Abstract
Melioidosis is a tropical disease endemic in southeast Asia and northern Australia caused by the gram-negative soil saprophyte Burkholderia pseudomallei. Although infection is often systemic, the lung is frequently involved. B. thailandensis is a closely related organism that at high doses causes lethal pneumonia in mice. We examined the role of Toll-like receptors (TLRs), essential components of innate immunity, in vitro and in vivo during murine B. thailandensis pneumonia. TLR2, TLR4, and TLR5 mediate NF-κB activation by B. thailandensis in transfected HEK293 or CHO cells. In macrophages, TLR4 and the adaptor molecule MyD88, but not TLR2 or TLR5, are required for tumor necrosis factor alpha production induced by B. thailandensis. In low-dose airborne infection, TLR4 is needed for early, but not late, bacterial containment, and MyD88 is essential for control of infection and host survival. TLR2 and TLR5 are not necessary to contain low-dose infection. In high-dose airborne infection, TLR2 deficiency confers a slight survival advantage. Lung and systemic inflammatory responses are induced by low-dose inhaled B. thailandensis independently of individual TLRs or MyD88. These findings suggest that redundancy in TLR signaling or other MyD88-dependent pathways may be important in pneumonic B. thailandensis infection but that MyD88-independent mechanisms of inflammation are also activated. TLR signaling in B. thailandensis infection is substantially comparable to signaling induced by virulent B. pseudomallei. These studies provide additional insights into the host-pathogen interaction in pneumonic Burkholderia infection.
Burkholderia pseudomallei is a gram-negative, flagellated soil saprophyte that causes the tropical disease melioidosis. Endemic in southeast Asia and northern Australia, melioidosis results from the inhalation or cutaneous inoculation of B. pseudomallei. Although there is a myriad of clinical presentations, the lung is the organ most commonly involved (30). B. pseudomallei is considered a CDC category B pathogen because of concerns about its use as a bioweapon (5). Burkholderia thailandensis is a closely related organism that also is isolated from soil in the tropics (24) and is largely avirulent to humans but is lethal at high doses to mice (28, 29). Study of B. thailandensis does not require the strict biocontainment conditions necessary for research with B. pseudomallei, and because of its genomic similarity with B. pseudomallei (33), has been used as a surrogate organism to study melioidosis.
Toll-like receptors (TLRs) are membrane-associated pathogen recognition receptors that provide essential host defense against infection by inducing nuclear transcription factor (NF)-κB translocation and activation of a proinflammatory response (17). TLR2, in conjunction with TLR1 or TLR6, is stimulated by bacterial cell wall lipopeptides and peptidoglycan. TLR4, in association with coreceptors CD14 and MD-2, recognizes the lipid A component of lipopolysaccharide (LPS) of most gram-negative organisms. TLR5 is activated by bacterial flagellin. All these TLRs signal via adaptor molecule MyD88, although TLR4 may alternatively signal through TRIF. Various TLRs and their downstream adaptor molecules are important in both experimental and human bacterial lung infections (10, 14, 18, 19, 21). We and others have previously shown that TLR2 and TLR4 mediate host recognition of B. pseudomallei in vitro (15, 27, 31), and we have shown that B. pseudomallei LPS (and subcomponent lipid A) is a TLR4 agonist (27). However, despite the lethality of B. thailandensis when aerosolized at high doses to mice (28), little is known about innate immunity in B. thailandensis infection.
In this study of B. thailandensis infection, we examined the roles of three TLRs that recognize conserved motifs of gram-negative, flagellated bacteria—TLR2, TLR4, and TLR5—as well as the TLR adaptor molecule MyD88. In vitro, we found that TLR2, TLR4, and TLR5 recognize B. thailandensis and mediate activation of NF-κB, but that only TLR4 or MyD88 deficiency impairs tumor necrosis factor alpha (TNF-α) secretion by macrophages. In vivo, TLR4 is required only for early bacterial control in low-dose infection. Absence of TLR2 prolongs survival slightly after high-dose infection. MyD88 deficiency results in exponential bacterial replication, dissemination, and death. Lack of TLR2, TLR4, TLR5, or MyD88 does not impair the host inflammatory response to inhaled B. thailandensis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Burkholderia thailandensis E264 was provided by Donald Woods (University of Calgary). Bacteria were grown from frozen glycerol stock in LB broth at 37°C for 9 (log phase) or 18 (stationary phase) hours, isolated by centrifugation, washed twice, and suspended in Dulbecco's phosphate-buffered saline (PBS) to the desired concentration. An optical density of 0.20 at 600 nm yielded approximately 1 × 108 CFU/ml. Bacteria were heat-killed by heating to 65°C for 40 to 45 min.
Cell transfections and stimulations.
HEK293 cells were used as previously described (27). Cells were cultured in a 96-well flat-bottomed tissue culture plate at ∼5 × 104 cells/well in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (FBS). The following day, cells were transiently transfected with 5 μl of transfection reagent comprised of a 1:1 mix of 0.25 M CaCl2 containing 2× BBS buffer [50 mM N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), 280 mM NaCl, and 1.5 mM NaH2PO4] and the following DNA: NF-κB-dependent firefly ELAM luciferase and control β-actin-dependent Renilla luciferase, and murine CD14 and MD-2, with TLR2, TLR2 and TLR1, TLR2 and TLR6, or TLR4. CD14 enhances TLR2-dependent responses to B. pseudomallei (31) and, when cotransfected with CD14, MD-2 is necessary for TLR4-dependent signaling (27). For simplicity, both coreceptors were transfected together with either TLR2 or TLR4. DNA was added in the following amounts to each well: 0.0025 μg MD-2, 0.0025 μg CD14, 0.01 μg ELAM luciferase, and 0.0003 μg Renilla luciferase. When transfected alone, the following amounts of DNA were added: 0.0025 μg TLR2 or 0.0003 μg TLR4. When cotransfected with TLR1 or TLR6, 0.0025 μg TLR2 was used in addition to 0.025 μg TLR1 or 0.0025 μg TLR6. All transfections were normalized to 0.05 μg total DNA with the addition of an empty vector. Transfected cells were washed once after 4 hours and were stimulated the following day with experimental ligands, nonspecific stimulus recombinant human interleukin-1β (IL-1β; Pierce Endogen, Rockford, IL), control TLR4 ligand ultrapure Escherichia coli O111:B4 LPS (Invivogen, San Diego, CA), or control TLR2 ligand Pam3CSK4 (Invivogen and EMC Microcollections, Tübingen, Germany). After 4 hours, cells were lysed with passive lysis buffer (Promega, Madison, WI), and NF-κB activation was determined in 10 μl of lysate by the ratio of firefly to Renilla luciferase light emission by the use of the Dual-Luciferase reporter system (Promega, Madison, WI). CHO cells stably transfected with human TLR5 were used as previously described (14). Cell populations expressing TLR5, NF-κB-dependent firefly ELAM luciferase, and control thymidine kinase-driven Renilla luciferase maintained in Ham's F-12 medium with l-glutamine, 10% FBS, and 100 μg/ml G418 were washed and added onto a 96-well plate at ∼6 × 104 cells/well before stimulation the following day with experimental ligands, recombinant human IL-1β, E. coli O111:B4 LPS, or control TLR5 ligand Salmonella enterica serovar Typhimurium FliC flagellin purified as previously described (12). After 5 hours, cells were lysed, and NF-κB activation was determined as described above.
Animals.
TLR2−/−, TLR4−/−, TLR5−/−, and MyD88−/− mice were derived by Shizuo Akira (University of Osaka) and backcrossed for 8 to 10 generations to C57BL/6 (16, 25, 26). Specific-pathogen-free C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). All animals were housed in laminar flow cages and were permitted ad lib access to sterile food and water. Euthanasia was performed by injection of intraperitoneal pentobarbital followed by exsanguination from a cardiac puncture. The Institutional Animal Care and Use Committee of the University of Washington approved all experimental procedures.
Macrophage stimulations.
Femurs and tibias were harvested under sterile conditions from euthanized mice. Marrow was flushed out using a 26-gauge needle through a 0.2-μm strainer and cultured in petri dishes in RPMI medium supplemented with 1% l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, 10% FBS, and 20% L929 cell-conditioned medium at 37°C under 5% CO2 for 5 to 10 days to allow macrophages to predominate. The monolayer was washed twice with Hank's balanced salt solution or medium, and macrophages were resuspended in RPMI medium supplemented with 1% l-glutamine, 1% HEPES, and 10% FBS. Cells were added to a 96-well flat-bottomed tissue culture plate at 3 × 104 to 2 × 105 cells/well, depending on the experiment. The following day, cells were stimulated with experimental ligands in fresh medium added to each well. Alveolar macrophages were harvested by cannulating the tracheas of euthanized mice with a 20-gauge catheter and lavaging both lungs with 4 ml 0.9% NaCl-0.6 mM EDTA in divided aliquots. Cells were pelleted by centrifugation, resuspended in RPMI medium supplemented with 1% l-glutamine, 1% HEPES, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% FBS and were added to a 96-well plate at 7 × 104 cells/well. The following day, cells were washed three times and stimulated with experimental ligands or control Salmonella serovar Typhimurium FliC flagellin in fresh antibiotic-free medium. After stimulation for 16 to 24 h, macrophage supernatants were removed and stored at −80°C until assayed.
Murine model of pneumonia.
Mice were exposed to aerosolized bacteria by the use of a snout-only inhalation system (In-Tox Products, Moriarty, NM) as previously described (28). Aerosols were generated from a MiniHeart Hi-Flo nebulizer (Westmed, Tucson, AZ) driven at 40 lb/in2. Airflow through the system was maintained for 10 min at 24 liters/min followed by 5 minutes purge with air. Bacterial deposition in each experiment was determined from a quantitative culture of lung tissue from sentinel mice sacrificed immediately after infection. Animals were examined one to three times daily for illness or death, and abdominal surface temperatures were measured using a Ranger MX4P digital infrared thermometer (Raytek, Santa Cruz, CA). Ill animals with temperatures of <22.5°C or ruffled fur, eye crusting, hunched posture, and lack of resistance to handling were deemed terminal and were euthanized (spontaneous death was not required as an endpoint). At specific time points after infection, mice were sacrificed, and the left pulmonary hilum was tied off; the left lung, median hepatic lobe, and spleen were each homogenized in 1 ml sterile Dulbecco's PBS, and serial dilutions were plated onto LB agar. Colonies were counted after 2 to 4 days of incubation at 37°C in humid air under 5% CO2. Bronchoalveolar lavage (BAL) was performed by cannulating the trachea with a 20-gauge catheter and lavaging the right lung with four 0.5-ml aliquots of 0.9% NaCl-0.6 mM EDTA. Cell counts in BAL fluid specimens were measured in a hemocytometer. Differentials were determined from examination of cytocentrifuge slides (Thermo Shandon, Pittsburgh, PA) that were stained with a modified Wright-Giemsa technique (Diff-Quik; Dade Behring, Dudingen, Switzerland). Left lung homogenates in Dulbecco's PBS were diluted 1:1 in lysis buffer containing 2× protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), incubated on ice for 30 min, and then centrifuged at 1,500 × g. Supernatants were harvested and stored at −80°C until assayed for cytokines. Whole blood was centrifuged, and serum was removed and stored at −80°C until assayed.
Measurements of cytokines.
TNF-α was quantified in the supernatants of stimulated macrophages by using the DuoSet enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). TNF-α, IL-1β, IL-6, KC, monocyte chemoattractant protein 1 (MCP-1), and MIP-2 levels in lung homogenates and sera were measured using a multiplex bead assay (Luminex, Austin, TX) and reagents purchased from R&D Systems.
Statistical analyses.
Combined data that follow a normal distribution are reported as mean ± standard deviation. Comparisons between two groups of normally distributed data were made using the t test and between three or more groups by using analysis of variance (ANOVA) and either the Dunnett or Bonferroni post-tests. Data displayed on a logarithmic scale in Fig. 4 to 7 were first transformed to log10 before testing. Survival analyses were performed with the log rank test. All statistics were performed with Stata 9.0 (College Station, TX) or GraphPad Prism 4.0 (San Diego, CA). A two-sided P value of ≤0.05 was considered significant.
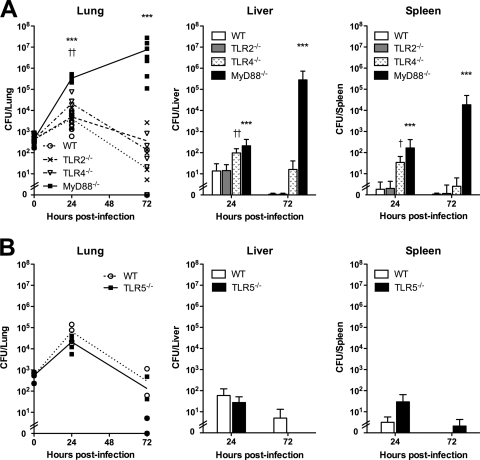
FIG. 4.
Containment of B. thailandensis infection is dependent on TLR4 and MyD88. Approximately 400 CFU/lung B. thailandensis was deposited by aerosol in the lungs of WT, TLR2−/−, TLR4−/−, and MyD88−/− mice (A), and ∼550 CFU/lung B. thailandensis was deposited by aerosol in the lungs of WT and TLR5−/− mice (B). Lung, liver, and spleen were harvested and quantitatively cultured at 24 and 72 h after infection. (A) Eight mice total per strain were sampled at each time point (seven for TLR2−/− mice at 24 h), combining data from two comparable experiments performed independently. *** indicates a P value of <0.001 for MyD88−/− versus WT. † indicates a P value of <0.05, and †† indicates a P value of <0.01 for TLR4−/− versus WT. (B) Four mice per strain were sampled at each time point, and the data displayed represent one of two comparable experiments performed independently. For lung CFU, the lines connect mean values at each time point; otherwise, data are plotted as means ± standard deviations. Statistical testing was performed after log transformation of the data with ANOVA and Bonferroni's post-test (A) or the t test (B).
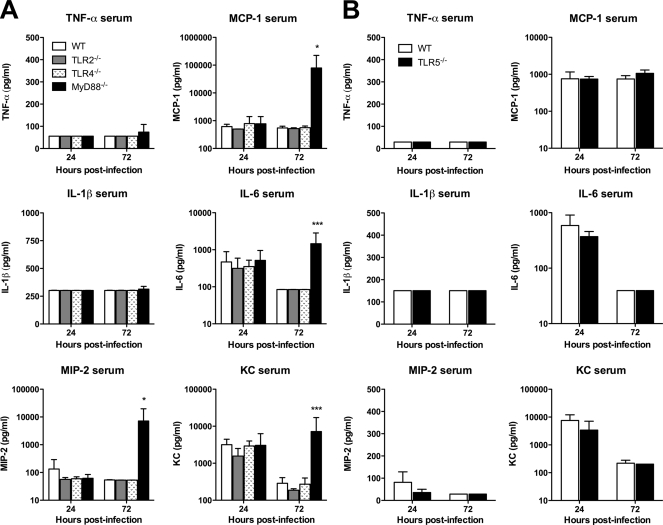
FIG. 7.
Systemic cytokine responses to B. thailandensis are not dependent on individual TLRs or MyD88. Mice were infected as described in the legend for Fig. 4 after deposition of ∼400 CFU/lung (A) or ∼550 CFU/lung (B). Serum cytokines were measured in duplicate at 24 and 72 h after infection. (A) Seven mice total per strain were sampled at 24 h, and eight mice total per strain were sampled at 72 h; data from the two comparable experiments performed independently were combined. (B) Four mice per strain were sampled at each time point, and the data displayed represent one of two comparable experiments performed independently. Data are plotted as means ± standard deviations. Statistical testing was performed after log transformation of data when displayed on a log scale with ANOVA and Bonferroni's post-test (A) or the t test (B) after. * indicates a P value of <0.05, and *** indicates a P value of <0.001 for MyD88−/− versus WT. Mean values (± standard deviations) for serum cytokines in naïve C57BL/6 mice (n = 4) in pg/ml are as follows: TNF-α, 55 (± 0); MCP-1, 468 (± 0); IL-1β, 400 (± 0); IL-6, 72 (± 0); MIP-2, 50 (± 0); and KC, 155 (± 43).
RESULTS
TLR2 and TLR4 mediate activation of NF-κB by B. thailandensis.
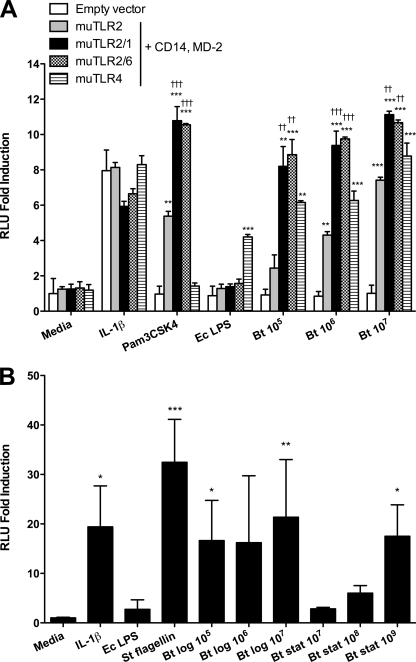
To determine whether B. thailandensis stimulates TLR2 and TLR4, we transfected HEK293 cells with TLR2, TLR2/1, TLR2/6, or TLR4, and CD14/MD-2. We stimulated the cells with stationary-phase, heat-killed B. thailandensis and quantified NF-κB activation by using a luciferase reporter assay (Fig. 1A). We observed 2.4- to 7.4-fold TLR2- and 6.2- to 8.8-fold TLR4-mediated induction of NF-κB activation upon stimulation with 105 to 107 CFU/ml B. thailandensis. The responses were dose dependent, and TLR2-mediated signaling was enhanced by cotransfection of either TLR1 or TLR6.
FIG. 1.
TLR2, TLR4, and TLR5 mediate activation of NF-κB by B. thailandensis (Bt). (A) HEK293 cells were transiently transfected with murine TLR2, TLR2/1, TLR2/6, or TLR4 and coreceptors CD14 and MD-2, or with an empty vector and NF-κB-dependent firefly ELAM luciferase and control β-actin-dependent Renilla luciferase. Cells were stimulated with medium alone, 20 ng/ml IL-1β, 100 ng/ml Pam3CSK4, 10 ng/ml E. coli (Ec) O111:B4 LPS, or various concentrations of stationary-phase heat-killed B. thailandensis (CFU/ml). †† indicates a P value of <0.01, and ††† indicates a P value of <0.001, compared to TLR2-transfected cells stimulated with the same ligand. (B) CHO cells stably transfected with human TLR5, NF-κB-dependent firefly ELAM luciferase, and control thymidine kinase-driven Renilla luciferase were stimulated with medium alone, 20 ng/ml IL-1β, 10 ng/ml E. coli O111:B4 LPS, 100 ng/ml Salmonella serovar Typhimurium (St) FliC flagellin, or various concentrations of log- or stationary-phase heat-killed B. thailandensis (CFU/ml). NF-κB activation was measured by light emission (n-fold induction of relative light units over that of cells transfected with empty vector and stimulated with media). Data plotted are means ± standard deviations of duplicate conditions that represent one of two similar experiments performed independently (A) or quadruplicate conditions that represent one of six experiments performed independently (B) using either or both stationary- and/or log-phase heat-killed bacteria. Statistical testing was performed with ANOVA and Bonferroni's post-test (A) or Dunnett's post-test (B). *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared to empty vector-transfected cells stimulated by the same ligand (A) or medium (B).
TLR5 mediates activation of NF-κB by B. thailandensis.
To determine whether B. thailandensis activates NF-κB in a TLR5-dependent manner, we stimulated TLR5-transfected CHO cells with heat-killed B. thailandensis and quantified NF-κB activation by light emission (Fig. 1B). Because of concern that B. pseudomallei may lose or reduce expression of flagella at stationary phase (Sharon Peacock, personal communication), we used both log- and stationary-phase B. thailandensis cells. We detected NF-κB activation in transfected cells in response to both preparations of B. thailandensis, indicating that TLR5 mediates recognition of B. thailandensis. The signal was more robust on average (16.6- to 21.4-fold) but more variable (2.8- to 17.5-fold) in response to 105 to 107 CFU/ml log-phase bacteria than to 107 to 109 CFU/ml stationary-phase bacteria, suggesting diminished presence of flagellin in stationary-phase organisms. Similar TLR5-dependent activation of NF-κB was observed upon stimulation with live B. thailandensis (data not shown).
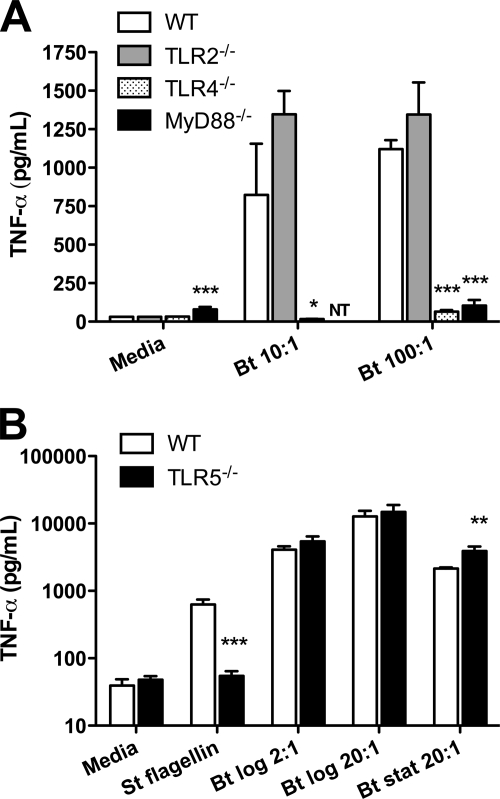
B. thailandensis-induced TNF-α production by bone marrow-derived macrophages requires TLR4 and MyD88 but not TLR2.
Having determined roles for TLR2, TLR4, and TLR5 in mediating B. thailandensis-induced NF-κB activation in HEK293 or CHO cells, we sought to characterize B. thailandensis-induced cytokine production in primary cells in the absence of specific TLRs or the adaptor molecule MyD88. We stimulated bone marrow-derived macrophages from wild-type (WT), TLR2−/−, TLR4−/−, and MyD88−/− mice with heat-killed B. thailandensis and quantified TNF-α in the supernatants after 16 h (Fig. 2A). Compared to WT macrophages, we found that TNF-α production was dramatically diminished in the absence of TLR4 or MyD88 but was not impaired in the presence of TLR2. In fact, we observed a nonsignificant trend toward higher TNF-α secretion in the absence of TLR2.
FIG. 2.
B. thailandensis-induced TNF-α production by macrophages requires TLR4 and MyD88 but not TLR2 or TLR5. (A) Bone marrow-derived macrophages from WT, TLR2−/−, TLR4−/−, or MyD88−/− mice were stimulated with medium or various bacterium/cell ratios of heat-killed B. thailandensis (Bt). Data plotted are means ± standard deviations of the results for triplicate samples (duplicate for MyD88−/− cells) and represent one of three similar experiments performed independently comparing WT to TLR2−/− and TLR4−/− cells and one of two similar experiments performed independently comparing WT to MyD88−/− cells. (B) Alveolar macrophages harvested from WT or TLR5−/− mice were stimulated with medium alone, 100 ng/ml Salmonella serovar Typhimurium (St) FliC flagellin, or various bacterium/cell ratios of log- or stationary-phase heat-killed B. thailandensis. Data plotted are means ± standard deviations of triplicate samples and represent one of three independently performed experiments using either or both log- or stationary-phase bacteria. Supernatants were harvested after 16 h (A) or 24 h (B), and TNF-α production was measured by enzyme-linked immunosorbent assay. Statistical testing was performed with ANOVA and Bonferroni's post-test (A) or the t test (B). *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared to WT cells stimulated with the same ligand.
B. thailandensis-induced TNF-α production by alveolar macrophages does not require TLR5.
To assess the TLR5 dependence of cytokine production in primary cells, we compared TNF-α production induced by stationary- or log-phase heat-killed B. thailandensis by WT alveolar macrophages to that by TLR5−/− macrophages. We used alveolar macrophages because we had previously determined that these cells mediate TLR5-dependent TNF-α secretion in response to flagellin stimulation, unlike bone marrow-derived macrophages (12). Regardless of bacterial phase, upon stimulation with B. thailandensis, we found no reduction in TNF-α levels produced by alveolar macrophages at 24 h in the absence of TLR5. Instead, we noted increased production of TNF-α in TLR5−/− cells stimulated with stationary-phase bacteria (Fig. 2B).
Survival after low-dose infection with B. thailandensis is dependent on MyD88, whereas TLR2 deficiency extends survival in high-dose infection.
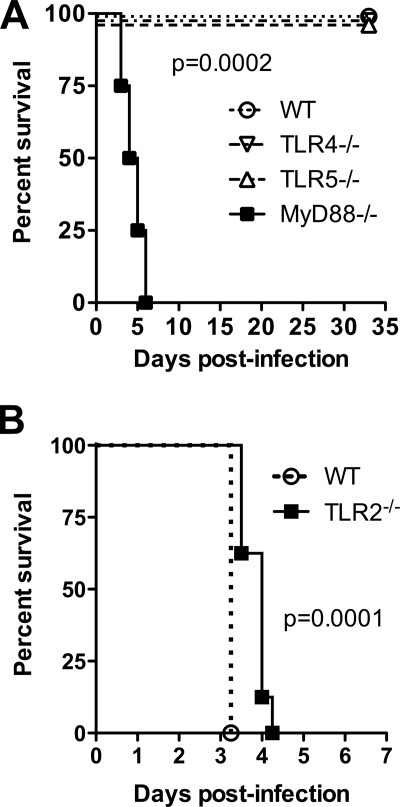
Having determined that specific TLRs mediate differential activation of the host response to B. thailandensis in vitro, we examined the roles of these TLRs in B. thailandensis infection in vivo. We first characterized TLR-dependent survival in a murine model of airborne B. thailandensis infection. We have previously observed that deposition of ≥104 CFU/lung is uniformly lethal to C57BL/6 mice (28), so we targeted a sublethal dose to identify any difference between WT and TLR-deficient mice. After deposition of ∼800 CFU/lung of B. thailandensis, we observed no impairment in survival in TLR4−/− or TLR5−/− mice (Fig. 3A). However, we did identify 100% lethality within a week in mice deficient in adaptor molecule MyD88. Cultures of lung, liver, and spleen at 33 days postinfection in WT, TLR4−/−, and TLR5−/− mice yielded no persistent bacteria (data not shown). Because Wiersinga et al. (31) reported a protective effect of TLR2 deficiency in intranasal B. pseudomallei infection, we then infected WT and TLR2−/− mice with a lethal dose of B. thailandensis and measured survival (Fig. 3B). After deposition of 2 × 104 CFU/lung, survival of TLR2−/− mice was statistically greater than that of WT mice but the absolute difference in median survival was less than 1 day.
FIG. 3.
Survival after pneumonic infection with B. thailandensis is dependent on MyD88, whereas TLR2 deficiency extends survival. (A) Approximately 800 CFU/lung B. thailandensis was deposited by aerosol in the lungs of WT, TLR4−/−, TLR5−/−, and MyD88−/− mice. (B) A total of 2 × 104 CFU/lung B. thailandensis was deposited in the lungs of WT and TLR2−/− mice. Survival of four (A) or eight (B) mice per strain was recorded. P values were calculated with the log rank test.
Containment of B. thailandensis infection is dependent on TLR4 and MyD88.
We further characterized the roles of TLRs in B. thailandensis infection in vivo by examining bacterial replication and dissemination in TLR- and MyD88-deficient mice. For these experiments, we chose a low deposition dose that should not be lethal but that would ensure detectable replication (and accompanying inflammatory markers) in WT mice. After deposition of ∼400 CFU/lung B. thailandensis, we observed similar replications in the lungs of WT and TLR2−/− mice at 24 h that was contained by 72 h (Fig. 4A). In MyD88−/− mice, however, mean bacterial replication was about 2 logs higher than that in WT mice at 24 h and continued to increase exponentially by 72 h. In TLR4−/− mice, bacterial loads measured in the lung at 24 h were 1 log higher than those in WT mice, but there was no difference at 72 h. Bacterial burdens in liver and spleen 24 h after infection were minimal in WT and TLR2−/− mice but were slightly higher in TLR4−/− and MyD88−/− mice. There were no differences in liver and spleen bacterial counts between WT, TLR2−/−, and TLR4−/− mice at 72 h, but bacterial loads in these organs were >4 logs higher in MyD88−/− mice at this time point. We then undertook a similar experiment comparing TLR5−/− mice to WT mice, depositing ∼550 CFU/lung B. thailandensis, but observed no difference in bacterial replication or dissemination (Fig. 4B).
After identifying a pronounced requirement of MyD88 for bacterial containment and survival, we evaluated the early host dependence on MyD88 by infecting WT and MyD88−/− mice in two additional experiments with 3 × 103 CFU/lung or 5 × 103 CFU/lung. We compared bacterial burdens at 4 h but observed no strain-dependent difference in the bacterial counts in the lung and did not detect any dissemination to liver or spleen in either experiment (data not shown).
Pulmonary and systemic inflammation induced by B. thailandensis does not require individual TLRs or MyD88.
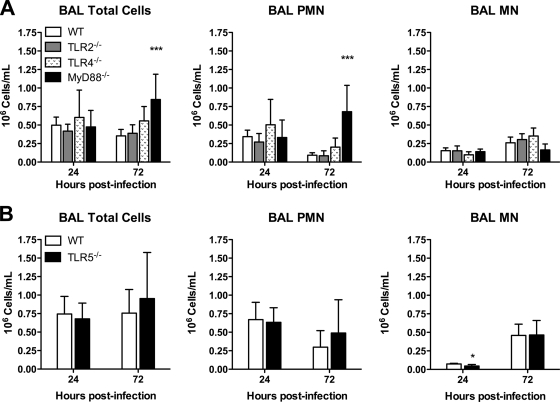
To determine whether the host inflammatory response to B. thailandensis is dependent on specific TLRs, we measured pulmonary leukocyte recruitment and cytokine responses in the mice infected as described in the legend for Fig. 4. We first compared BAL fluid cell counts in WT and TLR-deficient mice (Fig. 5). We found that neutrophils (normally absent from BAL fluid of naïve mice) were elevated in the lavage samples from all mice at 24 h, without any TLR- or MyD88-dependent differences. At 72 h, while neutrophil counts decreased slightly in WT, TLR2−/−, TLR4−/−, and TLR5−/− mice, counts were further increased in MyD88−/− mice. These data suggest that in this system, individual TLRs and MyD88 are not required for bronchoalveolar neutrophil recruitment.
FIG. 5.
Bronchoalveolar inflammation induced by B. thailandensis is not dependent on individual TLRs or MyD88. BAL was performed in the mice infected as described in the legend for Fig. 4 after deposition of ∼400 CFU/lung (A) or ∼550 CFU/lung (B). At 24 and 72 h after infection, total cells, polymorphonuclear leukocytes (PMN), and mononuclear cells (MN) in the BAL fluid were enumerated. Data are displayed as means ± standard deviations. (A) Eight mice total per strain were sampled at each time point (seven for TLR2−/− and MyD88−/− mice at 24 h), and data from two comparable experiments performed independently are combined. (B) Four mice per strain were sampled at each time point, and the data displayed represent one of two comparable experiments performed independently. Statistical testing was performed with ANOVA and Bonferroni's post-test (A) or the t test (B). * indicates a P value of <0.05 for TLR5−/− versus WT, and *** indicates a P value of <0.001 for MyD88−/− versus WT. Mean values (± standard deviations) for BAL fluid cell counts in naïve C57BL/6 mice (n = 4) are 0 (± 0) × 106 PMN/ml and 0.09 (± 0.03) × 106 MN/ml.
To determine if MyD88-dependent pulmonary host responses were evident at an early time point after higher-dose infection, we also compared BAL fluid cell counts in the MyD88−/− and WT mice 4 h after infection with 3 × 103 or 5 × 103 CFU/lung. We found that at these doses, both strains of mice had uniform populations of mononuclear cells in BAL fluid (data not shown). Thus, at these doses in the very early phase of pneumonic infection, inhalation of B. thailandensis does not appear to induce a measurable neutrophilic inflammatory response in the lung.
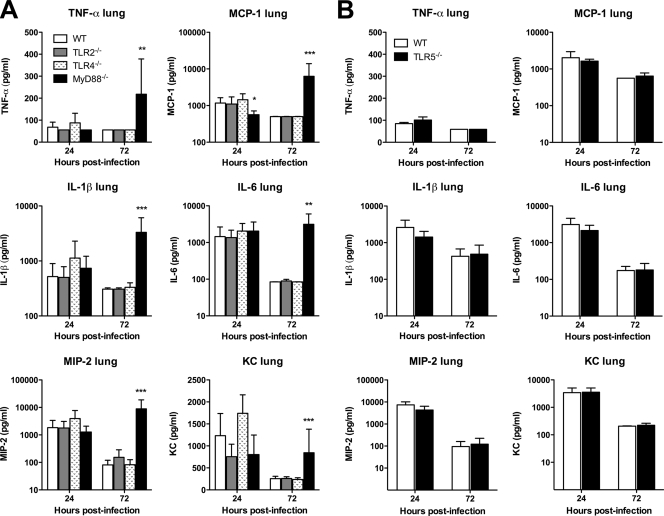
We then examined the TLR dependence of cytokine and chemokine production induced by pneumonic B. thailandensis infection in WT and TLR-deficient mice. We measured a panel of mediators in homogenized lung tissue and serum samples from mice infected as described in the legend for Fig. 4. We found universal elevation in cytokines IL-1β and IL-6 and chemokines MIP-2 and KC in the lung homogenates of WT, TLR2−/−, TLR4−/−, TLR5−/−, and MyD88−/− mice at 24 h (Fig. 6A and B). Only MCP-1 production in the lung at this time point was significantly lower in MyD88−/− mice. TNF-α production was low in all mice. At 72 h, values of all mediators had diminished (or, for TNF-α, remained low) in WT, TLR2−/−, TLR4−/−, and TLR5−/− mice. However, in MyD88−/− mice, levels of all cytokines and chemokines increased further. Serum cytokine measurements were notable for increased IL-6 and KC levels by 24 h in all mice (Fig. 7A and B). While these decreased in WT, TLR2−/−, TLR4−/−, and TLR5−/− mice by 72 h, there was a significant increase in MCP-1, IL-6, MIP-2, and KC serum levels in MyD88−/− mice at 72 h after infection. These data provide further evidence for largely unimpaired host inflammatory responses in the absence of individual TLRs or MyD88.
FIG. 6.
Pulmonary cytokine responses to B. thailandensis are not dependent on individual TLRs or MyD88. Mice were infected as described in the legend for Fig. 4 after deposition of ∼400 CFU/lung (A) or ∼550 CFU/lung (B). Lung homogenate cytokines were measured in duplicate at 24 and 72 h after infection. (A) Eight mice total per strain were sampled at each time point (seven for TLR2−/− mice at 24 h), and data from two comparable experiments performed independently are combined. (B) Four mice per strain were sampled at each time point, and the data displayed represent one of two comparable experiments performed independently. Data are plotted as means ± standard deviations. Statistical testing was performed after log transformation of data when displayed on a log scale with ANOVA and Bonferroni's post-test (A) or the t test (B). * indicates a P value of <0.05, ** P values of <0.01, and *** P values of <0.001 for MyD88−/− versus WT. Mean values (± standard deviations) for lung homogenate cytokines in naïve C57BL/6 mice (n = 4) in pg/ml are as follows: TNF-α, 55 (± 0); MCP-1, 468 (± 0); IL-1β, 400 (± 0); IL-6, 84 (± 37); MIP-2, 50 (± 0); and KC, 137 (± 38).
DISCUSSION
The main finding of this study is that TLR2, TLR4, and TLR5 recognize B. thailandensis, but that only deficiency of TLR4 or adaptor molecule MyD88 impairs cytokine production by macrophages. In a murine model of inhaled infection, the adaptor molecule MyD88 is essential for survival and containment of bacterial outgrowth. However, apart from a modest TLR4-dependent effect on bacterial containment at 24 h, absence of individual TLRs does not significantly impair survival or bacterial replication and dissemination. TLR2 may actually accelerate death in high-dose infection. Pulmonary and systemic inflammation occurs in the absence of individual TLRs or MyD88.
The importance of TLRs in mediating host defense against infection has been well established in animal models of disease as well as in human populations (10, 13, 18, 19, 21). A better understanding of the role of individual TLRs is of particular relevance when developing targeted therapeutic strategies. Earlier work by several groups has demonstrated that TLR2 and TLR4 are involved in host recognition of B. pseudomallei in vitro (15, 27, 31), but the logistical limitations of the study of B. pseudomallei have driven the use of B. thailandensis as a surrogate research organism. While it is essentially nonpathogenic to humans, B. thailandensis replicates, disseminates, and is lethal in high doses to mice (28, 29) and shares significant homology with B. pseudomallei (33). Accordingly, study of TLRs in murine B. thailandensis infection may shed light on the host-pathogen interaction in human melioidosis.
B. thailandensis is a gram-negative, flagellated organism, comprising the prototypical TLR2, TLR4, and TLR5 ligands—lipopeptide, LPS, and flagellin, respectively. Thus, our observations of the roles for TLR2, TLR4, and TLR5 in the recognition of B. thailandensis, as assessed by measures of NF-κB activation, are not surprising. While studies using heat-killed B. thailandensis are potentially limited because heat may conceivably destroy TLR ligands, our measurements of TLR-dependent signaling in transfected cells stimulated with heat-killed B. thailandensis support the persistent functionality of these ligands. We also observed similar patterns of TLR5-dependent activation of CHO cells stimulated with heat-killed or live B. thailandensis.
Our transfection studies indicate that either TLR1 or TLR6 augments TLR2-mediated NF-κB activation. Although each of these TLRs functions in a MyD88-dependent fashion, the TLR-dependent variation in TNF-α production by primary cells points to specific and contrasting roles for individual TLRs in this infection. These findings of B. thailandensis-induced TLR2- and TLR4-dependent NF-κB activation and the specific effect of TLR2 and TLR4 on cytokine production in macrophages are similar to our previously published findings using B. pseudomallei (27), lending credence to our supposition that study of B. thailandensis may provide relevant insights into the pathogenesis of virulent B. pseudomallei infection. This prompted us to extend our investigations to a murine model of B. thailandensis pneumonia.
We did not observe a significant increase in TNF-α production in response to B. thailandensis by macrophages in the absence of TLR2, or identify any alteration in bacterial counts or inflammation in TLR2−/− mice after a low-dose inhalation challenge. After high-dose infection, however, the presence of TLR2 accelerates death. A unifying explanation is that at high, but not low, infecting doses, an overexuberant proinflammatory response may harm the host. This parallels the observations by Wiersinga et al. (31) that TLR2−/− mice display enhanced survival, decreased pulmonary cytokine levels, and decreased bacterial growth at 72 h compared to WT mice in an intranasal model of B. pseudomallei infection. The etiology of this deleterious effect of TLR2 in Burkholderia infection is unclear, but our in vitro studies suggest it is not an IL-10-mediated phenomenon (27).
We have previously determined that B. pseudomallei LPS and lipid A are TLR4 agonists and that TLR4 deficiency abrogates macrophage cytokine production induced by B. pseudomallei in vitro (27). We speculate that B. thailandensis LPS is also a TLR4 agonist and probably accounts for TLR4-dependent macrophage cytokine production induced by B. thailandensis. TLR4−/− mice infected with B. thailandensis have a defect in their ability to contain the organism at 24 h, but this is overcome by 72 h when the phenotype is comparable to that of WT. These findings suggesting a transient role for TLR4 are similar to Wiersinga et al.'s report (31), in which bacterial growth after intranasal B. pseudomallei infection of mice was greater in TLR4−/− spleen and blood only at 24 h after infection. In that study, TLR4 deficiency also did not alter survival. In vivo, we did not identify any differences in pulmonary or systemic inflammation that would explain a TLR4-dependent effect, despite our observation that TLR4 is required for TNF-α secretion by macrophages. Pulmonary and systemic TNF-α levels remained close to baseline levels throughout the experiment in all strains of infected mice. Higher-dose infection of TLR4−/− mice may elucidate the role of TNF-α in vivo, but other cell types (or more differentiated macrophages) may compensate for the lack of TNF-α production by bone marrow-derived macrophages.
TLR5 recognizes B. thailandensis, and the TLR5-mediated signal is more pronounced upon stimulation with log-phase bacteria than with stationary-phase bacteria. This suggests that expression of B. thailandensis flagellin—the likely TLR5 ligand and a putative virulence factor (7)—is increased during log-phase growth. Alternatively, flagella may be lost during stationary-phase growth and in preparation of heat-killed bacteria. Despite this recognition signal, however, macrophage cytokine production is not mitigated in the absence of TLR5, and there is no difference between TLR5 and WT mice in their abilities to contain B. thailandensis infection or in the inflammatory response. It seems plausible that a TLR5-specific effect on alveolar macrophages stimulated with whole B. thailandensis is obscured by concurrent TLR2 or TLR4 activation. In the absence of TLR5, we speculate that greater compensatory stimulation of these TLR2- and TLR4-dependent pathways may be induced by less-flagellated stationary-phase B. thailandensis. This could account for the higher TNF-α concentrations detected in TLR5−/− macrophages than in WT macrophages. In the lung, bronchial epithelial cells also express TLR5, and in these cells, it is known that activation of NF-κB in response to the opportunistic pathogen Burkholderia cenocepacia is mediated by TLR5 (8). Further study of these cells in B. thailandensis infection may be informative. The in vitro response to B. pseudomallei is likely to be similar to that induced by B. thailandensis. In preliminary studies, we have found B. pseudomallei-induced NF-κB activation in TLR5-transfected CHO cells and no difference in cytokine release between WT and TLR5−/− alveolar macrophages stimulated with B. pseudomallei (our unpublished data). Others have also identified TLR5-dependent NF-κB activation induced by B. pseudomallei (15).
In studies of murine pneumonia caused by Legionella pneumophila, a gram-negative, flagellated organism, we have observed a defect in neutrophil recruitment in TLR5−/− mice 4 h following infection that was not attributable to reduced chemokine production. By 24 h, neutrophil counts were comparable (12). We speculated that early TLR5-mediated neutrophil recruitment might also occur in B. thailandensis infection. However, even depositing 5 × 103 CFU/lung (1 log greater than the 550 CFU/lung used in our TLR5−/− experiments), we could not measure neutrophils in the bronchoalveolar space of WT mice at 4 h. Only at deposition doses approaching 105 CFU/lung do we detect a BAL fluid neutrophilia at this time point following infection (28). Conceivably, if TLR5 plays a similar role in the first few hours of B. thailandensis infection, much-higher-dose inoculation would be required to uncover the phenotype. Furthermore, compared to Burkholderia infection, TLR5 may be particularly important in early Legionella infection because of a weak Legionella LPS that signals via TLR2 (11). Cytokine production by bone marrow-derived macrophages stimulated with L. pneumophila is not impaired by TLR4 deficiency, unlike stimulation of these cells with B. thailandensis or B. pseudomallei (1, 2, 27).
MyD88 is a key adaptor molecule for each of the TLRs studied in this work. We show that it is essential for host containment of B. thailandensis even at doses that are readily contained in WT mice. Similarly, MyD88 is necessary for host defense against intranasal B. pseudomallei infection, where it is required for neutrophil recruitment to the lung and activation but not for phagocytosis or macrophage killing ability (32). In our model of B. thailandensis pneumonia, we did not identify any impairment in neutrophil recruitment to the alveolar space or any differences in neutrophil chemoattractant KC and MIP-2 levels in the lung in the absence of MyD88. The mechanism underlying the MyD88-dependent mortality in B. thailandensis infection therefore remains unclear. We have observed a comparable essential dependence on MyD88 in our studies of inhaled Pseudomonas aeruginosa, another gram-negative, flagellated pathogen (23). This contrasts with inhaled Staphylococcus aureus infection, which is contained and cleared by the murine host independently of MyD88. However, the absence of individual MyD88-dependent TLRs (apart from the modest phenotype observed in TLR4−/− mice) does not explain the MyD88−/− phenotype in pneumonic B. thailandensis infection. Furthermore, the slight survival advantage in the absence of TLR2 is opposite of the effect of MyD88 deficiency. This suggests a possible role for TLR9 in host defense against B. thailandensis infection. TLR9 recognizes CpG DNA and also signals via MyD88 (17). Preliminary studies of TLR9 in B. pseudomallei infection by others do not support this speculation (32), but pulmonary host defense in infection with gram-negative pathogens L. pneumophila or Klebsiella pneumoniae is partially TLR9 dependent (3, 4). Our observations also point to likely critical redundancy in multiple coordinated TLR signaling pathways, and a better understanding of these mechanisms may require systematic study of combined TLR gene deletions. MyD88 serves as an adaptor molecule for IL-1- and IL-18-dependent signaling pathways, so the pronounced phenotype may alternatively be explained by a dependence on these receptors. In support of this hypothesis, caspase-1, a component of the host inflammasome that processes IL-1β and IL-18 production, promotes resistance to B. pseudomallei infection (6).
Although MyD88 is essential for bacterial containment and host survival in vivo, and for TNF-α production by macrophages, the host inflammatory response in MyD88−/− mice at 24 h is largely the same as those in WT and TLR-deficient mice. This is in part explained by the greater bacterial burdens in the MyD88−/− mice, but is notable because of the presence of substantial MyD88-independent inflammation. The sole mediator that was significantly lower in the lungs of MyD88−/− mice at 24 h was MCP-1, a C-C chemokine produced by a variety of cell types that governs migration and infiltration of monocytes, memory T lymphocytes, and NK cells (9). MCP-1 induction may be MyD88 dependent or independent in different experimental systems (20, 22). We did not observe a reduction in bronchoalveolar macrophage recruitment at this time point, however. More marked pulmonary and systemic inflammation is apparent in MyD88−/− mice at 72 h when bacterial loads have increased further. Substantial pulmonary inflammation has also been reported following intranasal B. pseudomallei infection in the absence of MyD88 (32), yet we have observed abrogated inflammation in the lungs of highly susceptible MyD88−/− mice infected with P. aeruginosa (23). Thus, non-MyD88-mediated pathways of host defense seem to be particularly relevant in airborne Burkholderia infection and may involve cell types other than macrophages.
In conclusion, our studies add to the growing literature about B. thailandensis, specifically demonstrating the distinct and complex roles of TLR2, TLR4, TLR5, and adaptor molecule MyD88 in host defense against this organism. Many of our findings with B. thailandensis mirror published reports of virulent B. pseudomallei, confirming the utility of B. thailandensis as a surrogate research organism for B. pseudomallei in elucidating the host-pathogen interaction in melioidosis.
Acknowledgments
This work was supported by NIH grants U54 AI057141 (T.E.W. and S.J.S.), K08 HL094759 (T.E.W.), and R01 AI061464 (T.R.H.) and by a Parker B. Francis Fellowship for Pulmonary Research (T.E.W.).
Malinka Jansson-Hutson and Ravi Iyer provided expert technical assistance. Christopher Wilson and Alan Aderem provided the TLR-deficient mice.
We declare that we have no conflicts of interest.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 21 September 2009.
REFERENCES
- 1.Akamine, M., F. Higa, N. Arakaki, K. Kawakami, K. Takeda, S. Akira, and A. Saito. 2005. Differential roles of Toll-like receptors 2 and 4 in in vitro responses of macrophages to Legionella pneumophila. Infect. Immun. 73:352-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer, K. A., and C. R. Roy. 2006. MyD88-dependent responses involving Toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infect. Immun. 74:3325-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhan, U., N. W. Lukacs, J. J. Osterholzer, M. W. Newstead, X. Zeng, T. A. Moore, T. R. McMillan, A. M. Krieg, S. Akira, and T. J. Standiford. 2007. TLR9 is required for protective innate immunity in gram-negative bacterial pneumonia: role of dendritic cells. J. Immunol. 179:3937-3946. [DOI] [PubMed] [Google Scholar]
- 4.Bhan, U., G. Trujillo, K. Lyn-Kew, M. W. Newstead, X. Zeng, C. M. Hogaboam, A. M. Krieg, and T. J. Standiford. 2008. Toll-like receptor 9 regulates the lung macrophage phenotype and host immunity in murine pneumonia caused by Legionella pneumophila. Infect. Immun. 76:2895-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossi, P., A. Tegnell, A. Baka, F. Van Loock, J. Hendriks, A. Werner, H. Maidhof, and G. Gouvras. 2004. Bichat guidelines for the clinical management of glanders and melioidosis and bioterrorism-related glanders and melioidosis. Euro Surveill. 9:E17-E18. [PubMed] [Google Scholar]
- 6.Breitbach, K., G. W. Sun, J. Kohler, K. Eske, P. Wongprompitak, G. Tan, Y. Liu, Y. H. Gan, and I. Steinmetz. 2009. Caspase-1 mediates resistance in murine melioidosis. Infect. Immun. 77:1589-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua, K. L., Y. Y. Chan, and Y. H. Gan. 2003. Flagella are virulence determinants of Burkholderia pseudomallei. Infect. Immun. 71:1622-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de C. Ventura, G. M., R. Le Goffic, V. Balloy, M. C. Plotkowski, M. Chignard, and M. Si-Tahar. 2008. TLR 5, but neither TLR2 nor TLR4, is involved in lung epithelial cell response to Burkholderia cenocepacia. FEMS Immunol. Med. Microbiol. 54:37-44. [DOI] [PubMed] [Google Scholar]
- 9.Deshmane, S. L., S. Kremlev, S. Amini, and B. E. Sawaya. 2009. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29:313-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerold, G., A. Zychlinsky, and J. L. de Diego. 2007. What is the role of Toll-like receptors in bacterial infections? Semin. Immunol. 19:41-47. [DOI] [PubMed] [Google Scholar]
- 11.Girard, R., T. Pedron, S. Uematsu, V. Balloy, M. Chignard, S. Akira, and R. Chaby. 2003. Lipopolysaccharides from Legionella and Rhizobium stimulate mouse bone marrow granulocytes via Toll-like receptor 2. J. Cell Sci. 116:293-302. [DOI] [PubMed] [Google Scholar]
- 12.Hawn, T. R., W. R. Berrington, I. A. Smith, S. Uematsu, S. Akira, A. Aderem, K. D. Smith, and S. J. Skerrett. 2007. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J. Immunol. 179:6981-6987. [DOI] [PubMed] [Google Scholar]
- 13.Hawn, T. R., E. A. Misch, S. J. Dunstan, G. E. Thwaites, N. T. Lan, H. T. Quy, T. T. Chau, S. Rodrigues, A. Nachman, M. Janer, T. T. Hien, J. J. Farrar, and A. Aderem. 2007. A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur. J. Immunol. 37:2280-2289. [DOI] [PubMed] [Google Scholar]
- 14.Hawn, T. R., A. Verbon, K. D. Lettinga, L. P. Zhao, S. S. Li, R. J. Laws, S. J. Skerrett, B. Beutler, L. Schroeder, A. Nachman, A. Ozinsky, K. D. Smith, and A. Aderem. 2003. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J. Exp. Med. 198:1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hii, C. S., G. W. Sun, J. W. Goh, J. Lu, M. P. Stevens, and Y. H. Gan. 2008. Interleukin-8 induction by Burkholderia pseudomallei can occur without Toll-like receptor signaling but requires a functional type III secretion system. J. Infect. Dis. 197:1537-1547. [DOI] [PubMed] [Google Scholar]
- 16.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115-122. [DOI] [PubMed] [Google Scholar]
- 17.Kawai, T., and S. Akira. 2006. TLR signaling. Cell Death Differ. 13:816-825. [DOI] [PubMed] [Google Scholar]
- 18.Khor, C. C., S. J. Chapman, F. O. Vannberg, A. Dunne, C. Murphy, E. Y. Ling, A. J. Frodsham, A. J. Walley, O. Kyrieleis, A. Khan, C. Aucan, S. Segal, C. E. Moore, K. Knox, S. J. Campbell, C. Lienhardt, A. Scott, P. Aaby, O. Y. Sow, R. T. Grignani, J. Sillah, G. Sirugo, N. Peshu, T. N. Williams, K. Maitland, R. J. Davies, D. P. Kwiatkowski, N. P. Day, D. Yala, D. W. Crook, K. Marsh, J. A. Berkley, L. A. O'Neill, and A. V. Hill. 2007. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat. Genet. 39:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korbel, D. S., B. E. Schneider, and U. E. Schaible. 2008. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 10:995-1004. [DOI] [PubMed] [Google Scholar]
- 20.Moon, S. K., J. I. Woo, H. Y. Lee, R. Park, J. Shimada, H. Pan, R. Gellibolian, and D. J. Lim. 2007. Toll-like receptor 2-dependent NF-κB activation is involved in nontypeable Haemophilus influenzae-induced monocyte chemotactic protein 1 up-regulation in the spiral ligament fibrocytes of the inner ear. Infect. Immun. 75:3361-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salomão, R., P. S. Martins, M. K. Brunialti, L. Fernandes Mda, L. S. Martos, M. E. Mendes, N. E. Gomes, and O. Rigato. 2008. TLR signaling pathway in patients with sepsis. Shock 30(Suppl 1):73-77. [DOI] [PubMed] [Google Scholar]
- 22.Serbina, N. V., W. Kuziel, R. Flavell, S. Akira, B. Rollins, and E. G. Pamer. 2003. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity 19:891-901. [DOI] [PubMed] [Google Scholar]
- 23.Skerrett, S. J., H. D. Liggitt, A. M. Hajjar, and C. B. Wilson. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 172:3377-3381. [DOI] [PubMed] [Google Scholar]
- 24.Smith, M. D., B. J. Angus, V. Wuthiekanun, and N. J. White. 1997. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect. Immun. 65:4319-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 26.Uematsu, S., M. H. Jang, N. Chevrier, Z. Guo, Y. Kumagai, M. Yamamoto, H. Kato, N. Sougawa, H. Matsui, H. Kuwata, H. Hemmi, C. Coban, T. Kawai, K. J. Ishii, O. Takeuchi, M. Miyasaka, K. Takeda, and S. Akira. 2006. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 7:868-874. [DOI] [PubMed] [Google Scholar]
- 27.West, T. E., R. K. Ernst, M. J. Jansson-Hutson, and S. J. Skerrett. 2008. Activation of Toll-like receptors by Burkholderia pseudomallei. BMC Immunol. 9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West, T. E., C. W. Frevert, H. D. Liggitt, and S. J. Skerrett. 2008. Inhalation of Burkholderia thailandensis results in lethal necrotizing pneumonia in mice: a surrogate model for pneumonic melioidosis. Trans. R. Soc. Trop. Med. Hyg. 102(Suppl. 1):S119-S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiersinga, W. J., A. F. de Vos, R. de Beer, C. W. Wieland, J. J. Roelofs, D. E. Woods, and T. van der Poll. 2008. Inflammation patterns induced by different Burkholderia species in mice. Cell. Microbiol. 10:81-87. [DOI] [PubMed] [Google Scholar]
- 30.Wiersinga, W. J., T. van der Poll, N. J. White, N. P. Day, and S. J. Peacock. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 4:272-282. [DOI] [PubMed] [Google Scholar]
- 31.Wiersinga, W. J., C. W. Wieland, M. C. Dessing, N. Chantratita, A. C. Cheng, D. Limmathurotsakul, W. Chierakul, M. Leendertse, S. Florquin, A. F. de Vos, N. White, A. M. Dondorp, N. P. Day, S. J. Peacock, and T. van der Poll. 2007. Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (melioidosis). PLoS Med. 4:e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiersinga, W. J., C. W. Wieland, J. J. Roelofs, and T. van der Poll. 2008. MyD88 dependent signaling contributes to protective host defense against Burkholderia pseudomallei. PLoS ONE 3:e3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu, Y., H. S. Kim, H. H. Chua, C. H. Lin, S. H. Sim, D. Lin, A. Derr, R. Engels, D. DeShazer, B. Birren, W. C. Nierman, and P. Tan. 2006. Genomic patterns of pathogen evolution revealed by comparison of Burkholderia pseudomallei, the causative agent of melioidosis, to avirulent Burkholderia thailandensis. BMC Microbiol. 6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]