Abstract
Interleukin-12 (IL-12) is critical for resistance to Toxoplasma gondii during both the acute and chronic stages of infection. However, the cellular and molecular pathways that regulate IL-12 production during chronic toxoplasmosis are incompletely defined. We recently discovered that 12/15-lipoxygenase (12/15-LOX), which oxidizes unsaturated lipids in macrophages, is a novel and selective regulator of IL-12 production. We now demonstrate the essential role of this enzyme in the chronic phase of toxoplasmosis. Although 12/15-LOX-deficient mice were resistant to acute T. gondii infection, 80% of 12/15-LOX-deficient mice died during chronic toxoplasmosis, compared to no deaths in wild-type controls. The morbidity of chronically infected 12/15-LOX mice was associated with an increase in brain inflammation and parasite burden. These data suggest that the evolution of the immune response to T. gondii is accompanied by an increasing requirement for 12/15-LOX-mediated signaling. Consistent with this conclusion, 12/15-LOX activity was enhanced during chronic, but not acute, toxoplasmosis. Furthermore, the enhanced susceptibility of 12/15-LOX-deficient mice to chronic toxoplasmosis was associated with reduced production of IL-12 and gamma interferon (IFN-γ) that was not evident during acute infection. Importantly, ex vivo IFN-γ production by 12/15-LOX-deficient splenocytes could be rescued by the addition of recombinant IL-12. These data establish that 12/15-LOX is a critical mediator of the chronic type 1 inflammatory response and that immune mediators can be subject to distinct cellular and/or molecular mechanisms of regulation at different stages of inflammation.
Lipoxygenase and cyclooxygenase families are critical regulators of chronic inflammation that seem to play little role in acute processes (26, 37). Because of this potential specificity, these lipid-metabolizing enzymes have been targeted for years in the search for pathways that selectively impact chronic inflammatory disease. Several studies demonstrated an important role for cyclooxygenase and lipoxygenase products in regulating interleukin-12 (IL-12) production, and lipoxygenases in particular contribute toward the response to Toxoplasma gondii. Specifically, lipoxin A4 (LxA4), a product of both 15-lipoxygenase (15-LOX) and 5-lipoxygenase (5-LOX) (45), downregulates IL-12 produced by toxoplasma antigen-activated dendritic cells (DCs), thereby tempering the immune response (3, 42, 57). Indeed, 5-LOX-deficient mice infected with T. gondii die early in the chronic stage of this infection as a consequence of an overwhelming inflammatory response (3).
The human enzymes 12-LOX and 15-LOX (together referred to as 12/15-LOX) metabolize arachidonic acid and linoleic acid into products such as 12(S)-hydroxyeicosatetraenoic acid [12(S)-HETE], 15(S)-HETE, and 13(S)-hydroxyoctadecadienoic acid [13(S)-HODE] via short-lived peroxidated intermediates (9, 49). We recently demonstrated that 12/15-LOX is required for macrophage production of IL-12/23p40 (but not other inflammatory mediators tested) in response to select Toll-like receptor ligands in vitro (27). The impact of 12/15-LOX on this innate pathway translated to decreased levels of IL-12 and gamma interferon (IFN-γ) expression in atherosclerotic plaques in mice deficient in 12/15-LOX compared to littermate controls (58). In contrast to this setting of macrophage-dominated chronic inflammation, 12/15-LOX-deficient mice produced comparable levels of IL-12 upon acute stimulation with lipopolysaccharide (LPS) in vivo (27). This apparent paradox may be explained by our observation that, in contrast to macrophages, LPS-induced IL-12 production by DCs and neutrophils is 12/15-LOX independent (27) and by the fact that DCs tend to dominate during the acute inflammatory response (36). The selectivity of the 12/15-LOX pathway is underscored by the fact that although 12/15-LOX is expressed in some nonhematopoietic cell types, such as vascular smooth muscle cells and endothelial cells (21, 33), 12/15-LOX expression is only detectable in mature macrophages among several leukocyte subsets tested (28, 43, 49, 56, 57). Thus, the 12/15-LOX-dependent pathway to IL-12 may be selectively invoked during chronic inflammation by virtue of its differential expression in various cell types. To investigate this hypothesis, we compared the contribution of 12/15-LOX in resistance to acute versus chronic infection with Toxoplasma gondii.
T. gondii is an intracellular parasitic protozoan that invokes acute and chronic inflammation in mouse models (29). The acute stage of toxoplasmosis is characterized by the replication of tachyzoites, while cerebral bradyzoite cysts, which are kept relatively latent by constant immune pressure, dominate the chronic stage of the infection (17). The type 1 cytokines IL-12 and IFN-γ are essential for an effective immune response during both phases of toxoplasmosis (11, 41, 50, 57). The importance of IL-12/23p40 is demonstrated by the observation that IL-12/23p40-deficient mice die during the acute stage of toxoplasmosis, secondary to overwhelming parasite burden (11). Furthermore, IL-12/23p40 is required for survival during the chronic stage of toxoplasmosis, as evidenced by the fact that IL-12/23p40-deficient animals rescued from acute infection by injections of recombinant IL-12 later succumb if this treatment is withdrawn during the chronic stage (57). Animals deficient in IFN-γ are similarly vulnerable to both acute and chronic toxoplasmosis (41, 50). IL-23, which shares the IL-12/23p40 subunit with IL-12, appears to have a comparatively minor role in resistance to acute toxoplasmosis (22).
Interestingly, mouse strains that are relatively resistant to acute toxoplasmosis may not be as resilient during chronic toxoplasmosis, and vice versa (51). The fact that genetic susceptibilities do not always overlap indicates that there are distinct mechanisms involved in the host response to T. gondii during these two stages. A critical question remains as to the cellular and molecular basis for the divergence in immune behavior during acute versus chronic infection. In this regard, several studies have indicated a selective role for macrophages in many models of chronic inflammation (1, 18, 25). Thus, the cell types involved in each stage may provide a mechanistic distinction between acute and chronic IL-12 production, including in the context of immune-mediated control of T. gondii. Consistent with a selective role for macrophages in toxoplasmosis is the fact that nitric oxide, largely a macrophage product, is only required for resistance to the chronic stage of the infection (42).
To test the hypothesis that 12/15-LOX, a macrophage-selective regulator of IL-12 production, preferentially impacts chronic inflammation, we compared the acute and chronic immune responses of C57BL/6 wild-type and 12/15-LOX-deficient (Alox15) C57BL/6 mice during infection with the ME49 strain of T. gondii. We found that survival during the chronic, but not the acute, phase of toxoplasmosis by C57BL/6 mice is dependent on 12/15-LOX-mediated IL-12 and IFN-γ production in vivo.
MATERIALS AND METHODS
Animals.
C57BL/6 and Alox15 mice on a C57BL/6 background (backcrossed 11 generations) were purchased from Jackson Laboratories (Bar Harbor, ME) and housed and bred in the Wistar Institute Animal Facility (Philadelphia, PA) under an IACUC-approved protocol.
Bone marrow-derived cells.
Single-cell suspensions of bone marrow cells were prepared by flushing the femurs of 6- to 9-week-old male and female mice with ice-cold phosphate-buffered saline (PBS) and a 23-gauge needle. Bulk bone marrow cells were cultured at 2 × 105 cells/ml in RPMI supplemented with 10% fetal calf serum, 50 μM β-mercaptoethanol, 1% penicillin, streptomycin, and amphotericin B (Fungizone), and 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ) or 30% of the supernatant harvested from a GM-CSF-producing cell line (kindly provided by Yvonne Paterson at the University of Pennsylvania School of Medicine, Philadelphia). Dendritic cells were prepared as described elsewhere (46). Briefly, the GM-CSF concentration was halved at day 10, nonadherent cells were aspirated, and loosely adherent cells were harvested at days 11 to 12. The purity of CD11c+ cells was determined by flow cytometric analysis to be between 85 and 95%. Soluble toxoplasma antigen (STAg) was pretreated with 25 μg/ml polymyxin B (Sigma) for 20 min at room temperature before being added to cells in order to inhibit any contaminating endotoxin. All cultures were normalized to viability by the metabolic MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay as described previously (20).
Thioglycolate-elicited macrophages.
C57BL/6 and Alox15 mice were injected intraperitoneally with 1 ml of sterile 3% Brewer's thioglycolate broth (Sigma). At 4 days, cells were harvested by peritoneal lavage with Ca2+/Mg2+-free PBS and cultured in RPMI supplemented with 10% fetal calf serum, 50 μM β-mercaptoethanol, 1% penicillin, streptomycin, and amphotericin B in 5% CO2 as previously described (13). The purity of CD11b+ cells was consistently greater than 95% by flow cytometric analysis. PD146176, a selective inhibitor of 12/15-LOX (6, 27), was purchased from Sigma.
Lipid quantification.
Unperfused fresh spleens were cut into sections, stimulated for 20 min with 200 nM phorbol myristate acetate in serum-free medium in duplicate, and then centrifuged at 1,500 rpm to isolate the supernatant. An aliquot of the supernatant was used for lipoxin A4 analysis by enzyme-linked immunosorbent assay (ELISA; Oxford Biomedical Research, Inc., Oxford, MI). The remainder was extracted for analysis by stable isotope dilution normal-phase chiral liquid chromatography coupled with electron capture atmospheric pressure chemical ionization/mass spectrometry as described previously (23). Briefly, samples were spiked with deuterium-labeled internal standards, adjusted to pH 3 with 2.5 N hydrochloric acid, and extracted with diethyl ether (4 ml, twice). The organic layer was evaporated to dryness under nitrogen, derivatized with 2,3,4,5,6-pentafluorobenzyl bromide in the presence of diisopropylethylamine, and evaporated to dryness under a stream of nitrogen. Derivatized samples were reconstituted in 100 μl of hexane-ethanol (97:3, vol/vol), and 20 μl was analyzed by using a liquid chromatography/electron capture atmospheric pressure chemical ionization/mass spectrometry system. Quantitation was performed by comparison of peak area ratios of the analytes to their relevant stable isotope internal standard and interpolation of area ratios from a standard curve (49).
Peritoneal counts.
Slides were prepared for cells isolated from murine peritoneum by adding 0.5 ml of 104 cells/ml to a cytospin apparatus (ThermoScientific) and centrifuging for 10 min at 1,200 rpm. Slides were dried and stained with Kwik-diff (Sigma), and cells were quantified by microscopy.
Parasites.
STAg was prepared from the RH strain tachyzoites as previously described (55). The RH strain tachyzoites were maintained in human foreskin fibroblasts. Cysts of the ME49 strain of T. gondii were harvested from brains of CBA/CaJ mice infected for 1 to 2 months. For experimental infections, mice were given 20 ME49 cysts intraperitoneally in a volume of 0.2 ml.
Parasite killing assay.
Thioglycolate-elicited macrophages (see above) were primed with IFN-γ for 16 h or left unstimulated overnight in polystyrene tubes before addition of 4 RH strain tachyzoites/macrophage for 8 h. After infection, parasites were washed off and macrophages were resuspended in their original medium overnight. Slides were prepared by cytospin analysis with subsequent Kwik-Diff staining, and parasite burden was quantified under a light microscope.
Brain mononuclear cells.
To isolate brain mononuclear cells, animals were first anesthetized and perfused with sterile phosphate-buffered saline to remove peripheral blood from the brain. Following excision, brains were minced with scissors and then digested for 1 h at 37°C with 300 μg of collagenase/dispase (Boehringer Mannhein, Indianapolis, IN) and 600 μg of DNase I (Boehringer Mannheim) per ml in complete RPMI medium. The dissociated brain tissue was pelleted at 200 × g for 10 min, resuspended in a 60% isotonic Percoll solution (Sigma), and overlaid with a 30% Percoll solution. Discontinuous gradients were centrifuged for 25 min at 1,000 × g. After removal of the myelin layer on top of the gradient, brain-associated mononuclear cells (BMNCs) were harvested from the 30%-60% interphase and washed twice in complete RPMI medium before further analysis.
Flow cytometry.
Single-cell suspensions were prepared and depleted of red blood cells by lysis in ammonium chloride buffer, and cells were stained using 2 μg/ml directly labeled specific monoclonal antibodies and isotype-matched antibodies as controls. Compensation was performed using anti-CD4 or Mac-1 (fluorescein isothiocyanate) and anti-CD8 or Mac-1 (phycoerythrin) staining. All flow cytometric antibodies were purchased from BD Biosciences (San Diego, CA). Cells were washed, fixed in 3.7% formaldehyde in calcium- and magnesium-free PBS, and analyzed using a BD FACSCalibur machine (BD Biosciences). For sorting, cells were stained as above and sorted for the indicated populations at the Wistar Institute Flow Cytometry Core Facility.
Reverse transcription reaction and quantitative real-time PCR.
Total RNA was extracted from splenocytes using TRIzol (Invitrogen) according to the manufacturer's instructions. RNA was treated with Turbo DNase (Ambion, Austin, TX) according to the manufacturer's instructions to remove any contaminating genomic DNA, and the absence of appreciable genomic DNA was confirmed by real-time PCR of the treated RNA. RNA was normalized by the optical density at 260 nm, and the reverse transcription reaction was performed using a cDNA synthesis kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Quantitative real-time PCR analysis was performed using Sybr green master mix and analyzed using an ABI 7000 machine (Applied Biosystems). Gene-specific primers (Table 1) were designed using Primer Express (Applied Biosystems), and gene expression levels were normalized using β-actin RNA as an internal control. To determine parasite burden, one-fourth portions of each brain was incubated in 1 mg/ml proteinase K overnight, and 10% of the supernatant was subjected to phenol-chloroform extraction and isopropanol precipitation. DNA was normalized by the optical density at 280 nm and subjected to real-time PCR using the primers listed below and β-actin as an internal control. Specificity was confirmed using both water and uninfected samples.
TABLE 1.
Primer sequencesa
| Primer | Sequence (5′ to 3′) |
|
|---|---|---|
| Forward | Reverse | |
| β-Actin | TCAGCAAGCAGGAGTACGATG | AACAGTCCGCCTAGAAGCACTT |
| 12/15-LOX | ACCCCACCGCCGATTTT | AGCTTCGGACCCAGCATTT |
| IL-4 | CGCCATGCACGGAGATG | ACGAGCTCACTCTCTGTGGTGTT |
| T-bet | GCCAGGGAACCGCTTATA TG | GCCAGGGAACCGCTTATATG |
| WSX | CAAGAAGAGGTCCCGTGCTG | TTGAGCCCAGTCCACCACAT |
| Arginase-1 | GCTGTCTTCCCAAGAGTTGGG | ATGGAAGAGACCTTCAGCTAC |
| IL-12/23p40 | AGACCCTGCCCATTGAACTG | GAAGCTGGTGCTGTAGTTCTCATATT |
| IL-12p35 | CACCCTTGCCCTCCTAAAC | CACCTGGCAGGTCCAGAG |
| IL-23p19 | GCCCCGTATCCAGTGTGAAG | CGGATCCTTTGCAAGCAGAA |
| IL-10 | CAGCCGGGAAGACAATAACTG | CCGCAGCTCTAGGAGCATGT |
| IL-18 | GCTTGAATCTAAATTATCAGTC | GAAGATTCAAATTGCATCTTAT |
Primer sequences were synthesized by Integrated DNA Technologies.
Determination of inflammatory mediator production.
The IL-12/23p40 and IFN-γ OptEIA ELISA kits were purchased from BD Bioscience (San Jose, CA), and supernatant was analyzed according to the manufacturer's instructions. Nitrite levels were measuring using a Griess reagent assay kit purchased from Cayman Chemicals (Ann Arbor, MI). The IL-23 ELISA was performed as previously described (22).
Other reagents.
Recombinant murine IL-12 was kindly provided by Wyeth Pharmaceuticals (Madison, NJ). 12(S)-HETE was purchased from Cayman Chemicals.
Statistical analysis.
One-way analysis of variance (for comparing more than two groups) and t tests (for comparing two groups) were performed using Prism software (Graphpad, San Diego, CA) according to the program's designation of the most statistically valid test for each experiment. The α-level was set at 0.05 for all tests, and P values below this were considered statistically significant. All error bars represent standard deviations.
RESULTS
Macrophage, but not dendritic cell, IL-12 production in response to in vitro stimulation with toxoplasma antigen is 12/15-LOX dependent.
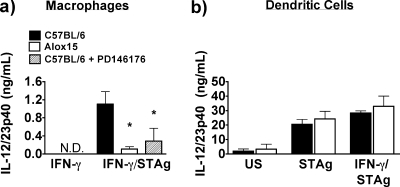
We previously reported that macrophages, but not dendritic cells, require 12/15-LOX for IL-12 production in vitro in response to a subset of Toll-like receptor ligands (27). In the case of stimulation with T. gondii, macrophages do not produce significant amounts of IL-12 without IFN-γ priming (39) (data not shown), consistent with the fact that CD8α+ DCs reportedly dominate in terms of IL-12 production during the acute response to T. gondii antigen (36). However, IL-12 produced by T. gondii-stimulated macrophages in the context of sufficient IFN-γ (i.e., once the immune response is already under way) may play a role in the chronic response. Thus, we investigated whether 12/15-LOX activity is required for IFN-γ-primed IL-12 production in response to T. gondii. We primed thioglycolate-elicited macrophages with IFN-γ and stimulated these cells with STAg. Macrophages isolated from 12/15-LOX-deficient mice produced markedly reduced levels of IL-12/23p40 compared to wild-type controls (Fig. 1a). STAg-induced IL-12 production in IFN-γ-primed wild-type macrophages pretreated with the 12/15-LOX inhibitor PD146176 was also impaired (Fig. 1a). In contrast, bone marrow-derived DCs did not require IFN-γ priming for maximal IL-12/23p40 production in response to STAg, nor was IL-12 production by DCs dependent on 12/15-LOX (Fig. 1b). Thus, as we previously demonstrated for LPS (27), 12/15-LOX mediates the in vitro IL-12 response to STAg in a cell-type-selective manner. Although macrophages produced an order of magnitude less IL-12/23p40 than dendritic cells in this in vitro model, the preponderance of macrophages in infected tissue, in addition to suppressors of DC-mediated IL-12 production (2, 4), such as LXA4, may transform the relative contributions to IL-12 production during chronic toxoplasmosis.
FIG. 1.
Macrophages, but not dendritic cells, produce IL-12 in response to T. gondii antigen in vitro in a 12/15-LOX-dependent manner. (a) Thioglycolate-elicited macrophages (105 cells/μl) from wild-type and Alox15 mice were primed with IFN-γ for 16 h; wild-type cells were pretreated for 45 min with a 10 μM ceoncentration of the 12/15-LOX inhibitor PD146176 or the dimethyl sulfoxide vehicle control before being left in medium alone or stimulated with 25 μg/ml STAg for 24 h. (b) Bone marrow-derived dendritic cells (105 cells/μl) from wild-type or Alox15 mice were unprimed or primed with IFN-γ for 16 h and stimulated for 24 h with 25 μg/ml STAg. IL-12/23p40 levels in the supernatant were quantified by ELISA. n ≥ 3. *, P < 0.05. US, unstimulated; N.D., not detectable.
12/15-Lipoxygenase is critical for resistance to chronic, but not acute, toxoplasmosis.
Given the data above demonstrating cell-type-selective regulation of IL-12 production by 12/15-LOX, we employed a T. gondii infection model to test the hypothesis that the macrophage-selective defect in IL-12 production would differentially impact the acute versus chronic inflammatory response to this pathogen.
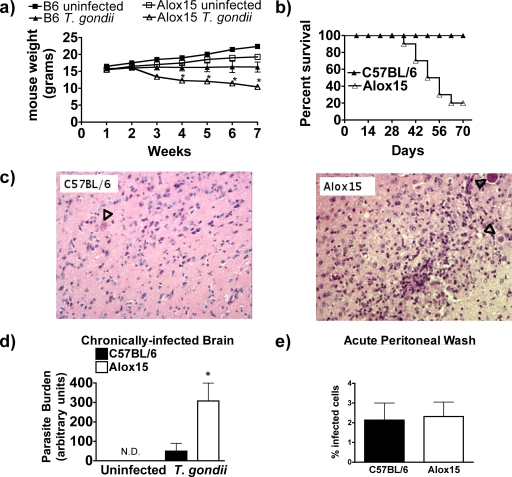
We infected Alox15 mice and C57BL/6 wild-type controls intraperitoneally with 20 cysts of the ME49 strain of T. gondii. While Alox15 mice progressed normally through the acute phase of toxoplasmosis (the first 7 to 10 days), upon transition to the chronic phase, Alox15 mice began losing weight and 80% succumbed to the infection within 60 days postinfection, compared to 0% mortality in wild-type controls (Fig. 2a and b). Examination of the brain parenchyma of chronically infected mice revealed a striking increase in toxoplasmic encephalitis in Alox15 mice compared to controls, with periventricular inflammation and clusters of lymphocyte and myeloid infiltrates throughout the parenchyma (Fig. 2c). Despite this inflammatory response, brains isolated from Alox15 mice exhibited a substantially greater parasite burden than wild-type mice as assessed by quantitative PCR and microscopic examination of peritoneal cells (Fig. 2d). The increased parasitism was selective for the chronic stage of toxoplasmosis, as peritoneal cells from 7-day-infected wild-type and Alox15 mice displayed comparable parasite burdens (Fig. 2e). As expected, there was no detectable parasite signal in the brains of either murine genotype during the acute phase of the infection. Our data demonstrate that 12/15-LOX is dispensable for control of acute infection but is essential for resistance to chronic toxoplasmosis.
FIG. 2.
12/15-lipoxygenase is critical for resistance to chronic, but not acute, toxoplasmosis. (a) Chart of weekly weighed C67BL/6 (B6) uninfected, Alox15 uninfected, and T. gondii-infected C57BL/6 and Alox15 mice (n = 4 for uninfected groups and 7 for T. gondii-infected groups). (b) Kaplan-Meier survival curve of T. gondii-infected B6 and Alox15 mice (n = 10 in each group) (P = 0.003. (c) Representative 20× magnifications of hematoxylin and eosin stains of paraffin-sectioned brains isolated from C57BL/6 and Alox15 mice at day 40 postinfection with T. gondii. Arrowheads indicate T. gondii cysts. (d) Real-time PCR quantification of parasite burdens in the brains of B6 and Alox15 mice after 40 days of infection with T. gondii compared to burdens in uninfected controls. (e) Percentage of infected macrophages isolated from wild-type and Alox15 peritonea 7 days postinfection. Data are averages of three fields in three different animals. *, P < 0.05.
12/15-LOX activity is selectively invoked during chronic toxoplasmosis.
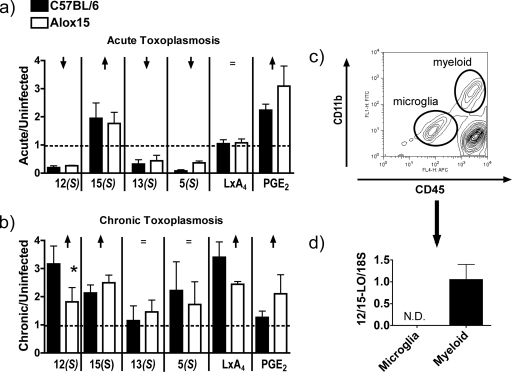
We previously reported that basal levels of some 12/15-LOX products are diminished in spleens isolated from 12/15-LOX-deficient mice, demonstrating a role for this enzyme in vivo (28). We hypothesized that 12/15-LOX activity is induced during infection with T. gondii. 12/15-LOX, along with other enzymes, catabolizes the oxidation of certain fatty acids (9). To detect 12/15-LOX-dependent increases in oxidized lipid formation, we quantified oxidized polyunsaturated fatty acid metabolites produced by splenocytes isolated from wild-type and Alox15 mice during the acute and chronic stages of infection. The levels of fatty acid metabolites were expressed as the change compared to levels produced by splenocytes isolated from uninfected mice to differentiate augmentation of 12/15-LOX activity from basal differences between the animals. In spleens isolated from wild-type or Alox15 mice on day 7 postinfection, there was no change in the levels of LxA4 (a 5-LOX and 15-LOX product) (Fig. 3a). While both 15(S)-HETE (a 12/15-LOX and cyclooxygenase product) and prostaglandin E2 (a cyclooxygenase product) were increased relative to uninfected controls, there was no difference between wild-type and Alox15 mice (Fig. 3a). On the other hand, there was reduced production of 12(S)-HETE (arachidonic acid product of 12/15-LOX and nonspecific oxidation), 13(S)-HODE (linoleic acid-derived product of 12/15-LOX and nonspecific oxidation), and 5(S)-HETE (arachidonic acid product of 5-LOX) relative to uninfected controls in both wild-type and Alox15 mice (Fig. 3a), suggesting that lipoxygenase activity is suppressed during acute toxoplasmosis, the uptake or metabolism of the products is increased, and/or the enzyme activity is exhausted by the time of restimulation.
FIG. 3.
12/15-LOX activity is selectively invoked during the chronic response to T. gondii. Liquid chromatography mass spectrometric analysis of 12(S)-HETE [12(S)], 15(S)-HETE [15(S)], 13(S)-HODE [13(S)], 5(S)-HETE [5(S)], LxA4, and prostaglandin E2 (PGE2) released by phorbol myristate acetate-stimulated brain and spleen fragments isolated from C57BL/6 and Alox15 mice at 7 days (acute) (a) or 40 days (chronic) (b) postinfection. The fold increase was calculated over uninfected controls. The dashed line represents relative abundance in uninfected mice. ↑, P < 0.05 for the fold increase compared to uninfected controls; ↓, P < 0.05 for the decrease compared to uninfected controls; =, no difference compared to uninfected controls; *, P < 0.05 for the difference in induction between wild-type and Alox15 mice. (c and d) Infiltrating myeloid cells (myeloid) and microglia were isolated from 60-day postinfection brain mononuclear cells using fluorescence-activated cell sorting based on CD45 and CD11b expression, and RNA was extracted (c) subjected to real-time PCR (d) using primers specific for 12/15-LOX. n = 4. N.D., not detectable.
During the chronic phrase of infection, there were statistically significant increases in lipid metabolite production compared to uninfected controls in the cases of 12(S)-HETE, 15(S)-HETE, and LxA4 (Fig. 3b). The increases in 15(S)-HETE and LxA4 production were comparable between Alox15 mice and wild-type controls, indicating that their upregulation in the context of T. gondii infection is independent of 12/15-LOX (Fig. 3b). Importantly, chronically infected wild-type mice demonstrated increased production of 12(S)-HETE that was largely 12/15-LOX dependent (Fig. 3b). These data are consistent with earlier in vitro reports which indicated that the exposure of macrophages to T. gondii triggers an increase in arachidonic acid release and lipoxygenase activity that largely favors the 12/15-LOX product 12(S)-HETE (37). However, since 12(S)-HETE is the major product of murine 12/15-LOX (24, 52), it is possible that the apparently selective nature of the 12/15-LOX-dependent increased 12(S)-HETE production in wild-type mice may be related to the sensitivity of our assay. Also, the increased abundance of this 12/15-LOX product could represent a decrease in metabolism/uptake or an increased proportion of 12/15-LOX-expressing cells rather than an induction of expression or activity in a given cell type.
Although we tested 12/15-LOX activity as described above using brain tissue, there was no enhanced product elaboration in wild-type animals compared to uninfected controls (data not shown). This could possibly be due to the relatively small contribution of leukocytes to total brain tissue, as well as the high basal levels of 12/15-LOX expression found in brain parenchyma (8). Therefore, we investigated whether infiltrating myeloid cells and or microglia in the brain at least expressed 12/15-LOX during chronic toxoplasmosis. BMNCs from chronically infected wild-type mice were isolated using fluorescence-activated cell sorting based on CD11b and CD45 expression to distinguish between microglia and infiltrating macrophages (Fig. 3c). 12/15-LOX expression in these enriched populations was assessed by real-time PCR. Although resident microglia (CD45int CD11bint) did not express detectable levels of 12/15-LOX, preparations of infiltrating myeloid cells (CD45hi CD11bhi) did express this enzyme, similar to peripheral mature macrophages (Fig. 3d). Taken together, these data demonstrate that 12/15-LOX is selectively enhanced in the periphery and that 12/15-LOX-expressing myeloid cells are enriched in the brain during chronic toxoplasmosis.
12/15-LOX does not play a role in macrophage killing of T. gondii in vitro.
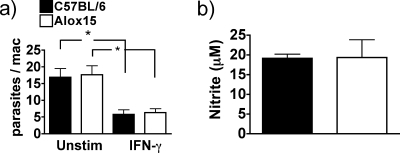
One of the principal mechanisms by which macrophages are thought to contribute toward resistance to T. gondii infection is the inhibition of parasite replication due to cytotoxic or cytostatic effects of reactive species such as nitric oxide (48). Thus, it was possible that the increased parasite burden in Alox15 mice was due to an intrinsic defect in macrophage parasite killing mechanisms. To test this possibility, we compared the ability of thioglycolate-elicited macrophages isolated from wild-type and Alox15 mice to kill tachyzoites in vitro. Unstimulated and IFN-γ-primed Alox15 macrophages were able to eliminate parasites comparably to wild-type controls and also produced equivalent amounts of nitric oxide (Fig. 4a and b). Although other factors may influence parasite control in vivo, these data indicate that 12/15-LOX is not directly involved in the pathogen killing machinery in macrophages.
FIG. 4.
12/15-LOX-deficient macrophages are as efficient as the wild type at parasite control in vitro. (a) Elicited peritoneal macrophages left unstimulated or primed for 16 h with 50 U/ml IFN-γ were incubated with RH strain T. gondii tachyzoites overnight, and the number of parasites per infected macrophage was quantified by microscopy. (b) Nitrite production as measured by Griess assay from the IFN-γ-primed supernatant of the experiment shown in panel a. n = 4. *, P = 0.01.
Absence of 12/15-LOX impacts leukocyte composition and activation during chronic toxoplasmosis.
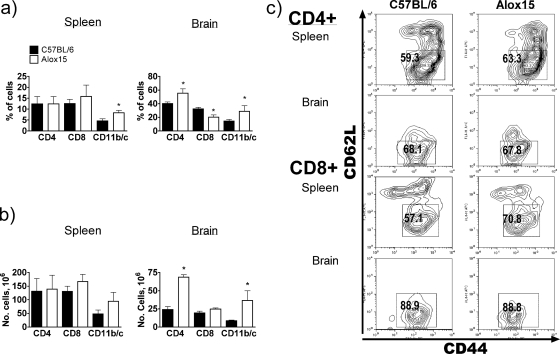
Other researchers have demonstrated that 12/15-LOX can upregulate chemokines and facilitate leukocyte extravasation into inflamed pancreas and atherosclerotic plaques (21, 31, 35). To determine how the loss of 12/15-LOX affects immune cell recruitment and activation during toxoplasmosis, we assessed the leukocyte populations in spleens and brains isolated from chronically infected Alox15 mice versus wild-type controls. Alox15 mice displayed increased proportions of CD11b/CD11c double-positive cells in both spleen and brain compared to wild-type mice and an increase in the ratio of CD4+ to CD8+ cells in the brain (Fig. 5a). When adjusted for the total cell numbers recovered from the tissue, the proportion of these cell types in spleen were comparable, whereas CD4+ lymphocyte and macrophage infiltration levels were augmented in brain (Fig. 5b and 2d).
FIG. 5.
12/15-LOX impacts leukocyte composition and activation during chronic toxoplasmosis. (a and b) The proportion (a) and number (b) of CD4+, CD8+, and CD11b+ CD11c+ (CD11b/c) cells in spleen and brains isolated from chronically infected C57BL/6 and Alox15 mice. (c) Flow cytometric analysis of CD62L and CD44 expression levels on CD4- and CD8-gated cells isolated from spleen or brain of chronically infected C57BL/6 and Alox15 mice. The percentage of the most activated cells (CD62lo CD44hi) are quantified in boxes. Four (spleen) or two (brain) separate flow cytometry experiments were conducted (n = 4 in each). *, P < 0.05.
CD4+ activation, as assessed by CD62L and CD44 expression (where CD62lo CD44hi populations are the most activated), was comparable between infected wild-type and Alox15 mice both centrally and peripherally (Fig. 5c). However, we detected a greater percentage of activated CD8+ cells in the spleens of chronically infected Alox15 mice compared to wild-type controls (Fig. 5c). Despite these differences, it is important to note that deletion of 12/15-LOX did not lead to impaired recruitment of leukocytes during chronic toxoplasmosis. Therefore, reduced leukocyte recruitment is unlikely to account for the overwhelming parasite burden in these animals.
12/15-LOX regulates Th1 cytokine production during chronic, but not acute, toxoplasmosis.
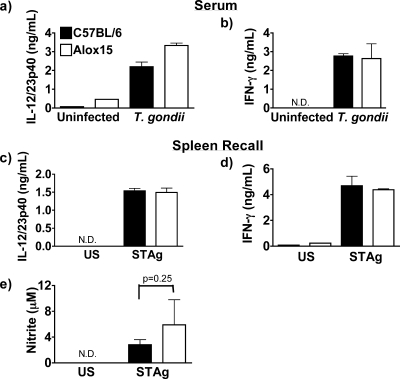
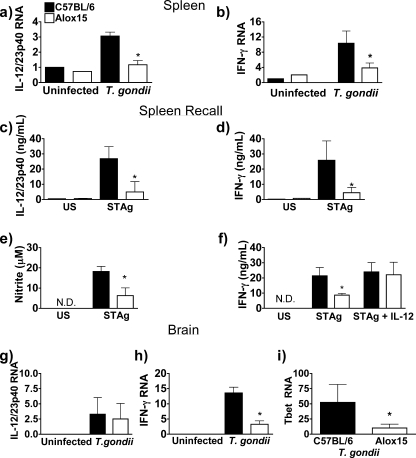
We tested the hypothesis that the loss of 12/15-LOX impacts the type 1 cytokine response to toxoplasmosis. Serum samples and splenocytes isolated from Alox15 mice during the acute phase (7 days postinfection) and restimulated with STAg displayed no defect in the production of IL-12/23p40, IFN-γ, or nitric oxide compared to wild-type controls (Fig. 6). This observation is in keeping with the fact that DCs, the principle IL-12 producers during acute toxoplasmosis (36), are not subject to regulation by 12/15-LOX in vitro (Fig. 1b). We next measured cytokine production during the chronic stage of infection. Ex vivo RNA levels of IL-12/23p40 and IFN-γ in spleen, as well as protein production during recall experiments, were markedly reduced in chronic-stage Alox15 mice compared to wild-type animals (Fig. 7a to d). Consistent with the diminished IFN-γ levels, we detected lower levels of nitric oxide production as well (Fig. 7e). Importantly, the addition of recombinant IL-12 was able to restore IFN-γ production in 12/15-LOX-deficient splenocytes isolated from chronically infected mice but had no impact on the levels of cytokines produced by wild-type cells (Fig. 7f). Thus, wild-type IL-12 levels are maximal for IFN-γ stimulation, whereas reduced IFN-γ production by splenocytes isolated from chronically infected Alox15 mice is attributable to a primary IL-12 defect.
FIG. 6.
12/15-LOX is dispensable for inflammatory mediator production during the acute phase of toxoplasmosis. (a and b) Serum levels of IL-12/23p40 (a) and IFN-γ (b) in C57/BL6 wild-type and Alox15 mice 7 days postinfection with T. gondii as assessed by ELISA. (c and d) IL-12/23p40 (c) and IFN-γ (d) production by splenocytes isolated from B6 and Alox15 mice that were restimulated with STAg for 48 h, as assessed by ELISA. (e) Nitric oxide (nitrite) production as assessed by Griess assay in restimulated B6 and Alox15 splenocytes. N.D., not detectable.
FIG. 7.
The 12/15-LOX-dependent pathway to IL-12 production predominates during chronic toxoplasmosis. (a and b) RNA levels of IL-12/23p40 (a) and IFN-γ (b) expression in spleens isolated from C57BL/6 wild-type and Alox15 mice 35 to 40 days postinfection with T. gondii, as assessed by real-time PCR. (c to e) IL-12/23p40 (c), IFN-γ (d), and NO (e) production by splenocytes isolated from B6 and Alox15 mice that were restimulated with STAg for 48 h, as assessed by ELISA. (f) IFN-γ production in cells stimulated as above except for the addition of 5 pg/ml recombinant IL-12 every 24 h where indicated. (g to i) RNA levels of IL-12/23p40 (g), IFN-γ (h), and T-bet (i) expression in 40 days postinfection with T. gondii in C57BL/6 and Alox15 mice (n ≥ 4). *, P < 0.05. N.D., not detectable.
In brain, although IL-12/23p40 RNA levels of expression were comparable between tissue isolated from wild-type and Alox15 mice, the expression would be considerably less in Alox15 animals if adjusted for total leukocyte or macrophage number (Fig. 7f; see also Fig. 5b and 8f). Moreover, the abundance of IFN-γ transcripts was reduced in chronically infected Alox15 brain tissue compared to controls (Fig. 7g). In keeping with this observation, T-bet, a transcription factor that is induced by IFN-γ and directs the Th1 response, was markedly reduced in brain tissue isolated from chronically infected Alox15 mice compared to controls (Fig. 7h). Despite the diminished type 1 cytokine production, we detected no differences in the expression of either IL-4 (a canonical type 2 cytokine) or arginase 1 (a marker of alternatively activated macrophages) in brain tissue (data not shown), indicating that the absence of 12/15-LOX did not skew the inflammatory response.
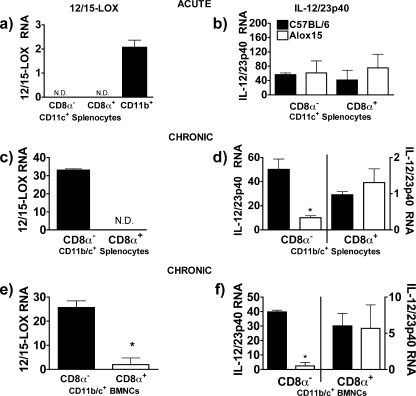
FIG. 8.
12/15-LOX is required for IL-12 generation by the principal IL-12-producing myeloid cells during chronic toxoplasmosis. (a, c, and e) 12/15-LOX expression in sorted cells from splenocytes isolated from mice infected with T. gondii for 7 days (a), splenocytes isolated from mice infected with T. gondii for 40 days (c), or BMNCs isolated from mice infected with T. gondii for 40 days (e). (b, d, and f) IL-12/23p40 expression in cells sorted based on CD11c and CD8α expression from wild-type and Alox15 splenocytes at 7 days postinfection (b), splenocytes at 40 days postinfection (d), or BMNCs at 40 days postinfection (f). Note the differences in the scales. *, P < 0.05. N.D., not detectable.
12/15-LOX is required for maximal IL-12 generation by the principal IL-12-producing myeloid cell during chronic toxoplasmosis.
As our data above indicated reduced T. gondii-stimulated IL-12 production in the absence of 12/15-LOX, we next investigated whether this enzyme is expressed in the cells that produce IL-12 during chronic toxoplasmosis. We sorted splenocytes from uninfected, acutely infected, and chronically infected mice based on CD11b, CD11c, and CD8α expression. We also sorted BMNCs from chronically infected mice and quantified the levels of 12/15-LOX and IL-12p40 transcripts by real-time PCR. During chronic toxoplasmosis, all CD11c+ cells were also CD11b+ (data not shown) Only CD11b+ CD8α− CD11c− subsets expressed 12/15-LOX in uninfected and acutely infected mice (Fig. 8a and data not shown), and these cells displayed negligible IL-12p40 expression during acute infection (data not shown) (36). No CD11c+ CD8α+ cells expressed 12/15-LOX during acute toxoplasmosis. As would be expected, there was no difference in IL-12 levels of expression between CD11c+ CD8α+ cells isolated from wild-type and Alox15 mice (Fig. 8b). 12/15-LOX transcripts were induced in CD11c+ CD8α− cells but not CD11c+ CD8α+ cells in spleen and brain (Fig. 8c and e). We also found that CD11c+ CD8α− IL-12/23p40 expression dominated over that of CD11c+ CD8α+ splenocytes and BMNCs by approximately 50-fold and 5-fold, respectively (Fig. 8d and f; note the different scales used). Thus, 12/15-LOX is expressed in the cells that produce IL-12 during chronic toxoplasmosis. Furthermore, the level of IL-12/23p40 was markedly reduced in CD11c+ CD8α− splenocytes and BMNCs isolated from chronically infected Alox15 mice compared to those isolated from wild-type animals (Fig. 8d and f). We did detect a small amount of 12/15-LOX expression in CD8α+ cells. These findings may be due to the induction of 12/15-LOX expression, but not the 12/15-LOX-dependent pathway to IL-12 in CD8α+ cells upon infiltration into brain, or a small amount of contamination during sorting. Overall, the above data indicate that 12/15-LOX mediates IL-12 production in the predominant IL-12-producing cells during chronic toxoplasmosis.
DISCUSSION
This study demonstrates for the first time that the absence of 12/15-LOX leads to selective reduction of IL-12 and IFN-γ production in the brain and periphery of mice during the chronic, but not acute, stage of toxoplasmosis, resulting in profound susceptibility to this phase of T. gondii infection.
Due to its restricted impact, the 12/15-LOX pathway to IL-12 production may thus prove useful in immunotherapy for chronic inflammatory disease.
In this report, we have identified a significant dependence on the 12/15-LOX pathway for IL-12/23p40 and subsequent IFN-γ production during chronic T. gondii infection. Consistent with other knockout models susceptible to toxoplasmosis, we observed an apparent disconnect between the levels of IL-12/23p40 and IFN-γ (5, 34), in our case only with regard to levels of expression in brain tissue. This may simply be due to the marked increase in IL-12-producing cells in Alox15 brain. Additionally, other 12/15-LOX-independent producers of IL-12/23p40, such as astrocytes and microglia, may dilute differences in brain IL-12/23p40 expression. In either case, priming of T lymphocytes for cerebral IFN-γ production may primarily occur in the periphery. Others have shown that once T lymphocytes home to brain, they downregulate proliferation and upregulate survival factors, such that the Th1 response during chronic toxoplasmosis is long-lived and requires IL-12 stimulation infrequently (43, 57). Indeed, 14-day administration of antibodies to IL-12/23p40 is unable to reverse preestablished T lymphocyte memory responses to T. gondii (44), and even the withdrawal of recombinant IL-12 from IL-12/23p40-deficient mice chronically infected with T. gondii does not lead to pathology until 2 weeks later (57). These data, together with our own, suggest that IL-12 is required for long-term IFN-γ production and T. gondii resistance during chronic toxoplasmosis, although it is yet unclear whether IL-12 is necessary in the brain. In addition to IL-12 and IL-23, deficiency in IL-12/23p40 itself may impact the susceptibility of Alox15 mice to toxoplasmosis, as it may have functions independent of heterodimer formation (38).
IL-12 is needed for the initiation of the Th1 inflammatory response to toxoplasma and appears to be intermittently required for maintenance of the chronic response. Others have shown that once T lymphocytes home to brain, they become less proliferative and upregulate survival factors (43, 57). The decreased turnover of these effector cells results in a long-lived immune response that likely requires IL-12 stimulation infrequently. Indeed, administration of antibodies against IL-12/23p40 for 14 days was not sufficient to reverse preestablished T lymphocyte memory responses to T. gondii (44), and the withdrawal of recombinant IL-12 from IL-12/23p40-deficient mice chronically infected with T. gondii did not lead to increased parasite numbers until 2 weeks later (57). These data, together with our own, suggest that IL-12 is required for long-term IFN-γ production and T. gondii resistance during chronic toxoplasmosis.
The mechanism by which 12/15-LOX regulates IL-12 production has not been completely elucidated. We previously demonstrated that 12/15-LOX may regulate IL-12 production by inducing the association of NF-κB and interferon regulatory factor-8 (IRF-8)/interferon consensus sequence binding protein (ICSBP) with the IL-12/23p40 promoter (27). In this regard, it is important to note that IRF-8/ICSBP-deficient mice succumb to T. gondii infection during the acute stage of toxoplasmosis, likely due to the fact that IRF-8/ICSBP is required for IL-12 production in both macrophages and dendritic cells, as well as for the development of critical dendritic cell subsets (40, 53). 12/15-LOX-deficient mice may be spared these sequelae due to the cell-type-restricted nature of 12/15-LOX expression (27, 49).
As we and others have reported, another similarity between 12/15-LOX-deficient and IRF-8/ICSBP-deficient mice is the fact that both genotypes develop a myeloproliferative disorder (14, 28), with the Alox15 phenotype becoming manifest with age. Importantly, we employed young mice in this study. Although Alox15 mice did demonstrate increased Gr-1-positive cells in the spleen, consistent with our prior report (average of 4.3% versus 8.7%) (28), we observed no increase in Gr-1-positive cells in the brain or atypical cells in the blood at this age (M. Middleton and E. Puré, unpublished results). Together with the fact that wild-type macrophages treated with the 12/15-LOX inhibitor PD146176 elaborate reduced levels of IL-12 in response to T. gondii antigen, it is clear that the myeloproliferative disorder apparent in older Alox15 mice is not the underlying cause of the immune defect observed in these animals during chronic toxoplasmosis.
The balance of arachidonic acid products appears to be an important determinant of chronic inflammation, especially with regard to type 1 cytokine production. Given the established interplay between 5-LOX and 12/15-LOX (49), obtaining a better understanding of the mechanisms by which these enzymes and their products impact chronic inflammatory disease is of import. The 12/15-LOX pathway to IL-12 production appears to act in a cell-type-restricted manner by selectively mediating IL-12p40 expression in macrophages (27), just as 5-LOX seems to exert most of its effects on dendritic cells (12, 47). Thus, differential expression and activity of 5-LOX and 12/15-LOX in dendritic cells and macrophages, respectively, may underlie some of the divergence between these two cell types in the context of cytokine production. Our prior work has demonstrated that individual stable end products of 12/15-LOX-mediated metabolism, such as 12(S)-HETE, 15(S)-HETE, and 13(S)-HODE, are unlikely to be directly involved in its ability to regulate IL-12 production (27). Also, supplementation of splenocytes isolated from chronically infected 12/15-LOX-deficient mice with 12(S)-HETE was unable to restore IL-12 production (Middleton and Puré, unpublished). Therefore, 12(S)-HETE may be a reflection of increased 12/15-LOX activity but unrelated to the impact of 12/15-LOX on IL-12 production. Another explanation may lie with the biologically active peroxidated products of 12/15-LOX, such as 12(S)-HpETE, 15(S)-HpETE, and 13(S)-HpODE, or their physiologically active metabolites (54). Moreover, the plethora of recently discovered 12/15-LOX products, such as its phospholipid derivatives (30), or even the ability of 12/15-LOX to generate reactive oxygen species may explain or at least contribute to the regulation of IL-12 production by this enzyme (7, 16, 32). Should stable analogs of these compounds be developed, it will be of interest to determine whether, like LxA4, such short-lived metabolites may be responsible for the impact of 12/15-LOX on chronic toxoplasmosis. Finally, we must consider the possibility that 12/15-LOX products, including many possible lipid mediators and reactive oxygen species, have a combinatorial effect, such that no one mediator can restore IL-12 production.
We previously demonstrated that the 12/15-LOX-dependent pathway to IL-12 is the predominant source of IL-12 production in atherosclerotic plaques (58). Together with the data we have presented in this report with regard to toxoplasmosis, as well as the fact that macrophage activity is important for many other chronic diseases (10, 15, 19), it may be that IL-12 production by macrophages is preferentially involved during the chronic response. Studies employing macrophage-specific knockouts of IL-12 will be required to formally test this hypothesis. However, with the growing recognition that there is a critical role for a macrophage-restricted pathway to IL-12 production in chronic inflammation, better understanding of the pathways that regulate macrophage cytokine production may provide opportunities to develop selective therapies for chronic inflammatory disease.
Acknowledgments
We acknowledge the generous support of the following members of the Wistar Institute: Irene Crichton for lab management, and colleagues in the Animal, Flow Cytometry Microscopy, and Histology core facilities and in Information Systems.
These studies were funded by grants AI45813 (to E.P.), AI41158 and AI71302 (to C.A.H.), CA095586 (to I.A.B.), T32CA01940 (to M.K.M.), 5-T32-GM07229-32 and 5-T32-CA09171-31 (to M.K.) provided by the National Institutes of Health and a grant from the Pennsylvania Department of Health. C.A.H. is a member of the Mari Lowe Center.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 12 October 2009.
REFERENCES
- 1.Aikawa, M., and P. Libby. 2004. Atherosclerotic plaque inflammation: the final frontier? Can. J. Cardiol. 20:631-634. [PubMed] [Google Scholar]
- 2.Aliberti, J., S. Hieny, C. Reis e Sousa, C. N. Serhan, and A. Sher. 2002. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat. Immunol. 3:76-82. [DOI] [PubMed] [Google Scholar]
- 3.Aliberti, J., C. Serhan, and A. Sher. 2002. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J. Exp. Med. 196:1253-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliberti, J., and A. Sher. 2002. Positive and negative regulation of pathogen induced dendritic cell function by G-protein coupled receptors. Mol. Immunol. 38:891-893. [DOI] [PubMed] [Google Scholar]
- 5.Blass, S. L., E. Pure, and C. A. Hunter. 2001. A role for CD44 in the production of IFN-gamma and immunopathology during infection with Toxoplasma gondii. J. Immunol. 166:5726-5732. [DOI] [PubMed] [Google Scholar]
- 6.Bocan, T. M., W. S. Rosebury, S. B. Mueller, S. Kuchera, K. Welch, A. Daugherty, and J. A. Cornicelli. 1998. A specific 15-lipoxygenase inhibitor limits the progression and monocyte-macrophage enrichment of hypercholesterolemia-induced atherosclerosis in the rabbit. Atherosclerosis 136:203-216. [DOI] [PubMed] [Google Scholar]
- 7.Brys, L., A. Beschin, G. Raes, G. H. Ghassabeh, W. Noel, J. Brandt, F. Brombacher, and P. De Baetselier. 2005. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J. Immunol. 174:6095-6104. [DOI] [PubMed] [Google Scholar]
- 8.Chinnici, C. M., Y. Yao, T. Ding, C. D. Funk, and D. Pratico. 2005. Absence of 12/15 lipoxygenase reduces brain oxidative stress in apolipoprotein E-deficient mice. Am. J. Pathol. 167:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad, D. J. 1999. The arachidonate 12/15 lipoxygenases. A review of tissue expression and biologic function. Clin. Rev. Allergy Immunol. 17:71-89. [DOI] [PubMed] [Google Scholar]
- 10.Gabaglia, C. R., E. E. Sercarz, Y. Diaz-De-Durana, M. Hitt, F. L. Graham, J. Gauldie, and T. A. Braciak. 2004. Life-long systemic protection in mice vaccinated with L. major and adenovirus IL-12 vector requires active infection, macrophages and intact lymph nodes. Vaccine 23:247-257. [DOI] [PubMed] [Google Scholar]
- 11.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 12.Hedi, H., and G. Norbert. 2004. 5-Lipoxygenase pathway, dendritic cells, and adaptive immunity J. Biomed. Biotechnol. 2004:99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodge-Dufour, J., P. W. Noble, M. R. Horton, C. Bao, M. Wysoka, M. D. Burdick, R. M. Strieter, G. Trinchieri, and E. Pure. 1997. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J. Immunol. 159:2492-2500. [PubMed] [Google Scholar]
- 14.Holtschke, T., J. Lohler, Y. Kanno, T. Fehr, N. Giese, F. Rosenbauer, J. Lou, K. P. Knobeloch, L. Gabriele, J. F. Waring, M. F. Bachmann, R. M. Zinkernagel, H. C. Morse III, K. Ozato, and I. Horak. 1996. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87:307-317. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann, S. H. 1991. The macrophage in tuberculosis: sinner or saint? The T cell decides. Pathobiology 59:153-155. [DOI] [PubMed] [Google Scholar]
- 16.Kim, Y. S., M. A. Reddy, L. Lanting, S. G. Adler, and R. Natarajan. 2003. Differential behavior of mesangial cells derived from 12/15-lipoxygenase knockout mice relative to control mice. Kidney Int. 64:1702-1714. [DOI] [PubMed] [Google Scholar]
- 17.Lang, C., U. Gross, and C. G. Luder. 2007. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitol. Res. 100:191-203. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence, T., M. Bebien, G. Y. Liu, V. Nizet, and M. Karin. 2005. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature 434:1138-1143. [DOI] [PubMed] [Google Scholar]
- 19.Leemans, J. C., T. Thepen, S. Weijer, S. Florquin, N. van Rooijen, J. G. van de Winkel, and T. van der Poll. 2005. Macrophages play a dual role during pulmonary tuberculosis in mice. J. Infect. Dis. 191:65-74. [DOI] [PubMed] [Google Scholar]
- 20.Levitz, S. M., and R. D. Diamond. 1985. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J. Infect. Dis. 152:938-945. [DOI] [PubMed] [Google Scholar]
- 21.Li, S. L., R. S. Dwarakanath, Q. Cai, L. Lanting, and R. Natarajan. 2005. Effects of silencing leukocyte-type 12/15-lipoxygenase using short interfering RNAs. J. Lipid Res. 46:220-229. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman, L. A., F. Cardillo, A. M. Owyang, D. M. Rennick, D. J. Cua, R. A. Kastelein, and C. A. Hunter. 2004. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J. Immunol. 173:1887-1893. [DOI] [PubMed] [Google Scholar]
- 23.Liu, B., L. J. Marnett, A. Chaudhary, C. Ji, I. A. Blair, C. R. Johnson, C. A. Diglio, and K. V. Honn. 1994. Biosynthesis of 12(S)-hydroxyeicosatetraenoic acid by B16 amelanotic melanoma cells is a determinant of their metastatic potential. Lab. Investig. 70:314-323. [PubMed] [Google Scholar]
- 24.Locksley, R. M., J. Fankhauser, and W. R. Henderson. 1985. Alteration of leukotriene release by macrophages ingesting Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 82:6922-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahida, Y. R. 2000. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm. Bowel Dis. 6:21-33. [DOI] [PubMed] [Google Scholar]
- 26.Makheja, A. N. 1992. Atherosclerosis: the eicosanoid connection. Mol. Cell. Biochem. 111:137-142. [DOI] [PubMed] [Google Scholar]
- 27.Middleton, M. K., T. Rubinstein, and E. Puré. 2006. Cellular and molecular mechanisms of the selective regulation of IL-12 production by 12/15-lipoxygenase. J. Immunol. 176:265-274. [DOI] [PubMed] [Google Scholar]
- 28.Middleton, M. K., A. M. Zukas, T. Rubinstein, M. Jacob, P. Zhu, L. Zhao, I. Blair, and E. Puré. 2006. Identification of 12/15-lipoxygenase as a suppressor of myeloproliferative disease. J. Exp. Med. 203:2529-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 30.Morgan, A. H., V. Dioszeghy, B. H. Maskrey, C. P. Thomas, S. R. Clark, S. A. Mathie, C. M. Lloyd, H. Kühn, N. Topley, B. C. Coles, P. R. Taylor, S. A. Jones, and V. B. O'Donnell. 2009. Phosphatidylethanolamine-esterified eicosanoids in the mouse: tissue localization and inflammation-dependent formation in Th-2 disease. J. Biol. Chem. 284:21185-21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natarajan, R., and J. L. Nadler. 2003. Lipoxygenases and lipid signaling in vascular cells in diabetes. Front. Biosci. 8:s783-s795. [DOI] [PubMed] [Google Scholar]
- 32.Rankin, S. M., S. Parthasarathy, and D. Steinberg. 1991. Evidence for a dominant role of lipoxygenase(s) in the oxidation of LDL by mouse peritoneal macrophages. J. Lipid Res. 32:449-456. [PubMed] [Google Scholar]
- 33.Reddy, M. A., Y. S. Kim, L. Lanting, and R. Natarajan. 2003. Reduced growth factor responses in vascular smooth muscle cells derived from 12/15-lipoxygenase-deficient mice. Hypertension 41:1294-1300. [DOI] [PubMed] [Google Scholar]
- 34.Reichmann, G., W. Walker, E. N. Villegas, L. Craig, G. Cai, J. Alexander, and C. A. Hunter. 2000. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 68:1312-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reilly, K. B., S. Srinivasan, M. E. Hatley, M. K. Patricia, J. Lannigan, D. T. Bolick, G. Vandenhoff, H. Pei, R. Natarajan, J. L. Nadler, and C. C. Hedrick. 2004. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J. Biol. Chem. 279:9440-9450. [DOI] [PubMed] [Google Scholar]
- 36.Reis e Sousa, C., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R. N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocha, P. N., T. J. Plumb, and T. M. Coffman. 2003. Eicosanoids: lipid mediators of inflammation in transplantation. Springer Semin. Immunopathol. 25:215-227. [DOI] [PubMed] [Google Scholar]
- 38.Russell, T. D., Q. Yan, G. Fan, A. P. Khalifah, D. K. Bishop, S. L. Brody, and M. J. Walter. 2003. IL-12 p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta 1. J. Immunol. 171:6866-6874. [DOI] [PubMed] [Google Scholar]
- 39.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997-6001. [DOI] [PubMed] [Google Scholar]
- 40.Scharton-Kersten, T., C. Contursi, A. Masumi, A. Sher, and K. Ozato. 1997. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J. Exp. Med. 186:1523-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharton-Kersten, T. M., T. A. Wynn, E. Y. Denkers, S. Bala, E. Grunvald, S. Hieny, R. T. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045-4054. [PubMed] [Google Scholar]
- 42.Scharton-Kersten, T. M., G. Yap, J. Magram, and A. Sher. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schluter, D., T. Meyer, L. Y. Kwok, M. Montesinos-Rongen, S. Lutjen, A. Strack, M. L. Schmitz, and M. Deckert. 2002. Phenotype and regulation of persistent intracerebral T cells in murine Toxoplasma encephalitis. J. Immunol. 169:315-322. [DOI] [PubMed] [Google Scholar]
- 44.Seder, R. A., B. L. Kelsall, and D. Jankovic. 1996. Differential roles for IL-12 in the maintenance of immune responses in infectious versus autoimmune disease. J. Immunol. 157:2745-2748. [PubMed] [Google Scholar]
- 45.Serhan, C. N., M. Hamberg, and B. Samuelsson. 1984. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. USA 81:5335-5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Son, Y. I., S. Egawa, T. Tatsumi, R. E. Redlinger, Jr., P. Kalinski, and T. Kanto. 2002. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J. Immunol. Methods 262:145-157. [DOI] [PubMed] [Google Scholar]
- 47.Spanbroek, R., M. Hildner, A. Kohler, A. Muller, F. Zintl, H. Kuhn, O. Radmark, B. Samuelsson, and A. J. Habenicht. 2001. IL-4 determines eicosanoid formation in dendritic cells by down-regulation of 5-lipoxygenase and up-regulation of 15-lipoxygenase 1 expression. Proc. Natl. Acad. Sci. USA 98:5152-5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subauste, C. S., and J. S. Remington. 1993. Immunity to Toxoplasma gondii. Curr. Opin. Immunol. 5:532-537. [DOI] [PubMed] [Google Scholar]
- 49.Sun, D., and C. D. Funk. 1996. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J. Biol. Chem. 271:24055-24062. [PubMed] [Google Scholar]
- 50.Suzuki, Y., F. K. Conley, and J. S. Remington. 1989. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol. 143:2045-2050. [PubMed] [Google Scholar]
- 51.Suzuki, Y., M. A. Orellana, S. Y. Wong, F. K. Conley, and J. S. Remington. 1993. Susceptibility to chronic infection with Toxoplasma gondii does not correlate with susceptibility to acute infection in mice. Infect. Immun. 61:2284-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thardin, J. F., C. M'Rini, M. Beraud, J. Vandaele, M. F. Frisach, M. H. Bessieres, J. P. Seguela, and B. Pipy. 1993. Eicosanoid production by mouse peritoneal macrophages during Toxoplasma gondii penetration: role of parasite and host cell phospholipases. Infect. Immun. 61:1432-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsujimura, H., T. Tamura, C. Gongora, J. Aliberti, C. Reis e Sousa, A. Sher, and K. Ozato. 2003. ICSBP/IRF-8 retrovirus transduction rescues dendritic cell development in vitro. Blood 101:961-969. [DOI] [PubMed] [Google Scholar]
- 54.Wei, C., P. Zhu, S. J. Shah, and I. A. Blair. 2009. 15-oxo-Eicosatetraenoic acid, a metabolite of macrophage 15-hydroxyprostaglandin dehydrogenase that inhibits endothelial cell proliferation. Mol. Pharmacol. 76:516-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson, E. H., U. Wille-Reece, F. Dzierszinski, and C. A. Hunter. 2005. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J. Neuroimmunol. 165:63-74. [DOI] [PubMed] [Google Scholar]
- 56.Yang, X. Y., L. H. Wang, K. Mihalic, W. Xiao, T. Chen, P. Li, L. M. Wahl, and W. L. Farrar. 2002. Interleukin (IL)-4 indirectly suppresses IL-2 production by human T lymphocytes via peroxisome proliferator-activated receptor gamma activated by macrophage-derived 12/15-lipoxygenase ligands. J. Biol. Chem. 277:3973-3978. [DOI] [PubMed] [Google Scholar]
- 57.Yap, G., M. Pesin, and A. Sher. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165:628-631. [DOI] [PubMed] [Google Scholar]
- 58.Zhao, L., C. A. Cuff, E. Moss, U. Wille, T. Cyrus, E. A. Klein, D. Pratico, D. J. Rader, C. A. Hunter, E. Puré, and C. D. Funk. 2002. Selective interleukin-12 synthesis defect in 12/15-lipoxygenase-deficient macrophages associated with reduced atherosclerosis in a mouse model of familial hypercholesterolemia. J. Biol. Chem. 277:35350-35356. [DOI] [PubMed] [Google Scholar]