Abstract
Knowledge of the in vivo physiology and metabolism of Streptococcus pneumoniae is limited, even though pneumococci rely on efficient acquisition and metabolism of the host nutrients for growth and survival. Because the nutrient-limited, hypoxic host tissues favor mixed-acid fermentation, we studied the role of the pneumococcal pyruvate formate lyase (PFL), a key enzyme in mixed-acid fermentation, which is activated posttranslationally by PFL-activating enzyme (PFL-AE). Mutations were introduced to two putative pfl genes, SPD0235 and SPD0420, and two putative pflA genes, SPD0229 and SPD1774. End-product analysis showed that there was no formate, the main end product of the reaction catalyzed by PFL, produced by mutants defective in SPD0420 and SPD1774, indicating that SPD0420 codes for PFL and SPD1774 for putative PFL-AE. Expression of SPD0420 was elevated in galactose-containing medium in anaerobiosis compared to growth in glucose, and the mutation of SPD0420 resulted in the upregulation of fba and pyk, encoding, respectively, fructose 1,6-bisphosphate aldolase and pyruvate kinase, under the same conditions. In addition, an altered fatty acid composition was detected in SPD0420 and SPD1774 mutants. Mice infected intranasally with the SPD0420 and SPD1774 mutants survived significantly longer than the wild type-infected cohort, and bacteremia developed later in the mutant cohort than in the wild type-infected group. Furthermore, the numbers of CFU of the SPD0420 mutant were lower in the nasopharynx and the lungs after intranasal infection, and fewer numbers of mutant CFU than of wild-type CFU were recovered from blood specimens after intravenous infection. The results demonstrate that there is a direct link between pneumococcal fermentative metabolism and virulence.
Streptococcus pneumoniae is the leading cause of pneumonia in children and adults, and it is a major cause of otitis media, meningitis, and septicemia (22). Despite considerable progress in pneumococcal vaccine development, the serotype specificity and the genomic plasticity of the pneumococcus will hamper its success (36). In addition, the increasing frequency of antibiotic resistance (14) makes it important to understand the mechanisms of pathogenesis of pneumococcal disease.
Probably one of the most understudied fields of pneumococcal biology is that of how the pathogen generates its metabolic energy. The pneumococcus is strictly fermentative, and sugars are the major sources of energy for biosynthesis and growth (19, 52). Therefore, in vivo fitness is determined to a large extent by the processes governed by sugar metabolism. Accumulating evidence suggests that there is a direct link between pneumococcal virulence and sugar metabolism (6, 16, 20, 21, 50). This is not limited to proteins involved in polysaccharide degradation, transport, and regulation (16, 31) but also includes those involved in redox metabolism, such as NADH oxidase (Nox) (6), and in pyruvate metabolism (42, 50). For example, mutation of nox caused diminished virulence in a systemic infection model, and this was attributed to a probable change in the NADH/NAD+ ratio or the increased sensitivity of S. pneumoniae to oxidative stress (6). Mutation of spxB (pyruvate oxidase) led to a reduction in virulence in both pneumonia and sepsis models with mice, which was linked to a decrease in acetyl phosphate levels and downregulation of adhesive proteins (45, 50).
Pneumococcus is known to maintain a fermentative metabolism regardless of oxygen, since it lacks a complete set of genes required for respiration (19, 52). Breakdown of carbohydrates by the classical Embden-Meyerhof pathway results in generation of pyruvate, NADH, and a net gain of two ATP per mole of substrate (40). In homolactic bacteria, NAD+ regeneration is accomplished mainly via the lactate dehydrogenase-catalyzed conversion of pyruvate to lactate (37) (Fig. 1). However, under certain conditions, such as aerobiosis, sugar limitation, or the presence of sugars less preferred than glucose, such as galactose, there is a metabolic shift from homolactic (lactate production) to mixed-acid fermentation, with the formation of products other than lactate (e.g., ethanol, acetate, and formate) (34, 37, 40) (Fig. 1). Mixed-acid fermentation is mediated in part by the activities of the pyruvate dehydrogenase complex (PDHC) or pyruvate formate lyase (PFL) (34, 37, 40). While PDHC catalyzes the oxidative decarboxylation of pyruvate to form acetyl-coenzyme A (CoA) and CO2 in aerobiosis, under microaerobic and anaerobic conditions, most of the pyruvate is converted to acetyl-CoA and formate by the oxygen-sensitive PFL (40, 62). Further metabolism of acetyl-CoA by phosphotransacetylase/acetate kinase or by aldehyde and alcohol dehydrogenases leads to the generation of acetate or ethanol, respectively. While acetate formation generates an additional molecule of ATP, ethanol production from acetyl-CoA regenerates two molecules of NAD+ (34, 40).
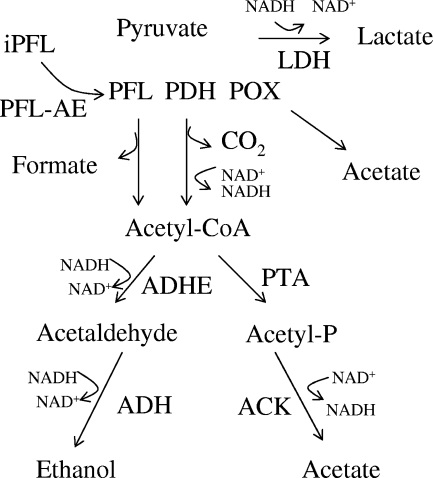
FIG. 1.
Schematic representation of reactions downstream of pyruvate in lactic acid bacteria. After entering bacteria, galactose is converted to pyruvate, which is then further catabolized by homolactic or mixed-acid fermentation pathways. iPFL, inactive PFL; PDH, pyruvate dehydrogenase; POX, pyruvate oxidase; ADH, alcohol dehydrogenase; ACK, acetate kinase; PTA, phosphotransacetylase.
Unlike in other lactic acid bacteria (2, 56), in S. pneumoniae there are no genes for PDHC nor any pyruvate dehydrogenase activity in cell extracts (19, 49, 52), therefore, mixed-acid fermentation is expected to occur mainly by the action of PFL. The regulation of PFL activity in other bacteria has been reported to arise at three levels (27, 46). PFL is synthesized as an inactive enzyme and then posttranslationally converted to an active form by accepting an electron from the PFL-activating enzyme (PFL-AE) (7). PFL may also be regulated transcriptionally and allosterically (4). In Lactococcus lactis, transcription of pfl was found to be significantly higher when the organism was grown in galactose compared to glucose-containing media (2, 34). In addition, in other lactic acid bacteria, low pH and excess supplies of energy reduced pfl mRNA levels, while the level of ldh mRNA increased (3). The regulation of PFL is allosterically controlled in L. lactis by the glycolytic intermediates fructose 1,6-bisphosphate, glyceraldehyde-3-phosphate (GAP), and dihydroxyacetone phosphate, which are strong inhibitors of PFL (33).
The sequenced genome of the S. pneumoniae D39 strain has two copies of both pfl genes, annotated as SPD0235 and SPD0420, and pflA homologs, annotated as SPD0229 and SPD1774 (19, 28, 52). However, the role of the PFL/PFL-AE system in pneumococci is not known, despite the fact that SPD0420 and SPD1774 were predicted to be among the highest expressed genes, based on codon usage (24). In addition, it is not clear why the pneumococcus has multiple copies of these genes. Given that host tissues are limited environments for free, readily fermented sugars (44), such as glucose, but are rich in glycoproteins with O- and N-linked glycans that contain monosaccharides, including galactose (48), and because deep tissue sites are hypoxic, both conditions that favor mixed-acid fermentation (38), we hypothesized that the PFL/PFL-AE system must be important for the in vivo fitness of the pneumococcus. Hence, we identified the genes responsible for pneumococcal PFL activity and studied the impact of these genes on the expression of selected genes involved in glycolysis. Furthermore, we demonstrated that the lack of PFL results in altered lipid composition in the cell membrane and attenuates pneumococcal virulence.
MATERIALS AND METHODS
Bacterial growth conditions.
S. pneumoniae type 2 strain D39 and its isogenic mutants were used in this work. Routinely, pneumococci were grown at 37°C in microaerophilic conditions either in brain heart infusion broth (BHI) (Oxoid, Basingstoke, United Kingdom) or on blood agar base (Oxoid) supplemented with 5% (vol/vol) horse blood. When appropriate, the growth medium was supplemented with 100 μg/ml spectinomycin or 500 μg/ml kanamycin.
Bacteria were also grown in chemically defined medium (CDM) (39) containing disodium β-glycerophosphate (21 g/liter), sodium pyruvate (0.01% [wt/vol]), and choline (0.001% [wt/vol]) at 37°C without pH control (initial pH 6.5). Glucose (1% [wt/vol]) or galactose (1% [wt/vol]) was used as the carbon source. S. pneumoniae was grown under anaerobic conditions in rubber-stoppered bottles (200 ml). Aerobic growth was established under low oxygen tension in Erlenmeyer flasks with an agitation of 50 rpm. Growth was monitored by measuring the optical density at 600 nm (OD600). Growth rates (μ) were calculated through linear regressions of the plots of ln(OD600) versus time during the exponential growth phase.
Mariner mutagenesis.
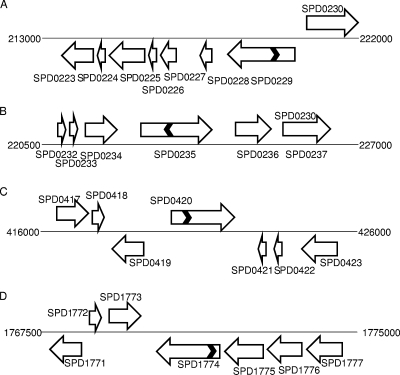
In vitro mariner mutagenesis was used to construct the mutants (32). Approximately 2-kb genomic regions containing the target genes were amplified with the appropriate primers (Table 1). For transposition reactions, approximately 200 ng of PCR fragments was mixed with 200 to 400 ng of donor mariner plasmid pR412, which contains a spectinomycin resistance cassette, and incubated in the presence of purified Himar1 transposase, as described previously (32). Gaps in transposition products were repaired with T4 DNA polymerase (New England Biolabs, Ipswich, MA) and subsequently by Escherichia coli ligase (New England Biolabs). Repaired transposition products were transformed into S. pneumoniae D39 using synthetic competence-inducing peptide (1). Transformants isolated from selective medium were tested for the presence of mariner minitransposons through PCR and sequencing. The PCR used one of the transposon-specific primers, MP127 and MP128 (Table 1), together with an appropriate chromosomal primer. In addition, chromosomal primers were also used to confirm the absence of an intact copy of the gene that may result from duplication. Typical PCR conditions consisted of 1 cycle of 95°C denaturation, 35 cycles of amplification (30-s denaturation at 94°C, 60-s annealing at 53 to 55°C, and 3-min extension at 72°C) with a 3-min final extension at 72°C. The amplification products were analyzed by agarose (1% [wt/vol]) gel electrophoresis. In addition, the exact insertion sites and direction of the antibiotic cassette were also determined by sequencing. The amplicons for sequencing were prepared by three rounds of PCR using MP128 primer as described previously (25), and then the purified products were sequenced using MP127 primer. Sequencing indicated that the spectinomycin cassette had been inserted 608 bp, 833 bp, 650 bp, and 116 bp away from the 5′ end of SPD0229, SPD0235, SPD0420, and SPD1774, respectively. SPD0229M, SPD0235M, SPD0420M, and SPD1774M (mutated in SPD0229, SPD0235, SPD0420, and SPD1774, respectively) (Fig. 2) were selected for further study.
TABLE 1.
Oligonucleotide primers used in this studya
| Primer | Primer sequence (5′-3′) | Target gene in D39 (reference) |
|---|---|---|
| SPD0229F | ACCTCCTGATAAAGTTAAACC | SPD0229 |
| SPD0229R | ACGACTAGCGAACCACCG | |
| SPD0235F | CAGCCTTACTGAAAGGATG | SPD0235 |
| SPD0232R | GGGTTGCCTTAGAAAGAAC | |
| SPD0420F | GACAGTTGTTGAAGCACAAG | SPD0420 |
| SPD0420R | CTCAATGCATCCAAGGCATC | |
| SPD0420CF | GTCCATGGATGGTTGTTAAGACAGTTGTTGAAG | SPD0420 |
| SPD0420CR | GTGGATCCTTAGCTCAATGCATCCAAGG | |
| MalF | GCTTGAAAAGGAGTATACTT | NA* |
| PCEPR | AGGAGACATTCCTTCCGTATC | NA* |
| SPD0420RTF | TGGTGTTTACGCACGTCTTG | SPD0420 |
| SPD0420RTR | CATCAACCCCGTAAAGGTCAC | |
| SPD0526RTF | AATCGTATTGCACGGTGGAT | fba |
| SPD0526RTR | TCGTTTGCTTCGTAGTCACG | |
| SPD0709RTF | TCGTGTGGCTGCCAAGCGTG | gyrB |
| SPD0709RTR | GGCTGATCCACCAGCTGAGTC | |
| SPD0722RTF | CGTCACCTTCACATGACACC | spxB |
| SPD0722RTR | CATGTTGAATGCTCCGTCAC | |
| SPD0880RTF | AGAAGTAATGGCTTCTGCTG | pfk |
| SPD0880RTR | TGCTAAGATGTCAGCATTTG | |
| SPD1078RTF | TGCAGCTCAATACTCTGAC | ldh |
| SPD1078RTR | GTTAGCAGCAACAAGGAAG | |
| SPD1366RTF | CACTGACCCCATGGAAAATC | aldB |
| SPD1366RTR | TGCATGTGCGTATGATTCCT | |
| SPD1774F | AGACACTTGGGCTATGGA | SPD1774 |
| SPD1774R | GTTCCTTAGCCTTGGTGA | |
| SPD1774RTF | TCCCTATCCTCCTTCAAATGC | |
| SPD1774RTR | AAGAAGTTCTTACGTCAGCAGG | |
| SPD1823RTF | TTCTGGATCACGTTCAGCAG | gap |
| SPD1823RTR | CGCATCAACGACCTTACAGA | |
| MP 127 | CCGGGGACTTATCAGCCAACC | pR412 specific (32) |
| MP 128 | TACTAGCGACGCCATCTATGTG | pR412 specific (32) |
Primers indicated by an “F” or “R” tag were used for amplification of gene targets for mutational work, while “RTF” and “RTR” primers were utilized for gene expression analysis. The underlined sequences in the SPD0420F and SPD0420R complementation primers indicate NcoI and BamHI recognition sites, respectively. NA, not applicable; *, the recognition sites for MalF and PCEPR are in pCEP (18).
FIG. 2.
Schematic representation of genomic regions containing putative pfl genes (SPD0235 and SPD0420) (B and C) and pflA genes (SPD0229 and SPD1774) (A and D). The chromosome is represented with a thin solid line, and genes are shown with a block arrow. The chevrons in SPD0229, SPD0235, SPD0420, and SPD01774 represent the approximate positions of insertion (see the text for details), and the direction of chevrons represents the orientation of the spectinomycin cassette. The diagram is not drawn to scale.
Complementation of SPD0420M.
To eliminate the possibility of polar effect, SPD0420M was complemented by introduction of an intact copy of SPD0420 using pCEP, which is a nonreplicative plasmid and allows controlled gene expression following ectopic integration into the chromosome (18). The plasmid integrates immediately downstream of the well-studied ami operon (1). This site is believed to be transcriptionally silent and, as far as it is known, does not affect any cellular functions (18). The intact copy of SPD0420 was amplified with SPD0420CF and SPD0420CR primers, which incorporate the NcoI and BamHI sites to the 5′ and 3′ ends of the gene, respectively (Table 1). The amplicons were digested with NcoI and BamHI, and the digested products were ligated with NcoI/BamHI-digested pCEP. An aliquot of ligation mixture was directly transformed into SPD0420M as described previously (1), and the transformants were selected in the presence of spectinomycin and kanamycin. The successful introduction of the intact copy of the gene was confirmed by PCR using the malF and pCEPR primers (Table 1), whose recognition sites are localized immediately up- and downstream, respectively, of the cloning site. One of the positive transformants, SPD0420Comp, was selected for further analysis.
Quantification of fermentation products during growth.
Strains were grown as described above. Culture samples (2 ml) were taken at the beginning of the stationary phase of growth and centrifuged (16,000 × g, 2 min, 4°C), and the supernatants were stored at −20°C until analysis by high-performance liquid chromatography or 1H nuclear magnetic resonance (NMR) (40). End products were quantified using a high-performance liquid chromatography apparatus equipped with an HPX-87H anion exchange column (Bio-Rad Laboratories Inc., CA) and a refractive index detector (Shodex RI-101; Showa Denko K. K., Japan) operating at 60°C, with 5 mM H2SO4 as the eluent at a flow rate of 0.5 ml/min (40). Alternatively, quantification of metabolites in the supernatants was performed by 1H NMR using a Bruker Avance II 500 MHz spectrometer. 1H-NMR spectra were acquired with water presaturation, a 5.5-μs-pulse width corresponding to a 70° flip angle, and a repetition delay of 50 s. Spectra were referenced to the resonance of 3-(trimethylsilyl) propanesulfonic acid (sodium salt), designated 0 ppm. Formic acid (sodium salt) was added to the samples as an internal concentration standard.
Fatty acid extraction and analysis.
Streptococcus pneumoniae strains were grown anaerobically as described above. Lipids were transmethylated with 2% (vol/vol) H2SO4 in methanol at 80°C for 1 h (43). The resulting fatty acid methyl esters were analyzed by gas chromatography on a Supelcowax 10 capillary column using a temperature gradient from 180 to 225°C, as previously described (10). A known amount of an internal standard (C17:0) was added to each sample to enable fatty acid quantitation. Fatty acid methyl esters were identified by cochromatography with authentic standards (Sigma Co., St Louis, MO) (43). A P value of <0.05 was considered significant, except as otherwise noted.
Quantitative reverse transcriptase-PCR.
The extraction of RNA was done by the Trizol method using mid-log-phase cultures as described previously (51). Before use, the RNA was treated with amplification-grade DNase I (Qiagen, Crawley, United Kingdom) and subsequently purified with an RNeasy minikit (Qiagen). First-strand cDNA synthesis was performed on approximately 1 μg DNase-treated total RNA immediately after isolation using 200 U of SuperScript II reverse transcriptase (Invitrogen, Paisley, United Kingdom) at 42°C for 55 min and random hexamers (59). The transcription level of specific genes was normalized to gyrB transcription and amplified in parallel with the SPD0709RTF and SP0709RTR primers. To reduce the bias in the quantitative reverse transcriptase-PCR, we used primer pairs with similar PCR efficiencies. The results were analyzed by the comparative threshold cycle method (30).
In vivo virulence studies.
Female MFI outbred mice (Harlan Olac) were used for virulence testing. A standardized inoculum was prepared as described previously (58, 60). Briefly, after overnight growth in BHI (Oxoid) in microaerophilic conditions, the OD500 of the pneumococcal cultures was adjusted to 1.6 with phosphate-buffered saline (PBS), and 100 μl of this was administered intraperitoneally to mice. Once the animals reached the lethargic state, blood specimens were collected by cardiac puncture under deep anesthesia with 5% (vol/vol) fluothane (Astra Zeneca, Macclesfield, United Kingdom) over oxygen (1.5 to 2 liter/min), and an aliquot of blood specimens was used to inoculate BHI containing 20% bovine serum. The cultures were allowed to grow until they reached an OD500 of 1.6, at which point the growth ceased and bacteria were stored at −80°C until needed.
To determine the virulence of pneumococcal strains, mice were lightly anesthetized with 2.5% (vol/vol) fluothane over oxygen (1.5 to 2 liter/min). A 50-μl sample of PBS containing approximately 5 × 105 prepassaged S. pneumoniae CFU was given through the nostrils. The inoculum dose was confirmed by viable counting on blood agar plates. Mice were monitored for disease signs (hunched, piloerect, or lethargic) for 7 days (35), and those that reached the severely lethargic stage were considered to have reached the end point of the assay and were killed humanely. The time to this point was defined as “survival time.” Mice that were alive 7 days after infection were deemed to have survived the infection. To express the disease signs numerically, a mouse was given a score of 2 if it was found hunched, a score of 4 if it had a starry coat (piloerection), and a score of 6 if it was lethargic. To determine the development of bacteremia in each mouse, approximately 20 μl of venous blood specimens was obtained from intranasally infected mice at predetermined time points after infection. Viable counts in blood specimens were determined by serial dilution in sterile PBS and plating onto blood agar plates supplemented with 5% (vol/vol) defibrinated horse blood with appropriate antibiotic. Median survival times were analyzed using the Mann-Whitney U test.
Growth of bacteria in the nasopharynx and lungs was also determined. To do this, at predetermined time intervals following intranasal infection, groups of mice were deeply anesthetized as described previously (58, 60), and subsequently the mice were killed by cervical dislocation. The lungs and nasopharynx were transferred separately into 10 ml of sterile PBS, weighed, and then homogenized in a stomacher lab blender (Seward Medical, London, United Kingdom) (58, 60). Viable counts in homogenates were determined as described above. For intravenous infections, 1 × 105 CFU/ml S. pneumoniae in 100 μl PBS, pH 7.0, were administered via the dorsal tail vein. The inoculum was confirmed by plating on blood agar as described above. Data were analyzed by analysis of variance followed by the Bonferroni posttest. Statistical significance was considered at P values of <0.05.
RESULTS
Bioinformatic analysis.
The putative pfl genes, SPD0235 and SPD0420, are found in all sequenced pneumococcal genomes (19, 28, 52). The predicted amino acid sequences of SPD0235 and SPD0420 exhibit 20% identity over 770 amino acids. While the SPD0420 protein has conserved adjacent cysteinyl residues characteristic of a PFL active site (EMSCISCCVSPLD) (47), as well as the highly conserved peptide sequence around the C-terminal glycyl residue (RISGY) (47), the SPD0235 protein does not have the conserved cysteine residues. This implies that SPD0235 is unlikely to encode a PFL, in spite of the annotation. However, it has a sequence motif containing the C-terminal glycyl residue (RVAGY), which is converted to a free radical in the PFL of E. coli (53, 55). Thus, the SPD0235 protein may be a member of a distinct class of glycyl radical enzymes with a conserved pentapeptide region containing the C-terminal glycyl residue, albeit without PFL activity. This class of proteins is also found in E. coli, but so far no function has been attributed to them (47).
In the vicinity of SPD0235 there are cellobiose-specific sugar phosphotransferase (SPD0234) and fructose-6-phosphate aldolase (FBA) (SPD0236) genes (Fig. 2) in all sequenced pneumococcal genomes, suggestive of a conserved nature for this locus (19, 28, 52). On the other hand, SPD0420 is surrounded by a DNA-polymerase gene (dinP, SPD0419) and a hypothetical gene of unknown function (SPD0421) (19, 28, 52). The genes adjacent to SPD0420 are transcribed in opposite directions (Fig. 2), implying that the mutation of SPD0420 is unlikely to create a polar effect, and furthermore, a strong rho-independent transcription terminator with an estimated ΔGf of −12.3 kcal/mol is located 17 bp downstream of the SPD0420 stop codon. While dinP is conserved universally in all sequenced pneumococcal genomes, the hypothetical gene (SPD0421) either exhibits sequence variation or is absent in certain pneumococcal genomes, such as in TIGR4 or Hungary19A_6, indicating that recent evolutionary genetic events have taken place in the region (www.ncbi.nlm.nih.gov). In contrast to the genes in E. coli, Haemophilus influenzae, and Clostridium pasteurianum (15, 46, 54), in S. pneumoniae, none of the putative pfl and pflA genes are adjacently located, which is also a feature of the pfl/pflA system in lactococci (2).
The predicted amino acid sequences of the putative PFL-AE proteins SPD0229 and SPD1774 share 32% identity over 236 amino acids, and they are found in all sequenced pneumococcal genomes (www.ncbi.nlm.nih.gov). Both of these proteins have a CXXXCXXC consensus sequence motif close to the N terminus of the protein, which is reported to be the catalytic site in the E. coli enzyme (7). SPD1774 is the last gene of a predicted operon (13) and surrounded by genes responsible for protein export (SPD1773) and pH homeostasis (SPD1775), while the locus encompassing SPD0229 contains genes coding for transcriptional regulators.
In vitro growth characteristics of pfl and pflA mutants.
The growth characteristics of strains varied, depending on aeration and the carbon source (Table 2 and Fig. 3). When glucose was used as the carbon substrate, all strains grew better under aerobic rather than anaerobic conditions (P < 0.01, for all comparisons). With glucose, there was no difference in growth rates and yields between mutant strains and the parent D39 either in anaerobiosis or in aerobiosis (P > 0.05).
TABLE 2.
In vitro growth rates of pneumococcal strains
| Strain | Growth rate for conditiona: |
|||
|---|---|---|---|---|
| Glucose aerobic | Glucose anaerobic | Galactose aerobic | Galactose anaerobic | |
| D39 | 0.63 (0.02) | 0.45 (0.02) | 0.41 (0.01) | 0.33 (0.02) |
| SPD0420 M | 0.56 (0.06) | 0.4 (0.04) | 0.4 (0.03) | 0.1 (0) |
| SPD0420Comp | 0.55 (0.03) | 0.47 (0) | 0.35 (0.02) | 0.2 (0.03) |
| SPD1774 M | 0.6 (0) | 0.41 (0.008) | 0.22 (0.01) | 0.3 (0) |
| SPD0229 M | 0.6 (0) | 0.45 (0) | 0.41 (0.03) | 0.3 (0.03) |
| SPD0235 M | 0.62 (0.06) | 0.44 (0.07) | 0.43 (0.04) | 0.35 (0.03) |
Pneumococcal strains were grown in CDM containing the indicated sugars as the sole carbon source in aerobiosis or anaerobiosis. The mean rate, where rate is defined as the increase in optical density per hour, was calculated from three independent experiments. Values are the mean rate, with the standard deviation given in parentheses.
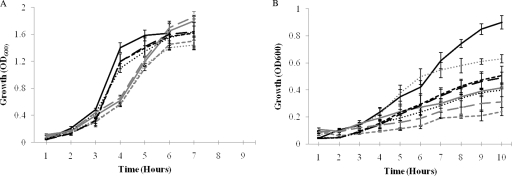
FIG. 3.
In vitro growth characteristics of pneumococcal strains in CDM supplemented with glucose (A) and galactose (B). D39, SPD0420M, SPD0420Comp, and SPD1774M are represented by solid; short, dashed; long, dashed; or dotted lines, respectively. Black lines are for aerobiosis, and gray lines are for anaerobiosis. Error bars show the standard error of the mean for three independent experiments.
Galactose stimulated the growth rates of all strains under aerobic conditions relative to anaerobiosis, (P < 0.01), with the exception of SPD1774M, which grew better in anaerobiosis than aerobiosis (P < 0.01). The growth rates of the mutants did not differ from those of D39 in aerobiosis and anaerobiosis (P > 0.05), with the exception of SPD1774M, which grew with a lower rate in aerobiosis (P < 0.01), and SPD0420M, which had a lower rate than D39 in anaerobiosis (P < 0.01). The growth rate of complemented strain SPD0420Comp was greater than that of SPD0420M (P < 0.05) in anaerobiosis. The growth yields of the strains were higher aerobically than anaerobically, with the exception of SPD1774M, whose yield was higher anaerobically than aerobically. In addition, SPD0420M exhibited an extended lag phase during anaerobic growth. With galactose, the growth rate that was observed for all strains in aerobiosis and anaerobiosis (P < 0.01) was lower than that for glucose-containing medium.
End-product analysis.
The PFL enzyme can be measured directly (57) or indirectly (2). The indirect assay relies on formate detection in spent bacterial culture supernatants to indicate the presence of active enzyme. In this study we used the indirect method to assay PFL activity. The fermentation end products of pneumococcal strains in aerobiosis and anaerobiosis, in CDM with glucose or galactose as the sole carbon source, were analyzed using late-exponential-culture supernatants. Regardless of aeration, when glucose was used, the main fermentation product of D39 was lactate, with a small amount of acetate (Fig. 4A). However, replacement of glucose with galactose led to the generation of mixed fermentation products, under both aerobic and anaerobic conditions, with formate being the most abundant metabolite in both cases (Fig. 4A). Additionally, acetate and ethanol were also formed, and the ratio of products was approximately 2:1:1 for formate, acetate, and ethanol, both in aerobiosis and anaerobiosis.
FIG. 4.
Fermentation end product analysis for strains D39 (A), SPD0420M (B), SPD0420Comp (C), and SPD1774M (D). The pneumococcal strains were grown aerobically or anaerobically in CDM containing glucose or galactose. Glu, glucose; Gal, galactose. Each metabolite concentration was measured in duplicate with supernatants of three independently grown cultures.
The end product analysis of SPD0229M and SPD0235M was principally similar to that of D39 in the presence of glucose and galactose regardless of aeration, ruling out their involvement in active PFL synthesis (data not shown). On the other hand, in SPD0420M and SPD1774M culture supernatants the main end product was lactate in all growth conditions, and no formate was detected under any of the conditions employed, indicating that these two genes are responsible for active PFL synthesis (Fig. 4B and D). Introduction of an intact copy of SPD0420 to SPD0420M restored formate production (Fig. 4C). In the light of sequence homology and end-product analysis, it is likely that SPD0420 is responsible for PFL synthesis and that the SPD1774 protein is putative PFL-AE.
Gene expression analysis.
The glycolytic pathway is composed of an integrated network of enzymes whose activity is dependent on environmental, allosteric, and transcriptional regulation. Disruption of a route leading to end products in a strictly fermentative organism can be expected to affect expression of the glycolytic enzymes, as the flux through glycolysis is intimately associated with the conversion of pyruvate to end products, which ultimately allows glycolysis to proceed by replenishing NAD+ pools. To further understand the role of PFL in the regulation of pneumococcal glycolysis, the expression of certain genes coding for glycolytic enzymes was determined for bacteria cultured under conditions of aerobiosis and anaerobiosis, using glucose or galactose as a carbon source (Table 3). Specifically, the expression of genes coding for FBA (fba), pyruvate kinase (pyk), pyruvate oxidase (spxB), GAP dehydrogenase (gap), lactate dehydrogenase (ldh), and α-acetolactate dehydrogenase (aldB) was studied, along with SPD0420 and SPD1774. Differences in expression twofold or greater were considered significant, although some differences less than twofold are known to be biologically important (9).
TABLE 3.
Fold change in gene expression under different conditionsa
| Gene | Fold change in gene expression |
|||||
|---|---|---|---|---|---|---|
| Galactose vs glucose |
SPD0420M vs D39 in galactose |
|||||
| Anaerobic |
Aerobic |
|||||
| D39 | SPD0420M | D39 | SPD0420M | Anaerobic | Aerobic | |
| Glycolytic | ||||||
| fba | 0.7 (0.12) | 5.7(1.5) | 1.1 (0.06) | 1.6 (0.19) | 18.3 (5.16) | 2.5 (0.29) |
| pyk | 1.3 (0.05) | 4.8 (1.7) | 1.2 (0.07) | 0.6 (0.14) | 4.4(1.32) | 0.8 (0.17) |
| gap | 1.3 (0.01) | 2.2 (0.96) | 1.7 (0.03) | 0.8 (0.17) | 3.5 (0.82) | 0.7 (0.14) |
| Pyruvate breakdown | ||||||
| spxB | 1.2 (0.04) | 0.7 (0.16) | 1.1 (0.1) | 2.3 (0.26) | 1.3 (0.27) | 1.3 (0.12) |
| ldh | 1.8 (0.17) | 1.9 (0.14) | 1.0 (0.06) | 0.6 (0.04) | 2.2 (0.17) | 1 (0.05) |
| aldB | 1.6 (0.09) | 1.4 (0.33) | 1 (0.21) | 1.0 (0.13) | 1.8 (0.38) | 1.3 (0.28) |
| SPD1774 | 1.5 (0.21) | 2.0 (0.47) | 2.4 (0.1) | 1.2 (0.13) | 3.5 (0.82) | 3.5 (0.82) |
| SPD0420 | 8.6 (0.35) | 3.5 (0.32) | ||||
Pneumococcal strains grown in CDM containing the indicated sugars as the sole carbon source in aerobiosis or anaerobiosis. See the text for gene names. The relative expression was calculated from three independent experiments, and the standard deviation is indicated in parentheses.
pfl expression in other bacteria is known to be influenced by galactose and anaerobiosis (33, 34). Indeed, in strain D39 in anaerobiosis, SPD0420 expression increased nearly ninefold in galactose relative to that for glucose-grown bacteria. In addition, in aerobiosis, SPD0420 and SPD1774 were upregulated by 2.4- and 3.5-fold, respectively, in D39 grown in galactose relative to growth in glucose. The overexpression of these genes in aerobiosis reflects the readiness of the pneumococcus to adjust its metabolism when a shift from aerobiosis to anaerobiosis occurs. The expression of other genes did not show significant change under the experimental conditions employed.
In SPD0420M grown anaerobically in galactose, the expression of fba and pyk went up 5.7-fold (±1.4) and 4.8-fold (±1.7), respectively, compared to anaerobic growth in glucose. The expression of other genes in SPD0420M was similar to that for D39 in anaerobiosis, regardless of the carbon source (P > 0.05). When the expression of fba in SPD0420M grown anaerobically with galactose was compared to that for D39 cultured under the same conditions, the difference in expression levels was further pronounced, to >18-fold (Table 3).
Fatty acid composition of bacterial cell membranes.
Because PFL activity leads to formation of acetyl-CoA in addition to formate and because acetyl-CoA is an important precursor of fatty acid biosynthesis (8), we hypothesized that in SPD0420M and SPD1774M, the fatty acid composition would exhibit altered patterns. Analysis of the fatty acid composition was done with D39 and the two mutant strains SPR0420M and SPD1774M. For each strain the weighted average of the number of double bonds per fatty acid, i.e., the unsaturation index, was calculated. Since the mean value of the unsaturation index is one or two orders of magnitude smaller than the proportions of fatty acids, we adjusted the critical P value by using a Bonferroni correction that considers the number of constituent fatty acids on which the unsaturation index is based; i.e., the initial critical P value, 0.05, was divided by 5, giving a new critical P value of 0.01.
As shown in Table 4, the absence of PFL activity resulted in a decreased unsaturation index, from 0.31 (±0.01) in the wild-type strain (D39) to 0.25 (±0.02) (P = 0.029) and 0.24 (±0.01) (P = 0.0056) in SPR0420M and SPD1774M, respectively, when bacteria were grown in BHI. Fatty acid analysis of bacteria grown in CDM supplemented with galactose resulted in only a slight change in SPD1774M (data not shown). Since CDM contains a high acetate concentration (7.3 mM), the organism can bypass the need for PFL/PFL-AE by converting acetate to acetyl-CoA via acetate kinase and phosphotransacetylase (50), supplying the PFL mutants with sufficient acetyl-CoA for fatty acid biosynthesis.
TABLE 4.
The fatty acid composition of pneumococcal strains grown in BHI
| Strain | Fatty acid compositione |
P value for 18:1b | UIc | P value for UId | ||||
|---|---|---|---|---|---|---|---|---|
| 14:0a | 16:0 | 16:1 | 18:0 | 18:1 | ||||
| D39 | 20.0 (0.6) | 36.8 (1.5) | 13.0 (0.2) | 7.1 (0.9) | 14.1 (0.6) | 0.31 (0.01) | ||
| SPD0420M | 19.1 (0.3) | 38.7 (1.4) | 13.0 (0.5) | 6.8 (1.4) | 9.4 (0.5) | 0.014 | 0.25 (0.02) | 0.029 |
| SPD1774M | 16.2 (0.5) | 42.0 (0.9) | 13.1 (0.1) | 6.9 (0.8) | 8.2 (0.8) | 0.016 | 0.24 (0.01) | 0.0056 |
The first figure shows the number of carbons, and the second the number of double bonds.
The significance of difference was calculated in comparison to D39 by Student's t test; P < 0.05.
UI, unsaturation index, defined as the weighted average of the number of double bonds per fatty acid.
P < 0.05 based on the Bonferroni correction.
Values are percentages of the five major fatty acyl residues; standard deviations are given in parentheses.
In vivo studies.
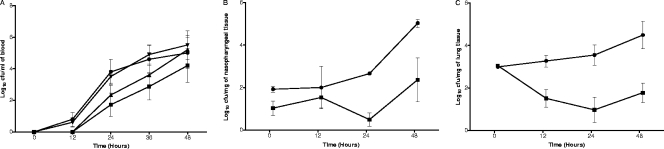
The SPD0420M and SPD1774M strains were tested for their ability to cause disease in mice after intranasal infection. It was found that the SPD0420M- and SPD1774M-infected groups (108 ± 14 h, n = 10, and 90 ± 13 h, n = 10, respectively) survived significantly longer than the wild-type-infected group (51 ± 2 h, n = 28) (P < 0.01 and P < 0.05, respectively). There was no difference in the survival times of the SPD1774M- and SPD0420M-infected groups (P > 0.05). SPD0420Comp-infected mice did not survive (61 ± 8 h, n = 10) significantly longer than the wild-type-infected group (P > 0.05). Furthermore, the disease sign scores for the groups infected with mutants at 24, 36, and 48 h postinfection (for SPD0420M, 0 ± 0 at 24 h, 1.6 ± 0.3 at 36, and 3.4 ± 0.2 at 48 h; for SPD1774M, 0 ± 0, 1.7 ± 0.2, and 3.6 ± 0.2, respectively; n = 10 for both) were lower than those for the wild-type group (1.1 ± 0.3, 3.3 ± 0.4, and 4.8 ± 0.3, respectively; n = 20) (P < 0.01 for 24 and 36 h, and P < 0.05 for 48 h postinfection). For example, by 24 h postinfection, signs of disease could be detected only in the wild-type-infected cohort, and the signs increased swiftly thereafter. However, the signs of disease emerged after 24 h postinfection in the groups infected with SPD0420M and SPD1774M. Moreover, by sampling blood at predetermined time intervals following intranasal infection, it was demonstrated that bacteremia in the groups infected with SPD0420M or SPD1774M (between 12 and 24 h postinfection) occurred later than with D39 or SPD0420Comp (0 to 12 h postinfection) (P < 0.05) (Fig. 5A). However, once in the blood the mutants grew as well as the wild type.
FIG. 5.
Growth of D39 (•), SPD0420M (▪), SPD1774M (▴), and SPD0420Comp (▾) in blood specimens (A), nasopharynx (B), and lung (C) after intranasal infection. Each point is the mean of data from five mice. Error bars show the standard error of the mean.
To further investigate the nature of impaired virulence in strain SPD0420M, we went on to determine the growth of bacteria in the nasopharynx (Fig. 5B) and lungs (Fig. 5C) after intranasal infection. The D39 numbers in the nasopharynx were significantly greater than those for the mutant at 24 h (P < 0.01) and 48 h (P < 0.05) postinfection. D39 numbers progressively increased in the nasopharynx 12 h postinfection, whereas numbers for SPD0420M did not significantly change over 48 h. In the lungs, the wild-type numbers progressively increased after infection, from log10 2.99 ± 0.08 at the beginning of infection to log10 4.49 ± 0.65 at 48 h postinfection (P < 0.05) (Fig. 5C). However, SPD0420M was not as successful as the wild type in its ability to survive in the lungs, and by 24 h after infection an approximately 2-log decrease was observed with the numbers of SPD0420M (P < 0.05).
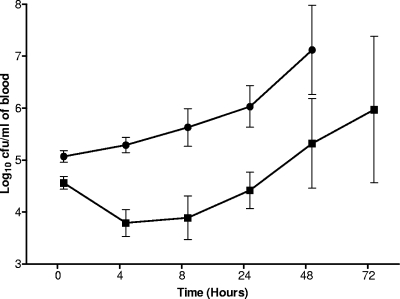
When the intravenous route was used for infection, the wild-type numbers increased from log10 5.07 ± 0.11 CFU/ml at the beginning of the infection to log10 7.12 ± 0.86 CFU/ml at 48 h postinfection, without apparent lag (Fig. 6). However, the mutant numbers started to increase only after 8 h postinfection, following an initial decline in viable counts. From this point onward the mutant grew as well as the wild type, suggesting the presence of alternative mechanisms to compensate the absence of PFL.
FIG. 6.
Time course of bacterial growth in blood specimens from mice infected intravenously with D39 (•) or SPD0420M (▪). Each point is the mean of data from 6 to 10 mice, except 72 h for SPD0420M, which is from 3 mice. Error bars show the standard error of the mean.
DISCUSSION
The transition from aerobiosis to an oxygen-depleted environment signifies passage from mucosal surfaces to deep-tissue sites for the pneumococcus; hence, it is integral to pneumococcal virulence (22). This move is expected to trigger a fundamental change in energy metabolism due to differences in nutrient availability and oxygen concentration (44). We investigated biochemical aspects of these changes and the implication of this metabolic shift on pneumococcal virulence.
In many microorganisms, PFL is one of the key enzymes for anaerobic energy metabolism, especially in the metabolic shift to mixed-acid fermentation (2, 4, 11, 34). We demonstrated through mutational studies and subsequent metabolite analysis and by an in silico approach that the pneumococcal SPD0420 and SPD1774 genes are responsible for active PFL protein. The mutant strains did not produce formate, which is generated only in the presence of active PFL, and a homology search indicated conserved residues that are found in other PFLs (47).
SPD0420 and SPD1774 mutations led to reduced rates of growth in galactose-containing medium compared to glucose in anaerobiosis, consistent with PFL's role in mixed-acid fermentation. In addition, the mutation of SPD0420 and SPD1774 led to a reduction in virulence, as manifested by the increased survival times of animals infected with SPD0420M and SPD1774M compared to the parental strain, and the bacteremia in cohorts infected with SPD0420M and SPD1774M was developed later than that in D39. Moreover, SPD0420M attenuated in its growth in the nasopharynx and lungs, showing that PFL contributes to pneumococcal virulence in several tissue sites. Initially, we predicted that the main impact of PFL would be seen in the hypoxic environment of blood specimens (29), and no role was envisaged to be present during infection of the nasopharynx and lungs, because PFL was expected to be inactivated in these oxygenated sites. Our results indicate that the pneumococcal PFL may not be as susceptible to oxygen as other bacterial PFLs (26). Indeed, it has been reported previously that PFL in Streptococcus mutans and L. lactis is relatively resistant to inactivation by oxygen compared to the E. coli enzyme (2, 56). This could be due to the presence of a deactivation system that may protect the PFL (34, 41). PFL deactivation was suggested to occur through removal of the glycyl free radical, which is required for the active PFL, by alcohol dehydrogenase during the transition from anaerobic to aerobic growth (47). Hence, future detailed structural and biochemical characterization is required to establish the mechanism of the oxygen-resistant nature of pneumococcal PFL. The second reason for attenuated virulence of strains lacking active PFL could be differences in overall sugar and oxygen compositions in different tissue sites. For example, mucosal sites, the nasopharynx and lung, are rich in galactose, whereas blood is rich in its glucose content (44). Hence, as galactose triggers mixed-acid fermentation mediated by PFL, it is expected that the lack of PFL will attenuate virulence in mucosal sites because of the composition of sugars and in blood because of the low oxygen concentration.
A detailed analysis of reduction in SPD0420M and SPD1774M virulence was beyond the scope of this study. However, we speculate that the decreased virulence was due to reduction in ATP generation, which would have an adverse impact on diverse metabolic functions. Alternatively, the defect in PFL synthesis may affect purine synthesis (11), as was reported for Streptococcus thermophilus (11). In addition, diminished synthesis of acetyl-CoA may influence virulence through its impact on choline and fatty acid biosynthesis (8, 11). We detected a small but significant (12, 17) change in membrane fatty acid composition in both SPD0420M and SPD1774M, due most probably to a shortage of acetyl-CoA in the mutant strains. Other studies have also shown that small changes in fatty acid composition are meaningful and account for membrane fatty acid adaptation to stress conditions, such as temperature, H2O2, and pH (12, 17). Since the growth rate of bacteria depends on membrane fluidity (61), the lower fatty acid unsaturation level is likely to explain the reduced growth rate of the mutant strains compared to that of the wild type, which affected their virulence in mice.
The expression of SPD0420 was shown to be elevated in anaerobiosis in galactose-containing medium, which is in line with other studies (33, 34, 47). In the absence of PFL, the pneumococcus upregulates its expression of fba and pyk during anaerobic growth in galactose-containing medium, compared to growth in glucose. In Streptococcus bovis, overexpression of fba increases pfl expression when grown in lactose-containing medium (5). This may indicate concerted regulation of pfl, since the products of FBA, in addition to GAP and dihydroxyacetone phosphate, are allosteric regulators of PFL. It can be suggested that by increasing fba expression, the pneumococcal cell machinery is “optimized” to operate the PFL pathway. In addition, we observed upregulation of pyk. The increased pyk expression in an E. coli pfl mutant was suggested to be due to an increase in glycolytic flux. Higher glycolytic flux may be used to achieve the energy requirement (62).
PFL is posttransitionally regulated by PFL-AE, which activates PFL by generation of a stable and catalytically essential glycyl radical at G734 of PFL (based on the E. coli PFL amino acid sequence). In E. coli, induction of pfl in anaerobiosis has been shown to involve both FNR and ArcA/ArcB regulators (33, 34, 47). The global transcription factor FNR was reported to have a role in the expression of pfl via two recognition sequences located in the promoter region of this gene (23). However, there is no fnr homolog in S. pneumoniae, but the pneumococcal genomes contain homologs of ArcA/ArcB transcriptional regulators (data not shown).
In addition to SPD0420 and SPD1774, SPD0235 and SPD0229 closely resemble the pfl and pflA genes, respectively. However, under the conditions tested, these genes are not involved in the synthesis of an active PFL. Genomic analysis of E. coli indicated the presence of several pfl- and pflA-like genes (46, 47). Many of these PFL-like enzymes are unlikely to have a role in the dissimilation of 2-keto acids, and therefore, they may represent new classes of glycyl radical enzymes with novel enzymatic activities (46, 47).
In this study we identified the pneumococcal pfl and pflA genes and demonstrated their involvement in mixed-acid fermentation. It was found that the mutation of pfl results in reduced pneumococcal virulence, indicating a strong link between the ability to have flexible fermentative metabolism and virulence. The reduced virulence was probably due to a defect in ATP and acetyl-CoA biosynthesis, which affect fitness and fatty acid composition, respectively. At mucosal surfaces the concentration of glucose is low (44), but the pneumococcus is exposed to mucin at these sites, and mucin is rich in galactose-containing glycosides (48, 59). We recently demonstrated the mucin utilization ability of the pneumococcus (59). In light of available data, we conclude that the pneumococcus maintains mixed-acid fermentation in the nutrient-limited niches of the host, and active PFL plays a vital role in this metabolic event. Thus, future studies investigating pneumococcal virulence should not define virulence solely from the host perspective, but the intricacies of the environmental settings surrounding the microorganism should also be considered.
Acknowledgments
This work was supported by The Wellcome Trust (078763/Z/05/Z) and by Fundação para a Ciência e a Tecnologia, Portugal (FCT), project PDCT/BIA-MIC/56235/2004.
We thank Marc Prudhomme for kindly providing pCEP and pR412.
Editor: A. Camilli
Footnotes
Published ahead of print on 14 September 2009.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Alloing, G., C. Granadel, D. A. Morrison, and J. P. Claverys. 1996. Competence pheromone, oligopeptide permease, and induction of competence in Streptococcus pneumoniae. Mol. Microbiol. 21:471-478. [DOI] [PubMed] [Google Scholar]
- 2.Arnau, J., F. Jorgensen, S. M. Madsen, A. Vrang, and H. Israelsen. 1997. Cloning, expression, and characterization of the Lactococcus lactis pfl gene, encoding pyruvate formate-lyase. J. Bacteriol. 179:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanuma, N., M. Iwamoto, and T. Hino. 1999. Structure and transcriptional regulation of the gene encoding pyruvate formate-lyase of a ruminal bacterium, Streptococcus bovis. Microbiology 145:151-157. [DOI] [PubMed] [Google Scholar]
- 4.Asanuma, N., T. Yoshii, and T. Hino. 2004. Molecular characteristics and transcription of the gene encoding a multifunctional alcohol dehydrogenase in relation to the deactivation of pyruvate formate-lyase in the ruminal bacterium Streptococcus bovis. Arch. Microbiol. 181:122-128. [DOI] [PubMed] [Google Scholar]
- 5.Asanuma, N., T. Yoshii, M. Kikuchi, and T. Hino. 2004. Effects of the overexpression of fructose-1,6-bisphosphate aldolase on fermentation pattern and transcription of the genes encoding lactate dehydrogenase and pyruvate formate-lyase in a ruminal bacterium, Streptococcus bovis. J. Gen. Appl. Microbiol. 50:71-78. [DOI] [PubMed] [Google Scholar]
- 6.Auzat, I., S. Chapuy-Regaud, G. Le Bras, D. Dos Santos, A. D. Ogunniyi, I. Le Thomas, J. R. Garel, J. C. Paton, and M. C. Trombe. 1999. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol. Microbiol. 34:1018-1028. [DOI] [PubMed] [Google Scholar]
- 7.Buis, J. M., and J. B. Broderick. 2005. Pyruvate formate-lyase activating enzyme: elucidation of a novel mechanism for glycyl radical formation. Arch. Biochem. Biophys. 433:288-296. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, J. W., and J. E. Cronan, Jr. 2001. Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery. Annu. Rev. Microbiol. 55:305-332. [DOI] [PubMed] [Google Scholar]
- 9.Chaussee, M. S., G. L. Sylva, D. E. Sturdevant, L. M. Smoot, M. R. Graham, R. O. Watson, and J. M. Musser. 2002. Rgg influences the expression of multiple regulatory loci to coregulate virulence factor expression in Streptococcus pyogenes. Infect. Immun. 70:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, Z., H. A. Norman, and Y. M. Heimer. 1995. Microalgae as a source of omega 3 fatty acids. World Rev. Nutr. Diet. 77:1-31. [PubMed] [Google Scholar]
- 11.Derzelle, S., A. Bolotin, M. Y. Mistou, and F. Rul. 2005. Proteome analysis of Streptococcus thermophilus grown in milk reveals pyruvate formate-lyase as the major upregulated protein. Appl. Environ. Microbiol. 71:8597-8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Pasqua, R., N. Hoskins, G. Betts, and G. Mauriello. 2006. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 54:2745-2749. [DOI] [PubMed] [Google Scholar]
- 13.Ermolaeva, M. D., O. White, and S. L. Salzberg. 2001. Prediction of operons in microbial genomes. Nucleic Acids Res. 29:1216-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felmingham, D., A. R. White, M. R. Jacobs, P. C. Appelbaum, J. Poupard, L. A. Miller, and R. N. Gruneberg. 2005. The Alexander Project: the benefits from a decade of surveillance. J. Antimicrob. Chemother. 56(Suppl. 2):ii3-ii21. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, et al. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 16.Giammarinaro, P., and J. C. Paton. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 70:5454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerzoni, M. E., R. Lanciotti, and P. S. Cocconcelli. 2001. Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiology 147:2255-2264. [DOI] [PubMed] [Google Scholar]
- 18.Guiral, S., V. Henard, M. H. Laaberki, C. Granadel, M. Prudhomme, B. Martin, and J. P. Claverys. 2006. Construction and evaluation of a chromosomal expression platform (CEP) for ectopic, maltose-driven gene expression in Streptococcus pneumoniae. Microbiology 152:343-349. [DOI] [PubMed] [Google Scholar]
- 19.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyer, R., N. S. Baliga, and A. Camilli. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 187:8340-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyer, R., and A. Camilli. 2007. Sucrose metabolism contributes to in vivo fitness of Streptococcus pneumoniae. Mol. Microbiol. 66:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadioglu, A., J. N. Weiser, J. C. Paton, and P. W. Andrew. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288-301. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser, M., and G. Sawers. 1995. Fnr activates transcription from the P6 promoter of the pfl operon in vitro. Mol. Microbiol. 18:331-342. [DOI] [PubMed] [Google Scholar]
- 24.Karlin, S., J. Theriot, and J. Mrazek. 2004. Comparative analysis of gene expression among low G+C gram-positive genomes. Proc. Natl. Acad. Sci. USA 101:6182-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlyshev, A. V., M. J. Pallen, and B. W. Wren. 2000. Single-primer PCR procedure for rapid identification of transposon insertion sites. BioTechniques 28:1078, 1080, 1082. [DOI] [PubMed] [Google Scholar]
- 26.Knappe, J., S. Elbert, M. Frey, and A. F. Wagner. 1993. Pyruvate formate-lyase mechanism involving the protein-based glycyl radical. Biochem. Soc. Trans. 21:731-734. [DOI] [PubMed] [Google Scholar]
- 27.Knappe, J., and G. Sawers. 1990. A radical-chemical route to acetyl-CoA: the anaerobically induced pyruvate formate-lyase system of Escherichia coli. FEMS Microbiol. Rev. 6:383-398. [DOI] [PubMed] [Google Scholar]
- 28.Lanie, J. A., W. L. Ng, K. M. Kazmierczak, T. M. Andrzejewski, T. M. Davidsen, K. J. Wayne, H. Tettelin, J. I. Glass, and M. E. Winkler. 2007. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 189:38-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy, M. M. 2005. Pathophysiology of oxygen delivery in respiratory failure. Chest 128:547S-553S. [DOI] [PubMed] [Google Scholar]
- 30.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 31.Manco, S., F. Hernon, H. Yesilkaya, J. C. Paton, P. W. Andrew, and A. Kadioglu. 2006. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect. Immun. 74:4014-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, B., M. Prudhomme, G. Alloing, C. Granadel, and J. P. Claverys. 2000. Cross-regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol. Microbiol. 38:867-878. [DOI] [PubMed] [Google Scholar]
- 33.Melchiorsen, C. R., N. B. Jensen, B. Christensen, K. Vaever Jokumsen, and J. Villadsen. 2001. Dynamics of pyruvate metabolism in Lactococcus lactis. Biotechnol. Bioeng. 74:271-279. [DOI] [PubMed] [Google Scholar]
- 34.Melchiorsen, C. R., K. V. Jokumsen, J. Villadsen, M. G. Johnsen, H. Israelsen, and J. Arnau. 2000. Synthesis and posttranslational regulation of pyruvate formate-lyase in Lactococcus lactis. J. Bacteriol. 182:4783-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morton, D. B. 1985. Pain and laboratory animals. Nature 317:106. [DOI] [PubMed] [Google Scholar]
- 36.Mulholland, K., O. Levine, H. Nohynek, and B. M. Greenwood. 1999. Evaluation of vaccines for the prevention of pneumonia in children in developing countries. Epidemiol. Rev. 21:43-55. [DOI] [PubMed] [Google Scholar]
- 37.Neijssel, O. M., J. L. Snoep, and M. J. Teixeira de Mattos. 1997. Regulation of energy source metabolism in streptococci. Soc. Appl. Bacteriol. Symp. Ser. 26:12S-19S. [PubMed] [Google Scholar]
- 38.Neves, A. R., W. A. Pool, J. Kok, O. P. Kuipers, and H. Santos. 2005. Overview on sugar metabolism and its control in Lactococcus lactis—the input from in vivo NMR. FEMS Microbiol. Rev. 29:531-554. [DOI] [PubMed] [Google Scholar]
- 39.Neves, A. R., A. Ramos, M. C. Nunes, M. Kleerebezem, J. Hugenholtz, W. M. de Vos, J. Almeida, and H. Santos. 1999. In vivo nuclear magnetic resonance studies of glycolytic kinetics in Lactococcus lactis. Biotechnol. Bioeng. 64:200-212. [DOI] [PubMed] [Google Scholar]
- 40.Neves, A. R., R. Ventura, N. Mansour, C. Shearman, M. J. Gasson, C. Maycock, A. Ramos, and H. Santos. 2002. Is the glycolytic flux in Lactococcus lactis primarily controlled by the redox charge? Kinetics of NAD(+) and NADH pools determined in vivo by 13C NMR. J. Biol. Chem. 277:28088-28098. [DOI] [PubMed] [Google Scholar]
- 41.Nnyepi, M. R., Y. Peng, and J. B. Broderick. 2007. Inactivation of E. coli pyruvate formate-lyase: role of AdhE and small molecules. Arch. Biochem. Biophys. 459:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pericone, C. D., S. Park, J. A. Imlay, and J. N. Weiser. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 185:6815-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pesakhov, S., R. Benisty, N. Sikron, Z. Cohen, P. Gomelsky, I. Khozin-Goldberg, R. Dagan, and N. Porat. 2007. Effect of hydrogen peroxide production and the Fenton reaction on membrane composition of Streptococcus pneumoniae. Biochim. Biophys. Acta 1768:590-597. [DOI] [PubMed] [Google Scholar]
- 44.Philips, B. J., J. X. Meguer, J. Redman, and E. H. Baker. 2003. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 29:2204-2210. [DOI] [PubMed] [Google Scholar]
- 45.Regev-Yochay, G., K. Trzcinski, C. M. Thompson, M. Lipsitch, and R. Malley. 2007. SpxB is a suicide gene of Streptococcus pneumoniae and confers a selective advantage in an in vivo competitive colonization model. J. Bacteriol. 189:6532-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauter, M., and R. G. Sawers. 1990. Transcriptional analysis of the gene encoding pyruvate formate-lyase-activating enzyme of Escherichia coli. Mol. Microbiol. 4:355-363. [DOI] [PubMed] [Google Scholar]
- 47.Sawers, G., and G. Watson. 1998. A glycyl radical solution: oxygen-dependent interconversion of pyruvate formate-lyase. Mol. Microbiol. 29:945-954. [DOI] [PubMed] [Google Scholar]
- 48.Sheehan, J. K., P. S. Richardson, D. C. Fung, M. Howard, and D. J. Thornton. 1995. Analysis of respiratory mucus glycoproteins in asthma: a detailed study from a patient who died in status asthmaticus. Am. J. Respir. Cell Mol. Biol. 13:748-756. [DOI] [PubMed] [Google Scholar]
- 49.Smith, A. W., H. Roche, M. C. Trombe, D. E. Briles, and A. Hakansson. 2002. Characterization of the dihydrolipoamide dehydrogenase from Streptococcus pneumoniae and its role in pneumococcal infection. Mol. Microbiol. 44:431-448. [DOI] [PubMed] [Google Scholar]
- 50.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idanpaan-Heikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 51.Stewart, G. R., L. Wernisch, R. Stabler, J. A. Mangan, J. Hinds, K. G. Laing, D. B. Young, and P. D. Butcher. 2002. Dissection of the heat-shock response in Mycobacterium tuberculosis using mutants and microarrays. Microbiology 148:3129-3138. [DOI] [PubMed] [Google Scholar]
- 52.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 53.Vey, J. L., J. Yang, M. Li, W. E. Broderick, J. B. Broderick, and C. L. Drennan. 2008. Structural basis for glycyl radical formation by pyruvate formate-lyase activating enzyme. Proc. Natl. Acad. Sci. USA 105:16137-16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weidner, G., and G. Sawers. 1996. Molecular characterization of the genes encoding pyruvate formate-lyase and its activating enzyme of Clostridium pasteurianum. J. Bacteriol. 178:2440-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong, K. K., B. W. Murray, S. A. Lewisch, M. K. Baxter, T. W. Ridky, L. Ulissi-DeMario, and J. W. Kozarich. 1993. Molecular properties of pyruvate formate-lyase activating enzyme. Biochemistry 32:14102-14110. [DOI] [PubMed] [Google Scholar]
- 56.Yamada, T., S. Takahashi-Abbe, and K. Abbe. 1985. Effects of oxygen on pyruvate formate-lyase in situ and sugar metabolism of Streptococcus mutans and Streptococcus sanguis. Infect. Immun. 47:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto, Y., Y. Sato, S. Takahashi-Abbe, N. Takahashi, and H. Kizaki. 2000. Characterization of the Streptococcus mutans pyruvate formate-lyase (PFL)-activating enzyme gene by complementary reconstitution of the in vitro PFL-reactivating system. Infect. Immun. 68:4773-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yesilkaya, H., A. Kadioglu, N. Gingles, J. E. Alexander, T. J. Mitchell, and P. W. Andrew. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yesilkaya, H., S. Manco, A. Kadioglu, V. S. Terra, and P. W. Andrew. 2008. The ability to utilize mucin affects the regulation of virulence gene expression in Streptococcus pneumoniae. FEMS Microbiol. Lett. 278:231-235. [DOI] [PubMed] [Google Scholar]
- 60.Yesilkaya, H., S. Soma-Haddrick, S. J. Crennell, and P. W. Andrew. 2006. Identification of amino acids essential for catalytic activity of pneumococcal neuraminidase A. Res. Microbiol. 157:569-574. [DOI] [PubMed] [Google Scholar]
- 61.Zaritsky, A., A. H. Parola, M. Abdah, and H. Masalha. 1985. Homeoviscous adaptation, growth rate, and morphogenesis in bacteria. Biophys. J. 48:337-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu, J., and K. Shimizu. 2004. The effect of pfl gene knockout on the metabolism for optically pure d-lactate production by Escherichia coli. Appl. Microbiol. Biotechnol. 64:367-375. [DOI] [PubMed] [Google Scholar]