Abstract
Culture filtrate proteins (CFP) are potential targets for tuberculosis vaccine development. We previously showed that despite the high level of gamma interferon (IFN-γ) production elicited by homologous immunization with CFP plus CpG oligodeoxynucleotides (CFP/CpG), we did not observe protection when these mice were challenged with Mycobacterium tuberculosis. In order to use the IFN-γ-inducing ability of CFP antigens, in this study we evaluated a prime-boost heterologous immunization based on CFP/CpG to boost Mycobacterium bovis BCG vaccination in order to find an immunization schedule that could induce protection. Heterologous BCG-CFP/CpG immunization provided significant protection against experimental tuberculosis, and this protection was sustained during the late phase of infection and was even better than that conferred by a single BCG immunization. The protection was associated with high levels of antigen-specific IFN-γ and interleukin-17 (IL-17) and low IL-4 production. The deleterious role of IL-4 was confirmed when IL-4 knockout mice vaccinated with CFP/CpG showed consistent protection similar to that elicited by BCG-CFP/CpG heterologous immunization. These findings show that a single dose of CFP/CpG can represent a new strategy to boost the protection conferred by BCG vaccination. Moreover, different immunological parameters, such as IFN-γ and IL-17 and tightly regulated IL-4 secretion, seem to contribute to the efficacy of this tuberculosis vaccine.
The attenuated Mycobacterium bovis strain bacillus Calmette-Guérin (BCG) is the currently used vaccine against tuberculosis (TB). In spite of its wide use, the BCG vaccine only protects against severe forms of childhood TB and generally does not prevent adult pulmonary TB (11, 30, 47).
Considering that one-third of the world population is thought to be infected with Mycobacterium tuberculosis and that only a small proportion of these individuals will develop active disease, new vaccine candidates to prevent the establishment of infection could also boost and improve the cellular immunity of already latently infected individuals. Vaccine candidates currently in clinical trials include improved recombinant BCG vaccines, virus-based recombinant vaccines, and subunit vaccines comprised of dominant secreted antigens (1, 32). Secreted proteins, regularly described as culture filtrate proteins (CFP), are the main targets of the T-cell response in mice, both at the height of infection and in a state of memory immunity, as well as in humans with active TB (1, 4, 5, 7, 23). Immunization with these antigens in the presence of different adjuvants provided protection in mice challenged with M. tuberculosis, and protection was mediated by gamma interferon (IFN-γ)-producing CD4+ cells (29, 38).
We previously showed that a homologous immunization schedule based on three doses of CFP antigens plus CpG oligodeoxynucleotide adjuvant stimulated significant IFN-γ production by spleen cells and in the lungs of challenged mice. In spite of high IFN-γ concentrations, immunized and challenged mice were not protected and indeed had extensive lung damage (16).
Since IFN-γ is the best indicator of protective immunity defined thus far, we changed the schedule of homologous immunization to heterologous immunization, also known as a prime-boost regimen, to induce protection.
Several studies have demonstrated the efficacy of prime-boost vaccination strategies in generating cellular immunity to a variety of pathogens (3, 10, 14, 17, 34, 36, 44, 45, 49). Recently, our group also showed that a single dose of a DNA-HSP65 vaccine booster significantly enhanced the protection conferred against TB by a single subcutaneous dose of BCG (18). In addition, secreted antigens such as the 6-kDa early-secretion antigen target (ESAT-6), 85A or 85B antigens, and Mtb72F have proven to be promising candidates for BCG-boosting vaccines in mice, guinea pigs, and nonhuman primates (6, 9, 12, 19, 33, 37, 46, 48). Because a single dominant antigen may not confer the same level of protection to all vaccinated individuals, and based on high CFP antigen-mediated IFN-γ production in the presence of CpG adjuvant, in this study we used CFP plus CpG oligodeoxynucleotides to boost BCG vaccination in order to improve protection and lung preservation following M. tuberculosis challenge.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female BALB/c or C57BL/6 mice, 6 to 8 weeks old, were obtained from the local breeding facility of the University of São Paulo at Ribeirão Preto School of Medicine. Interleukin-4 knockout (IL-4 KO) mice were donated by João Santana da Silva, University of São Paulo at Ribeirão Preto School of Medicine. Mice were housed under barrier conditions in a level III biohazard laboratory and provided with food and sterile water. Experiments were conducted according to the local ethical guidelines.
CFP.
CFP were obtained using an M. tuberculosis 14-day culture, as previously described (16).
Immunizations.
We employed three immunization schedules: (i) a single subcutaneous BCG dose (105 bacilli, Moreau strain); (ii) three subcutaneous injections of CFP (50 μg) plus CpG 1826 (50 μg) (Invitrogen, San Diego, CA) (CFP/CpG) at 7-day intervals; and (iii) prime-boost immunizations, with one group receiving a BCG prime and a single CFP/CpG boost 15 days later, both by subcutaneous route (BCG-CFP/CpG group), and another group receiving a single CFP/CpG prime and a BCG boost 15 days later (CFP/CpG-BCG group).
Bacteria and challenge.
M. tuberculosis H37Rv (ATCC 27294; American Type Culture Collection, Rockville, MD) was grown in 7H9 Middlebrook broth (Difco Laboratories, Detroit, MI) for 7 days at 37°C and used as previously described (8, 16). Sixty days after the last immunization, mice were challenged with 105 bacilli via the intratracheal route.
Preparation of lung and spleen cell suspension for CFU assay and cultures.
The right lower and middle lobes of lungs were digested using Liberase Blendzyme 2 solution, 0.5 μg/ml (Roche, Indianapolis, IN), as previously described (8, 16). For the CFU assay, serial dilutions of digested lungs and macerated spleens were plated on 7H11 agar medium (Difco). The CFU were counted 28 days after incubation at 37°C. Lung cell suspensions were then passed through a nylon screen and resuspended in complete RPMI 1640 medium (10% fetal bovine serum, gentamicin, penicillin/streptomycin, and polymyxin B). Total cell counts were determined in a Neubauer chamber, and 2 × 106 cells/ml were cultured in complete RPMI 1640 medium for 48 h at 37°C in 5% CO2 in the presence of 10 μg/ml of CFP antigens. For negative and positive controls, lung cells were cultured with RPMI 1640 medium or with 40 μg/ml of concanavalin A (Sigma), respectively.
Cytokine production.
IFN-γ, IL-4, IL-5, IL-6, IL-10, IL-12, transforming growth factor β (TGF-β), and IL-17 concentrations were determined in lung culture supernatants and lung homogenates (left lobes) by enzyme-linked immunosorbent assay according to the manufacturer's instructions. Anti-mouse purified monoclonal antibodies (MAb) to the following cytokines were used (1 μg/ml): IFN-γ (R4-6A2), IL-4 (11B11), IL-5 (TRKF5), IL-6 (MP5-20F3), IL-10 (JES5-2A5), IL-12 (C15.6), and TGF-β (A75-2.1) (BD Biosciences-PharMingen, San Diego, CA). Cytokine-antibody complexes were detected via the addition of 0.5 μg/ml of anti-mouse biotinylated MAb to the following cytokines: IFN-γ (XMG1.2), IL-4 (BVD6-24G2), IL-5 (TRFK4), IL-6 (MP5-32C11), IL-10 (SXC-1), IL-12 (C17.8), and TGF-β (A75-3.1) (BD Biosciences-PharMingen, San Diego, CA). For IL-17 detection, an enzyme-linked immunosorbent assay kit (R&D) was used.
Histology and immunohistochemistry.
For histopathological analysis, the right upper lobes of the lungs were fixed in 10% formalin, embedded in paraffin blocks, prepared routinely, and then sectioned for light microscopy. Five-micrometer sections were stained with hematoxylin-eosin. All samples were analyzed by a pathologist in a double-blind test.
For the immunohistochemistry staining, the left lobes were frozen in O.C.T. compound (Sakura Finetek, Torrance, CA). Immunohistochemistry was performed using the avidin-biotin-peroxidase method with rat anti-mouse MAb to IFN-γ (XMG1.2), IL-4 (BVD6-24G2), and IL-10 (SXC-1) (BD Biosciences-PharMingen, San Diego, CA), according to the manufacturer's instructions. For a control, we used a purified mouse IgG1 isotype control (BD Biosciences-PharMingen, San Diego, CA). The lung sections were microphotographed in different areas with a final magnification of ×200, and brown-stained areas were quantified using ImageJ software.
Statistical analysis.
All values were expressed as means ± standard deviation. Data were compared using analysis of variance and PRISMA software. When the values indicated the presence of a significant difference, the Tukey test was used. P values of <0.05 were considered significant.
RESULTS
BCG protection was enhanced by CFP/CpG booster.
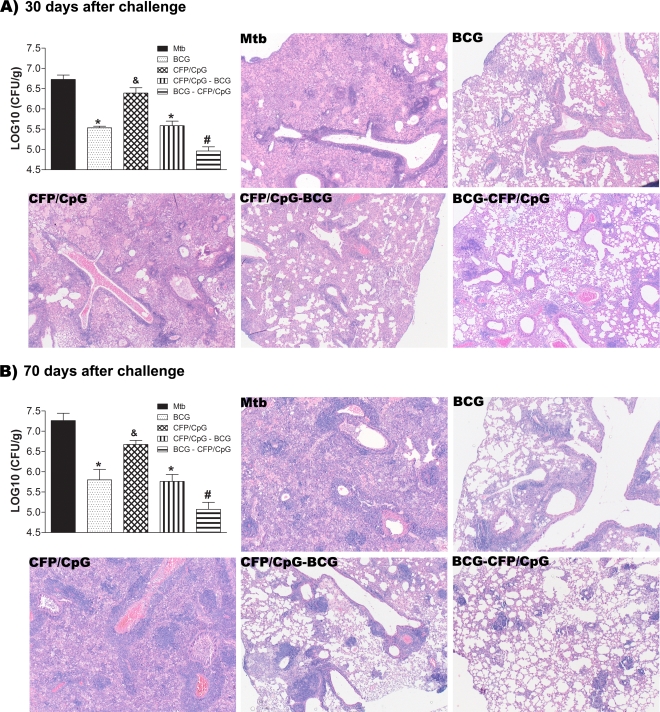
BCG is used as the gold standard of protection against TB in mouse models. In an attempt to improve BCG protection, and in order to evaluate a heterologous immunization schedule using CFP antigens plus CpG (CFP/CpG), we evaluated the progression of TB in mice subjected to the different vaccination schedules. BCG immunization significantly protected the challenged mice compared to the nonimmunized, infected mice (MTB group) (P < 0.05). Protection was evidenced 30 (Fig. 1A) and 70 (Fig. 1B) days after infection, which correspond to initial and late phases of infection, respectively. When we used the heterologous immunization comprised of a BCG prime and a CFP/CpG booster (BCG-CFP/CpG), CFU counts were significantly lower than in samples obtained after a single BCG immunization, either 30 or 70 days after challenge (P < 0.05). However, when we changed the order of immunization and used a CFP/CpG prime followed by a BCG booster (CFP/CpG-BCG), we noted no additional protection. CFP/CpG homologous immunization did not confer protection against TB, as previously reported (16).
FIG. 1.
Protection against experimental TB conferred by the different vaccination schedules. BALB/c mice (n = 8 to 10) were immunized subcutaneously with a single dose of BCG (BCG group), three doses of CFP/CpG (CFP/CpG group) at 7-day intervals, one dose of BCG followed by one dose of CFP/CpG after 15 days (BCG-CFP/CpG group), or one dose of CFP/CpG followed by one dose of BCG after 15 days (CFP/CpG-BCG group). Sixty days after vaccination, mice were challenged with a virulent strain of M. tuberculosis. At 30 (A) or 70 (B) days after infection, the lungs were processed for the CFU assay and histopathological analysis. Bacterial load is expressed as log10 CFU/g of lung from the means ± standard deviations of serial dilutions individually counted for each group. Results are from experiments repeated twice. #, P < 0.05 versus other groups; *, P < 0.05 versus nonimmunized, infected mice (MTB group); &, P < 0.05 versus the BCG-vaccinated group. For histological representation of the lungs from infected mice, sections (5 μm) of 30-day or 70-day infected lungs were stained with hematoxylin and eosin. Magnification, ×50.
At 30 and 70 days after infection, mice from the MTB group had pronounced pulmonary parenchyma involvement, characterized by isolated and confluent granulomas and an intense infiltration of macrophages, lymphocytes, and neutrophils. These mice also manifested bronchus-associated lymphoid tissue hyperplasia and exudates in the parenchyma (Fig. 1 and Table 1). It is worth mentioning that the lung injury increased at 70 days after infection, when we also observed the presence of foamy macrophages and neutrophil aggregates. In agreement with the CFU counts, the injury of lung tissue in mice immunized with CFP/CpG was similar to that seen in the MTB group, and in some areas, the inflammation was worse. Compared to the lung tissue of the MTB group, the lung tissue of the BCG and CFP/CpG-BCG groups had more preserved areas, as well as lower inflammation. We observed more isolated granulomas, with a predominance of lymphocytes and macrophages. In contrast, the lung tissue of BCG-CFP/CpG-immunized mice was better preserved than that of other immunized groups. We observed a predominant lymphocyte influx and isolated granulomas but no neutrophil infiltration. Moreover, while the infection seemed to progress in all the other groups, this group showed a reduction in lung injury at 30 to 70 days after infection. Thus, CFP/CpG vaccination did not confer protection when used in a homologous immunization schedule but increased the protection conferred by BCG vaccination. Moreover, this additional protective effect was observed only when CFP/CpG was used as a booster of a BCG prime and not the reverse. This protection was sustained even in the late phase of infection.
TABLE 1.
Qualitative histopathological analysisa
| Group and infection time (days) | Degree of parenchyma damage | Description of granulomas | Infiltration of macrophage (other cells) | Infiltration of neutrophil | Infiltration of lymphocyte | Degree of disease progression |
|---|---|---|---|---|---|---|
| MTB | ||||||
| 30 | +++ | Isolated and confluents | +++ | + | +++ | ++ |
| 70 | ++++ | Confluents | ++++ (foam cells) | ++ | +++ | |
| BCG | ||||||
| 30 | + | Isolated | ++ | − | +++ | + |
| 70 | ++ | Isolated and confluents | ++ | + | +++ | |
| CFP/CpG | ||||||
| 30 | +++ | Confluents | +++ | ++ | +++ | ++ |
| 70 | ++++ | Confluents | ++++ (foam cells) | +++ | +++ | |
| CFP/CpG-BCG | ||||||
| 30 | + | Isolated | +++ | − | +++ | + |
| 70 | ++ | Isolated and confluents | ++ | + | +++ | |
| BCG-CFP/CpG | ||||||
| 30 | + | Isolated | + | − | ++ | − |
| 70 | + | Isolated | + | − | ++ |
Mice (n = 8 to 10) were vaccinated with BCG, CFP/CpG, BCG-CFP/CpG, or CFP/CpG-BCG, as described in the legend to Fig. 1. Sixty days after the last immunization, we performed an intratracheal challenge with M. tuberculosis, and at 30 and 70 days after infection, the lungs were collected and processed for the histopathological analysis. The symbols indicate the absence (−) or the very low (+), low (++), intermediate (+++), or intense (++++) presence of each parameter evaluated.
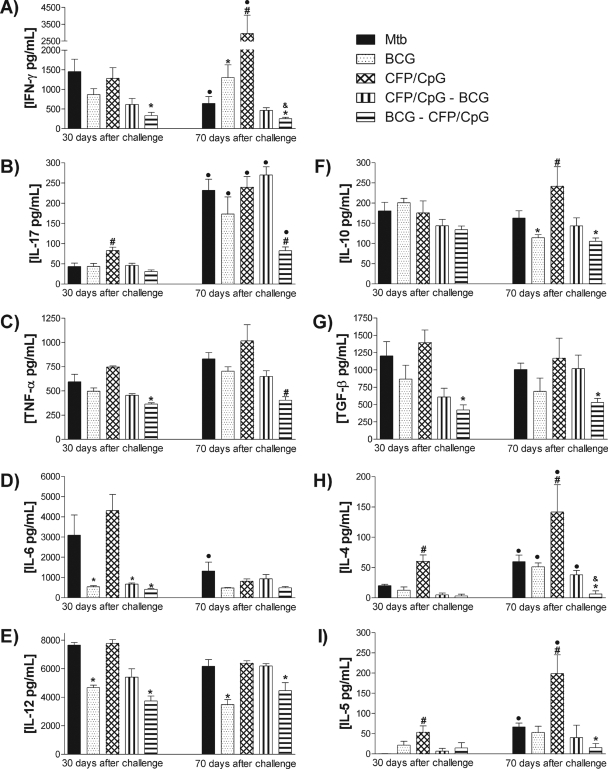
Protection conferred by BCG-CFP/CpG did not induce high concentrations of IFN-γ but did result in low concentrations of IL-4 ex vivo.
As we observed the differential protection conferred by the distinct vaccination schedules, we evaluated ex vivo cytokine production in lung homogenates and sections. Thirty days after infection, IFN-γ production levels were similar between the MTB group and the CFP/CpG-vaccinated mice (Fig. 2A). However, 70 days after infection, while the MTB group exhibited a decrease in local IFN-γ concentrations compared to the concentrations at 30 days (P < 0.05), the CFP/CpG group exhibited high IFN-γ concentrations (P < 0.05). Mice immunized with a single BCG dose, CFP/CpG-BCG, or BCG-CFP/CpG had reductions in IFN-γ concentrations compared to the MTB group 30 days after infection. This reduction was statistically significant only in mice vaccinated with BCG-CFP/CpG. Seventy days after infection, BCG-, CFP/CpG-BCG-, or BCG-CFP/CpG-immunized mice sustained the production of this cytokine beyond that at 30 days after infection. However, the IFN-γ was higher in single-dose BCG-immunized mice than in mice receiving prime-boost BCG-CFP/CpG immunization.
FIG. 2.
Ex vivo cytokine detection in the lungs. Mice (n = 8 to 10) were vaccinated with BCG, CFP/CpG, BCG-CFP/CpG, or CFP/CpG-BCG, as described in the legend to Fig. 1. Sixty days after the last immunization, we performed an intratracheal challenge with M. tuberculosis, and at 30 and 70 days after infection, local cytokine production was evaluated ex vivo in lung homogenates. Results are expressed as means ± standard deviations of cytokine concentrations individually analyzed and obtained in experiments repeated twice. •, P < 0.05 versus the same group at 30 days postchallenge. The following symbols indicate significance at the same time of infection: #, P < 0.05 versus other groups; *, P < 0.05 versus nonimmunized, infected mice (MTB group); &, P < 0.05 versus the BCG-vaccinated group.
The induction of a Th17 response was assayed by the detection of IL-17 (Fig. 2B). Thirty days after infection, the CFP/CpG-immunized group exhibited a significant increase in IL-17 production compared to other groups. However, at 70 days after infection, the concentrations of IL-17 were similar among the MTB-, BCG-, CFP/CpG-, and CFP/CpG-BCG-immunized groups and increased significantly in all experimental groups compared to the respective infected groups at day 30. However, the IL-17 concentrations were significantly lower in BCG-CFP/CpG-vaccinated mice (P < 0.05).
The concentrations of inflammatory cytokines tumor necrosis factor alpha (TNF-α), IL-6, and IL-12 (Fig. 2C, D, and E, respectively) were similar between the BCG, CFP/CpG-BCG, and BCG-CFP/CpG groups after 30 days. At the late phase, IL-6 and IL-12 concentrations were still similar between these groups. However, the BCG-CFP/CpG group exhibited a decrease in TNF-α concentrations compared to all other groups.
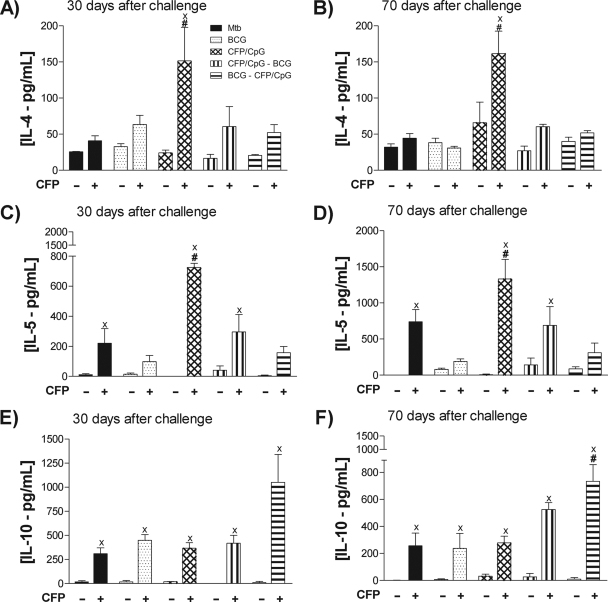
Figure 2F shows no difference in IL-10 concentrations detected after 30 days. However, 70 days after challenge, while single-dose BCG and BCG-CFP/CpG groups showed a significant decrease in IL-10 compared to that of the MTB group, the CFP/CpG group exhibited higher IL-10 concentrations than the other groups (P < 0.01). Moreover, only BCG-CFP/CpG-immunized mice exhibited TGF-β concentrations lower than those detected in the MTB group (Fig. 2G).
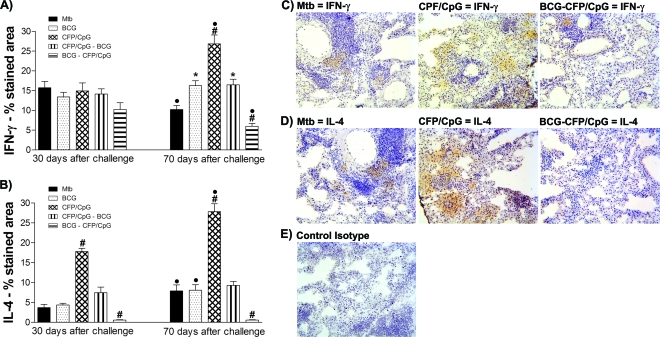
We also observed an increase in IL-4 and IL-5 in the lungs of the MTB group 70 days after infection, compared to that seen after 30 days (P < 0.05) (Fig. 2H and 2I). Mice immunized with a single BCG dose also exhibited increased concentrations of IL-4 at 70 days compared to 30 days after infection. In spite of the increased levels of IFN-γ detected in CFP/CpG-immunized mice, this group also showed the highest concentrations of IL-4 and IL-5, either 30 or 70 days after challenge (P < 0.01). In the prime-boost immunization protocols, with BCG-CFP/CpG and CFP/CpG-BCG, very low concentrations of IL-4 and IL-5 were detected in both evaluated periods, 30 and 70 days postinfection (P < 0.05). To confirm these data, and to determine the localization of the produced cytokines in lung tissue, IFN-γ and IL-4 were detected by immunohistochemistry. As expected, the results obtained were similar to those observed in lung homogenates. Figure 3 shows that at 30, and mainly at 70, days after infection, lung parenchyma of CFP/CpG-immunized mice exhibited a marked increase in IFN-γ and IL-4 levels compared to those in the other groups. Very low detection of IL-4 and weak staining for IFN-γ were observed in the lung parenchyma of BCG-CFP/CpG- and BCG-immunized mice compared to those for the MTB group (P < 0.05). However, IFN-γ was found over a greater area along the granulomas than was IL-4.
FIG. 3.
Ex vivo cytokine detection in the lungs. Mice (n = 8 to 10) were vaccinated with BCG, CFP/CpG, BCG-CFP/CpG, or CFP/CpG-BCG, as described in the legend to Fig. 1. Sixty days after the last immunization, we performed an intratracheal challenge with M. tuberculosis, and at 30 and 70 days after infection, local cytokine production was evaluated ex vivo in lung sections by immunohistochemistry assay. The results were obtained from experiments repeated twice. (A and B) Morphometric analyses of stained areas. •, P < 0.05 versus the same group at 30 days postchallenge. The following symbols indicate significance at the same time of infection: #, P < 0.05 versus other groups; *, P < 0.05 versus nonimmunized, infected mice (MTB group). (C and D) Representative sections of lungs from mice after 70 days of infection. (E) Control isotype. Magnification, ×200.
In conclusion, these results show that although CFP/CpG-immunized mice exhibited an increase of IFN-γ, IL-17, and inflammatory cytokines, they also secreted high concentrations of Th2 cytokines ex vivo. On the other hand, in the BCG-CFP/CpG-immunized mice, we detected lower levels of IFN-γ, IL-17, and inflammatory cytokines, as well as Th2 and regulatory cytokines, than in the other groups. Thus, although vaccination with BCG-CFP/CpG did not increase IFN-γ production (as seen in BCG and CFP/CpG groups), induction of IL-4 was impaired.
The BCG-CFP/CpG-immunized mice secreted higher levels of IFN-γ and IL-17 than other groups after in vitro lung cell stimulation.
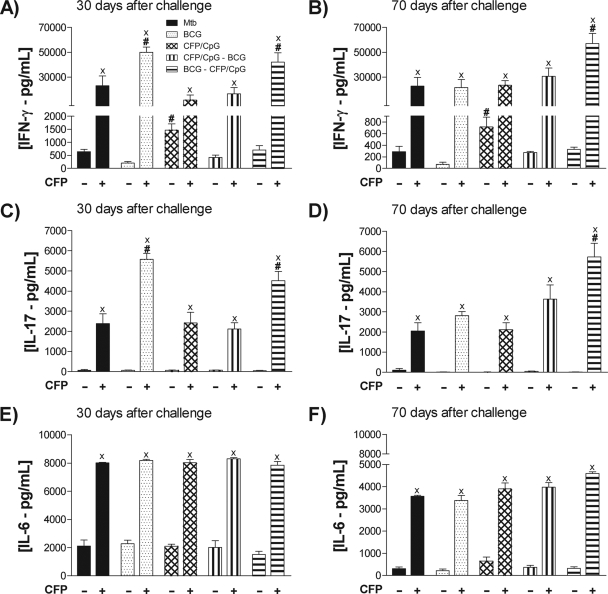
To evaluate the pattern of cytokines secreted by CFP-specific cells, we evaluated supernatants of lung cell cultures restimulated with these antigens.
Only lung cells obtained from CFP/CpG-immunized mice were able to release IFN-γ spontaneously (without CFP stimulation) (P < 0.05) (Fig. 4A). However, after restimulation, cells obtained from CFP/CpG-immunized mice secreted lower concentrations of IFN-γ than those from mice immunized with a single BCG dose and those vaccinated with BCG-CFP/CpG. In fact, these two latter groups exhibited significantly higher IFN-γ concentrations than were detected in other groups after in vitro stimulation. Seventy days after challenge, the BCG-CFP/CpG group still sustained higher IFN-γ concentrations than other groups (Fig. 4B). We found exactly the same pattern of response when we evaluated IL-17 concentrations (Fig. 3C and D). IL-6 secretion levels were similar among experimental groups (Fig. 4E and F).
FIG. 4.
Cytokine production by lung cells. Mice (n = 8 to 10) were vaccinated with BCG, CFP/CpG, BCG-CFP/CpG, or CFP/CpG-BCG, as described in the legend to Fig. 1. Sixty days after the last immunization, we performed an intratracheal challenge with M. tuberculosis, and at 30 and 70 days after infection, the CFP-specific cytokine production was evaluated in vitro in lung cell cultures when left unstimulated or when stimulated with CFP antigens. Results are expressed as means ± standard deviations of cytokine concentrations individually analyzed and obtained in experiments repeated twice. ×, P < 0.001 versus nonstimulated cells; #, P < 0.05 versus other groups.
Homologous immunization with CFP/CpG induced high levels of IL-4 and IL-5 secretion, while BCG-CFP/CpG immunization did not.
Production of IL-4 and IL-5 was upregulated by lung cells of CFP/CpG-immunized mice after in vitro CFP stimulation, either 30 (Fig. 5A) or 70 (Fig. 5B) days after challenge, while it was not in the other groups.
FIG. 5.
Cytokine production by lung cells. Mice (n = 8 to 10) were vaccinated with BCG, CFP/CpG, BCG-CFP/CpG, or CFP/CpG-BCG, as described in the legend to Fig. 1. Sixty days after the last immunization, we performed an intratracheal challenge with M. tuberculosis, and at 30 and 70 days after infection, the CFP-specific cytokine production was evaluated in vitro in lung cell cultures when left unstimulated or when stimulated with CFP antigens. Results are expressed as means ± standard deviations of cytokine concentrations individually analyzed and obtained in experiments repeated twice. ×, P < 0.05 versus nonstimulated cells; #, P < 0.05 versus other groups.
Only the BCG-CFP/CpG group showed antigen-specific IL-10 upregulation (Fig. 5E and F). In contrast to data obtained in ex vivo cytokine analyses, we verified that, while CFP-specific cells of the BCG-CFP/CpG group sustained the ability to produce IFN-γ in vitro, CFP-specific cells of the CFP/CpG group did not and, indeed, secreted significant concentrations of IL-4 and IL-5.
CFP/CpG homologous immunization conferred protection in the absence of IL-4.
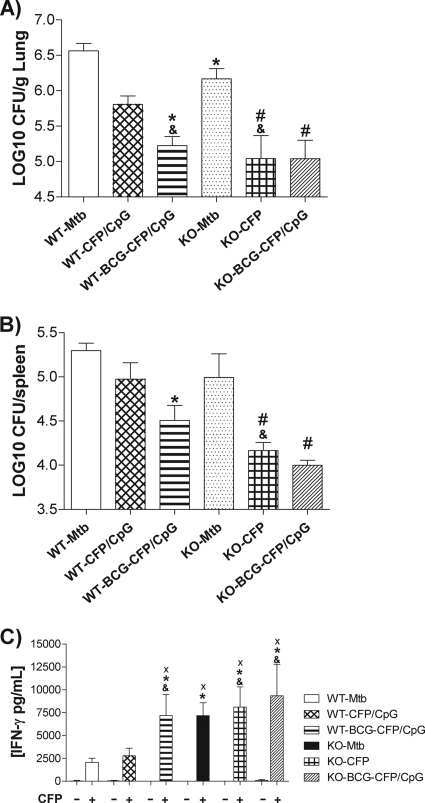
In an effort to confirm whether IL-4 plays a role in the inability of CFP/CpG homologous immunization to induce protection, two groups of IL-4 KO mice were vaccinated with CFP/CpG (KO-CFP/CpG) or BCG-CFP/CpG (KO-BCG-CFP/CpG). An additional group comprised nonvaccinated, infected KO mice (KO-MTB). Again, we observed that even with the change in genetic background, wild-type C57BL/6 mice vaccinated with CFP/CpG (WT-CFP/CpG) were not protected after challenge, while the prime-boost-immunized WT group was (Fig. 6A). The lungs of the KO-MTB group had lower CFU counts than the lungs of infected WT mice (WT-MTB) (P < 0.05). Nevertheless, the lack of IL-4 conferred significant protection in CFP/CpG-immunized mice (KO-CFP/CpG) (P < 0.01). We observed that in IL-4 KO mice, the protection induced by CFP/CpG was comparable to that induced by BCG-CFP/CpG vaccination in lungs (Fig. 6A) and spleens (Fig. 6B). The lack of IL-4 had no effect on the protection conferred by BCG-CFP/CpG vaccination. Additionally, there was a significant decrease in CFU counts in lungs from the KO-CFP/CpG group compared to those from the WT-CFP/CpG group.
FIG. 6.
Protection against experimental TB conferred by the different vaccination schedules. C57BL/6 WT or IL-4 KO mice (n = 5) were immunized subcutaneously with three doses of CFP/CpG (CFP/CpG group) at 7-day intervals or with one dose of BCG followed by one dose of CFP/CpG after 15 days (BCG-CFP/CpG group). Sixty days after vaccination, mice were challenged with a virulent strain of M. tuberculosis. At 30 or 70 days after infection, the lungs were processed for the CFU assay and lung cell cultures. Bacterial load is expressed as log10 CFU/g of lung (A) or CFU/spleen (B) from the means ± standard deviations of serial dilutions individually counted for each group and obtained in experiments repeated twice. (C) Cytokine secretion by lung cells stimulated with mycobacterial antigens. *, P < 0.05 versus nonimmunized, infected WT mice (WT-MTB group); #, P < 0.05 versus nonimmunized, infected KO mice (KO-MTB group); &, P < 0.05 versus CFP/CpG-immunized WT mice; ×, P < 0.05 versus nonstimulated cells.
When we performed a lung cell culture, we observed that, after antigen stimulation, there was no difference in IFN-γ secretion among cells from all IL-4 KO groups. However, in WT groups, we observed that WT-BCG-CFP/CpG-immunized mice secreted increased concentrations of IFN-γ compared to those of the WT-MTB and WT-CFP/CpG groups, according to the results found for BALB/c mice (Fig. 4). In addition, the IFN-γ secretion was increased in lung cell cultures from KO-CFP/CpG-immunized mice, compared to those from the WT-CFP/CpG group.
Taken together, these results strongly suggest that IL-4 plays a role in dampening protection conferred by CFP/CpG immunization.
DISCUSSION
In this work, we showed that it is possible to use the IFN-γ-inducing ability of CFP/CpG immunization to design a new vaccine against TB. Our data confirm that a change from a homologous immunization schedule with three doses of CFP/CpG to a prime-boost strategy using CFP/CpG to boost BCG vaccination (BCG-CFP/CpG) conferred significant protection against experimental TB. Aside from establishing this protective effect, which is not observed with homologous immunization, we also verified that heterologous immunization improved the protection conferred by a single BCG immunization. Indeed, BCG-CFP/CpG immunization sustained the restriction of bacillus growth 70 days after challenge, while vaccination with BCG only did not.
BCG immunization is the gold standard to evaluate the protective efficacy of a vaccine in experimental models. Moreover, considering that a BCG homologous booster in humans does not affect protection induced by this vaccine, a new vaccine that can boost immunity in BCG-vaccinated individuals should be very beneficial. It could certainly have an impact on the number of new TB cases in the adult population, especially given that the exclusion of BCG vaccination could have a negative effect on the control of severe forms of childhood TB.
A major impact on vaccine-induced protection in our study was associated with the order of stimulation. When we changed the order of immunization and used a CFP/CpG prime followed by a BCG booster (CFP/CpG-BCG), we noted no additional protection compared to that provided by a single BCG immunization. Considering the larger antigen diversity in live microorganisms than in a protein suspension, we hypothesize that a BCG prime expands a high number of specific T-cell clones and, following a CFP/CpG booster, only CFP-specific clones will be selected. In contrast, when we used a CFP/CpG prime and a BCG booster, the diversity of expanded T-cell clones was more restricted than that of clones which would be expanded following the booster.
An important issue worth mentioning is our concern about Koch's phenomenon (40). Hernandez-Pando et al. reported the occurrence of Koch's phenomenon when BALB/c mice were vaccinated with large doses of killed Mycobacterium vaccae but not with low doses (24). Based on this experimental evidence, we assumed that previous contact with CFP antigens in the presence of a powerful inflammatory property adjuvant (CpG oligodeoxynucleotides) would result in extensive inflammation and necrosis, as observed with a homologous immunization schedule (16). Indeed, homologous immunization increased the production of IFN-γ locally but did not modify TNF-α secretion. Aside from a Th1 and inflammatory response, we also observed increased levels of IL-4 and IL-5 in the lungs. When we reduced CFP antigen exposure by using a single dose to boost BCG, the magnitude of the inflammatory response decreased, and the pattern shifted from mixed Th1/Th2 to Th1. We suggest that IFN-γ plays a key role in exacerbating inflammation in the presence of IL-4 and IL-5. Indeed, IFN-γ has also been associated with the development of mycobacterium-induced caseous necrosis, with excessive amounts of this cytokine contributing to the pathology observed in tuberculous patients (2, 13, 15, 22, 43).
According to Rook et al., progressive TB occurs because the potentially protective Th1 response is converted to an immunopathological response, normally associated with IL-4, which fails to eliminate the bacteria (39, 41). In experimental murine TB, the presence of at least 10% of IL-4-producing cells exacerbates the toxic effects of TNF-α, including fibrosis and necrosis (25). Furthermore, in vitro studies have demonstrated that Th2 cytokines mediate an alternative pathway of macrophage activation not aimed at the elimination of intracellular pathogens, because these macrophages increase the expression of transferrin and DC-SIGN receptors, increase IL-10 and TGF-β secretion, and decrease TNF-α and IL-12 production (26, 31). It has also been reported that Th2 cytokines inhibit apoptotic and autophagic control of intracellular M. tuberculosis induced by IFN-γ secretion (20, 21, 41). The interaction of IL-4 and IL-13 with IL-4 receptor alpha induces a signaling via IRS-1 (insulin receptor substrate-1) and STAT6. The IRS-1 signaling activates the Akt pathway, which inhibits the autophagy in murine and human macrophages. The STAT-6 signaling upregulates Bcl-2, which inhibits apoptosis and sequestrates beclin-1, impairing the initiation of autophagy (20, 21, 41). IL-4 also impairs the effector mechanisms of cytotoxic T cells (35). In addition, IL-4 also influences the apoptosis of mycobacterium-reactive human lymphocytes in the presence of TNF-α (42). The increased expression of IL-4 in M. tuberculosis-activated lymphocytes promotes CD30 expression, which sensitizes the lymphocytes to TNF-α-mediated apoptosis via TRAF2 depletion, and this may be associated with the immunopathology in human TB.
In this way, confirming this detrimental role of IL-4, our data show that in IL-4 KO mice the CFP/CpG homologous immunization conferred significant protection compared to that in nonimmunized mice and also to that in CFP/CpG-immunized WT mice. However, this effect seems not to be mediated by an increase in IFN-γ secretion, once the production levels of this cytokine were similar among all IL-4 KO mice: nonimmunized, CFP/CpG immunized, and BCG-CFP/CpG immunized. It is possible that other factors, such as cytokine receptor modulation or lipid mediator production, might be involved in this IL-4-mediated detrimental role.
Our experiments suggest that the presence of IL-4 in the microenvironment corrupted the Th1 immune response. We hypothesized that, for the reasons described above, the IL-4 secretion impaired the clearance of bacilli that is dependent on the cooperation of Th1 and Th17 immune responses. The persistence of mycobacteria exacerbated the inflammatory response and in addition to the increased influx of IL-4-producing Th2 cells generated a process like a feedback of mixed immune responses. The excess inflammatory response caused tissue damage without bacterial clearance. On the other hand, BCG-CFP/CpG-immunized mice did not exhibit significant IL-4 secretion. We believe that, in this group, low concentrations of IL-4 allowed the effective elimination of the bacteria. This hypothesis can be explained by the fact that we found low ex vivo concentrations of Th1, Th17, and inflammatory cytokines in this group.
Therefore, the protection induced by a BCG-CFP/CpG vaccine was not associated with increased IFN-γ and IL-17 concentrations ex vivo, but it was associated with decreased IL-4, IL-5, IL-10, TNF-α, and TGF-β concentrations. To evaluate whether the reduction in IFN-γ detected ex vivo in lung tissue was due to a reduced migration or a deficiency of the functional ability of Th1 antigen-specific cells, we performed a lung cell culture. Seventy days after challenge, in vitro production of IFN-γ and IL-17 was sustained only in the BCG-CFP/CpG-vaccinated group. In contrast, lung cells of the CFP/CpG group secreted more IL-4 and IL-5 and less IFN-γ than BCG-CFP/CpG-immunized mice did. These data show that the specific Th1 and Th17 cells migrated into the lungs of BCG-CFP/CpG-immunized mice and that the CFP/CpG booster may have acted to sustain the recruitment and activation of these cell populations in a later phase of infection.
Although IFN-γ represents the best protective biomarker, the role of Th17 cells in TB has been described only recently. A protective role against this infection has been attributed to IL-17, one that is strongly associated with the recruitment of Th1-specific cells to the lung in the initial and late phases of infection (27, 28).
It is important to emphasize that the low ex vivo detection of IFN-γ and IL-17 in the BCG-CFP/CpG group might be due to the very low production of Th2 and regulatory cytokines locally. In this microenvironment, even low concentrations of IFN-γ could sustain the functional activity of this cytokine and the elimination of bacilli, effects that could impair further and unnecessary IFN-γ and IL-17 production. On the other hand, in the CFP/CpG group, the effects of IFN-γ may have been corrupted by Th2 and regulatory cytokines, and the persistence of bacilli likely induced more IFN-γ secretion.
Finally, experiments employing immunization of IL-4 KO mice confirmed the detrimental role of this cytokine, as the lack of IL-4 appeared to be protective in CFP/CpG-immunized mice after challenge. Thus, beyond IFN-γ, the characterization of additional immunological parameters associated with protection, or which may predict progression of infection, is essential to define levels of protection conferred by distinct vaccine formulations. In this study, we confirmed the essential role of IFN-γ, and we suggest that IL-17 may contribute indirectly to restrict the growth of bacilli, while IL-4 may not. We believe that a better understanding of mechanisms underlying protection against TB, as well as the mechanisms involved in the failure of unsuccessful vaccines, will facilitate the rational design of new TB vaccines.
Acknowledgments
This study received financial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; Foundation for the Support of Research in the State of São Paulo; grants 03/11303-2 and 05/01995-0), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Council for Scientific and Technological Development), and the Rede Brasileira de Pesquisa em TB (Rede-TB; Brazilian Tuberculosis Research Network).
We thank Ana Flávia Gembre, Izaíra Tincani Brandão, Ana Paula Masson, and Elaine Medeiros Floriano for the general technical assistance provided, as well as João Santana da Silva for providing the IL-4 KO mice and Carlos Rodrigo Zárate-Bládes for helpful suggestions with the manuscript.
We declare that we do not have a commercial or other association that might pose a conflict of interest.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 14 September 2009.
REFERENCES
- 1.Aagaard, C., J. Dietrich, M. Doherty, and P. Andersen. 2009. TB vaccines: current status and future perspectives. Immunol. Cell Biol. 87:279-286. [DOI] [PubMed] [Google Scholar]
- 2.Aly, S., T. Laskay, J. Mages, A. Malzan, R. Lang, and S. Ehlers. 2007. Interferon-gamma-dependent mechanisms of mycobacteria-induced pulmonary immunopathology: the role of angiostasis and CXCR3-targeted chemokines for granuloma necrosis. J. Pathol. 212:295-305. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, P., D. Askgaard, L. Ljungqvist, M. W. Bentzon, and I. Heron. 1991. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect. Immun. 59:1558-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen, P., and I. Heron. 1993. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect. Immun. 61:844-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badell, E., F. Nicolle, S. Clark, L. Majlessi, F. Boudou, A. Martino, L. Castello-Branco, C. Leclerc, D. J. Lewis, P. D. Marsh, B. Gicquel, and N. Winter. 2009. Protection against tuberculosis induced by oral prime with Mycobacterium bovis BCG and intranasal subunit boost based on the vaccine candidate Ag85B-ESAT-6 does not correlate with circulating IFN-gamma producing T-cells. Vaccine 27:28-37. [DOI] [PubMed] [Google Scholar]
- 7.Bassey, E. O., P. F. Life, D. Catty, J. S. Gaston, and D. S. Kumararatne. 1996. T-cell response to mycobacterial proteins: a comparative study of tuberculous and control immunoblots of Mycobacterium tuberculosis and M. bovis BCG. Tuber. Lung Dis. 77:146-153. [DOI] [PubMed] [Google Scholar]
- 8.Bonato, V. L., E. D. Gonçalves, E. G. Soares, R. R. Santos Júnior, A. Sartori, A. A. Coelho-Castelo, and C. L. Silva. 2004. Immune regulatory effect of pHSP65 DNA therapy in pulmonary tuberculosis: activation of CD8+ cells, interferon-gamma recovery and reduction of lung injury. Immunology 113:130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt, L., Y. A. W. Skeiky, M. R. Alderson, Y. Lobet, W. Dalemans, O. C. Turner, R. J. Basaraba, A. A. Izzo, T. M. Lasco, P. L. Chapman, S. G. Reed, and I. M. Orme. 2004. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect. Immun. 72:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks, J. V., A. A. Frank, M. A. Keen, J. T. Bellisle, and I. M. Orme. 2001. Boosting vaccine for tuberculosis. Infect. Immun. 69:2714-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 12.Dietrich, J., C. Andersen, R. Rappuoli, T. M. Doherty, C. G. Jensen, and P. Andersen. 2006. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J. Immunol. 177:6353-6360. [DOI] [PubMed] [Google Scholar]
- 13.Ehlers, S., J. Benini, H. D. Held, C. Roeck, G. Alber, and S. Uhlig. 2001. Alphabeta T cell receptor-positive cells and interferon-gamma, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology. J. Exp. Med. 194:1847-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, C. G., U. Palendira, C. Demangel, J. M. Spratt, A. S. Malin, and W. J. Britton. 2001. Priming by DNA immunization augments protective efficacy of Mycobacterium bovis bacille Calmette-Guerin against tuberculosis. Infect. Immun. 69:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florido, M., A. M. Cooper, and R. Appelberg. 2002. Immunological basis of the development of necrotic lesions following Mycobacterium avium infection. Immunology 106:590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca, D. M., C. L. Silva, M. O. Paula, E. G. Soares, G. Marchal, C. Horn, and V. L. Bonato. 2007. Increased levels of interferon-gamma primed by culture filtrate proteins antigen and CpG-ODN immunization do not confer significant protection against Mycobacterium tuberculosis infection. Immunology 121:508-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert, S. C., J. Schneider, C. M. Hannan, J. T. Hu, M. Plebanski, R. Sinden, and A. V. Hill. 2002. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunization regimes. Vaccine 20:1039-1045. [DOI] [PubMed] [Google Scholar]
- 18.Gonçalves, E. D., V. L. Bonato, D. M. Fonseca, E. G. Soares, I. T. Brandão, A. P. Soares, and C. L. Silva. 2007. Improve protective efficacy of a TB DNA-HSP65 vaccine by BCG priming. Genet. Vaccines Ther. 22:5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goonetilleke, N. P., H. McShane, C. M. Hannan, R. J. Anderson, R. H. Brookes, and A. V. Hill. 2003. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. Immunology 171:1602-1609. [DOI] [PubMed] [Google Scholar]
- 20.Harris, J., S. A. De Haro, S. S. Master, J. Keane, E. A. Roberts, M. Delgado, and V. Deretic. 2007. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 27:505-517. [DOI] [PubMed] [Google Scholar]
- 21.Harris, J., S. S. Master, S. A. De Haro, M. Delgado, E. A. Roberts, J. C. Hope, J. Keane, and V. Deretic. 2009. Th1-Th2 polarisation and autophagy in the control of intracellular mycobacteria by macrophages. Vet. Immunol. Immunopathol. 128:37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan, Z., B. Jamil, J. Khan, R. Ali, M. A. Khan, N. Nasir, M. S. Yusuf, S. Jamil, M. Irfan, and R. Hussain. 2009. Relationship between circulating levels of IFN-gamma, IL-10, CXCL9 and CCL2 in pulmonary and extrapulmonary tuberculosis is dependent on disease severity. Scand. J. Immunol. 69:259-267. [DOI] [PubMed] [Google Scholar]
- 23.Havlir, D. V., R. S. Wallis, W. H. Boom, T. M. Daniel, K. Chervenak, and J. J. Ellner. 1991. Human immune responses to Mycobacterium tuberculosis antigens. Infect. Immun. 59:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Pando, R., L. Pavön, K. Arriaga, H. Orozco, V. Madrid-Marina, and G. Rook. 1997. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect. Immun. 65:3317-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez-Pando, R., and G. A. W. Rook. 1994. The role of TNFalpha in T cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology 82:591-595. [PMC free article] [PubMed] [Google Scholar]
- 26.Kahnert, A., P. Seiler, M. Stein, S. Bandermann, K. Hahnke, H. Mollenkopf, and S. H. Kaufmann. 2006. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur. J. Immunol. 36:631-647. [DOI] [PubMed] [Google Scholar]
- 27.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Cilley, F. Shen, S. M. Eaton, S. L. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper. 2007. IL-23 and IL-17 in establishment of protective pulmonary CD4+ T cell responses upon vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369-377. [DOI] [PubMed] [Google Scholar]
- 28.Khader, S. A., and A. M. Cooper. 2008. IL-23 and IL-17 in tuberculosis. Cytokine 41:79-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindblad, E. B., M. J. Elhay, R. Silva, R. Appelberg, and P. Andersen. 1997. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 65:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, J., V. Tran, A. S. Leung, D. C. Alexander, and B. Zhu. 2009. BCG vaccines: their mechanisms of attenuation and impact on safety and protective efficacy. Hum. Vaccin. 5:70-78. [DOI] [PubMed] [Google Scholar]
- 31.Martinez, F. O., L. Helming, and S. Gordon. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27:451-483. [DOI] [PubMed] [Google Scholar]
- 32.McShane, H. 2009. Vaccine strategies against tuberculosis. Swiss Med. Wkly. 139:156-160. [DOI] [PubMed] [Google Scholar]
- 33.McShane, H., A. A. Pathan, C. R. Sander, N. P. Goonetilleke, H. A. Fletcher, and A. V. Hill. 2005. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis 85:47-52. [DOI] [PubMed] [Google Scholar]
- 34.Meseda, C. A., K. L. Elkins, M. J. Merchlinsky, and J. P. Weir. 2002. Prime-boost immunization with DNA and modified vaccinia virus ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. J. Infect. Dis. 186:1065-1073. [DOI] [PubMed] [Google Scholar]
- 35.Olver, S., P. Groves, K. Buttigieg, E. S. Morris, M. L. Janas, A. Kelso, and N. Kienzle. 2006. Tumor-derived interleukin-4 reduces tumor clearance and deviates the cytokine and granzyme profile of tumor-induced CD8+ T cells. Cancer Res. 66:571-580. [DOI] [PubMed] [Google Scholar]
- 36.Pancholi, P., M. Perkus, N. Tricoche, Q. Liu, and A. M. Prince. 2003. DNA immunization with hepatitis C virus (HCV) polycistronic genes or immunization by HCV DNA priming-recombinant canarypox virus boosting induces immune responses and protection from recombinant HCV-vaccinia virus infection in HLA-A2.1-transgenic mice. J. Virol. 77:382-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed, S. G., R. N. Coler, W. Dalemans, E. V. Tan, E. C. DeLa Cruz, R. J. Basaraba, I. M. Orme, Y. A. Skeiky, M. R. Alderson, K. D. Cowgill, J. P. Prieels, R. M. Abalos, M. C. Dubois, J. Cohen, P. Mettens, and Y. Lobet. 2009. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc. Natl. Acad. Sci. USA 106:2301-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts, A. D., M. G. Sonnenberg, D. J. Ordway, S. K. Furney, P. J. Brennan, J. T. Belisle, and I. M. Orme. 1995. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology 85:502-507. [PMC free article] [PubMed] [Google Scholar]
- 39.Rook, G. A., K. Dheda, and A. Zumla. 2005. Immune responses to tuberculosis in developing countries: implications for new vaccines. Nat. Rev. Immunol. 5:661-667. [DOI] [PubMed] [Google Scholar]
- 40.Rook, G. A., and R. Hernandez-Pando. 1996. The pathogenesis of tuberculosis. Annu. Rev. Microbiol. 50:259-284. [DOI] [PubMed] [Google Scholar]
- 41.Rook, G. A., R. Hernández-Pando, and A. Zumla. 2009. Tuberculosis due to high-dose challenge in partially immune individuals: a problem for vaccination? J. Infect. Dis. 199:613-618. [DOI] [PubMed] [Google Scholar]
- 42.Seah, G. T., and G. A. Rook. 2001. IL-4 influences apoptosis of mycobacterium-reactive lymphocytes in the presence of TNF-alpha. J. Immunol. 167:1230-1237. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland, J. S., I. M. Adetifa, P. C. Hill, R. A. Adegbola, and M. O. Ota. 2009. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur. J. Immunol. 39:723-729. [DOI] [PubMed] [Google Scholar]
- 44.Takeda, A., H. Igarashi, H. Nakamura, M. Kano, A. Iida, T. Hirata, M. Hasegawa, Y. Nagai, and T. Matano. 2003. Protective efficacy of an AIDS vaccine, a single DNA priming followed by a single booster with a recombinant replication-defective Sendai virus vector, in a macaque AIDS model. J. Virol. 77:9710-9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanghe, A., S. D'Souza, V. Rosseels, O. Denis, T. H. Ottenhoff, W. Dalemans, C. Wheeler, and K. Huygen. 2001. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect. Immun. 69:3041-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tchilian, E. Z., C. Desel, E. K. Forbes, S. Bandermann, C. R. Sander, A. V. S. Hill, H. McShane, and S. H. E. Kaufmann. 2009. Immunogenicity and protective efficacy of prime-boost regimens with recombinant ΔureC hly+ Mycobacterium bovis BCG and modified vaccinia virus Ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infect. Immun. 77:622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trunz, B. B., P. Fine, and C. Dye. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367:1173-1180. [DOI] [PubMed] [Google Scholar]
- 48.Verreck, F. A., R. A. Vervenne, I. Kondova, K. W. van Kralingen, E. J. Remarque, G. Braskamp, N. M. van der Werff, A. Kersbergen, T. H. Ottenhoff, P. J. Heidt, S. C. Gilbert, B. Gicquel, A. V. Hill, C. Martin, H. McShane, and A. W. Thomas. 2009. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS One 4:e5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodland, D. L. 2004. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 25:98-104. [DOI] [PubMed] [Google Scholar]