, B. Beutler, L. Brossay, E. Vivier, and T. Walzer. 2008. Cutting edge: priming of NK cells by IL-18. J. Immunol. 181:1627-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng, W., P. Shivshankar, Z. Li, L. Chen, I. T. Yeh, and G. Zhong. 2008. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect. Immun. 76:515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobb, R. R., K. A. Felts, G. C. Parry, and N. Mackman. 1996. Proteasome inhibitors block VCAM-1 and ICAM-1 gene expression in endothelial cells without affecting nuclear translocation of nuclear factor-kappa B. Eur. J. Immunol. 26:839-845. [DOI] [PubMed] [Google Scholar]
- 14.Cotter, T. W., Q. Meng, Z. L. Shen, Y. X. Zhang, H. Su, and H. D. Caldwell. 1995. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4704-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darville, T., J. M. O'Neill, C. W. Andrews, Jr., U. M. Nagarajan, L. Stahl, and D. M. Ojcius. 2003. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J. Immunol. 171:6187-6197. [DOI] [PubMed] [Google Scholar]
- 16.Darville, T., L. Welter-Stahl, C. Cruz, A. A. Sater, C. W. Andrews, Jr., and D. M. Ojcius. 2007. Effect of the purinergic receptor P2X7 on Chlamydia infection in cervical epithelial cells and vaginally infected mice. J. Immunol. 179:3707-3714. [DOI] [PubMed] [Google Scholar]
- 17.Erridge, C., A. Pridmore, A. Eley, J. Stewart, and I. R. Poxton. 2004. Lipopolysaccharides of Bacteroides fragilis, Chlamydia trachomatis and Pseudomonas aeruginosa signal via Toll-like receptor 2. J. Med. Microbiol. 53:735-740. [DOI] [PubMed] [Google Scholar]
- 18.Fantuzzi, G., D. A. Reed, and C. A. Dinarello. 1999. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J. Clin. Investig. 104:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink, S. L., T. Bergsbaken, and B. T. Cookson. 2008. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. USA 105:4312-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franchi, L., A. Amer, M. Body-Malapel, T. D. Kanneganti, N. Ozoren, R. Jagirdar, N. Inohara, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7:576-582. [DOI] [PubMed] [Google Scholar]
- 21.Franchi, L., J. Stoolman, T. D. Kanneganti, A. Verma, R. Ramphal, and G. Nunez. 2007. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur. J. Immunol. 37:3030-3039. [DOI] [PubMed] [Google Scholar]
- 22.Frank, A., U. Rauen, and H. de Groot. 2000. Protection by glycine against hypoxic injury of rat hepatocytes: inhibition of ion fluxes through nonspecific leaks. J. Hepatol. 32:58-66. [DOI] [PubMed] [Google Scholar]
- 23.Gervassi, A., M. R. Alderson, R. Suchland, J. F. Maisonneuve, K. H. Grabstein, and P. Probst. 2004. Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect. Immun. 72:7231-7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghayur, T., S. Banerjee, M. Hugunin, D. Butler, L. Herzog, A. Carter, L. Quintal, L. Sekut, R. Talanian, M. Paskind, W. Wong, R. Kamen, D. Tracey, and H. Allen. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386:619-623. [DOI] [PubMed] [Google Scholar]
- 25.Greten, F. R., M. C. Arkan, J. Bollrath, L. C. Hsu, J. Goode, C. Miething, S. I. Goktuna, M. Neuenhahn, J. Fierer, S. Paxian, N. Van Rooijen, Y. Xu, T. O'Cain, B. B. Jaffee, D. H. Busch, J. Duyster, R. M. Schmid, L. Eckmann, and M. Karin. 2007. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130:918-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurcel, L., L. Abrami, S. Girardin, J. Tschopp, and F. G. van der Goot. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135-1145. [DOI] [PubMed] [Google Scholar]
- 27.Heine, H., S. Muller-Loennies, L. Brade, B. Lindner, and H. Brade. 2003. Endotoxic activity and chemical structure of lipopolysaccharides from Chlamydia trachomatis serotypes E and L2 and Chlamydophila psittaci 6BC. Eur. J. Biochem. 270:440-450. [DOI] [PubMed] [Google Scholar]
- 28.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herx, L. M., S. Rivest, and V. W. Yong. 2000. Central nervous system-initiated inflammation and neurotrophism in trauma: IL-1 beta is required for the production of ciliary neurotrophic factor. J. Immunol. 165:2232-2239. [DOI] [PubMed] [Google Scholar]
- 30.Hvid, M., A. Baczynska, B. Deleuran, J. Fedder, H. J. Knudsen, G. Christiansen, and S. Birkelund. 2007. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell. Microbiol. 9:2795-2803. [DOI] [PubMed] [Google Scholar]
- 31.Kanneganti, T. D., M. Lamkanfi, Y. G. Kim, G. Chen, J. H. Park, L. Franchi, P. Vandenabeele, and G. Nunez. 2007. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26:433-443. [DOI] [PubMed] [Google Scholar]
- 32.Kuida, K., J. A. Lippke, G. Ku, M. W. Harding, D. J. Livingston, M. S. Su, and R. A. Flavell. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267:2000- 2003. [DOI] [PubMed] [Google Scholar]
- 33.Kuo, C. C. 1978. Cultures of Chlamydia trachomatis in mouse peritoneal macrophages: factors affecting organism growth. Infect. Immun. 20:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lad, S. P., E. Y. Fukuda, J. Li, L. M. de la Maza, and E. Li. 2005. Up-regulation of the JAK/STAT1 signal pathway during Chlamydia trachomatis infection. J. Immunol. 174:7186-7193. [DOI] [PubMed] [Google Scholar]
- 35.Lilo, S., Y. Zheng, and J. B. Bliska. 2008. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect. Immun. 76:3911-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu, H., C. Shen, and R. C. Brunham. 2000. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J. Immunol. 165:1463-1469. [DOI] [PubMed] [Google Scholar]
- 37.Mariathasan, S., D. S. Weiss, K. Newton, J. McBride, K. O'Rourke, M. Roose-Girma, W. P. Lee, Y. Weinrauch, D. M. Monack, and V. M. Dixit. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228-232. [DOI] [PubMed] [Google Scholar]
- 38.Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10:417-426. [DOI] [PubMed] [Google Scholar]
- 39.Martinon, F., V. Petrilli, A. Mayor, A. Tardivel, and J. Tschopp. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237-241. [DOI] [PubMed] [Google Scholar]
- 40.Meng, G., F. Zhang, I. Fuss, A. Kitani, and W. Strober. 2009. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity 30:860-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao, E. A., C. M. Alpuche-Aranda, M. Dors, A. E. Clark, M. W. Bader, S. I. Miller, and A. Aderem. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7:569-575. [DOI] [PubMed] [Google Scholar]
- 42.Miao, E. A., R. K. Ernst, M. Dors, D. P. Mao, and A. Aderem. 2008. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl. Acad. Sci. USA 105:2562-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muruve, D. A., V. Petrilli, A. K. Zaiss, L. R. White, S. A. Clark, P. J. Ross, R. J. Parks, and J. Tschopp. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103-107. [DOI] [PubMed] [Google Scholar]
- 44.Muschiol, S., L. Bailey, A. Gylfe, C. Sundin, K. Hultenby, S. Bergstrom, M. Elofsson, H. Wolf-Watz, S. Normark, and B. Henriques-Normark. 2006. A small-molecule inhibitor of type III secretion inhibits different stages of the infectious cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 103:14566-14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muschiol, S., S. Normark, B. Henriques-Normark, and A. Subtil. 2009. Small molecule inhibitors of the Yersinia type III secretion system impair the development of Chlamydia after entry into host cells. BMC Microbiol. 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagarajan, U. M., D. M. Ojcius, L. Stahl, R. G. Rank, and T. Darville. 2005. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J. Immunol. 175:450-460. [DOI] [PubMed] [Google Scholar]
- 47.O'Connell, C. M., R. R. Ingalls, C. W. Andrews, Jr., A. M. Scurlock, and T. Darville. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J. Immunol. 179:4027-4034. [DOI] [PubMed] [Google Scholar]
- 48.Ojcius, D. M., P. Souque, J. L. Perfettini, and A. Dautry-Varsat. 1998. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J. Immunol. 161:4220-4226. [PubMed] [Google Scholar]
- 49.Parr, M. B., and E. L. Parr. 1991. Langerhans cells and T lymphocyte subsets in the murine vagina and cervix. Biol. Reprod. 44:491-498. [DOI] [PubMed] [Google Scholar]
- 50.Perry, L. L., H. Su, K. Feilzer, R. Messer, S. Hughes, W. Whitmire, and H. D. Caldwell. 1999. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J. Immunol. 162:3541-3548. [PubMed] [Google Scholar]
- 51.Petrilli, V., S. Papin, C. Dostert, A. Mayor, F. Martinon, and J. Tschopp. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14:1583-1589. [DOI] [PubMed] [Google Scholar]
- 52.Prantner, D., and U. M. Nagarajan. 2009. Role for the chlamydial type III secretion apparatus in host cytokine expression. Infect. Immun. 77:76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prebeck, S., H. Brade, C. J. Kirschning, C. P. da Costa, S. Durr, H. Wagner, and T. Miethke. 2003. The Gram-negative bacterium Chlamydia trachomatis L2 stimulates tumor necrosis factor secretion by innate immune cells independently of its endotoxin. Microbes Infect. 5:463-470. [DOI] [PubMed] [Google Scholar]
- 54.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y. X. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren, Q., S. J. Robertson, D. Howe, L. F. Barrows, and R. A. Heinzen. 2003. Comparative DNA microarray analysis of host cell transcriptional responses to infection by Coxiella burnetii or Chlamydia trachomatis. Ann. N. Y. Acad. Sci. 990:701-713. [DOI] [PubMed] [Google Scholar]
- 56.Robinson, D., K. Shibuya, A. Mui, F. Zonin, E. Murphy, T. Sana, S. B. Hartley, S. Menon, R. Kastelein, F. Bazan, and A. O'Garra. 1997. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFκB. Immunity 7:571-581. [DOI] [PubMed] [Google Scholar]
- 57.Roshick, C., H. Wood, H. D. Caldwell, and G. McClarty. 2006. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect. Immun. 74:225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rund, S., B. Lindner, H. Brade, and O. Holst. 1999. Structural analysis of the lipopolysaccharide from Chlamydia trachomatis serotype L2. J. Biol. Chem. 274:16819-16824. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz, J., A. Owyang, E. Oldham, Y. Song, E. Murphy, T. K. McClanahan, G. Zurawski, M. Moshrefi, J. Qin, X. Li, D. M. Gorman, J. F. Bazan, and R. A. Kastelein. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479-490. [DOI] [PubMed] [Google Scholar]
- 60.Shao, W., G. Yeretssian, K. Doiron, S. N. Hussain, and M. Saleh. 2007. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 282:36321-36329. [DOI] [PubMed] [Google Scholar]
- 61.Stephens, R. S. 2003. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 11:44-51. [DOI] [PubMed] [Google Scholar]
- 62.Sutterwala, F. S., Y. Ogura, M. Szczepanik, M. Lara-Tejero, G. S. Lichtenberger, E. P. Grant, J. Bertin, A. J. Coyle, J. E. Galan, P. W. Askenase, and R. A. Flavell. 2006. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24:317-327. [DOI] [PubMed] [Google Scholar]
- 63.Thornberry, N. A., H. G. Bull, J. R. Calaycay, K. T. Chapman, A. D. Howard, M. J. Kostura, D. K. Miller, S. M. Molineaux, J. R. Weidner, J. Aunins, et al. 1992. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768-774. [DOI] [PubMed] [Google Scholar]
- 64.Walev, I., K. Reske, M. Palmer, A. Valeva, and S. Bhakdi. 1995. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 14:1607-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker, N. P., R. V. Talanian, K. D. Brady, L. C. Dang, N. J. Bump, C. R. Ferenz, S. Franklin, T. Ghayur, M. C. Hackett, L. D. Hammill, et al. 1994. Crystal structure of the cysteine protease interleukin-1 beta-converting enzyme: a (p20/p10)2 homodimer. Cell 78:343-352. [DOI] [PubMed] [Google Scholar]
- 66.Wolf, K., H. J. Betts, B. Chellas-Gery, S. Hower, C. N. Linton, and K. A. Fields. 2006. Treatment of Chlamydia trachomatis with a small molecule inhibitor of the Yersinia type III secretion system disrupts progression of the chlamydial developmental cycle. Mol. Microbiol. 61:1543-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wyrick, P. B., and E. A. Brownridge. 1978. Growth of Chlamydia psittaci in macrophages. Infect. Immun. 19:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia, M., R. E. Bumgarner, M. F. Lampe, and W. E. Stamm. 2003. Chlamydia trachomatis infection alters host cell transcription in diverse cellular pathways. J. Infect. Dis. 187:424-434. [DOI] [PubMed] [Google Scholar]
- 69.Yong, E. C., E. Y. Chi, and C. C. Kuo. 1987. Differential antimicrobial activity of human mononuclear phagocytes against the human biovars of Chlamydia trachomatis. J. Immunol. 139:1297-1302. [PubMed] [Google Scholar]