Abstract
We previously showed that a multiple antigenic peptide (MAP) displaying amino acids (aa) 305 to 319 from the 2β2-2β3 loop of protective antigen (PA) can elicit high-titered antibody that neutralizes lethal toxin (LeTx) in vitro and that this loop-neutralizing determinant (LND) specificity is absent in PA-immune rabbits. Some immune rabbits were, however, nonresponders to the MAP. We hypothesized that the immunogen elicited suboptimal major histocompatibility complex (MHC) class II-restricted T-cell help and that introduction of a functional helper T-cell epitope would increase MHC-restricted responsiveness and the magnitude and affinity of the antibody responses. In the current study, we characterized the T- and B-cell responses to LND peptides in mice, then designed second-generation MAP immunogens for eliciting LND-specific immunity, and tested them in rabbits. The 305-319 sequence was devoid of helper T-cell epitopes in three strains of mice; however, a T-B peptide comprising aa 305 to 319, colinearly synthesized with the P30 helper epitope of tetanus toxin, elicited robust LeTx-neutralizing immunity in mice. T-B MAPs displaying B-cell epitopes 304 to 319 (MAP304) or 305 to 319 (MAP305) elicited high-titer, durable antibody responses in rabbits which exhibited potent neutralization of LeTx in vitro. All MAP304-immune rabbits demonstrated neutralization titers exceeding that of hyperimmune sera of rabbits immunized with PA in Freund's adjuvant, with peak neutralization titers 23-, 6-, and 3-fold higher than that of the PA antiserum. Overall, immunization with MAPs containing the P30 epitope elicited higher antibody and toxin neutralization titers and peptide-specific affinity than immunization with an LND MAP lacking a helper epitope. P30-containing MAP304 represents a promising LND-specific vaccine for anthrax.
Bacillus anthracis is a gram-positive, spore-forming bacterium that naturally infects wildlife and livestock and, less frequently, humans. Since 2001, when spores of B. anthracis sent through the U.S mail resulted in infection in 22 individuals, including five fatal cases of inhalation anthrax, considerable effort has been directed toward reevaluating our preparedness for possible bioterrorist threats, including weaponized anthrax.
The morbidity and mortality associated with inhalation anthrax are largely a result of the elaboration of two toxins, lethal toxin (LeTx) and edema toxin. These toxins are classic A-B toxins, where lethal factor and edema factor represent the active moieties and protective antigen (PA) represents the cell-binding moiety (5, 6, 20). Humoral immunity to PA can successfully mediate protection from lethal challenges in animal models of inhalation anthrax, and the protection is correlated with the ability of PA-specific antibodies to neutralize LeTx in vitro in the toxin neutralization assay (TNA) (17, 21, 22, 35, 36, 41).
While PA-specific antibody titer has been shown to correlate with TNA titers in vitro, this relationship demonstrates variability among different studies and in different species (19, 37, 40, 43, 48, 50). Most PA-specific neutralizing monoclonal antibodies (MAbs) show a more invariable linear relationship between concentration and toxin neutralization (4, 46). These findings can be reconciled, in part, by the fact that only a fraction of the polyclonal antibody produced in response to vaccination with PA contributes to LeTx neutralization (4, 39, 40). Indeed, analyses of human and murine MAbs suggest that whereas immunization with whole PA elicits antibodies to a wide range of sequences within the protein, the repertoire of neutralizing antibodies is considerably more focused, limited perhaps to only a couple of major regions in PA, including the anthrax toxin receptor binding region in domain 4 and the lethal factor- and edema factor-binding regions in domain 1 (4, 24, 25, 40). Though PA-specific antibody may contribute to protection through mechanisms other than neutralization, for example, by facilitating opsonization of spores (49), a restricted neutralizing antibody repertoire represents a potential vulnerability in PA-based vaccines (42, 44).
We previously showed that immunization of rabbits with a multiple antigenic peptide (MAP) displaying amino acids (aa) 305 to 319 from the 2β2-2β3 loop in domain 2 of PA was capable of eliciting antibody specific for a linear determinant in the 2β2-2β3 loop, which mediated high-titer neutralization of LeTx in vitro (31). This potentially important antibody specificity, referred to as the loop-neutralizing determinant (LND), targets a critical molecular structure of PA involved in translocating edema and lethal factors into cells, where their enzymatic activities mediate their toxic effects. Importantly, antibodies to the LND appear to be virtually absent in PA-immune rabbits and may be present only at functionally insignificant levels in human anthrax vaccine adsorbed (AVA) serum (14, 31). Thus, the LND appears to be immunologically silent or cryptic in animals immunized with PA. Development of immunogens which effectively target this cryptic epitope therefore could be strategic in the event of malicious attempts to engineer PA so as to escape the neutralizing specificities elicited by AVA or other PA-based vaccines.
While capable of eliciting high-titer neutralization in responder rabbits, a first-generation LND-specific MAP appeared to exhibit major histocompatibility complex (MHC)-restricted nonresponsiveness, as it did not elicit antibody in two of six MAP-immune rabbits. We hypothesized that this was likely a result of deficient helper T-cell activity in the MAP construct in general and specifically within the 305-319 peptide sequence itself. We further hypothesized that the inclusion of a potent helper epitope in a second-generation MAP construct would result in immune responses characterized by decreased MHC-restricted nonresponsiveness, higher antibody titer, and higher antibody affinity, ultimately resulting in increased LeTx neutralizing titers in vitro compared to those observed in a previous study in which rabbits were immunized with a MAP displaying the 305-319 sequence but without a defined helper T-cell epitope (31). In the current study, we characterized the T- and B-cell responses to the LND peptide sequence through the analysis of linear peptides in the mouse model. Insight from these studies was then used to design and study second-generation MAP constructs in rabbits, with the goal of eliciting high-titer, high-affinity LND-specific antibody responses capable of neutralizing B. anthracis LeTx in vitro at levels consistent with protection of rabbits from an inhalation challenge with aerosolized spores of B. anthracis.
MATERIALS AND METHODS
Synthetic peptides and recombinant proteins.
Linear synthetic peptides used for the T-cell proliferation studies of mice included aa 305 to 319 (GNAEVHASFFDIGG) from PA and a peptide representing the P30 helper epitope (GNNFTVSFWLRVPKVSASHLE) from tetanus toxin (GenBank accession no. AAA23282) (7, 33). A panel of peptides was also synthesized that comprised the P30 peptide colinearly synthesized at the N terminus of peptide sequences from the LND region of domain 2 of PA, as outlined in single-letter code in Fig. 3. MAP304 and MAP305 are four-branched MAPs displaying P30 helper epitope colinearly synthesized to either aa 304 to 319 (HGNAEVHASFFDIGG) or 305 to 319 from PA. The T-B peptides are linked at their C terminus to the lysine core of the MAP. All synthetic peptides were synthesized commercially (Sigma-Genosys, The Woodlands, TX). A recombinant protein for use in assessing anti-LND peptide immunity by enzyme-linked immunosorbent assay (ELISA) was molecularly constructed, expressed, and purified as previously described (31). The recombinant protein displays two tandemly repeated copies of aa 299 to 327 (HTSEVHGNAEVHASFFDIGGSVSAGFSN) from domain 2 of PA linked to maltose binding protein.
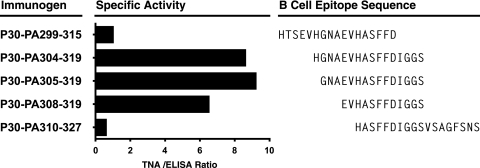
FIG. 3.
Comparative immunogenicity of P30 containing T-B peptides targeting the LND of PA. Groups of five B6C3F1 mice (C57BL/6 × C3H) were immunized s.c. four times at 2-week intervals with 12 nmol of a synthetic peptide comprised of the P30 helper T-cell epitope colinearly synthesized to an LND region peptide as described above. Sera were collected 10 days after the final immunization and analyzed by ELISA for reactivity with immobilized PA and by TNA. Specific activity = (geometric mean TNA ED50 titer/geometric mean EC50 antibody titer) × 100.
Immunization of animals and sample collection.
Eight- to 12-week-old female C57BL/6, SJL, C3H, BALB/c, or B6C3F1 (C57BL/6 × C3H) mice (Jackson Laboratory, Bar Harbor, ME) were used for all mouse experiments. For mouse antibody experiments, mice were immunized subcutaneously (s.c.) four times at 2-week intervals with 50 μg of PA (PA83; List Biological Laboratories, Inc., Campbell, CA), 12 nmol of a linear T-B peptide, or the P30 peptide alone mixed with 10 μg of Quil A adjuvant. New Zealand White rabbits were used for all rabbit experiments (Covance Research Products, Denver, PA). For immunization of rabbits, female New Zealand White rabbits were immunized on day 0 with 250 μg of MAP304 or MAP305 or the linear T-B peptide, P30-PA305-319, in an emulsion with complete Freund's adjuvant (CFA). Rabbits were then boosted at 2-week intervals with 125 μg of the respective MAP or linear peptide in an emulsion with incomplete Freund's adjuvant (IFA). Serum samples were collected prior to the first immunization (day 0) and approximately 10 to 12 days after each booster immunization. For MAP304-immune rabbits only, serial bleeds were obtained at 2- to 4-week intervals after the final booster immunizations until sacrifice 7 months later, as noted in Results. Positive control hyperimmune PA antiserum was generated and aliquoted from a previous study for use as a standard reference reagent in the ELISA and LeTx neutralization assay (31). These PA reference antisera have antibody titers (50% effective concentration [EC50]) of 34,291 and 37,225. All animal experiments and procedures were approved by an Institutional Animal Care and Use Committee, and all animals were cared for in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International.
ELISA analysis.
Individual rabbit antiserum was analyzed in duplicate by ELISA as described previously (26). For analysis of antibodies specific for PA, wells of microtiter plates (Immulon 2; Thermo Labsystems, Franklin, MA) were coated overnight at 4°C with 100 ng of PA in a 0.05 M carbonate buffer, pH 9.5. For analysis of antipeptide binding, wells were coated with 100 ng of a recombinant protein displaying two copies of the PA peptide spanning aa 299 to 327 as a fusion with maltose binding protein. Bound antibody was detected with secondary biotinylated antibody specific for either mouse or rabbit immunoglobulin G (Southern Biotechnology, Birmingham, AL), followed by streptavidin-alkaline phosphatase and 4-nitrophenylphosphate (Roche, Indianapolis, IN). Absorbance at 405 nm minus absorbance at 650 nm was determined using an ELISA reader (EMax microplate reader; Molecular Devices, Menlo Park, CA). Antibody titers were determined as previously described (31).
Antibody affinity.
For the determination of peptide 50% inhibitory concentration (IC50), serial twofold dilutions starting at 256 μM (2×) in dilution solution (phosphate-buffered saline, 2% bovine serum albumin, 0.05% Tween 20) were performed with either the P30-PA304-319 peptide or the P30 peptide. Experimental serum was added to the diluted peptides in duplicate at a predetermined dilution of one-half the reciprocal EC50 titer (2×). The serum-peptide mixtures were allowed to equilibrate at room temperature for 2 h and then overnight at 4°C. On the following day, the serum-peptide mixtures were analyzed in the standard ELISA as described above, and peptide IC50s were determined for each serum sample by using nonlinear regression to fit a variable slope sigmoidal equation to the serial dilution data with Prism 5.0 (GraphPad Software, Inc., San Diego, CA). Peptide IC50s were considered to represent good approximations of the antibody dissociation constant (Kd) (10).
Anthrax TNA.
The ability of antibody to block LeTx action in vitro was assessed using RAW 264.7 cells as described previously (31). Each TNA was validated by a contemporaneous PA titration. For neutralization studies, 110 ng/ml PA was used along with 150 ng of lethal factor. The reciprocal of the effective dilution at which 50% of the cells are protected from cytotoxicity (ED50) (16) was determined for each serum sample by using nonlinear regression to fit a variable slope sigmoidal equation to the serial dilution data set with Prism 5.0 (GraphPad Software, Inc., San Diego, CA). For comparative purposes, in some figures, a y axis is included to enable interpretation of the TNA ED50 relative to the neutralization detected in the reference PA antisera described above. The neutralization ED50 titers of the two PA-immune rabbits comprising the reference PA antisera fall within a range of titers validated to protect the rabbits from 100 to 200 50% lethal doses in a B. anthracis Ames strain aerosol spore challenge (22, 23, 36). The TNA has a lower limit of detection of 16. Samples with antibody or TNA titers below this limit were assigned a value of 16.
T-cell proliferation assay.
Mice were immunized s.c. at the base of the tail with 12 nmol of the 305-319 or P30 peptide in an emulsion with CFA. Seven days after priming, para-aortic and inguinal lymph nodes were aseptically removed, and single cell suspensions were prepared. Pooled lymph node cells, representing four to five mice per group, were cultured with the 305-319 peptide, P30 or an irrelevant peptide, or PA in 96-well microtiter plates (Costar, Cambridge, MA) at 4 × 105 cells/well in AIM V medium (Invitrogen, Carlsbad, CA) supplemented with 3% fetal bovine serum, 2 mM l-glutamine, and 50 × 10−6 M 2-mercaptoethanol. The proliferative response was assessed by measuring the incorporation of 1 μCi/well of [3H]thymidine during the final 16 h of a 4-day culture. The stimulation index for each replicate was calculated by dividing the geometric mean (in counts per minute) in the presence of test antigen by the geometric mean in the presence of medium alone.
Statistical analysis.
Student's t test was used to compare antibody affinities between cohorts of rabbits immunized with MAPs containing the P30 helper epitope or with no helper epitope. A P value of <0.05 was considered statistically significant. Spearman's ρ correlation was used to calculate correlations between IC50 data and antibody titers with normalized TNA levels. All statistical analysis was performed using GraphPad Prism software, version 5.0 (GraphPad Software, Inc., San Diego, CA).
RESULTS
The PA305-319 peptide lacks helper T-cell activity in three strains of mice.
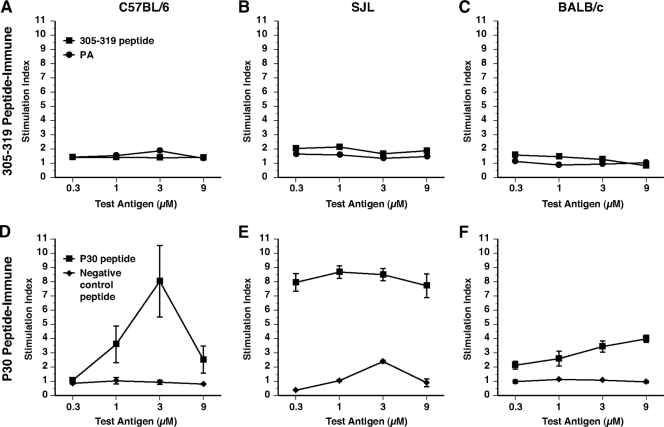
We suspected that the 305-319 sequence from the LND of PA might be devoid of helper T-cell epitopes and therefore evaluated the 305-319 peptide sequence in three strains of mice with diverse MHC class II alleles, using the lymph node proliferation assay. As shown in Fig. 1A to C, T cells from C57BL/6 (H-2b), SJL (H-2s), and BALB/c (H-2d) mice immunized with the 305-319 peptide failed to proliferate when restimulated with either the 305-319 peptide or PA, confirming that this sequence is devoid of helper T-cell activity in these strains. Separately, we also determined that the 305-319 peptide sequence fails to elicit T-cell responses in the C3H strain (not shown). Lacking intrinsic helper epitopes, the 305-319 sequence behaves as a hapten and therefore requires covalent linkage to a helper T-cell epitope for the stimulation of humoral immunity. For provision of such help, we evaluated the P30 helper T-cell epitope from tetanus toxin, which has been shown by others to stimulate immune responses in several strains of mice with distinct H-2 haplotypes and in outbred rabbits and primates (33). In contrast to the lack of proliferation observed with the 305-319 peptide, C57BL/6 and SJL mice immunized with the P30 peptide demonstrated robust proliferation to the P30 epitope in vitro (Fig. 1D and E); P30-immune BALB/c mice exhibited comparatively lower levels of proliferation (Fig. 1F). C3H mice were found to be nonresponders to P30 (not shown).
FIG. 1.
Lymph node proliferative responses to the PA305-319 and P30 peptides in three strains of mice. Lymph node proliferative responses to the PA305-319 peptide or to the P30 peptide in C57BL/6 (A), SJL (B), or BALB/c (C) mice. Five mice per group were immunized s.c. at the base of the tail with 12 nmol of the PA305-319 peptide or the P30 peptide in an emulsion with CFA. Ten days later, para-aortic and inguinal lymph nodes were restimulated with the indicated concentrations of test antigen for 72 h and proliferation was assessed by scintillation counting following incubation with tritiated thymidine. Stimulation indices represent the proliferation in the presence of test antigen divided by proliferation in the medium alone. Error bars represent standard errors of the means.
A synthetic T-B peptide elicits LeTx neutralizing immunity in mice.
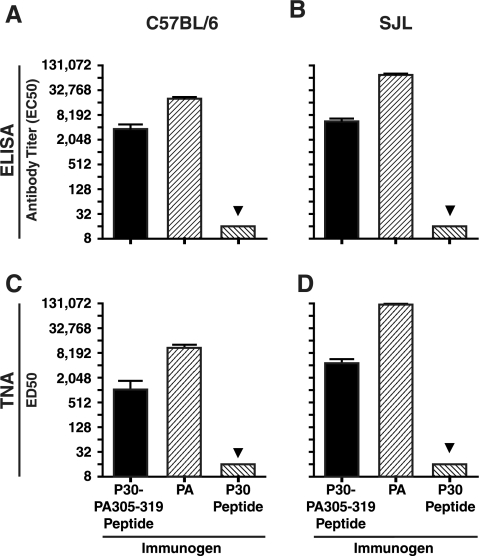
To evaluate a T-B peptide immunogen with the P30 helper T-cell epitope and the LND B-cell epitope, we prepared a peptide composed of the P30 epitope colinearly synthesized at the N terminus of the 305-319 peptide sequence and evaluated the T-B peptide for immunogenicity in the C57BL/6 and SJL strains. For these studies, separate groups of mice were immunized with the P30-PA305-319 peptide, the P30 peptide alone, or PA, using Quil A adjuvant in all cases. Immunization of both strains of mice with the P30-PA305-319 peptide elicited antibodies immunoreactive with immobilized PA by ELISA, whereas no PA-specific antibodies were detected in the sera of P30-immune control mice (Fig. 2A and B). As shown in Fig. 2, PA was also immunogenic in both strains. When evaluated in the TNA, the P30-PA305-319-specific antisera from both strains of mice were shown to neutralize LeTx in vitro (Fig. 2C and D). As expected, PA-specific antiserum exhibited high-titer neutralization in the TNA (Fig. 2C and D).
FIG. 2.
Antibody and TNA titers in C57BL/6 and SJL mice following immunization with PA, P30-PA305-319 peptide, or P30 peptide alone. Five mice per group of C57BL/6 (A and C) and SJL mice (B and D) were immunized four times at 2-week intervals with 50 μg of PA or 12 nmol of either the P30-PA305-319 peptide or the P30 peptide mixed with 10 μg of Quil A adjuvant. Ten days after the last immunization, mice were bled and sera were analyzed by ELISA for responses to immobilized PA (A and B) or by in vitro TNA (C and D), performed as described in Materials and Methods. The lower limit of detection for both assays is 16, and data below this level are indicated with a triangle. Bar charts represent geometric means. Error bars represent standard errors of the means.
Optimization of the LND peptide sequence for elicitation of neutralizing immunity.
We proceeded to more precisely define the optimal peptide sequence comprising the LND antibody target. To this end, we synthesized a panel of peptides representing sequences both N and C terminal to aa 305 to 319 from the 2β2-2β3 loop of PA, all colinear with the P30 helper sequence at their N termini. Separate groups of B6C3F1 mice (H-2b × H-2k), a strain we experimentally confirmed to be a responder to the P30 epitope (not shown), were then immunized four times at 2-week intervals with one of the T-B peptides, and antisera were evaluated by ELISA and TNA. Figure 3 demonstrates the specific activity, operationally defined as the quotient of the ED50 TNA titers and the EC50 antibody titers, associated with the group-specific antisera from mice immunized with each of the T-B peptides. As shown in Fig. 3, the peptide sequences associated with the highest specific activity were focused within the region delineated by aa 304 to 319 of PA, with little activity observed in the sera of mice immunized with the peptides comprising sequences within the N- and C-terminal portions of the 2β2-2β3 loop region studied.
MAPs displaying T-B peptides elicit potent neutralizing immunity in rabbits.
We next evaluated P30-containing T-B peptides in outbred rabbits. We first evaluated the immunogenicity of a linear T-B peptide in rabbits to allow us to determine what contributions, if any, could be attributed to the MAP architecture. Eight rabbits were immunized five times at 2-week intervals with the P30-PA305-319 linear T-B peptide, using CFA for priming immunizations and IFA for booster immunizations. Though the linear P30-PA305-319 T-B peptide stimulated antibody in seven of eight rabbits, only one rabbit had a high titer response (reciprocal EC50 titer, >8,000) (not shown). This rabbit, which had an antibody titer exceeding 32,000, developed significant TNA titers which approximated 50% of the mean neutralization observed in control PA reference antisera from two rabbits immunized five times with PA in CFA-IFA. Sera from the other six responder rabbits demonstrated only low or nondetectable levels of toxin neutralization in vitro.
We proceeded to evaluate two MAPs, each displaying four copies per molecule of either aa 304 to 319 or aa 305 to 319, each colinearly synthesized with the P30 helper epitope at the N terminus. Three rabbits per group were immunized five times at 2-week intervals with the respective MAP construct in emulsions with CFA for priming immunizations and IFA for booster immunizations. Both MAPs were highly immunogenic, rapidly inducing antibody responses that were immunoreactive with both immobilized LND peptide sequence and PA (Fig. 4A to D). Serum antibody responses from the MAP304-immune rabbits displayed less variability and were more consistently elevated compared to the responses seen in the MAP305 rabbits and achieved PA-specific antibody titers by their fifth immunization, equivalent to the polyclonal PA-specific antibody responses from the control PA antiserum (Fig. 4C and D).
FIG. 4.
Responses of rabbits immunized with MAP304 and MAP305. Antibody responses to immobilized peptide comprising aa 299 to 327 from PA (A and B) and to immobilized PA (C and D) and TNA activity (E and F) in sera from rabbits immunized at 2-week intervals with either MAP304 or MAP305. Sera were obtained 10 days after the indicated bleed. In panels E and F, the left y axis corresponds to the EC50 neutralization titer and the right y axis refers to the ED50 of the experimental serum divided by the mean EC50 neutralization titer of the positive control PA antiserum multiplied by 100. The triangles represent the antibody and neutralization titers from the PA control antisera of two rabbits, which were immunized as described in Materials and Methods. There was no detectable neutralization in the preimmune sera from any of the rabbits (not shown). Each circle or square represents an individual rabbit data point, and the horizontal lines are geometric means. The immunization protocol is diagrammed at the bottom of the figure.
In the TNA, sera from the MAP304 rabbits, in particular, were highly neutralizing, with all rabbits demonstrating neutralization titers considerably exceeding that of the PA control (Fig. 4E). One MAP304-immune rabbit and one MAP305-immune rabbit had peak neutralization titers that were 23- and 19-fold higher, respectively, than that of the control PA antiserum, confirming previous observations that antibodies to the LND can demonstrate significant potency (31).
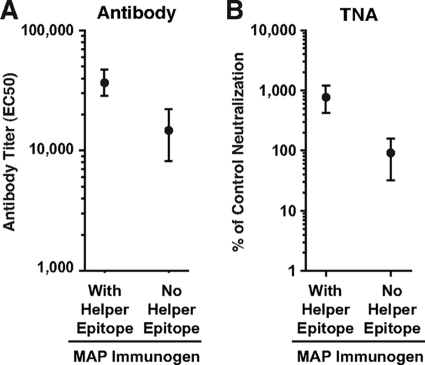
Antibody and TNA titers from the sera of MAP304- and MAP305-immune rabbits obtained after injection 6 (week 12) were compared to the antibody and TNA titers from sera of rabbits vaccinated in a prior study using the same immunization regimen but with a MAP displaying the 305-319 sequence without a helper epitope (31). The PA-specific antibody and TNA titers in the sera of the MAP304- and MAP305-immune rabbits were considerably higher than the titers in the sera of rabbits immunized with the MAP without a helper epitope (Fig. 5A and B).
FIG. 5.
Comparison of antibody and TNA titers among rabbits immunized with LND MAPs containing the P30 helper epitope or no helper epitope. Comparison of geometric mean antibody (A) or neutralization (B) titers in sera from rabbits immunized six times with either MAP304 or MAP305, each of which contains the P30 helper epitope, or a MAP displaying the 305-319 peptide without a helper epitope. For comparison of neutralization titers, all titers were normalized to neutralization from control PA antiserum, as described in Materials and Methods. Error bars represent standard errors of the means.
MAP304-immune rabbits display durable neutralizing responses.
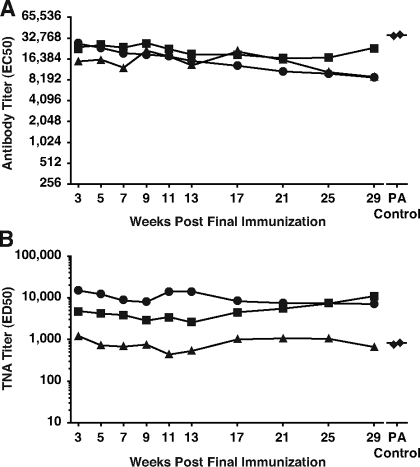
We had previously observed excellent durability of antibody and neutralization titers in rabbits immunized with a MAP displaying the 305-319 LND peptide but lacking a helper epitope. To assess the durability of the neutralizing responses from the three MAP304-immune rabbits, we obtained serial bleeds from the three rabbits following their sixth and final immunization and evaluated the sera by ELISA and TNA. Excellent durability of both antibody and neutralization titers was evident in the antisera from all three rabbits, with little diminution of the TNA titers over the 7 months following the last immunization (Fig. 6A and B). At the final serum analysis, 29 weeks after the last immunization, two of three MAP304-immune rabbits still demonstrated neutralization titers that were distinctly above the neutralization titers in the control PA antiserum, and the third rabbit had neutralization levels only marginally below the neutralization titers of the control PA antiserum.
FIG. 6.
Durability of neutralizing antibody responses in MAP304-immune rabbits. Serial analysis of the antibody (A) and TNA (B) titers from three MAP304 rabbits at the time points indicated following their sixth and final immunization. Diamonds represent the antibody and TNA titers from the two rabbits which comprise the PA control antisera.
Determination of peptide-specific antibody affinity in sera of MAP304- and MAP305-immune rabbits.
We previously showed that the neutralization present in the sera of rabbits immunized with an LND-displaying MAP was completely inhibitable with peptide (31) and found this to be true in the current study as well. As shown in Fig. 7, TNA activity in the sera of MAP304 rabbits is completely and specifically inhibitable with the P30-PA304-319 linear peptide but was unaffected by incubation with the P30 peptide alone. As expected, preincubation of control PA antiserum with the P30-PA304-319 peptide had no effect on the neutralization titers of the control PA antiserum.
FIG. 7.
Effect of preincubation of MAP304 and PA83 antisera with peptides in the in vitro TNA. Antisera from the three MAP304-immune rabbits procured after the fourth immunization and control PA antisera from two rabbits were incubated with 20 μM of the indicated peptides for 30 min prior to assessment in the TNA. The lower limit of detection for the TNA is 16, and data below this level are indicated with a triangle.
Since the antibody responsible for the TNA activity in the sera of MAP-immune rabbits was determined to be completely peptide specific, we determined the peptide-specific IC50s, which are good approximations of the affinity (Kd) of the LND-specific antibodies present in the rabbit antisera (38). This allowed us to evaluate (i) whether differences exist between the peptide-specific affinity of antibodies in the sera of rabbits immunized with a MAP containing the P30 helper epitope and that of rabbits immunized without a helper epitope from a prior study and (ii) whether a correlation exists between the affinity of the LND-specific antibody and neutralization.
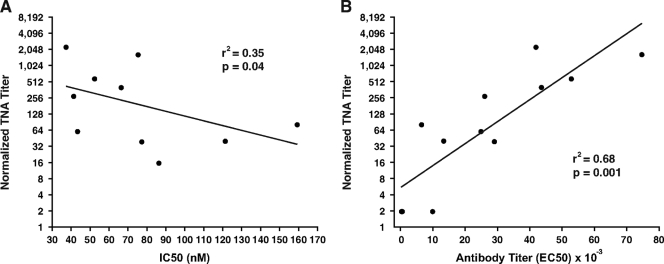
Sera from all six rabbits immunized with the MAP304 and MAP305 constructs were highly avid, with peptide-specific IC50s (Kds) ranging from 37 to 86 nM and a mean IC50 of 56 nM (Fig. 8A). Sera from the four helper-free MAP-responder rabbits from a previous study demonstrated significantly lower antibody affinity, with a mean IC50 of 105 nM (P = 0.019) (Fig. 8B and C). Peptide IC50s from all of the MAP-immune rabbits from the current study and previous study were found to be inversely correlated with those of the TNA, with a coefficient of determination (r2) of 0.35 (P = 0.04) (Fig. 9A). Antibody titer was found to be more highly correlated with that of the TNA, with an r2 value of 0.68 (P = 0.001) (Fig. 9B).
FIG. 8.
Determination and comparison of LND peptide-specific antibody affinities in sera from rabbits immunized with either LND MAPs containing the P30 helper T-cell epitope or an LND MAP without a helper T-cell epitope. LND peptide-specific IC50s were determined for antisera of rabbits immunized with MAP304 or MAP305 (A) and for antisera of rabbits immunized with a MAP without a helper epitope (B) from a previous study. Antisera from two rabbits immunized with MAP without a helper epitope which had no significant antibody responses were not considered in this analysis. Peptide IC50s were determined as described in Materials and Methods. (C) Mean peptide-specific IC50s in antisera from rabbits immunized with the LND MAPs containing the P30 helper epitope were significantly lower than mean IC50s from the antisera of rabbits immunized with MAP without a helper epitope (*, P = 0.019). Error bars represent standard errors of the means.
FIG. 9.
Correlation of peptide avidity and antibody titer with neutralization titers among MAP-immune rabbits. A significant negative correlation between peptide IC50s and normalized TNA titers (A) and a significant positive correlation between antibody titers and TNA titers (B) were observed among all rabbits immunized with LND-containing MAPs. TNA titers from all MAP-immune rabbits were normalized to the control PA antiserum, as described in Materials and Methods. Correlations were determined using Spearman's ρ correlation. Note that two data points overlap along the x axis at an antibody titer of approximately 0 in panel B.
DISCUSSION
We previously demonstrated the potential of peptide sequences in the 2β2-2β3 loop of PA as a target for an epitope-specific vaccine for anthrax (31). The criticality of this site for toxin formation, combined with the apparent absence of this potent specificity in the antibody repertoire elicited by whole PA, makes it an attractive and strategic target for an epitope-specific vaccine for anthrax.
In the current study, we targeted this site using engineered T-B peptides containing a broadly restricted helper T-cell epitope from tetanus toxin, P30, colinearly synthesized with a 15- or 16-aa haptenic peptide from the LND. In MAP form, the T-B peptides, as represented in MAP304 and MAP305, were shown to elicit rapid and potent immunity to the LND, in some rabbits demonstrating LeTx neutralization orders of magnitude greater than those observed in positive control PA antisera from rabbits which had been immunized with PA in Freund's adjuvant. Though the antibody titers in the control PA antiserum were essentially equivalent to the antibody titers in the sera of five of six of the LND-immune rabbits when tested on immobilized PA (Fig. 4), the control PA titers reflect immunoreactivity to a multitude of PA epitopes, only some of which are neutralizing. Conversely, the LND-specific antibodies exhibit considerably greater neutralizing potency, since they are specific for a linear neutralizing epitope within the 2β2-2β3 loop, which our data for mice suggest resides between aa 304 and 319 of PA. The mapping to the peptide region comprising aa 304 to 319 is inclusive of the 312SFFD315 sequences found to represent the critical neutralizing determinant for several MAbs specific for this site (13, 51).
MAP304 and MAP305 sera were found to exhibit considerably higher antibody and neutralization titers than sera of rabbits immunized with a MAP displaying the 305-319 LND sequence without a helper epitope from a previous study. The potentiation of hapten-specific humoral immunity through the use of helper T-cell epitopes parallels work with other models but has not previously been shown with anthrax (9, 12, 28, 32). The P30 helper T-cell epitope has been shown to potentiate responses to covalently linked B-cell epitopes in both linear and MAP formats (2, 30, 33, 45). The inclusion of the P30 helper T-cell epitope in MAP304 and MAP305 also likely reduced the incidence of MHC-restricted nonresponsiveness in the current study, since all rabbits demonstrated significant antibody production, whereas two of six rabbits in a previous study failed to produce meaningful levels of antibody when immunized with a MAP displaying the 305-319 peptide without a helper epitope (31).
Though all six MAP304- and MAP305-immune rabbits developed significant antibody titers, one rabbit failed to develop meaningful levels of neutralization. The explanation for the poor neutralizing response of this one MAP305 rabbit is unclear, but since this rabbit had the lowest PA- and LND-specific antibody titer and LND-specific antibody affinity among all the MAP-immune rabbits, it may have been a low responder to the P30 helper epitope. The mouse strains used to screen the P30 epitope were chosen to model the MHC restriction present in an outbred population, based on their relative diversity at the class II loci (18, 34). Though our in vivo screening of the P30 helper epitope in mice revealed that C57BL/6 and SJL mice were strong responders to the P30 epitope, BALB/c mice responded less well and we observed nonresponsiveness to the P30 epitope in C3H mice. The mouse results predict that in an outbred population, strong responses are possible and perhaps most likely, though low-responder animals will be seen as well. The rabbit with poor TNA titers may be one such animal. Among the five other MAP304 and MAP305 rabbits which did demonstrate neutralization, four would have been expected to survive a lethal aerosol challenge with B. anthracis administered subsequent to the fourth immunization and all five would have been expected to survive a challenge administered after the sixth immunization (22, 23, 36).
The neutralization observed in the MAP304-immune rabbits was found to be exceptionally durable, with TNA titers at 7 months after the last immunization still exceeding the PA control levels. Though not of the duration observed in the current study, we have noted durable antibody and neutralization responses to a MAP containing the LND sequence in a previous study (31). In both cases, rabbits received priming immunizations with MAPs in CFA and booster immunizations in IFA. It remains unclear to us whether the durability is a function of the MAP and/or the use of CFA, though exceptional durability has been demonstrated in other models using MAPs (47).
LND-specific antibody in the sera of MAP304- and MAP305-immune rabbits was shown to have a significantly higher affinity than the LND-specific antibody in the sera of rabbits immunized without a helper epitope from a prior study. This result is consistent with early seminal work with 2,4-dinitrophenol, highlighting the critical role of cognate T-cell help for the induction of high-affinity antibody to a covalently linked hapten (11). The increase in affinity of LND-specific antibodies in the sera of MAP304 and MAP305 rabbits may have contributed to the increased TNA titers observed in the sera of these animals compared to the neutralization titers observed in rabbits immunized with an LND MAP without a helper epitope. A few studies examining MAbs specific for PA have demonstrated associations between antibody affinity and anthrax toxin neutralization (27, 29). In the current study, antibody affinity was found to correlate with TNA titer, with an r2 value of 0.35. Not surprisingly, however, PA-specific antibody titer was shown to be more highly correlated to neutralization, with an r2 value of 0.68. We anticipate that the correlations between antibody titer and antibody affinity with TNA may be higher than currently observed once we determine the minimal epitope responsible for the TNA activity. Though we have not yet mapped the minimal epitope, we have observed that the correlations between peptide-specific antibody titer and neutralization determined from analysis of MAP304 and MAP305 antisera are higher when evaluating reactivity to the 299-315 peptide sequence than to the 304-319 peptide sequence (not shown). These data suggest that the minimal neutralizing epitope likely resides between aa 304 and 315.
A totally synthetic LND vaccine for anthrax might find utility in a number of potential scenarios where the induction of rapid, high-titer neutralization might be needed. These might include postexposure scenarios where the administration of a whole PA-containing vaccine might present safety concerns, unvaccinated but high-risk populations, individuals poorly responsive to AVA vaccination (15), and lastly, but perhaps most importantly, scenarios where the neutralizing specificities stimulated by AVA might be intentionally subverted (1, 3, 8).
Acknowledgments
This work was supported by award number U01-AI56580 from the National Institute of Allergy and Infectious Diseases. This work was also supported in part by resources and the use of facilities at the Veterans Administration Health Care System, Ann Arbor, MI. We have no financial conflicts of interest.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the Department of Veterans Affairs.
We thank Stephen Little, Arthur Friedlander, and Lanling Zou for their helpful discussions.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Albrecht, M. T., H. Li, E. D. Williamson, C. S. LeButt, H. C. Flick-Smith, C. P. Quinn, H. Westra, D. Galloway, A. Mateczun, S. Goldman, H. Groen, and L. W. Baillie. 2007. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect. Immun. 75:5425-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amir-Kroll, H., G. Nussbaum, and I. R. Cohen. 2003. Proteins and their derived peptides as carriers in a conjugate vaccine for Streptococcus pneumoniae: self-heat shock protein 60 and tetanus toxoid. J. Immunol. 170:6165-6171. [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer, R., E. Johnson, and R. Bollinger. 2004. Public health vaccination policies for containing an anthrax outbreak. Nature 432:901-904. [DOI] [PubMed] [Google Scholar]
- 4.Brossier, F., M. Levy, A. Landier, P. Lafaye, and M. Mock. 2004. Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect. Immun. 72:6313-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier, R. J., and J. A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 6.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 7.Fairweather, N. F., V. A. Lyness, D. J. Pickard, G. Allen, and R. O. Thomson. 1986. Cloning, nucleotide sequencing, and expression of tetanus toxin fragment C in Escherichia coli. J. Bacteriol. 165:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler, R. A., G. D. Sanders, D. M. Bravata, B. Nouri, J. M. Gastwirth, D. Peterson, A. G. Broker, A. M. Garber, and D. K. Owens. 2005. Cost-effectiveness of defending against bioterrorism: a comparison of vaccination and antibiotic prophylaxis against anthrax. Ann. Intern. Med. 142:601-610. [DOI] [PubMed] [Google Scholar]
- 9.Francis, M. J., G. Z. Hastings, A. D. Syred, B. McGinn, F. Brown, and D. J. Rowlands. 1987. Non-responsiveness to a foot-and-mouth disease virus peptide overcome by addition of foreign helper T-cell determinants. Nature 330:168-170. [DOI] [PubMed] [Google Scholar]
- 10.Friguet, B., A. F. Chaffotte, L. Djavadi-Ohaniance, and M. E. Goldberg. 1985. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 77:305-319. [DOI] [PubMed] [Google Scholar]
- 11.Gershon, R. K., and W. E. Paul. 1971. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J. Immunol. 106:872-874. [PubMed] [Google Scholar]
- 12.Good, M. F., W. L. Maloy, M. N. Lunde, H. Margalit, J. L. Cornette, G. L. Smith, B. Moss, L. H. Miller, and J. A. Berzofsky. 1987. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science 235:1059-1062. [DOI] [PubMed] [Google Scholar]
- 13.Gubbins, M. J., J. D. Berry, C. R. Corbett, J. Mogridge, X. Y. Yuan, L. Schmidt, B. Nicolas, A. Kabani, and R. S. Tsang. 2006. Production and characterization of neutralizing monoclonal antibodies that recognize an epitope in domain 2 of Bacillus anthracis protective antigen. FEMS Immunol. Med. Microbiol. 47:436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubbins, M. J., L. Schmidt, R. S. Tsang, J. D. Berry, A. Kabani, and D. I. Stewart. 2007. Development of a competitive enzyme linked immunosorbent assay to identify epitope specific antibodies in recipients of the U.S. licensed anthrax vaccine. J. Immunoassay Immunochem. 28:213-225. [DOI] [PubMed] [Google Scholar]
- 15.Hanson, J. F., S. C. Taft, and A. A. Weiss. 2006. Neutralizing antibodies and persistence of immunity following anthrax vaccination. Clin. Vaccine Immunol. 13:208-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hering, D., W. Thompson, J. Hewetson, S. Little, S. Norris, and J. Pace-Templeton. 2004. Validation of the anthrax lethal toxin neutralization assay. Biologicals 32:17-27. [DOI] [PubMed] [Google Scholar]
- 17.Ivins, B. E., M. L. Pitt, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, Jr., P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16:1141-1148. [DOI] [PubMed] [Google Scholar]
- 18.Klein, J. 1986. Natural history of the major histocompatibility complex. Wiley, New York, NY.
- 19.Klinman, D. M., H. Xie, S. F. Little, D. Currie, and B. E. Ivins. 2004. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine 22:2881-2886. [DOI] [PubMed] [Google Scholar]
- 20.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422-430. [DOI] [PubMed] [Google Scholar]
- 23.Little, S. F., B. E. Ivins, W. M. Webster, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2006. Duration of protection of rabbits after vaccination with Bacillus anthracis recombinant protective antigen vaccine. Vaccine 24:2530-2536. [DOI] [PubMed] [Google Scholar]
- 24.Little, S. F., S. H. Leppla, and E. Cora. 1988. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 56:1807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little, S. F., J. M. Novak, J. R. Lowe, S. H. Leppla, Y. Singh, K. R. Klimpel, B. C. Lidgerding, and A. M. Friedlander. 1996. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology 142(Pt. 3):707-715. [DOI] [PubMed] [Google Scholar]
- 26.Liu, T. H., J. Oscherwitz, B. Schnepp, J. Jacobs, F. Yu, K. B. Cease, and P. R. Johnson. 2009. Genetic vaccines for anthrax based on recombinant adeno-associated virus vectors. Mol. Ther. 17:373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maynard, J. A., C. B. Maassen, S. H. Leppla, K. Brasky, J. L. Patterson, B. L. Iverson, and G. Georgiou. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597-601. [DOI] [PubMed] [Google Scholar]
- 28.Milich, D. R., J. L. Hughes, A. McLachlan, G. B. Thornton, and A. Moriarty. 1988. Hepatitis B synthetic immunogen comprised of nucleocapsid T-cell sites and an envelope B-cell epitope. Proc. Natl. Acad. Sci. USA 85:1610-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed, N., M. Clagett, J. Li, S. Jones, S. Pincus, G. D'Alia, L. Nardone, M. Babin, G. Spitalny, and L. Casey. 2005. A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect. Immun. 73:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hern, P. A., Z. G. Liang, C. S. Bambra, and E. Goldberg. 1997. Colinear synthesis of an antigen-specific B-cell epitope with a ‘promiscuous’ tetanus toxin T-cell epitope: a synthetic peptide immunocontraceptive. Vaccine 15:1761-1766. [DOI] [PubMed] [Google Scholar]
- 31.Oscherwitz, J., F. Yu, J. L. Jacobs, T. H. Liu, P. R. Johnson, and K. B. Cease. 2009. Synthetic peptide vaccine targeting a cryptic neutralizing epitope in domain 2 of Bacillus anthracis protective antigen. Infect. Immun. 77:3380-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palker, T. J., T. J. Matthews, A. Langlois, M. E. Tanner, M. E. Martin, R. M. Scearce, J. E. Kim, J. A. Berzofsky, D. P. Bolognesi, and B. F. Haynes. 1989. Polyvalent human immunodeficiency virus synthetic immunogen comprised of envelope gp120 T helper cell sites and B cell neutralization epitopes. J. Immunol. 142:3612-3619. [PubMed] [Google Scholar]
- 33.Panina-Bordignon, P., A. Tan, A. Termijtelen, S. Demotz, G. Corradin, and A. Lanzavecchia. 1989. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur. J. Immunol. 19:2237-2242. [DOI] [PubMed] [Google Scholar]
- 34.Petkov, P. M., Y. Ding, M. A. Cassell, W. Zhang, G. Wagner, E. E. Sargent, S. Asquith, V. Crew, K. A. Johnson, P. Robinson, V. E. Scott, and M. V. Wiles. 2004. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 14:1806-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitt, M. L., S. Little, B. E. Ivins, P. Fellows, J. Boles, J. Barth, J. Hewetson, and A. M. Friedlander. 1999. In vitro correlate of immunity in an animal model of inhalational anthrax. J. Appl. Microbiol. 87:304. [DOI] [PubMed] [Google Scholar]
- 36.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 37.Pittman, P. R., S. F. Leitman, J. G. Oro, S. L. Norris, N. M. Marano, M. V. Ranadive, B. S. Sink, and K. T. McKee, Jr. 2005. Protective antigen and toxin neutralization antibody patterns in anthrax vaccinees undergoing serial plasmapheresis. Clin. Diagn. Lab. Immunol. 12:713-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rath, S., C. M. Stanley, and M. W. Steward. 1988. An inhibition enzyme immunoassay for estimating relative antibody affinity and affinity heterogeneity. J. Immunol. Methods 106:245-249. [DOI] [PubMed] [Google Scholar]
- 39.Reason, D., J. Liberato, J. Sun, W. Keitel, and J. Zhou. 2009. Frequency and domain specificity of toxin-neutralizing paratopes in the human antibody response to anthrax vaccine adsorbed. Infect. Immun. 77:2030-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reason, D. C., A. Ullal, J. Liberato, J. Sun, W. Keitel, and J. Zhou. 2008. Domain specificity of the human antibody response to Bacillus anthracis protective antigen. Vaccine 26:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosovitz, M. J., P. Schuck, M. Varughese, A. P. Chopra, V. Mehra, Y. Singh, L. M. McGinnis, and S. H. Leppla. 2003. Alanine-scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J. Biol. Chem. 278:30936-30944. [DOI] [PubMed] [Google Scholar]
- 43.Semenova, V. A., D. S. Schmidt, T. H. Taylor, Jr., H. Li, E. Steward-Clark, S. D. Soroka, M. M. Ballard, and C. P. Quinn. 2007. Analysis of anti-protective antigen IgG subclass distribution in recipients of anthrax vaccine adsorbed (AVA) and patients with cutaneous and inhalation anthrax. Vaccine 25:1780-1788. [DOI] [PubMed] [Google Scholar]
- 44.Sharma, S., D. Thomas, J. Marlett, M. Manchester, and J. A. Young. 2009. Efficient neutralization of antibody-resistant forms of anthrax toxin by a soluble receptor decoy inhibitor. Antimicrob. Agents Chemother. 53:1210-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valmori, D., A. Pessi, E. Bianchi, and G. Corradin. 1992. Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination. J. Immunol. 149:717-721. [PubMed] [Google Scholar]
- 46.Vitale, L., D. Blanset, I. Lowy, T. O'Neill, J. Goldstein, S. F. Little, G. P. Andrews, G. Dorough, R. K. Taylor, and T. Keler. 2006. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect. Immun. 74:5840-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, C. Y., D. J. Looney, M. L. Li, A. M. Walfield, J. Ye, B. Hosein, J. P. Tam, and F. Wong-Staal. 1991. Long-term high-titer neutralizing activity induced by octameric synthetic HIV-1 antigen. Science 254:285-288. [DOI] [PubMed] [Google Scholar]
- 48.Weiss, S., D. Kobiler, H. Levy, H. Marcus, A. Pass, N. Rothschild, and Z. Altboum. 2006. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect. Immun. 74:394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welkos, S., S. Little, A. Friedlander, D. Fritz, and P. Fellows. 2001. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 147:1677-1685. [DOI] [PubMed] [Google Scholar]
- 50.Xie, H., I. Gursel, B. E. Ivins, M. Singh, D. T. O'Hagan, J. B. Ulmer, and D. M. Klinman. 2005. CpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infect. Immun. 73:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, J., J. Xu, G. Li, D. Dong, X. Song, Q. Guo, J. Zhao, L. Fu, and W. Chen. 2006. The 2beta2-2beta3 loop of anthrax protective antigen contains a dominant neutralizing epitope. Biochem. Biophys. Res. Commun. 341:1164-1171. [DOI] [PubMed] [Google Scholar]